Abstract
Nuclear lipid metabolism is implicated in various processes, including transcription, splicing, and DNA repair. Sphingolipids play roles in numerous cellular functions, and an emerging body of literature has identified roles for these lipid mediators in distinct nuclear processes. Different sphingolipid species are localized in various subnuclear domains, including chromatin, the nuclear matrix, and the nuclear envelope, where sphingolipids exert specific regulatory and structural functions. Sphingomyelin, the most abundant nuclear sphingolipid, plays both structural and regulatory roles in chromatin assembly and dynamics in addition to being an integral component of the nuclear matrix. Sphingosine-1-phosphate modulates histone acetylation, sphingosine is a ligand for steroidogenic factor 1, and nuclear accumulation of ceramide has been implicated in apoptosis. Finally, nuclear membrane–associated ganglioside GM1 plays a pivotal role in Ca2+ homeostasis. This review highlights research on the factors that control nuclear sphingolipid metabolism and summarizes the roles of these lipids in various nuclear processes.
Keywords: sphingomyelin, ceramide, sphingosine-1-phosphate, ganglioside, nucleus
INTRODUCTION
The nucleus is an organelle with a high capacity for lipid metabolism (1). In recent years, many studies have established that nuclear lipids play distinct roles in many cellular processes, including DNA replication, RNA processing, chromatin structure, and Ca2+ homeostasis (reviewed in References 2-5). Phosphatidylinositol phosphates (PIPs), the most extensively characterized nuclear lipids, have pivotal roles in chromatin remodeling, gene transcription, and mRNA export (6-8). Most lipids are localized to the nuclear envelope (NE), where in addition to providing structural support they participate in multiple signaling cascades. However, bioactive lipids are localized in other nuclear compartments, including chromatin (9-11) and the nuclear matrix (12). Significantly, the concentration of nuclear lipids can be dynamically altered by metabolic flux in response to signaling cascades that are often uncoupled from cytosolic processes. Similarly, extracellular stimuli can elicit lipid metabolism and signaling exclusively in the nucleus. For example, insulin growth factor 1 (IGF-1) induces the phosphorylation and activation of nuclear phospholipase C β1 (PLCβ1) (13), which consequently results in nuclear diacylglycerol (DAG) accumulation with a corresponding decrease in phosphatidylinositol biphosphate (14, 15). Furthermore, accumulation of nuclear DAG in response to IGF-1 stimulation promotes protein kinase C (PKC) nuclear translocation (15-17). Activation of this signaling pathway modulates various nuclear processes, including gene expression and cell proliferation (4).
In addition to PIPs, other classes of phospholipids, including phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine, also have varied nuclear functions (5, 18-24). Roles for diacylglycerol kinases (DGKs), a family of enzymes that convert DAG to phosphatidic acid (PA), in varied nuclear processes are well documented (25-28). For example, nuclear DGK-ζ expression regulates A172 cell growth by decreasing DAG concentrations (29). DGK-ζ is localized in nuclear speckles (30) and is also activated by α-thrombin in IIC9 fibroblast nuclei (31) and by nerve growth factor in PC12 cells (32). We have shown that PA regulates steroidogenic gene transcription by serving as an agonist for the nuclear receptor steroidogenic factor 1 (SF-1) (33). SF-1 regulates the transcription of multiple genes in the endocrine system, including most genes that are required for steroid hormone biosynthesis and endocrine development (34, 35). DGK-ζ directly interacts with SF-1, and activation of the cAMP pathway stimulates DGK activity in the nucleus of H295R adrenocortical cells (33). Consistent with these findings establishing roles for DAG/PA in nuclear processes, lipins (proteins that have phosphatidate phosphatase activity and catalyze the formation of DAG in the glycerol-3-phosphate pathway) are also emerging as regulators of gene expression (36). Lipin-1 binds to peroxisome proliferator–activated receptor (PPAR)α and serves as a coactivator in the expression of genes involved in fatty acid uptake, mitochondrial function, and lipid metabolism (37). Finally, recent studies have identified cyclic PA as a PPARγ antagonist that binds to the receptor with nanomolar activity and inhibits the expression of PPARγ target genes and adipogenesis (38).
In addition to phospholipids, the nucleus is emerging as a hub for sphingolipid metabolism. Sphingolipids comprise a large family of phospholipids and glycolipids (Figure 1) that share a common sphingoid base backbone (Figure 2). These molecules participate in many signal transduction pathways (39-41). To date, various sphingolipid species have been identified in multiple nuclear compartments, including chromatin, NE, and nuclear matrix (1, 42-52). In this review, we summarize studies that have identified a role for this class of lipids in regulating nuclear processes.
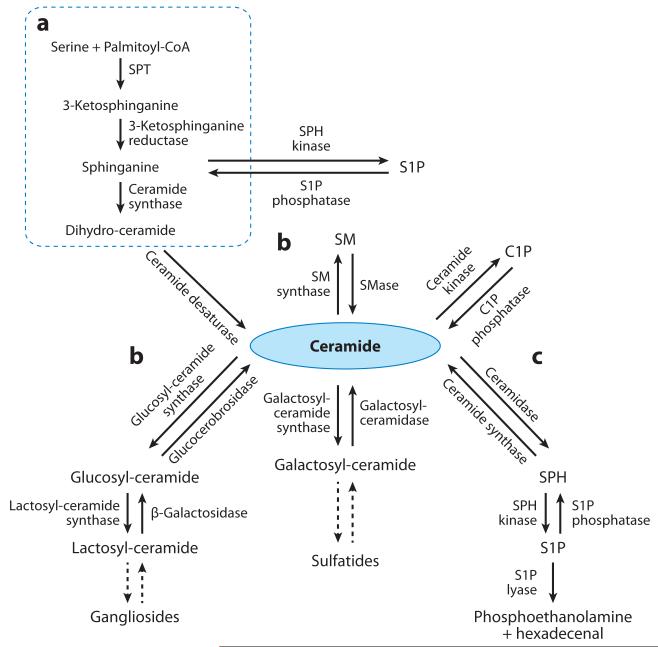
Figure 1.
The sphingolipid metabolic pathway. De novo biosynthesis begins with the condensation of serine and palmitoyl-CoA and various fatty acyl-CoAs. Ceramide can be generated through (a) de novo biosynthesis, (b) degradation of sphingomyelin (SM) or glucosyl-ceramides and galactosyl-ceramides, or (c) acylation of sphingosine (SPH). Ceramide can be phosphorylated into ceramide-1-phosphate (C1P) or hydrolyzed to form SPH, which is phosphorylated into sphingosine-1-phosphate (S1P). S1P can be either dephosphorylated to form SPH or irreversibly cleaved into phosphoethanolamine and hexadecenal. SPT denotes serine palmitoyltransferase.
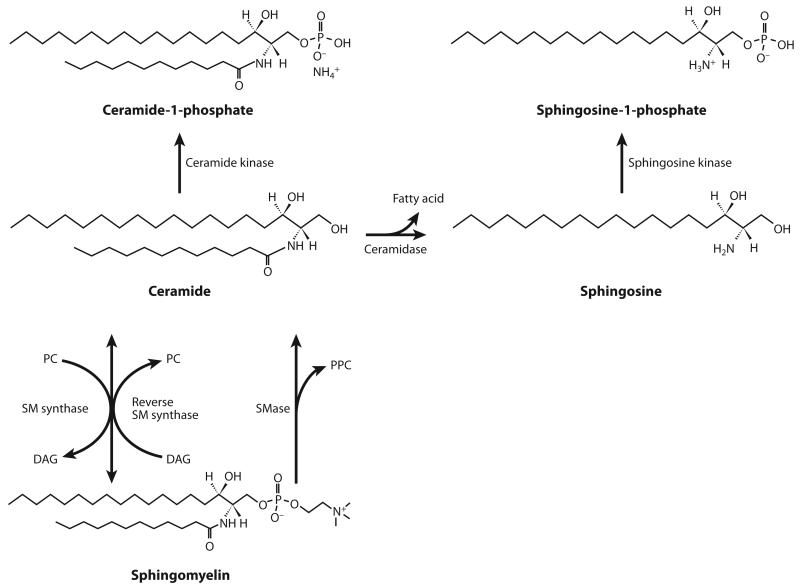
Figure 2.
Nuclear sphingomyelin (SM) metabolism. SM synthase catalyzes the formation of SM from ceramide and phosphatidylcholine (PC). SM can be degraded by sphingomyelinase (SMase), which generates phosphorylcholine (PPC) as a by-product, or by reverse SM synthase, which catalyzes the transfer of the PPC group in SM to diacylglycerol (DAG). Ceramide formed by SM hydrolysis can be phosphorylated into ceramide-1-phosphate or hydrolyzed into sphingosine, which can then be converted into its phosphorylated form, sphingosine-1-phosphate.
NUCLEUS ORGANIZATION AND ENDONUCLEAR DOMAINS
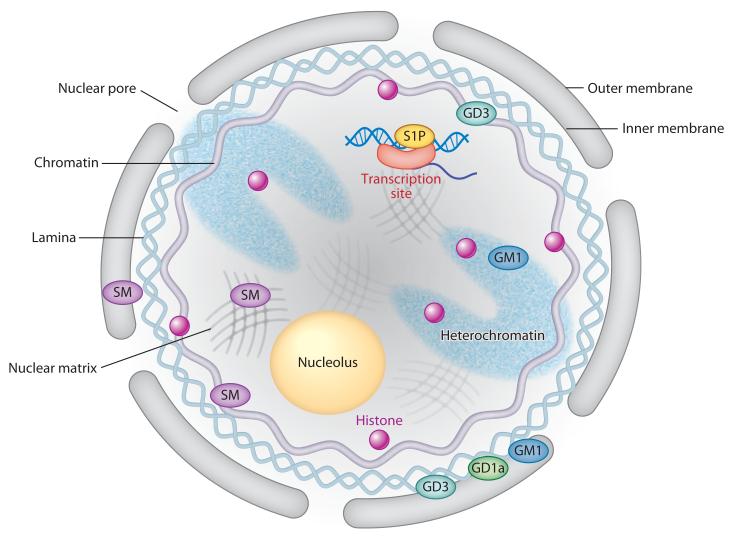
The nucleus is a well-organized substructure with a dynamic framework (53). It is composed of a well-defined NE that encapsulates several endonuclear domains, including the nuclear matrix, chromatin, and nucleolus. The NE is a bilayer whose outer and inner leaflets display unique lipid compositions. Although a detailed comparison of the relative distribution of lipid species between the two leaflets of the nuclear membrane has not been reported, cholesterol has been shown to reside in the outer membrane but not in the inner membrane (54), whereas the gangliosides GM1 and GD1a were detected in both (55). The outer membrane is continuous with the endoplasmic reticulum (ER) and thus shares certain lipidomic properties. Conversely, the inner membrane is closely associated with the nuclear lamina and has distinct lipid characteristics (Figure 3) (53).
Figure 3.
The localization of different sphingolipid species in subnuclear domains. The outer membrane is continuous with the endoplasmic reticulum, whereas the inner membrane is associated with the nuclear lamina. The nuclear pore allows passive flow of small molecules between the cytosol and the nucleoplasm. Abbreviations: S1P, sphingosine-1-phosphate; SM, sphingomyelin.
Like plasma membranes, nuclear membranes have been suggested to contain many types of receptors, including inositol 1,4,5-triphosphate, PPAR, and retinoic acid (RA) receptors (56, 57). Some agonists activate signaling exclusively through nuclear membrane–localized receptors. RA, for example, activates phospholipase A2 (PLA2), PLC, and phospholipase D (PLD) only in the nucleus (58-60). Additionally, compelling new evidence suggests that nuclear membrane–associated enzymes have physicochemical properties that are different from those of their plasma membrane and/or cytosolic counterparts. For example, the kinetic parameters of nuclear PLC differ from those of cytoplasmic or plasma membrane–associated PLC (61).
The nuclear matrix is often viewed as the basic organizing structure of the nucleus that is responsible for maintaining nuclear shape. However, the nuclear matrix is also the site at which many processes, including DNA replication, gene transcription, and protein phosphorylation, occur (62-64). Many enzymes linked to PIP metabolism associate with the nuclear matrix (65), suggesting that the matrix is actively involved in nuclear lipid signaling cascades. Chromatin is closely associated with the nuclear matrix and exhibits a dynamic structure that is actively regulated by multiple interconnected mechanisms, including DNA methylation and histone modification (66, 67). Heterochromatin regions, which are transcriptionally inactive but contain many specific nuclear proteins that regulate gene transcription (68), are similarly organized by the nuclear matrix (Figure 3).
SPHINGOLIPID BIOSYNTHESIS AND METABOLISM
Sphingolipids are synthesized de novo from serine and palmitoyl-CoA to form a sphingoid base, which is further N-acylated with various fatty acyl-CoAs to make N-acylsphinganine (dihydroce-ramide) and is sequentially desaturated to form ceramide (Figure 1). Ceramide can be metabolized into more complex sphingolipids by the incorporation of O-linked head groups such as phosphoryl-choline or carbohydrate moieties to form sphingomyelin (SM) (Figure 2) or glycosphingolipids (Figure 4), respectively. Alternatively, ceramide can be phosphorylated into ceramide-1-phosphate (C1P) by ceramide kinase (CERK) or hydrolyzed into sphingosine (SPH) by ceramidases (Figure 2). Sphingosine kinases (SKs) then phosphorylate SPH to form sphingosine-1-phosphate (S1P). Irreversible sphingolipid degradation occurs by the action of S1P lyase, which cleaves S1P into phosphoethanolamine and hexadecenal. Aside from being de novo synthesized, ceramide can also be formed by the hydrolysis of SM and glycosphingolipids through the salvage pathway or by the N-acylation of SPH through the action of ceramide synthases (Figure 1).
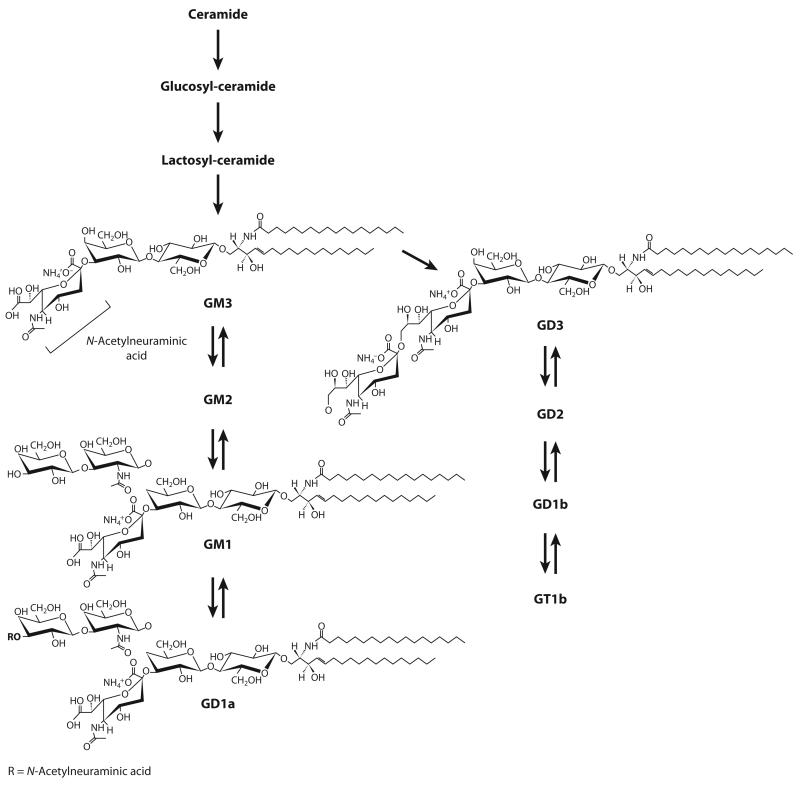
Figure 4.
Ganglioside structure and biosynthetic pathway. Lactosyl-ceramide, formed by the addition of two carbohydrate moieties (glucose and galactose) to the terminal OH group of ceramide, is the precursor of gangliosides. GM1 has one terminal N-acetylneuraminic acid (sialic acid) group. GD1a, which has two terminal sialic acid groups, is converted into GM1 by neuraminidase.
Cellular sphingolipid concentrations are tightly regulated by the actions of multiple enzymes that act to maintain sphingolipid homeostasis. Because several sphingolipid species have unique physiological functions, these enzymes are important not only to ensure optimal sphingolipid concentrations but also to regulate the capacity of these bioactive lipids to activate cell signaling. For example, because ceramidases control the ratio between ceramide and S1P (Figure 2), the activity of this family of hydrolases dictates whether a cell undergoes apoptosis or proliferates (69-71). Similarly, the activity of SK1 is strongly correlated with cell growth (72-75). Furthermore, sphingomyelinase (SMase), the enzyme that breaks the phosphodiester bond of SM to form ceramide (Figure 2), is activated by multiple apoptotic factors, including tumor necrosis factor α (TNFα) (76), ionizing radiation (77), and Fas ligand (78). Finally, because the length of the N-linked fatty acyl chain on ceramide is a key determinant of the cellular function of these molecules (53, 79-83), ceramide synthases play a central role in regulating the composition of sphingolipid pools (84).
Sphingolipid-metabolizing enzymes display distinct subcellular localizations and membrane topologies (85-89). Enzymes linked to de novo sphingolipid synthesis [e.g., serine palmitoyltransferase, ceramide synthase, dihydroceramide desaturase] are localized primarily in the ER lumen, whereas different isoforms of ceramidase and SMase are expressed at the plasma membrane, lysosomes, or mitochondria (90). Glycosphingolipids are synthesized by a series of enzymes residing in the Golgi apparatus. Due to the hydrophobic nature of most sphingolipid species, compart-mentalization of sphingolipid metabolism and subsequently signaling are important themes in sphingolipid biology (90). In this manner, ceramide localized at the plasma membrane, for example, participates in signaling pathways distinct from those of mitochondrial ceramide (91-96). Adding to this complexity, different isoforms of the same enzyme may have distinct substrate specificities (85, 97-102).
NUCLEAR SPHINGOLIPIDS
Biochemical, analytical, and microscopic techniques have been utilized to identify sphingolipid-metabolizing enzymes in nuclei and to quantify the concentrations of sphingolipid species (97). SM is the most prominent sphingolipid in nuclei; its concentration in nuclear matrix is three times higher than in chromatin (103). SM is a major component of chromatin (10), where it plays a role in DNA replication and chromatin architecture (43, 50, 104). The catabolism of SM gives rise to ceramide and subsequently to SPH, S1P, and C1P (Figure 2), all of which have specific nuclear functions. S1P, which has recognized roles in cell proliferation, migration, and differentiation (39), regulates gene transcription by specifically binding to histone deacetylases (HDACs) 1 and 2 and inhibiting their enzymatic activity (52). Gangliosides are also prominent in the outer and inner membranes of the NE. The ganglioside GM1 (Figure 4), in particular, has been extensively studied for its key role in nuclear Ca2+ homeostasis (105). Collectively, studies of nuclear sphingolipids not only illustrate the multifaceted regulatory capabilities of these lipid mediators, which in most cases differ from their cytoplasmic functions, but also highlight the importance of location (i.e., chromatin versus NE versus nuclear matrix) in determining their nuclear functions.
The localization of sphingolipid enzymes in various subnuclear compartments facilitates the dynamic nuclear metabolism of sphingolipids. To date, enzymes involved in SM, ceramide, SPH, and glycosphingolipid metabolism have been identified in nuclear extracts isolated from different cell types (42-44, 46, 48, 106-108). SM was the first sphingolipid identified as a component of the nuclear matrix (109). However, the likelihood of nuclear sphingolipid metabolism became apparent only after enzymes that metabolize sphingolipids were found in the nucleus (12, 45, 110). In fact, the nuclear levels of distinct sphingolipid subspecies change under different cellular physiological states (50, 78, 111, 112), illustrating the intrinsic capacity for dynamic nuclear sphin-golipid metabolism. For example, chromatin-associated SM synthase and SMase control dynamic oscillations in SM concentrations during the cell cycle (112-114). Furthermore, ganglioside-metabolizing enzymes, including neuraminidase (sialidase) (115) and GM2/GD2 synthase (116), play pivotal roles in regulating GM1 levels in the NE.
Sphingomyelin
SM was first identified in the NE of hepatocytes (117, 118). It was subsequently shown to be present in chromatin (10) and the nuclear matrix (12) and to be associated with double-stranded RNA (dsRNA) (Figure 3) (49). As discussed above, SM is the most abundant sphingolipid in the nucleus and the major phospholipid associated with chromatin (10), although it is enriched three times higher in the nuclear matrix (103). Studies have demonstrated that nuclear SM levels are dynamic and oscillate in response to different cellular states (43, 45, 50, 106, 112, 119). Additionally, distinct cellular cues differentially affect SM concentration in distinct subnuclear compartments (e.g., nuclear matrix versus chromatin) (103). For example, SM levels in the nuclear matrix increase at the beginning of S phase of the cell cycle during hepatic regeneration (103), whereas chromatin-associated SM decreases during the same period (119). These studies not only highlight the specificity of SM metabolism in different nuclear domains but also suggest that the amount of SM associated with DNA is proportional to the state of chromatin condensation (Table 1).
Table 1.
Summary of reported nuclear functions for different sphingolipid species and their endonuclear localizationa
| Sphingolipid | Nuclear localization | Function |
|---|---|---|
| Sphingomyelin | Nuclear envelope Nuclear matrix Chromatin |
DNA synthesis Chromatin assembly Membrane structure RNA stability |
| Ceramide | ? | Apoptosis |
| Ceramide-1-phosphate | Perinuclear region | cPLA2α translocation |
| Sphingosine | ? | SF-1 antagonist ligand |
| Sphingosine-1-phosphate | Chromatin | Histone acetylation |
| GM1 | Inner nuclear membrane | Ca2+ homeostasis |
| GD3 | ? | Histone H1 phosphorylation Apoptosis |
| GDla | Inner nuclear membrane | Reservoir for GM1 |
Question marks indicate that the specific endonuclear domain has not been determined. Abbreviations: cPLA2 α, α-type cytosolic phospholipase A2; SF-1, steroidogenic factor 1.
SMase, the enzyme that catalyzes the degradation of SM to form ceramide and phosphoryl-choline (Figure 2), has been detected in the nuclear matrix (42), the NE (43), and chromatin (112). SM synthase has also been identified in the NE and chromatin (113). These enzymes appear to have different physicochemical characteristics, depending on their intranuclear localization, and their activities change in response to different cellular cues, such as cell proliferation and apoptosis (50, 104, 107, 112). Chromatin-associated SM synthase differs in pH optimum and Km from the same isoform localized in the NE (113). Moreover, plasma membrane–localized SMase may be involved in axonal growth (120), whereas nuclear SMase activation is associated with apoptosis (46, 78). Different isoforms of the same enzyme can also reside in different intranuclear compartments, as is believed to be the case for neutral SMase. This ceramide-generating enzyme (Figure 2) was proposed to reside in the NE of rat liver cells and to translocate to the nuclear matrix during DNA synthesis (43). However, a subsequent study used biochemical and immunohistochemical approaches to demonstrate that SMase possesses a nuclear export signal and is enriched in the nuclear matrix (121). It is thus believed that distinct isoforms of SMase participate in each of these processes.
SM plays a role in stabilizing DNA during the cell cycle (112), and the dynamic changes in the amount of chromatin-associated SM are due to the opposing activities of SMase and SM synthase (112, 119). Concomitant activation of SMase and inhibition of SM synthase at the beginning of S phase may lead to a decrease in SM levels, which facilitates DNA unwinding (5). Increased SM synthase activity at the end of S phase then facilitates double-helix restoration after DNA synthesis ends (5). Notably, these changes in SM concentrations occur selectively in chromatin (104).
Interestingly, reverse SM synthase, an enzyme that catalyzes the reverse reaction as that of SM synthase (Figure 2), has also been identified in chromatin (114). Similar to SMase, reverse SM synthase catalyzes the degradation of SM but with the key difference that it catalyzes the transfer of the phosphorylcholine group from SM to DAG, forming ceramide and phosphatidyl-choline (Figure 2). Therefore, this enzyme not only promotes the accumulation of ceramide but also decreases DAG levels while increasing phosphatidylcholine. This is significant because the ceramide/DAG ratio is linked to cell proliferation and apoptosis. Although ceramide’s role in cell fate has been described predominantly in whole-cell studies (70), ceramide is a well-known mediator of apoptosis (122). Nonetheless, the induction of apoptosis in rat liver occurs through the selective accumulation of nuclear ceramide due to the activation of neutral SMase at the NE (46). In contrast, numerous studies have shown that an increase in nuclear DAG mediates the recruitment of various PKC isoforms into the nucleus (123, 124). PKC and DAG may be necessary for the transitions from G1 to S phase (125) and from G2 to M phase (126, 127) of the cell cycle. Although the identification of direct nuclear and cytoplasmic targets for ceramide is still an area of active investigation, by analogy to cytosolic signaling, it has been proposed that nuclear ceramide concentrations and PKC activity are also directly related (128).
Early studies pointed to an interaction between SM and RNA by observing a significant reduction in SM levels after nuclear digestion with RNase (129). This association was strengthened by studies identifying a complex containing RNA, proteins, SM, phosphatidylcholine, SM synthase, and neutral SMase from hepatocyte nuclei that were sequentially treated with Triton X-100 and a DNAse/RNAse cocktail (45). RNAse-resistant RNA became sensitive to enzymatic hydrolysis when it was pretreated with SMase, suggesting that SM protects RNA from degradation (15). Furthermore, because RNA digestion was temperature sensitive (i.e., higher temperatures yielded more undigested RNA that was hydrolyzed by RNAse), this RNA was assumed to be dsRNA (49). Although the precise role of dsRNA-bound SM is unclear, SM may play a role in RNA maturation by associating with newly synthesized RNA and protecting it from enzymatic digestion prior to its export from the nucleus (5). SM may also stabilize dsRNA by forming a bridge between the two strands (Table 1) (49).
Ceramide and Ceramide-1-Phosphate
As depicted in Figure 1, ceramide sits at the hub of the sphingolipid metabolic pathway because it not only serves as the building block for more complex sphingolipids (e.g., SM and glycosphin-golipids) but is also an intermediate metabolite for either sphingolipid degradation or the generation of phosphorylated species such as S1P and C1P. The presence of SMase and SM synthase in the nucleus suggests that ceramide is actively produced and consumed. Ceramidase activity in liver nuclear membranes was also reported (110), suggesting that the ceramide generated can be further metabolized. The nuclear localization of these enzymes suggests the existence of a nuclear SM cycle, although the regulation of ceramide concentrations in the nucleus is poorly understood. Akin to nuclear SM, spatially localized ceramide metabolism in different subnuclear domains may participate in distinct nuclear processes.
Nuclear ceramide accumulation has been associated with apoptosis in rat hepatocytes after hepatic vein ligation (46). [Portal vein ligation is a procedure that involves the ligation of hepatic lobes, which promotes atrophy of ligated lobes while inducing hypertrophy of nonligated lobes. This procedure can be used as a model of apoptosis of the ligated lobes, whereas hypertrophic hepatocytes can be studied for cell proliferation mechanisms (46, 47).] Increased SMase and ceramidase activity occurs after portal vein branch ligation, which correlates with DNA fragmentation and cell death (Table 1) (46). Additionally, SM degradation in chromatin at the beginning of S phase during hepatic regeneration suggests an accumulation of ceramide in this subnuclear domain (104). In Jurkat T cells, Fas ligand simultaneously stimulates neutral SMase and inhibits SM synthase activities in a caspase-3-dependent manner, which results in the time- and dose-dependent accumulation of nuclear ceramide (78). Although the precise molecular mechanisms involved have yet to be defined, these studies suggest a role for nuclear ceramide in cell proliferation. Recently, ceramide accumulation in rat hepatic nuclei as a result of neutral SMase activation was reported to occur in response to a high-fat diet (130). The basis for the accumulation of nuclear ceramide and the site at which this occurs are unknown. However, given that whole-cell studies have established a role for ceramide in insulin resistance, nuclear ceramide metabolism may have implications in insulin signaling (131, 132). Ceramide concentrations also increase in RAW 264.7 macrophages that have been activated by the Toll-like receptor 4–specific ligand Kdo2-lipid A (133). Finally, ceramide regulates nuclear protein import in smooth muscle cells by inducing p38 mitogen-activated protein kinase activation and the subsequent relocalization of two nuclear transport proteins, importin A and cellular apoptosis susceptibility gene (123). The inhibition of nuclear import by exogenously supplemented ceramide resulted in diminished expression of proliferation protein markers, including cyclin A and proliferating cell nuclear antigen, and in reduced proliferative capacity (123).
Rovina et al. (62) recently identified nuclear export and import signals in the primary sequence of CERK. This finding suggests that nuclear ceramide can be further metabolized into C1P, which may harbor unique nuclear functions. Although these functions are yet to be reported, there is compelling evidence for a regulatory role for C1P in α-type cytosolic phospholipase A2 (cPLA2α) activity and arachidonic acid release in many cell types (64). C1P binds to the Ca2+-binding regions in the C2 domain of cPLA2α and promotes its translocation to the perinuclear region of cells (134). Moreover, CERK activity is required for IL-1β-induced prostaglandin production (135), as does the interaction between C1P and cPLA2α (136). Nuclear C1P may represent a yet-to-be-established, key mediator of the inflammatory response.
Sphingosine and Sphingosine-1-Phosphate
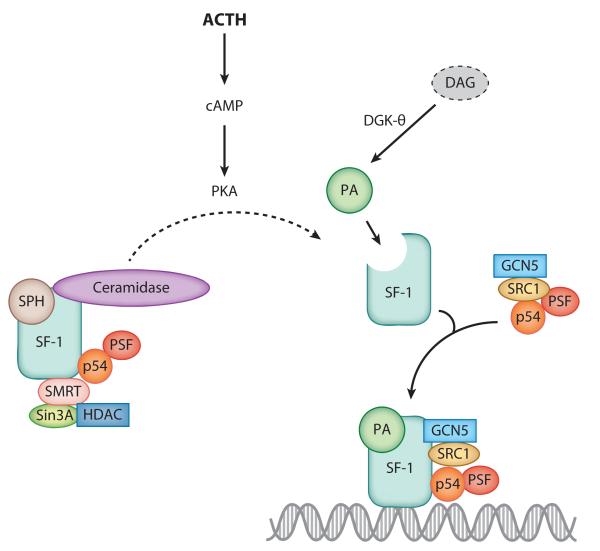
The nuclear localization of ceramidase (46) enables the local hydrolysis of ceramide to SPH. We demonstrated that SPH plays an important role in steroid hormone production in the human adrenal cortex by serving as a ligand for SF-1 (137). SPH is bound to SF-1 under basal conditions and antagonizes receptor function (137). Activation of the cAMP signaling pathway, the major regulatory cascade in adrenal steroidogenesis (138, 139), reduces the amount of receptor-bound SPH and enables the transcription of genes involved in the conversion of cholesterol to steroid hormones (Figure 5) (137). Significantly, we have found that acid ceramidase directly binds to SF-1 in the nucleus of H295R adrenocortical cells (N.C. Lucki & M.B. Sewer, unpublished observations). This suggests that ligand formation and delivery are facilitated by a direct interaction between enzyme and receptor and provides support for a novel coregulatory role of this enzyme in controlling gene expression. We also found that cAMP rapidly decreases nuclear concentrations of ceramide and stimulates the nuclear localization of SK1 while concomitantly increasing SPH and S1P levels (D. Li & M.B. Sewer, unpublished observations). Collectively, these studies implicate signal-induced nuclear sphingolipid metabolism as a critical regulator of gene transcription.
Figure 5.
Model for the role of sphingosine (SPH) in controlling the transactivation potential of steroidogenic factor 1 (SF-1). Under basal conditions, SPH is bound to SF-1 and corepressory proteins. Adrenocorticotropin hormone (ACTH) signaling activates protein kinase A (PKA), which promotes the release of SPH from the receptor’s ligand-binding pocket. Concomitantly, activation of the ACTH/cAMP pathway increases nuclear diacylglycerol kinase (DGK) activity, leading to increased phosphatidic acid (PA) biosynthesis. PA binding to SF-1 activates the receptor, thereby facilitating the recruitment of coactivator proteins and enabling interaction with the promoters of target genes. Abbreviations: GCN5, general control of amino acid synthesis protein 5; HDAC, histone deacetylase; PSF, polypyrimidine tract–binding protein–associated splicing factor; Sin3A, Sin3 homolog A; SMRT, silencing mediator for retinoid of thyroid hormone receptors; SRC1, steroid receptor coactivator 1.
The amount of intracellular SPH is regulated not only by the action of ceramidases and ceramide synthases (Figure 1) but also by its phosphorylation to S1P by SK (Figure 2). The two isoforms of SK have distinct subcellular localizations and physiological functions (72, 140). SK1 is cytoplasmic and is associated primarily with cell proliferation and growth, whereas SK2 is mainly nuclear and is linked to apoptosis. Nuclear SK activity was first described in the NE and nucleoplasm of Swiss 3T3 cells (108). Platelet-derived growth factor was shown to upregulate nucleoplasmic SK activity that correlated with progression through S phase (108). This study provided an early indication that nuclear S1P production might be involved in cell cycle regulation. Of note, we found that cAMP promotes the phosphorylation and nuclear translocation of SK1 in H295R adrenocortical cells (D. Li & M.B. Sewer, unpublished observations). More recently, Hait et al. (52) reported that SK2 interacts with histone H3 in chromatin of MCF-7 breast cancer cells, establishing a role for endonuclear S1P in the epigenetic regulation of gene transcription (52). Expression of SK2 induced histone acetylation, which correlated with the formation of S1P and dihydro-S1P in the nuclei of these cells (Figure 3). Furthermore, the authors demonstrated that S1P and dihydro-S1P inhibited the activity of HDAC1 and -2 by binding to their active site (52). SK2 interacted with HDAC1 and HDAC2 and thus facilitated S1P transfer to the enzymes. Finally, SK2 associated with HDACs at the promoters of the cyclin-dependent kinase inhibitor p21 and c-fos genes, where it induced histone acetylation and gene transcription (Table 1) (52). These findings not only identified HDACs as nuclear targets of S1P but also uncovered a novel role for this multifaceted sphingolipid molecule as a regulator of histone posttranslational modification and global gene expression programs.
Gangliosides
Gangliosides are formed by a ceramide molecule linked to an oligosaccharide chain containing hexose and N-acetylneuraminic acid (sialic acid) groups (Figure 4). Many studies have established that gangliosides are intrinsic components of the nucleus and occur in both membranes of the NE (Figure 3). The first evidence for nuclear ganglioside localization came from subcellular fractionation studies of bovine mammary gland and rat liver cells (141, 142), in which nuclear ganglioside concentrations were found to be 10% of those of the plasma membrane (143). The major species identified were GM1 and GM3 in rat liver (142) and GM3, GD3, and GTb1 in bovine mammary gland cells (143). Subsequent studies in neuroblastoma and primary neuronal cells demonstrated by cytochemical analysis with cholera toxin B subunit linked to horseradish peroxidase that GM1 occurs at high concentrations in the NE of differentiating, but not quiescent, cells (44, 144). Similarly, GM1, GM3, and c-series gangliosides were observed in mature rat brain (145). However, developing rat brain comprised relatively more GM3 and GD3 (145), indicating that the synthesis of distinct ganglioside subspecies in the nucleus is differentially regulated during development.
Nuclear quantification of gangliosides from cultured neuro2A cells by two successive high-density sucrose gradient purifications found that GM1 and GD1a are the predominant gangliosides found in nuclei and are localized primarily in the NE (44). A more detailed characterization of the nuclear localization of gangliosides became possible by subjecting the isolated nuclei to mild treatment with a sodium citrate solution, which allows the separation of the inner and outer nuclear membranes (146, 147). The use of this technique revealed that GM1 and GD1a are present in both membranes of the NE in primary neurons (48). Interestingly, GD1a is converted to GM1 by neuraminidase (Figure 4), a membrane-associated enzyme that is present in both membranes of the NE (148, 149). Nuclear GD1a may serve as a storage reserve precursor for GM1.
The characterization of gangliosides in other endonuclear domains is relatively unexplored. However, some studies have determined the presence of ganglioside subspecies in heterochromatin and chromatin. GM1 may associate with heterochromatin from mouse epithelial cells (150), whereas immunocytochemical studies showed that GD3 colocalizes with chromatin in rat cortical neurons subjected to β-amyloid peptide (151). GD3 accumulation is concomitant with reduced levels of SM and increased activity of 2,8-sialyltransferase (GD3 synthase) (151), the enzyme that forms GD3 from GM3 (Figure 4). GD3 synthase knockdown by RNA interference prevented β-amyloid peptide–induced entry into S phase and apoptosis (151), supporting a role for GD3 in cell cycle activation and cell death. In addition, translocation of GD3 from the cytosol into the nucleus was observed in HUT-78 T-lymphoma cells (51). This translocation strongly correlated with histone H1 phosphorylation after activation of apoptosis (51), which suggests that GD3 may have an epigenetic role in the transcriptional regulation of specific genes (Table 1).
GM1 and Ca2+ homeostasis
GM1 associated with the inner NE plays a prominent role in nuclear Ca2+ homeostasis. This nuclear function of GM1 emerged after researchers discovered that this ganglioside is tightly associated with a Na+/Ca2+ exchanger (NCX) (48). NCX mediates countertransport of three Na+ ions for one Ca2+ ion against a Ca2+ gradient. On the basis of the topology of plasma membrane–associated NCX (152), it has been proposed that in the NE, negatively charged sialic acid groups on the GM1 oligosaccharide chain interact with positively charged amino acid residues on the polypeptide loop between transmembrane segments 5 and 6 of NCX at the nucleoplasm (153, 154). This interaction is thought to facilitate Ca2+ transport from the nucleoplasm (low [Ca2+]) to the NE lumen (high [Ca2+]).
Xie et al. (48) demonstrated the association between GM1 and NCX by immunoprecipitation of NE extracts with an antibody against NCX followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blotting analysis of GM1. These investigators further determined by immunoblot analysis of the separate NE membranes that this association occurs specifically at the inner membrane of the NE (48). Interestingly, plasma membrane NCX showed no association with GM1, suggesting a difference between plasma membrane–localized NCX and NE-localized NCX (48). Given that splice variants of this exchanger have been described (155, 156), differential association between nuclear and cytoplasmic NCX with GM1 may be due to alternatively spliced NCX isoforms.
GM1 association with NCX potentiates Na+ and Ca2+ exchange between the nucleoplasm and the NE lumen. This theory was first demonstrated by Ca2+ uptake experiments with isolated nuclei (48) and more recently using genetically encoded chameleon Ca2+ sensors (157). Subsequent studies employed a comparison of NE/ER Ca2+ elevation in GM1-expressing NG108-15 and GM1-deficient NG-CR72 cells (154, 157), which showed significantly higher NE/ER Ca2+ elevation in cells containing GM1. Similar results were observed in C6 cells (158), which contain NCX/GM1 in the NE but not in the plasma membrane, whereas no NE/ER Ca2+ elevation was observed in NCX-deficient Jurkat T cells (153). Because the NE lumen is continuous with the ER, these data support a function for the NCX/GM1 complex as an alternate mechanism for transferring cytosolic Ca2+ to the ER. Studies using knockout (KO) mice engineered to lack GM2/GD2 synthase, which results in deficient synthesis of GM2, GD2, and GM1 (Figure 4), have demonstrated key regulatory roles for nuclear membrane–associated GM1 in Ca2+ homeostasis (159, 160). These KO mice develop late-onset neurological disease (161) and display deficient Ca2+ regulatory capabilities in their cerebellar granule neurons (162). Supplementation of neuronal cultures from GM2/GD2 synthase KO mice with exogenous GM1 restored a normal phenotype (116), supporting a role for this ganglioside in regulating Ca2+ homeostasis. Moreover, most cell types studied to date express nuclear NCX/GM1, which suggests that this complex is a ubiquitous mechanism for Ca2+ homeostasis employed by cells.
CONCLUSIONS AND FUTURE PERSPECTIVES
Nuclear sphingolipid metabolism is an area of research undergoing significant progress. Emerging new data are paving the way toward a more comprehensive understanding of the unique roles for these bioactive lipids in nuclear processes. Analogous to their cytosolic functions, distinct sphingolipid species have unique nuclear functions and act via temporally and spatially specific mechanisms. Future studies aimed at elucidating the contributions that sphingolipid-metabolizing enzymes play in nuclear processes and at quantifying the nuclear concentrations of different sphingolipid species under different cellular conditions will allow for a more thorough understanding of how nuclear lipid metabolism coordinates global changes in cell function.
SUMMARY POINTS.
Distinct subsets of sphingolipid-metabolizing enzymes catalyze sphingolipid turnover in varied subnuclear domains.
Sphingolipid concentrations at different intranuclear compartments fluctuate in response to diverse physiological cues. Some extracellular stimuli may affect nuclear sphingolipid turnover independently of cytosolic signaling.
Sphingomyelin plays structural and regulatory roles in chromatin architecture, DNA synthesis, and RNA stability.
Sphingosine regulates gene transcription by serving as a ligand for the nuclear receptor steroidogenic factor 1.
Sphingosine-1-phosphate plays an epigenetic role in gene expression by controlling hi-stone acetylation.
The GM1 ganglioside modulates nuclear Ca2+ homeostasis by forming a complex with a Na+/Ca2+ exchanger in the inner membrane of the nuclear envelope.
Nuclear matrix: a filamentous protein network in the nucleus
Cyclic phosphatidic acid (PA): a naturally occurring analog of lysophosphatidic acid, cyclic PA differs from phosphatidic acid in having a cyclic phosphate at the sn-2 and sn-3 positions of the glycerol carbons. This structure is critical for its biological activity (163)
Steroidogenic factor 1 (SF-1): a nuclear receptor that regulates the transcription of genes involved in steroid hormone biosynthesis and endocrine development
Sphingolipids: a family of glycolipids and phospholipids that are characterized by the presence of a common sphingoid base backbone
Ganglioside: a glycosphingolipid with one or more sialic acids (n-acetylneuraminic acid) linked to the carbohydrate chain
GM1: the major ganglioside subspecies in the nuclear envelope that binds to NCX. Regulates nuclear Ca2+ homeostasis
Heterochromatin: a tightly packed form of DNA that is usually associated with silenced gene regions
Sphingomyelin (SM): a sphingolipid with phosphocholine attached to the terminal OH group of ceramide
Glycosphingolipid: a sphingolipid with carbohydrate groups attached to the terminal OH group of ceramide
Salvage pathway: the regeneration of ceramide from the breakdown of complex sphingolipids
Sphingomyelin (SM) cycle: the activation of sphingomyelinase by extracellular stimuli that leads to sphingomyelin turnover to form ceramide
NCX: a Na+/Ca2+ exchanger localized at the plasma and nuclear membranes
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Ledeen RW, Wu G. Nuclear sphingolipids: metabolism and signaling. J. Lipid Res. 2008;49:1176–86. doi: 10.1194/jlr.R800009-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ledeen RW, Wu G. Sphingolipids of the nucleus and their role in nuclear signaling. Biochim. Biophys. Acta. 2006;1761:588–98. doi: 10.1016/j.bbalip.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Tamiya-Koizumi K. Nuclear lipid metabolism and signaling. J. Biochem. 2002;132:13–22. doi: 10.1093/oxfordjournals.jbchem.a003190. [DOI] [PubMed] [Google Scholar]
- 4.Barlow CA, Laishram RS, Anderson RA. Nuclear phosphoinositides: a signaling enigma wrapped in a compartmental conundrum. Trends Cell Biol. 2011;20:25–35. doi: 10.1016/j.tcb.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albi E, Viola Magni M. The role of intranuclear lipids. Biol. Cell. 2004;96:657–67. doi: 10.1016/j.biolcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343–55. doi: 10.1016/j.cell.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Sablin EP, Blind RD, Krylova IN, Ingraham JG, Cai F, et al. Structure of SF-1 bound by different phospholipids: evidence for regulatory ligands. Mol. Endocrinol. 2009;23:25–34. doi: 10.1210/me.2007-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irvine RF. Nuclear lipid signalling. Nat. Rev. Mol. Cell Biol. 2003;4:349–60. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- 9.Pliss A, Kuzmin AN, Kachynski AV, Prasad PN. Nonlinear optical imaging and Raman microspectrometry of the cell nucleus throughout the cell cycle. Biophys. J. 2010;99:3483–91. doi: 10.1016/j.bpj.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albi E, Mersel M, Leray C, Tomassoni ML, Viola-Magni MP. Rat liver chromatin phospholipids. Lipids. 1994;29:715–19. doi: 10.1007/BF02538916. [DOI] [PubMed] [Google Scholar]
- 11.Boronenkov IV, Loijens JC, Umeda M, Anderson RA. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol. Biol. Cell. 1998;9:3547–60. doi: 10.1091/mbc.9.12.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neitcheva T, Peeva D. Phospholipid composition, phospholipase A2 and sphingomyelinase activities in rat liver nuclear membrane and matrix. Int. J. Biochem. Cell Biol. 1995;27:995–1001. doi: 10.1016/1357-2725(95)00087-6. [DOI] [PubMed] [Google Scholar]
- 13.Xu A, Suh PG, Marmy-Conus N, Pearson RB, Seok OY, et al. Phosphorylation of nuclear phospholipase C β1 by extracellular signal-regulated kinase mediates the mitogenic action of insulin-like growth factor I. Mol. Cell. Biol. 2001;21:2981–90. doi: 10.1128/MCB.21.9.2981-2990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocco L, Martelli AM, Gilmour RS, Ognibene A, Manzoli FA, Irvine RF. Rapid changes in phospholipid metabolism in the nuclei of Swiss 3T3 cells induced by treatment of the cells with insulin-like growth factor I. Biochem. Biophys. Res. Commun. 1988;154:1266–72. doi: 10.1016/0006-291x(88)90276-8. [DOI] [PubMed] [Google Scholar]
- 15.Divecha N, Banfic H, Irvine RF. The polyphosphoinositide cycle exists in the nuclei of Swiss 3T3 cells under the control of a receptor (for IGF-I) in the plasma membrane, and stimulation of the cycle increases nuclear diacylglycerol and apparently induces translocation of protein kinase C to the nucleus. EMBO J. 1991;10:3207–14. doi: 10.1002/j.1460-2075.1991.tb04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martelli AM, Gilmour RS, Neri LM, Manzoli L, Corps AN, Cocco L. Mitogen-stimulated events in nuclei of Swiss 3T3 cells. Evidence for a direct link between changes of inositol lipids, protein kinase C requirement and the onset of DNA synthesis. FEBS Lett. 1991;283:243–46. doi: 10.1016/0014-5793(91)80598-w. [DOI] [PubMed] [Google Scholar]
- 17.Banfic H, Zizak M, Divecha N, Irvine RF. Nuclear diacylglycerol is increased during cell proliferation in vivo. Biochem. J. 1993;290:633–36. doi: 10.1042/bj2900633. Pt. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raben DM, Baldassare JJ. Nuclear envelope signaling: role of phospholipid metabolism. Eur. J. Histochem. 2000;44:67–80. [PubMed] [Google Scholar]
- 19.Banno Y, Tamiya-Koizumi K, Oshima H, Morikawa A, Yoshida S, Nozawa Y. Nuclear ADP-ribosylation factor (ARF)- and oleate-dependent phospholipase D (PLD) in rat liver cells. Increases of ARF-dependent PLD activity in regenerating liver cells. J. Biol. Chem. 1997;272:5208–13. doi: 10.1074/jbc.272.8.5208. [DOI] [PubMed] [Google Scholar]
- 20.Manzoli FA, Capitani S, Mazzotti G, Barnabei O, Maraldi NM. Role of chromatin phospholipids on template availability and ultrastructure of isolated nuclei. Adv. Enzyme Regul. 1982;20:247–62. doi: 10.1016/0065-2571(82)90019-x. [DOI] [PubMed] [Google Scholar]
- 21.Capitani S, Caramelli E, Felaco M, Miscia S, Manzoli FA. Effect of phospholipid vesicles on endogenous RNA polymerase activity of isolated rat liver nuclei. Physiol. Chem. Phys. 1981;13:153–58. [PubMed] [Google Scholar]
- 22.Li Y, Choi M, Cavey G, Daugherty J, Suino K, et al. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol. Cell. 2005;17:491–502. doi: 10.1016/j.molcel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Ortlund EA, Lee Y, Solomon IH, Hager JM, Safi R, et al. Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nat. Struct. Biol. 2005;12:357–63. doi: 10.1038/nsmb910. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Zhang C, Marimuthu A, Krupka HI, Tabrizizad M, et al. 2005;102:7505–10. doi: 10.1073/pnas.0409482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martelli AM, Bortul R, Bareggi R, Manzoli L, Narducci, Cocco L. Diacylglycerol kinases in nuclear lipid-dependent signal transduction pathways. Cell Mol. Life Sci. 2002;59:1129–37. doi: 10.1007/s00018-002-8492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martelli AM, Fala F, Faenza I, Billi AM, Cappellini A, et al. Metabolism and signaling activities of nuclear lipids. Cell Mol. Life Sci. 2004;61:1143–56. doi: 10.1007/s00018-004-3414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topham MK, Epand RM. Mammalian diacylglycerol kinases: molecular interactions and biological functions of selected isoforms. Biochim. Biophys. Acta. 2009;1790:416–24. doi: 10.1016/j.bbagen.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wattenberg BW, Pitson SM, Raben DM. The sphingosine and diacylglycerol kinase superfamily of signaling kinases: localization as a key to signaling function. J. Lipid Res. 2006;47:1128–39. doi: 10.1194/jlr.R600003-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Topham MK, Bunting M, Zimmerman GA, McIntyre TM, Blackshear PJ, Prescott SM. Protein kinase C regulates the nuclear localization of diacylglycerol kinase-ζ. Nature. 1998;394:697–700. doi: 10.1038/29337. [DOI] [PubMed] [Google Scholar]
- 30.Tabellini G, Bortul R, Santi S, Riccio M, Baldini G, et al. Diacylglycerol kinase-θ is localized in the speckle domains of the nucleus. Exp. Cell Res. 2003;287:143–54. doi: 10.1016/s0014-4827(03)00115-0. [DOI] [PubMed] [Google Scholar]
- 31.Bregoli L, Baldassare JJ, Raben DM. Nuclear diacylglycerol kinase-θ is activated in response to α-thrombin. J. Biol. Chem. 2001;276:23288–95. doi: 10.1074/jbc.M101501200. [DOI] [PubMed] [Google Scholar]
- 32.Tabellini G, Billi AM, Fala F, Cappellini A, Evagelisti C, et al. Nuclear diacylglycerol kinase-θ is activated in response to nerve growth factor stimulation of PC12 cells. Cell Signal. 2004;16:1263–71. doi: 10.1016/j.cellsig.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Li D, Urs AN, Allegood J, Leon A, Merrill AH, Jr, Sewer MB. Cyclic AMP-stimulated interaction between steroidogenic factor 1 and diacylglycerol kinase θ facilitates induction of CYP17. Mol. Cell. Biol. 2007;27:6669–85. doi: 10.1128/MCB.00355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, et al. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8:2302–12. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- 35.Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, et al. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog. Horm. Res. 2002;57:19–36. doi: 10.1210/rp.57.1.19. [DOI] [PubMed] [Google Scholar]
- 36.Csaki LS, Reue K. Lipins: multifunctional lipid metabolism proteins. Annu. Rev. Nutr. 2010;30:257–72. doi: 10.1146/annurev.nutr.012809.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, et al. Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Tsukahara T, Tsukahara R, Fujiwara Y, Yue J, Cheng Y, et al. Phospholipase D2-dependent inhibition of the nuclear hormone receptor PPARγ by cyclic phosphatidic acid. Mol. Cell. 2010;39:421–32. doi: 10.1016/j.molcel.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 2010;688:141–55. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hannun YA, Obeid LM. Principles of bioactive lipid signaling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2009;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 41.Zeidan YH, Hannun YA. Translational aspects of sphingolipid metabolism. Trends Mol. Med. 2007;13:327–36. doi: 10.1016/j.molmed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Tamiya-Koizumi K, Umekawa H, Yoshida S, Kojima K. Existence of Mg2+-dependent, neutral sphingomyelinase in nuclei of rat ascites hepatoma cells. J. Biochem. 1989;106:593–98. doi: 10.1093/oxfordjournals.jbchem.a122901. [DOI] [PubMed] [Google Scholar]
- 43.Alessenko A, Chatterjee S. Neutral sphingomyelinase: localization in rat liver nuclei and involvement in regeneration/proliferation. Mol. Cell. Biochem. 1995;143:169–74. doi: 10.1007/BF01816950. [DOI] [PubMed] [Google Scholar]
- 44.Wu G, Lu ZH, Ledeen RW. GM1 ganglioside in the nuclear membrane modulates nuclear calcium homeostasis during neurite outgrowth. J. Neurochem. 1995;65:1419–22. doi: 10.1046/j.1471-4159.1995.65031419.x. [DOI] [PubMed] [Google Scholar]
- 45.Micheli M, Albi E, Leray C, Magni MV. Nuclear sphingomyelin protects RNA from RNase action. FEBS Lett. 1998;431:443–47. doi: 10.1016/s0014-5793(98)00810-2. [DOI] [PubMed] [Google Scholar]
- 46.Tsugane K, Tamiya-Koizumi K, Nagino M, Nimura Y, Yoshida S. A possible role of nuclear ceramide and sphingosine in hepatocyte apoptosis in rat liver. J. Hepatol. 1999;31:8–17. doi: 10.1016/s0168-8278(99)80158-5. [DOI] [PubMed] [Google Scholar]
- 47.Mizuno S, Nimura Y, Suzuki H, Yoshida S. Portal vein branch occlusion induces cell proliferation of cholestatic rat liver. J. Surg. Res. 1996;60:249–57. doi: 10.1006/jsre.1996.0039. [DOI] [PubMed] [Google Scholar]
- 48.Xie X, Wu G, Lu ZH, Ledeen RW. Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J. Neurochem. 2002;81:1185–95. doi: 10.1046/j.1471-4159.2002.00917.x. [DOI] [PubMed] [Google Scholar]
- 49.Rossi G, Magni MV, Albi E. Sphingomyelin-cholesterol and double stranded RNA relationship in the intranuclear complex. Arch. Biochem. Biophys. 2007;459:27–32. doi: 10.1016/j.abb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 50.Albi E, Cataldi S, Rossi G, Viola Magni M, Toller M, et al. The nuclear ceramide/diacylglycerol balance depends on the physiological state of thyroid cells and changes during UV-C radiation-induced apoptosis. Arch. Biochem. Biophys. 2008;478:52–58. doi: 10.1016/j.abb.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 51.Tempera I, Buchetti B, Lococo E, Gradini R, Mastronardi A, et al. GD3 nuclear localization after apoptosis induction in HUT-78 cells. Biochem. Biophys. Res. Commun. 2008;368:495–500. doi: 10.1016/j.bbrc.2007.12.196. [DOI] [PubMed] [Google Scholar]
- 52.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–57. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupuy F, Fanani ML, Maggio B. Ceramide N-acyl chain length: a determinant of bidimensional transitions, condensed domain morphology, and interfacial thickness. Langmuir. 2011;27:3783–91. doi: 10.1021/la105011x. [DOI] [PubMed] [Google Scholar]
- 54.Stiban J, Fistere D, Colombini M. Dihydroceramide hinders ceramide channel formation: implications on apoptosis. Apoptosis. 2006;11:773–80. doi: 10.1007/s10495-006-5882-8. [DOI] [PubMed] [Google Scholar]
- 55.Kotzerke J, Stibane C, Dralle H, Wiese H, Burchert W. Screening for pheochromocytoma in the MEN 2 syndrome. Henry Ford Hosp. Med. J. 1989;37:129–31. [PubMed] [Google Scholar]
- 56.Farooqui AA, Horrocks LA. Signaling and interplay mediated by phospholipases A2, C, and D in LA-N-1 cell nuclei. Reprod. Nutr. Dev. 2005;45:613–31. doi: 10.1051/rnd:2005049. [DOI] [PubMed] [Google Scholar]
- 57.Ondrias K, Lencesova L, Sirova M, Labudova M, Pastorekova S, et al. Apoptosis induced clustering of IP3R1 in nuclei of nondifferentiated PC12 cells. J. Cell. Physiol. 2011;226:3147–55. doi: 10.1002/jcp.22665. [DOI] [PubMed] [Google Scholar]
- 58.Martelli AM, Manzoli L, Cocco L. Nuclear inositides: facts and persepectives. Pharmacol. Ther. 2004;101:47–64. doi: 10.1016/j.pharmthera.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Cocco L, Martelli AM, Gilmour RS, Rhee SG, Manzolli FA. Nuclear phospholipase C and signaling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2001;1530:1–14. doi: 10.1016/s1388-1981(00)00169-4. [DOI] [PubMed] [Google Scholar]
- 60.Antony P, Freysz L, Horrocks LA, Farooqui AA. Effects of retinoic acid on the Ca2+-independent phospholipase A2 in nuclei of LA-N-1 neuroblastoma cells. Neurochem. Res. 2001;26:83–88. doi: 10.1023/a:1007636801035. [DOI] [PubMed] [Google Scholar]
- 61.Stiban J, Caputo L, Colombini M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J. Lipid Res. 2008;49:625–34. doi: 10.1194/jlr.M700480-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Rovina P, Schanzer A, Graf C, Mechtcheriakova D, Jaritz M, Bornancin F. Subcellular localization of ceramide kinase and ceramide kinase-like protein requires interplay of their Pleckstrin Homology domain-containing N-terminal regions together with C-terminal domains. Biochem. Biophys. Acta. 2009;1791:1023–30. doi: 10.1016/j.bbalip.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 63.Schimizu M, Tada E, Makiyama T, Yasufuku K, Moriyama Y, et al. Effects of ceramide, ceramidase inhibition and expression of ceramide kinase on cytosolic phospholipase A2α; additional role for ceramide-1-phosphate in phosphorylation and Ca2+ signaling. Cell Signal. 2009;21:440–47. doi: 10.1016/j.cellsig.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 64.Lamour N, Chalfant CE. Ceramide kinase and the ceramide-1-phosphate/cPLA2α interaction as a therapeutic target. Curr. Drug Targets. 2008;9:674–82. doi: 10.2174/138945008785132349. [DOI] [PubMed] [Google Scholar]
- 65.Samanta S, Stiban J, Maugel TK, Colombini M. Visualization of ceramide channels by transmission electron microscopy. Biochim. Biophys. Acta. 2011;1808:1196–201. doi: 10.1016/j.bbamem.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng X, Blumenthal RM. Coordinated chromatin control: structural and functional linkage of DNA and histone methylation. Biochemistry. 2010;49:2999–3008. doi: 10.1021/bi100213t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lelievre SA. Contributions of extracellular matrix signaling and tissue architecture to nuclear mechanisms and spatial organization of gene expression control. Biochim. Biophys. Acta. 2009;1790:925–35. doi: 10.1016/j.bbagen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lamond AI, Earnshaw WC. Structure and function in the nucleus. Science. 1998;280:547–53. doi: 10.1126/science.280.5363.547. [DOI] [PubMed] [Google Scholar]
- 69.Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphin-gosine, and sphingosine-1-phosphate. Biochim. Biophys. Acta. 2008;1781:424–34. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim. Biophys. Acta. 2002;1585:114–25. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 71.Strelow A, Bernardo K, Adam-Klages S, Linke T, Sandhoff K, et al. Overexpression of acid ceramidase protects from tumor necrosis factor-induced cell death. J. Exp. Med. 2000;192:601–12. doi: 10.1084/jem.192.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J. Biol. Chem. 2007;282:2125–29. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 73.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 2008;60:181–95. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sukocheva O, Wang L, Verrier E, Vadas MA, Xia P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology. 2009;150:4484–92. doi: 10.1210/en.2009-0391. [DOI] [PubMed] [Google Scholar]
- 75.Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, et al. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 2010;285:10477–86. doi: 10.1074/jbc.M109.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henkes LE, Sullivan BT, Lynch MP, Kolesnick R, Arsenault D, et al. Acid sphingomyelinase involvement in tumor necrosis factor α-regulated vascular and steroid disruption during luteolysis in vivo. Proc. Natl. Acad. Sci. USA. 2008;105:7670–75. doi: 10.1073/pnas.0712260105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kolesnick RN, Haimovitz-Friedman A, Fuks Z. The sphingomyelin signal transduction pathway mediates apoptosis for tumor necrosis factor, Fas, and ionizing radiation. Biochem. Cell Biol. 1994;72:471–74. doi: 10.1139/o94-063. [DOI] [PubMed] [Google Scholar]
- 78.Watanabe M, Kitano T, Kondo T, Yabu T, Taguchi Y, et al. Increase of nuclear ceramide through caspase-3-dependent regulation of the “sphingomyelin cycle” in Fas-induced apoptosis. Cancer Res. 2004;64:1000–7. doi: 10.1158/0008-5472.can-03-1383. [DOI] [PubMed] [Google Scholar]
- 79.Nybond S, Bjorkqvist YJ, Ramstedt B, Slotte JP. Acyl chain length affects ceramide action on sterol/sphingomyelin-rich domains. Biochim. Biophys. Acta. 2005;1718:61–66. doi: 10.1016/j.bbamem.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 80.Megha, Sawatzki P, Kolter T, Bittman R, London E. Effect of ceramide N-acyl chain and polar headgroup structure on the properties of ordered lipid domains (lipid rafts) Biochim. Biophys. Acta. 2007;1768:2205–12. doi: 10.1016/j.bbamem.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Senkal CE, Ponnusamy S, Rossi MJ, Bialewski J, Sinha D, et al. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol. Cancer Ther. 2007;6:712–22. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- 83.Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, et al. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal. 2010;22:1300–7. doi: 10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stiban J, Tidhar R, Futerman AH. Ceramide synthases: roles in cell physiology and signaling. Adv. Exp. Med. Biol. 2010;688:60–71. doi: 10.1007/978-1-4419-6741-1_4. [DOI] [PubMed] [Google Scholar]
- 85.Sun W, Jin J, Xu R, Hu W, Szulc ZM, et al. Substrate specificity, membrane topology, and activity regulation of human alkaline ceramidase 2 (ACER2) J. Biol. Chem. 2010;285:8995–9007. doi: 10.1074/jbc.M109.069203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levy M, Futerman AH. Mammalian ceramide synthases. IUBMB Life. 2010;62:347–56. doi: 10.1002/iub.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv. Exp. Med. Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. Molecular cloning and characterization of a human mitochondrial ceramidase. J. Biol. Chem. 2000;275:21508–13. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 89.Hwang YH, Tani M, Nakagawa T, Okino N, Ito M. Subcellular localization of human neutral ceramidase expressed in HEK293 cells. Biochem. Biophys. Res. Commun. 2005;331:37–42. doi: 10.1016/j.bbrc.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 90.Breslow DK, Weissman JS. Membranes in balance: mechanisms of sphingolipid homeostasis. Mol. Cell. 2010;40:267–79. doi: 10.1016/j.molcel.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mullen TD, Jenkins RW, Clarke CJ, Bielawski J, Hannun YA, Obeid LM. Ceramide synthase-dependent ceramide generation and programmed cell death: involvement of salvage pathway in regulating post-mitochondrial events. J. Biol. Chem. 2011;286:15929–42. doi: 10.1074/jbc.M111.230870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Novgorodov SA, Chudakova DA, Wheeler BW, Bielawski J, Kindy MS, et al. Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis. J. Biol. Chem. 2011;286:4644–58. doi: 10.1074/jbc.M110.164392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mimeault M. New advances on structural and biological functions of ceramide in apoptotic/necrotic cell death and cancer. FEBS Lett. 2002;530:9–16. doi: 10.1016/s0014-5793(02)03432-4. [DOI] [PubMed] [Google Scholar]
- 94.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6:118–25. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, et al. CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001;276:20589–96. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 96.Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta. 2005;1746:284–94. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 97.Gupta S, Maurya MR, Merrill AH, Jr, Glass CK, Subramaniam S. Integration of lipidomics and transcriptomics data towards a systems biology model of sphingolipid metabolism. BMC Syst. Biol. 2011;5:26. doi: 10.1186/1752-0509-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hornemann T, Penno A, Rutti MF, Ernst D, Kivrak-Pfiffner F, et al. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J. Biol. Chem. 2009;284:26322–30. doi: 10.1074/jbc.M109.023192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han G, Gupta SD, Gable K, Niranjanakumari S, Moitra P, et al. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl. Acad. Sci. USA. 2009;106:8186–91. doi: 10.1073/pnas.0811269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eichler FS, Hornemann T, McCampbell A, Kuljis D, Penno A, et al. Overexpression of the wild-type SPT1 subunit lowers desoxysphingolipid levels and rescues the phenotype of HSAN1. J. Neurosci. 2009;29:14646–51. doi: 10.1523/JNEUROSCI.2536-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pruett ST, Bushnev A, Hagedorn K, Adiga M, Haynes CA, et al. Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J. Lipid Res. 2008;49:1621–39. doi: 10.1194/jlr.R800012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Menaldino DS, Bushnev A, Sun A, Liotta DC, Symolon H, et al. Sphingoid bases and de novo ceramide synthesis: enzymes involved, pharmacology and mechanisms of action. Pharmacol. Res. 2003;47:373–81. doi: 10.1016/s1043-6618(03)00054-9. [DOI] [PubMed] [Google Scholar]
- 103.Albi E, Cataldi S, Rossi G, Magni MV. A possible role of cholesterol-sphingomyelin/phosphatidylcholine in nuclear matrix during rat liver regeneration. J. Hepatol. 2003;38:623–28. doi: 10.1016/s0168-8278(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 104.Albi E, Pieroni S, Viola Magni MP, Sartori C. Chromatin sphingomyelin changes in cell proliferation and/or apoptosis induced by ciprofibrate. J. Cell. Physiol. 2003;196:354–61. doi: 10.1002/jcp.10314. [DOI] [PubMed] [Google Scholar]
- 105.Ledeen RW, Wu G. Gangliosides of the nuclear membrane: a crucial locus of cytoprotective modulation. J. Cell Biochem. 2006;97:893–903. doi: 10.1002/jcb.20731. [DOI] [PubMed] [Google Scholar]
- 106.Albi E, Cataldi S, Villani M, Perrella G. Nuclear phosphatidylcholine and sphingomyelin metabolism of thyroid cells changes during stratospheric balloon flight. J. Biomed. Biotechnol. 20092009:125412. doi: 10.1155/2009/125412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Albi E, La Porta CA, Cataldi S, Magni MV. Nuclear sphingomyelin-synthase and protein kinase Cδ in melanoma cells. Arch. Biochem. Biophys. 2005;438:156–61. doi: 10.1016/j.abb.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 108.Kleuser B, Maceyka M, Milstien S, Spiegel S. Stimulation of nuclear sphingosine kinase activity by platelet-derived growth factor. FEBS Lett. 2001;503:85–90. doi: 10.1016/s0014-5793(01)02697-7. [DOI] [PubMed] [Google Scholar]
- 109.Cocco L, Maraldi NM, Manzoli FA, Gilmour RS, Lang A. Phospholipid interactions in rat liver nuclear matrix. Biochem. Biophys. Res. Commun. 1980;96:890–98. doi: 10.1016/0006-291x(80)91439-4. [DOI] [PubMed] [Google Scholar]
- 110.Shiraishi T, Imai S, Uda Y. The presence of ceramidase activity in liver nuclear membrane. Biol. Pharm. Bull. 2003;26:775–79. doi: 10.1248/bpb.26.775. [DOI] [PubMed] [Google Scholar]
- 111.Haines DS, Strauss KI, Gillespie DH. Cellular response to double-stranded RNA. J. Cell Biochem. 1991;46:9–20. doi: 10.1002/jcb.240460104. [DOI] [PubMed] [Google Scholar]
- 112.Albi E, Magni MP. Chromatin neutral sphingomyelinase and its role in hepatic regeneration. Biochem. Biophys. Res. Commun. 1997;236:29–33. doi: 10.1006/bbrc.1997.6803. [DOI] [PubMed] [Google Scholar]
- 113.Albi E, Magni MV. Sphingomyelin synthase in rat liver nuclear membrane and chromatin. FEBS Lett. 1999;460:369–72. doi: 10.1016/s0014-5793(99)01378-2. [DOI] [PubMed] [Google Scholar]
- 114.Albi E, Lazzarini R, Magni MV. Reverse sphingomyelin-synthase in rat liver chromatin. FEBS Lett. 2003;549:152–56. doi: 10.1016/s0014-5793(03)00810-x. [DOI] [PubMed] [Google Scholar]
- 115.Wang J, Wu G, Miyagi T, Lu ZH, Ledeen RW. Sialidase occurs in both membranes of the nuclear envelope and hydrolyzes endogenous GD1a. J. Neurochem. 2009;111:547–54. doi: 10.1111/j.1471-4159.2009.06339.x. [DOI] [PubMed] [Google Scholar]
- 116.Wu G, Lu ZH, Xie X, Ledeen RW. Susceptibility of cerebellar granule neurons from GM2/GD2 synthase-null mice to apoptosis induced by glutamate excitotoxicity and elevated KCl: rescue by GM1 and LIGA20. Glycoconj. J. 2004;21:305–13. doi: 10.1023/B:GLYC.0000046273.68493.f7. [DOI] [PubMed] [Google Scholar]
- 117.James JL, Clawson GA, Chan CH, Smuckler EA. Analysis of the phospholipid of the nuclear envelope and endoplasmic reticulum of liver cells by high pressure liquid chromatography. Lipids. 1981;16:541–45. doi: 10.1007/BF02535053. [DOI] [PubMed] [Google Scholar]
- 118.Keenan TW, Berezney R, Crane FL. Lipid composition of further purified bovine liver nuclear membranes. Lipids. 1972;7:212–15. doi: 10.1007/BF02533066. [DOI] [PubMed] [Google Scholar]
- 119.Albi E, Magni MV. The presence and the role of chromatin cholesterol in rat liver regeneration. J. Hepatol. 2002;36:395–400. doi: 10.1016/s0168-8278(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 120.Lein M, Stibane I, Mansour R, Hege C, Roigas J, et al. Complications, urinary continence, and oncologic outcome of 1000 laparoscopic transperitoneal radical prostatectomies—experience at the Charite Hospital Berlin, Campus Mitte. Eur. Urol. 2006;50:1278–82. doi: 10.1016/j.eururo.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 121.Schmidt GW, Stibane H, Ulbrich F, Griesse H. Edwards syndrome with double trisomy D and E (mosaicism) Klin. Wochenschr. 1967;45:634–38. doi: 10.1007/BF01745637. [DOI] [PubMed] [Google Scholar]
- 122.Thevissen K, Francois IE, Winderickx J, Pannecouque C, Cammue BP. Ceramide involvement in apoptosis and apoptotic diseases. Mini. Rev. Med. Chem. 2006;6:699–709. doi: 10.2174/138955706777435643. [DOI] [PubMed] [Google Scholar]
- 123.Fasutino R, Cheung P, Richard M, Dibrov E, Kneesch A, et al. Ceramide regulation of nuclear protein import. J. Lipid Res. 2008;49:654–62. doi: 10.1194/jlr.M700464-JLR200. [DOI] [PubMed] [Google Scholar]
- 124.Martelli AM, Evangelisti C, Nyakern M, Manzoli FA. Nuclear protein kinase C. Biochim. Biophys. Acta. 2006;1761:542–51. doi: 10.1016/j.bbalip.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 125.Evangelisti C, Bortul R, Fala F, Tabellini G, Goto K, Martelli AM. Nuclear diacylglycerol kinases: emerging downstream regulators in cell signaling networks. Histol. Histopathol. 2007;22:573–79. doi: 10.14670/HH-22.573. [DOI] [PubMed] [Google Scholar]
- 126.Sun B, Murray NR, Fields AP. A role for nuclear phosphatidylinositol-specific phospholipase C in the G2/M phase transition. J. Biol. Chem. 1997;272:26313–17. doi: 10.1074/jbc.272.42.26313. [DOI] [PubMed] [Google Scholar]
- 127.Deacon EM, Pettitt TR, Webb P, Cross T, Chahal H, et al. Generation of diacylglycerol molecular species through the cell cycle: a role for 1-stearoyl, 2-arachidonyl glycerol in the activation of nuclear protein kinase C-βII at G2/M. J. Cell Sci. 2002;115:983–89. doi: 10.1242/jcs.115.5.983. [DOI] [PubMed] [Google Scholar]
- 128.Abboushi N, El-Hed A, El-Assaad W, Kozhaya L, El-Sabban ME, et al. Ceramide inhibits IL-2 production by preventing protein kinase C-dependent NF-κB activation: possible role in protein kinase Cθ regulation. J. Immunol. 2004;173:3193–200. doi: 10.4049/jimmunol.173.5.3193. [DOI] [PubMed] [Google Scholar]
- 129.Albi E, Micheli M, Viola Magni MP. Phospholipids and nuclear RNA. Cell Biol. Int. 1996;20:407–12. doi: 10.1006/cbir.1996.0051. [DOI] [PubMed] [Google Scholar]
- 130.Chocian G, Chabowski A, Zendzian-Piotrowska M, Harasim E, Lukaszuk B, Gorski J. High fat diet induces ceramide and sphingomyelin formation in rat’s liver nuclei. Mol. Cell. Biochem. 2010;340:125–31. doi: 10.1007/s11010-010-0409-6. [DOI] [PubMed] [Google Scholar]
- 131.Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 132.Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. J. Biol. Chem. 2005;280:20148–53. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- 133.Andreyev AY, Fahy E, Guan Z, Kelly S, Li X, et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J. Lipid Res. 2010;51:2785–97. doi: 10.1194/jlr.M008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schimazu T, Horinouchi S, Yoshida M. Multiple histone deacetylases and the CREB-binding protein regulate pre-mRNA 3′-end processing. J. Biol. Chem. 2007;282:4470–78. doi: 10.1074/jbc.M609745200. [DOI] [PubMed] [Google Scholar]
- 135.Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE. Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J. Biol. Chem. 2003;278:38206–13. doi: 10.1074/jbc.M304816200. [DOI] [PubMed] [Google Scholar]
- 136.Lamour NF, Subramanian P, Wijesinghe DS, Stahelin RV, Bonventre JV, Chalfant CE. Ceramide 1-phosphate is required for the translocation of group IVA cytosolic phospholipase A2 and prostaglandin synthesis. J. Biol. Chem. 2009;284:26897–907. doi: 10.1074/jbc.M109.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Urs AN, Dammer E, Sewer M. Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology. 2006;147:5249–58. doi: 10.1210/en.2006-0355. [DOI] [PubMed] [Google Scholar]
- 138.Sewer MB, Waterman MR. ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc. Res. Tech. 2003;61:300–7. doi: 10.1002/jemt.10339. [DOI] [PubMed] [Google Scholar]
- 139.Sewer MB, Waterman MR. cAMP-dependent transcription of steroidogenic genes in the human adrenal cortex requires a dual-specificity phosphatase in addition to protein kinase A. J. Mol. Endocrinol. 2002;29:163–74. doi: 10.1677/jme.0.0290163. [DOI] [PubMed] [Google Scholar]
- 140.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J. Biol. Chem. 2005;280:37118–29. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 141.Keenan TW, Morre DJ, Huang CM. Distribution of gangliosides among subcellular fractions from rat liver and bovine mammary gland. FEBS Lett. 1972;24:204–8. doi: 10.1016/0014-5793(72)80768-3. [DOI] [PubMed] [Google Scholar]
- 142.Matyas GR, Morre DJ. Subcellular distribution and biosynthesis of rat liver gangliosides. Biochim. Biophys. Acta. 1987;921:599–614. doi: 10.1016/0005-2760(87)90089-0. [DOI] [PubMed] [Google Scholar]
- 143.Katoh N, Kira T, Yuasa A. Protein kinase C substrates and ganglioside inhibitors in bovine mammary nuclei. J. Dairy Sci. 1993;76:3400–9. doi: 10.3168/jds.S0022-0302(93)77678-X. [DOI] [PubMed] [Google Scholar]
- 144.Wu G, Lu ZH, Ledeen RW. Induced and spontaneous neuritogenesis are associated with enhanced expression of ganglioside GM1 in the nuclear membrane. J. Neurosci. 1995;15:3739–46. doi: 10.1523/JNEUROSCI.15-05-03739.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saito M, Sugiyama K. Characterization of nuclear gangliosides in rat brain: concentration, composition, and developmental changes. Arch. Biochem. Biophys. 2002;398:153–59. doi: 10.1006/abbi.2001.2725. [DOI] [PubMed] [Google Scholar]
- 146.Humbert JP, Matter N, Artault JC, Koppler P, Malviya AN. Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. Discrete distribution of inositol phosphate receptors to inner and outer nuclear membranes. J. Biol. Chem. 1996;271:478–85. doi: 10.1074/jbc.271.1.478. [DOI] [PubMed] [Google Scholar]
- 147.Gilchrist JS, Pierce GN. Identification and purification of a calcium-binding protein in hepatic nuclear membranes. J. Biol. Chem. 1993;268:4291–99. [PubMed] [Google Scholar]
- 148.Saito M, Hagita H, Ito M, Ando S, Yu RK. Age-dependent reduction in sialidase activity of nuclear membranes from mouse brain. Exp. Gerontol. 2002;37:937–41. doi: 10.1016/s0531-5565(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 149.Wang J, Wu G, Miyagi T, Lu ZH, Ledeen RW. Sialidase occurs in both membranes of the nuclear envelope and hydrolyzes endogenous GD1a. J. Neurochem. 2009;111:547–54. doi: 10.1111/j.1471-4159.2009.06339.x. [DOI] [PubMed] [Google Scholar]
- 150.Parkinson ME, Smith CG, Garland PB, van Heyningen S. Identification of cholera toxin-binding sites in the nucleus of intestinal epithelial cells. FEBS Lett. 1989;242:309–13. doi: 10.1016/0014-5793(89)80491-0. [DOI] [PubMed] [Google Scholar]
- 151.Copani A, Melchiorri D, Caricasole A, Martini F, Sale P, et al. β-Amyloid-induced synthesis of the ganglioside GD3 is a requisite for cell cycle reactivation and apoptosis in neurons. J. Neurosci. 2002;22:3963–68. doi: 10.1523/JNEUROSCI.22-10-03963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Philipson KD, Nicoll DA. Sodium-calcium exchange: a molecular perspective. Annu. Rev. Physiol. 2000;62:111–33. doi: 10.1146/annurev.physiol.62.1.111. [DOI] [PubMed] [Google Scholar]
- 153.Xie X, Wu G, Lu ZH, Rohowsky-Kochan C, Ledeen RW. Presence of sodium-calcium exchanger/GM1 complex in the nuclear envelope of non-neural cells: nature of exchanger-GM1 interaction. Neurochem. Res. 2004;29:2135–46. doi: 10.1007/s11064-004-6887-8. [DOI] [PubMed] [Google Scholar]
- 154.Ledeen R, Wu G. New findings on nuclear gangliosides: overview on metabolism and function. J. Neurochem. 2011;116:714–20. doi: 10.1111/j.1471-4159.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- 155.Kofuji P, Lederer WJ, Schulze DH. Mutually exclusive and cassette exons underlie alternatively spliced isoforms of the Na/Ca exchanger. J. Biol. Chem. 1994;269:5145–49. [PubMed] [Google Scholar]
- 156.He S, Ruknudin A, Bambrick LL, Lederer WJ, Schulze DH. Isoform-specific regulation of the Na+/Ca2+ exchanger in rat astrocytes and neurons by PKA. J. Neurosci. 1998;18:4833–41. doi: 10.1523/JNEUROSCI.18-13-04833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu G, Xie X, Lu ZH, Ledeen RW. Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2009;106:10829–34. doi: 10.1073/pnas.0903408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xie X, Wu G, Ledeen RW. C6 cells express a sodium-calcium exchanger/GM1 complex in the nuclear envelope but have no exchanger in the plasma membrane: comparison to astrocytes. J. Neurosci. Res. 2004;76:363–75. doi: 10.1002/jnr.20068. [DOI] [PubMed] [Google Scholar]
- 159.Wu G, Lu ZH, Kulkarni N, Amin R, Ledeen RW. Mice lacking major brain gangliosides develop Parkinsonism. Neurochem. Res. 2011;36:1706–14. doi: 10.1007/s11064-011-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ledeen R, Wu G. New findings on nuclear gangliosides: overview on metabolism and function. J. Neurochem. 2011;116:714–20. doi: 10.1111/j.1471-4159.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- 161.Liu Y, Wada R, Kawai H, Sango K, Deng C, et al. A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J. Clin. Investig. 1999;103:497–505. doi: 10.1172/JCI5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wu G, Xie X, Lu ZH, Ledeen RW. Cerebellar neurons lacking complex gangliosides degenerate in the presence of depolarizing levels of potassium. Proc. Natl. Acad. Sci. USA. 2001;98:307–12. doi: 10.1073/pnas.011523698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fujiwara Y. Cyclic phosphatidic acid—a unique phospholipid. Biochim. Biophys. Acta. 2008;1781(9):519–24. doi: 10.1016/j.bbalip.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]