Abstract
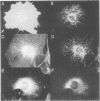
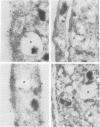
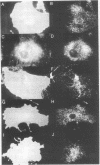
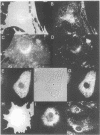
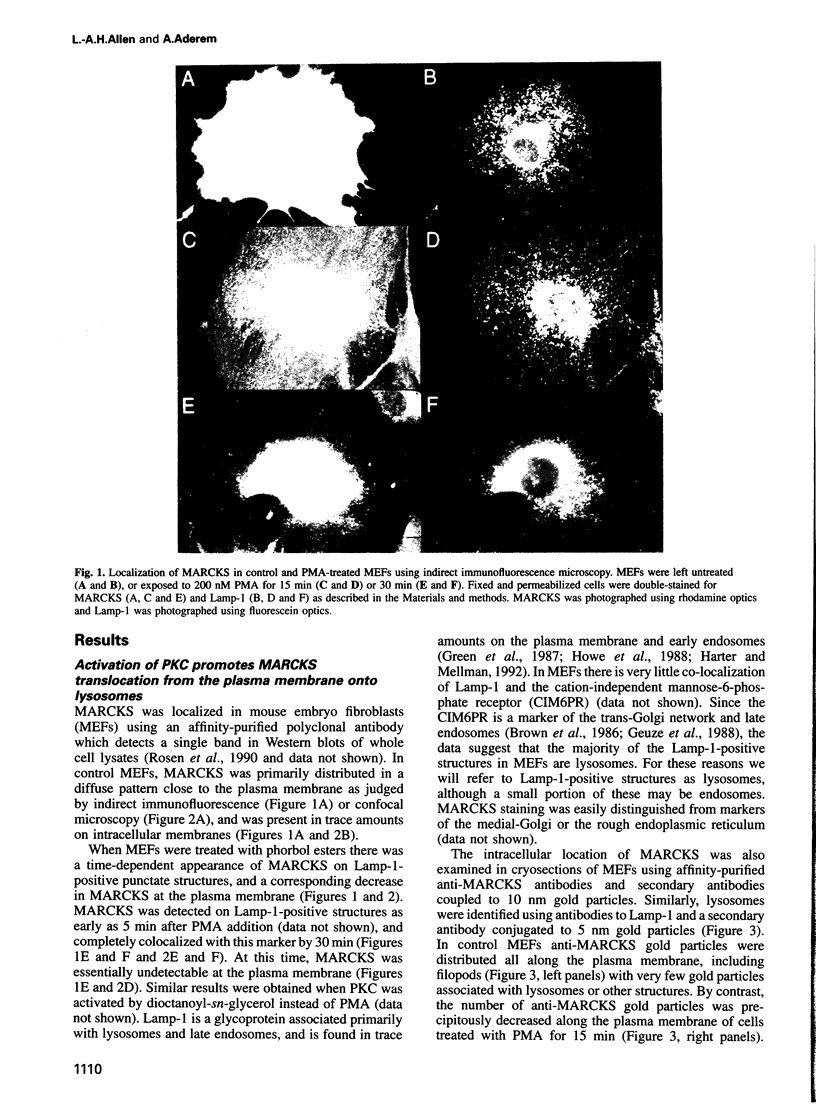
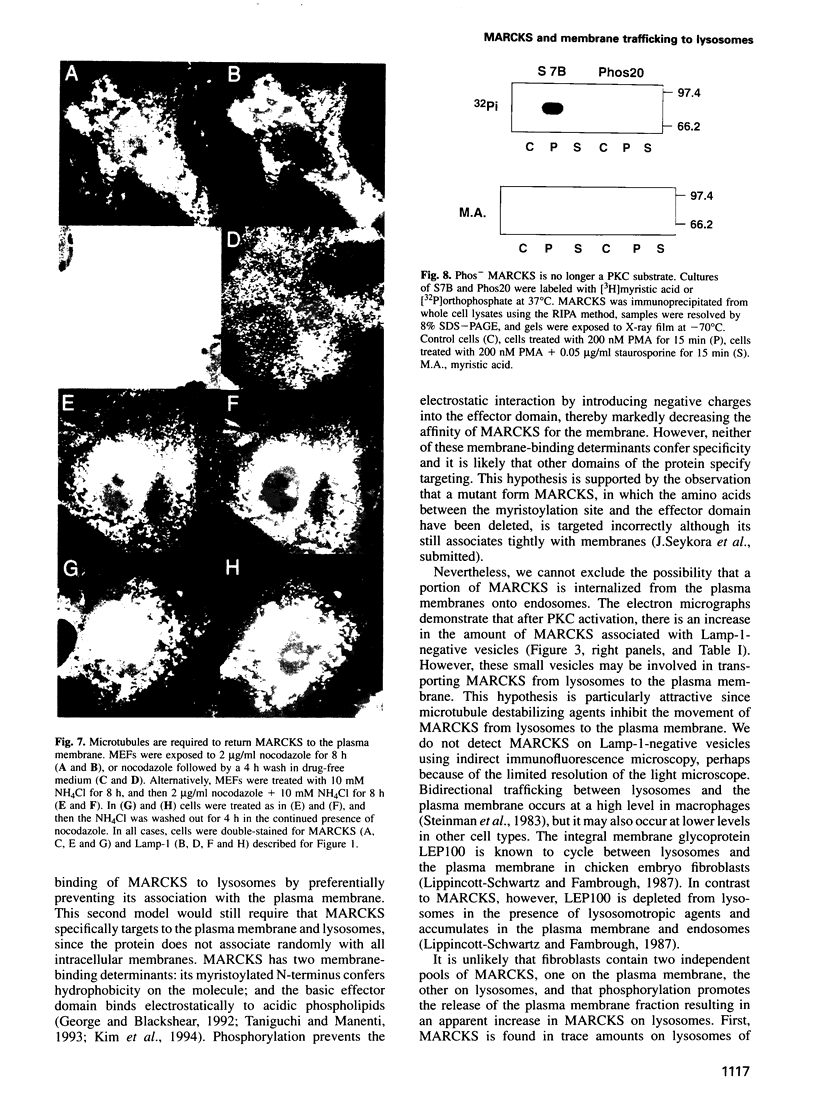
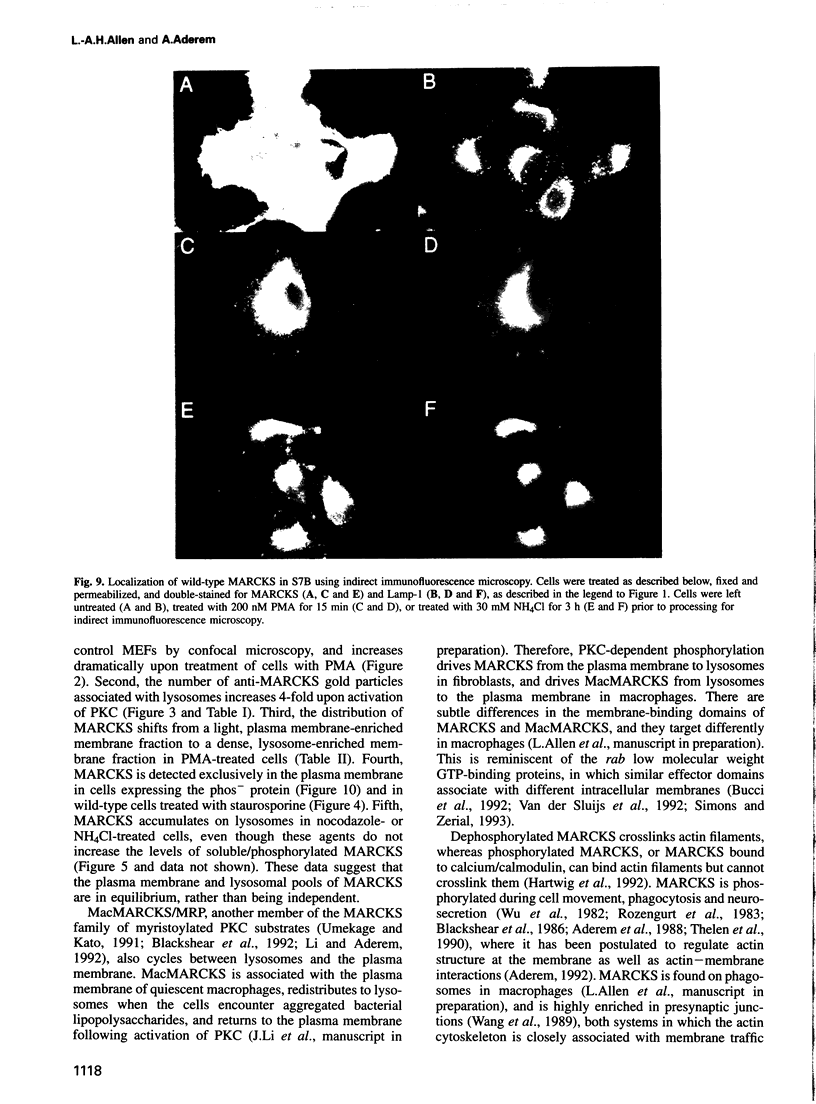
MARCKS is a protein kinase C (PKC) substrate that is phosphorylated during neurosecretion, phagocyte activation and growth factor-dependent mitogenesis. MARCKS binds calcium/calmodulin and crosslinks F-actin, and both these activities are regulated by PKC-dependent phosphorylation. We present evidence here that PKC-dependent phosphorylation also regulates the cycling of MARCKS between the plasma membrane and Lamp-1-positive lysosomes. Immuno-fluorescence and immunoelectron microscopy, and subcellular fractionation, demonstrated that MARCKS was predominantly associated with the plasma membrane of resting fibroblasts. Activation of PKC resulted in MARCKS phosphorylation and its displacement from the plasma membrane to Lamp-1-positive lysosomes. MARCKS phosphorylation is required for its translocation to lysosomes since mutating either the serine residues phosphorylated by PKC (phos-) or the PKC inhibitor staurosporine, prevented MARCKS phosphorylation, its release from the plasma membrane, and its subsequent association with lysosomes. In the presence of lysosomotropic agents or nocodazole, MARCKS accumulated on lysosomes and returned to the plasma membrane upon drug removal, further suggesting that the protein cycles between the plasma membrane and lysosomes. In contrast to wild-type MARCKS, the phos- mutant did not accumulate on lysosomes in cells treated with NH4Cl, suggesting that basal phosphorylation of MARCKS promotes its constitutive cycling between these two compartments.
Full text
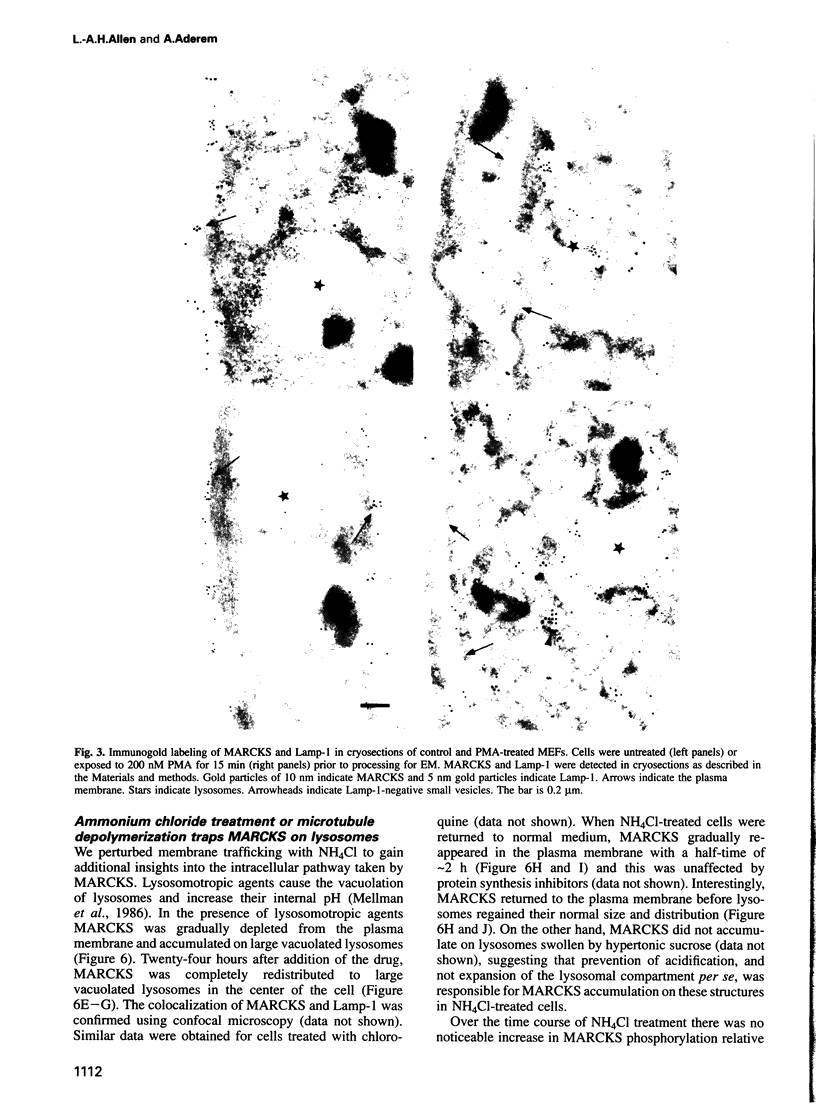
PDF


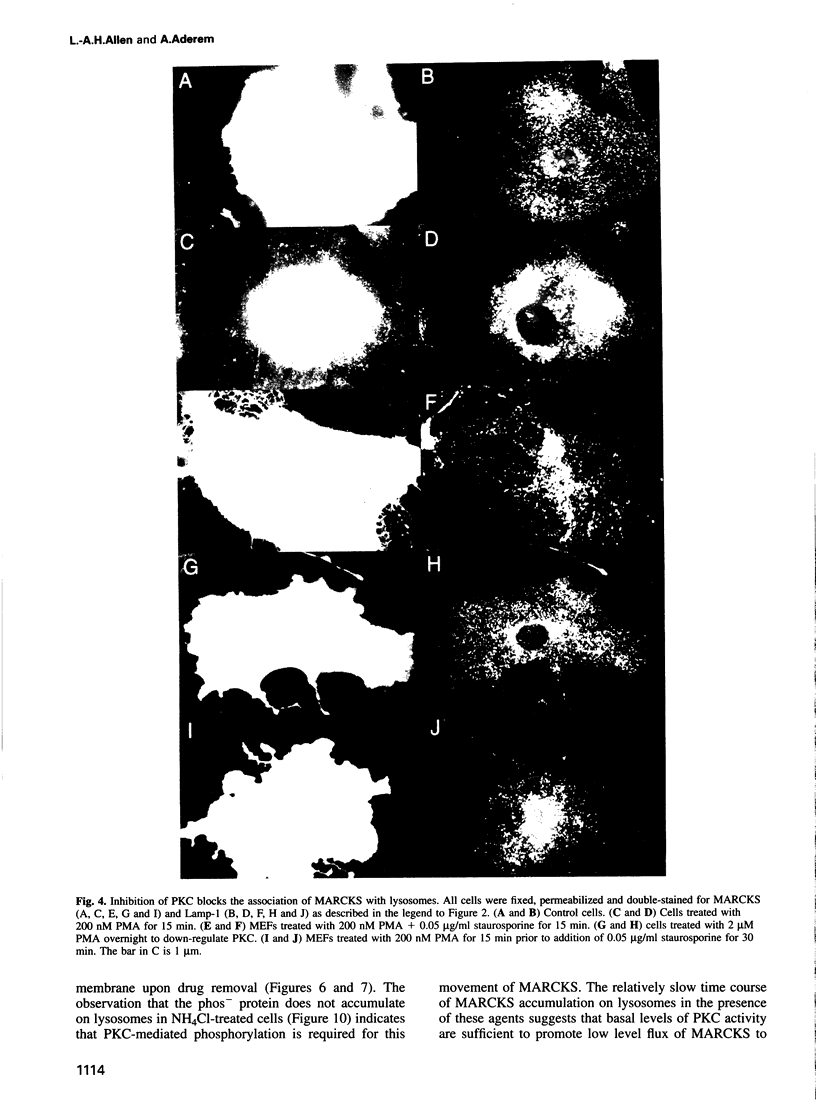



Images in this article
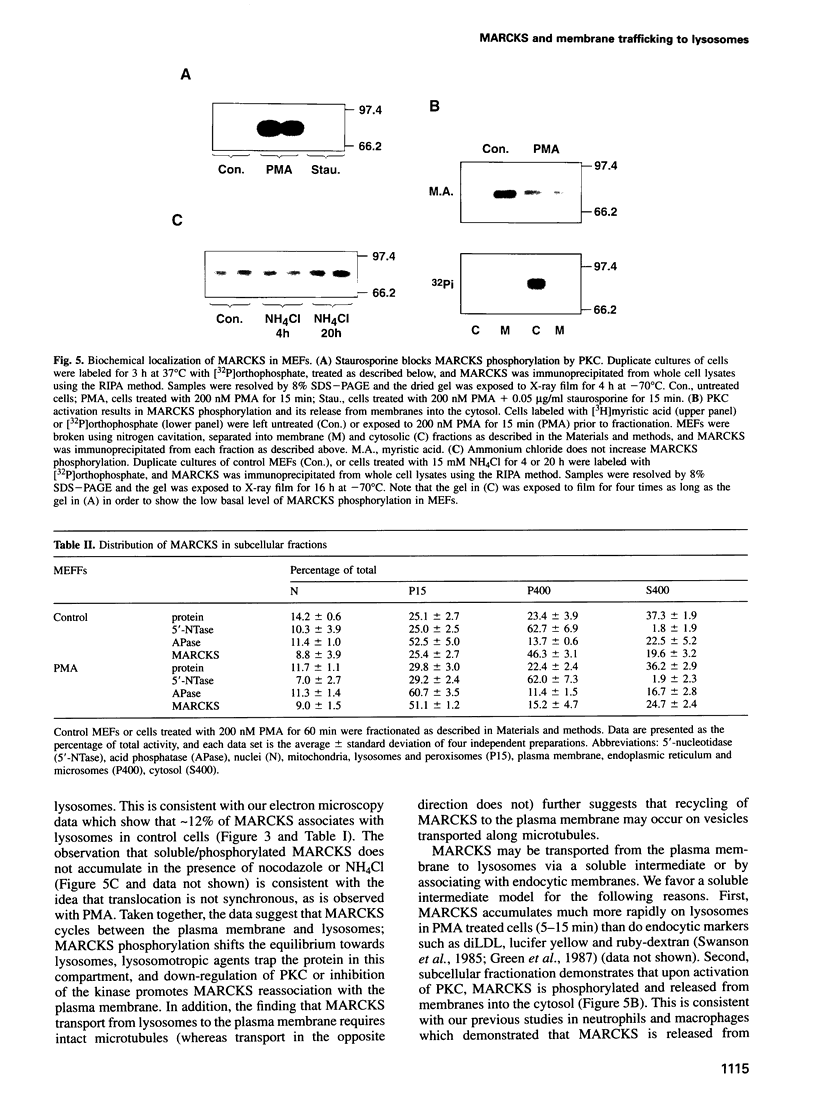
Selected References
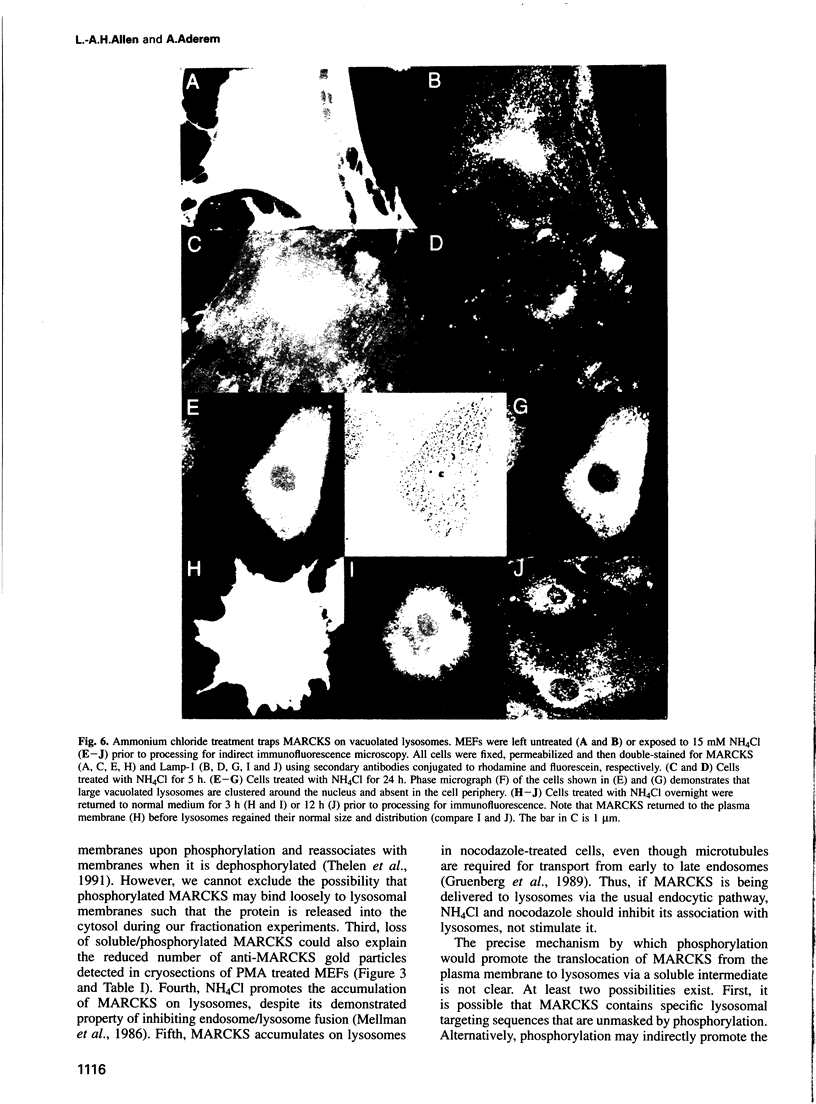
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. A., Albert K. A., Keum M. M., Wang J. K., Greengard P., Cohn Z. A. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988 Mar 24;332(6162):362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]
- Aderem A. A., Keum M. M., Pure E., Cohn Z. A. Bacterial lipopolysaccharides, phorbol myristate acetate, and zymosan induce the myristoylation of specific macrophage proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5817–5821. doi: 10.1073/pnas.83.16.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992 Nov 27;71(5):713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- Albert K. A., Walaas S. I., Wang J. K., Greengard P. Widespread occurrence of "87 kDa," a major specific substrate for protein kinase C. Proc Natl Acad Sci U S A. 1986 May;83(9):2822–2826. doi: 10.1073/pnas.83.9.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993 Jan 25;268(3):1501–1504. [PubMed] [Google Scholar]
- Blackshear P. J., Verghese G. M., Johnson J. D., Haupt D. M., Stumpo D. J. Characteristics of the F52 protein, a MARCKS homologue. J Biol Chem. 1992 Jul 5;267(19):13540–13546. [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Brown W. J., Goodhouse J., Farquhar M. G. Mannose-6-phosphate receptors for lysosomal enzymes cycle between the Golgi complex and endosomes. J Cell Biol. 1986 Oct;103(4):1235–1247. doi: 10.1083/jcb.103.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C., Parton R. G., Mather I. H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992 Sep 4;70(5):715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Clark S. W., Meyer D. I. Centractin is an actin homologue associated with the centrosome. Nature. 1992 Sep 17;359(6392):246–250. doi: 10.1038/359246a0. [DOI] [PubMed] [Google Scholar]
- George D. J., Blackshear P. J. Membrane association of the myristoylated alanine-rich C kinase substrate (MARCKS) protein appears to involve myristate-dependent binding in the absence of a myristoyl protein receptor. J Biol Chem. 1992 Dec 5;267(34):24879–24885. [PubMed] [Google Scholar]
- Geuze H. J., Stoorvogel W., Strous G. J., Slot J. W., Bleekemolen J. E., Mellman I. Sorting of mannose 6-phosphate receptors and lysosomal membrane proteins in endocytic vesicles. J Cell Biol. 1988 Dec;107(6 Pt 2):2491–2501. doi: 10.1083/jcb.107.6.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. S., Vale R. D. Cell biology. New cytoskeletal liaisons. Nature. 1992 Sep 17;359(6392):193–194. doi: 10.1038/359193a0. [DOI] [PubMed] [Google Scholar]
- Graff J. M., Young T. N., Johnson J. D., Blackshear P. J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21818–21823. [PubMed] [Google Scholar]
- Green S. A., Zimmer K. P., Griffiths G., Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol. 1987 Sep;105(3):1227–1240. doi: 10.1083/jcb.105.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P., Valtorta F., Czernik A. J., Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993 Feb 5;259(5096):780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K., Warren G., Tokuyasu K. T. Immunoelectron microscopy using thin, frozen sections: application to studies of the intracellular transport of Semliki Forest virus spike glycoproteins. Methods Enzymol. 1983;96:466–485. doi: 10.1016/s0076-6879(83)96041-x. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Griffiths G., Howell K. E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989 Apr;108(4):1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter C., Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol. 1992 Apr;117(2):311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Howe C. L., Granger B. L., Hull M., Green S. A., Gabel C. A., Helenius A., Mellman I. Derived protein sequence, oligosaccharides, and membrane insertion of the 120-kDa lysosomal membrane glycoprotein (lgp120): identification of a highly conserved family of lysosomal membrane glycoproteins. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7577–7581. doi: 10.1073/pnas.85.20.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Shishido T., Jiang X., Aderem A., McLaughlin S. Phosphorylation, high ionic strength, and calmodulin reverse the binding of MARCKS to phospholipid vesicles. J Biol Chem. 1994 Nov 11;269(45):28214–28219. [PubMed] [Google Scholar]
- Li J., Aderem A. MacMARCKS, a novel member of the MARCKS family of protein kinase C substrates. Cell. 1992 Sep 4;70(5):791–801. doi: 10.1016/0092-8674(92)90312-z. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1987 Jun 5;49(5):669–677. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- McIlroy B. K., Walters J. D., Blackshear P. J., Johnson J. D. Phosphorylation-dependent binding of a synthetic MARCKS peptide to calmodulin. J Biol Chem. 1991 Mar 15;266(8):4959–4964. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Rosen A., Keenan K. F., Thelen M., Nairn A. C., Aderem A. Activation of protein kinase C results in the displacement of its myristoylated, alanine-rich substrate from punctate structures in macrophage filopodia. J Exp Med. 1990 Oct 1;172(4):1211–1215. doi: 10.1084/jem.172.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen A., Nairn A. C., Greengard P., Cohn Z. A., Aderem A. Bacterial lipopolysaccharide regulates the phosphorylation of the 68K protein kinase C substrate in macrophages. J Biol Chem. 1989 Jun 5;264(16):9118–9121. [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Zerial M. Rab proteins and the road maps for intracellular transport. Neuron. 1993 Nov;11(5):789–799. doi: 10.1016/0896-6273(93)90109-5. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. A., Yirinec B. D., Silverstein S. C. Phorbol esters and horseradish peroxidase stimulate pinocytosis and redirect the flow of pinocytosed fluid in macrophages. J Cell Biol. 1985 Mar;100(3):851–859. doi: 10.1083/jcb.100.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H., Manenti S. Interaction of myristoylated alanine-rich protein kinase C substrate (MARCKS) with membrane phospholipids. J Biol Chem. 1993 May 15;268(14):9960–9963. [PubMed] [Google Scholar]
- Taniguchi H., Manenti S., Suzuki M., Titani K. Myristoylated alanine-rich C kinase substrate (MARCKS), a major protein kinase C substrate, is an in vivo substrate of proline-directed protein kinase(s). A mass spectroscopic analysis of the post-translational modifications. J Biol Chem. 1994 Jul 15;269(28):18299–18302. [PubMed] [Google Scholar]
- Thelen M., Rosen A., Nairn A. C., Aderem A. Regulation by phosphorylation of reversible association of a myristoylated protein kinase C substrate with the plasma membrane. Nature. 1991 May 23;351(6324):320–322. doi: 10.1038/351320a0. [DOI] [PubMed] [Google Scholar]
- Thelen M., Rosen A., Nairn A. C., Aderem A. Tumor necrosis factor alpha modifies agonist-dependent responses in human neutrophils by inducing the synthesis and myristoylation of a specific protein kinase C substrate. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5603–5607. doi: 10.1073/pnas.87.15.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umekage T., Kato K. A mouse brain cDNA encodes a novel protein with the protein kinase C phosphorylation site domain common to MARCKS. FEBS Lett. 1991 Jul 29;286(1-2):147–151. doi: 10.1016/0014-5793(91)80961-2. [DOI] [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Sihra T. S., Aderem A., Greengard P. Phosphorylation and associated translocation of the 87-kDa protein, a major protein kinase C substrate, in isolated nerve terminals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2253–2256. doi: 10.1073/pnas.86.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. C., Walaas S. I., Nairn A. C., Greengard P. Calcium/phospholipid regulates phosphorylation of a Mr "87k" substrate protein in brain synaptosomes. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5249–5253. doi: 10.1073/pnas.79.17.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs P., Hull M., Webster P., Mâle P., Goud B., Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992 Sep 4;70(5):729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]