Abstract
Hypothalamic orexin/hypocretin (Orx/Hcrt) peptides participate in the regulation of a wide range of physiological processes and are recruited by drugs of abuse. To advance our understanding of the potential of the Orx/Hcrt receptor-1 (Hcrt-r1) as a treatment target for cocaine addiction, the effect of SB334867, a specific Hcrt-r1 antagonist, on reinstatement elicited by cocaine-associated stimuli vs. stimuli associated with a highly palatable conventional reinforcer (sweetened condensed milk [SCM]) was tested. Two separate groups of male Wistar rats were trained to associate a discriminative stimulus (S+) with the response-contingent availability of cocaine (0.25 mg/0.1 ml/infusion) or SCM (2/1 [v/v]) and subjected to reinstatement tests following extinction, during which the reinforcers and S+ were withheld, of cocaine or SCM-reinforced behavior. Following extinction, presentation of the cocaine or SCM S+ produced comparable recovery of responding. Hcrt-r1 blockade by SB334867 (1–10 mg/kg, IP) dose-dependently and selectively reversed conditioned reinstatement induced by cocaine-related stimuli, without interfering with reward seeking produced by the same stimulus when conditioned to SCM. The findings implicate an important role for Hcrt-r1 in appetitive behavior controlled by reward-related stimuli with selectivity for cocaine seeking and identify Hcrt-r1 as a potential treatment target for cocaine relapse prevention.
Keywords: Orexin, hypocretin, cocaine-seeking behavior, natural reward, relapse
Introduction
Orexin A (Orx-A or hypocretin-1 [Hcrt-1]) and orexin B (Orx-B or hypocretin-2 [Hcrt-2]) are hypothalamic neuropeptides that bind two receptors, Hcrt-r1 and Hcrt-r2 [1] and regulate many physiological processes, including feeding, energy metabolism, and arousal [2]. Orx/Hcrt neurons in the lateral hypothalamus (LH) play a central role in drug-directed behavior [3]. Orx/Hcrt neurons in the LH become activated by stimuli associated with food, morphine, ethanol, and cocaine [3]. The Hcrt-r1 antagonist SB334867 blocks the acquisition of cocaine-induced behavioral sensitization and cocaine-induced potentiation of excitatory currents in ventral tegmental area (VTA) dopamine neurons [4]. Intra-VTA SB334867 administration reduces the motivation to self-administer cocaine and attenuates the cocaine-induced enhancement of dopamine signaling in the nucleus accumbens (NAC) [5]. Pharmacological manipulation of the Orx/Hcrt system is particularly effective in modifying the conditioned effects of drug cues in conditioned place preference and reinstatement studies [3,6]. The present study tested the effect of SB334867 on reinstatement elicited by cocaine-related stimuli vs. stimuli conditioned to a potent conventional reinforcer and whether this effect was specific to cocaine seeking.
Methods
Animals
Seventy-five male Wistar rats (Charles River, Wilmington, MA; 200–250 g upon arrival) were housed 2–3 per cage in a temperature- and humidity-controlled vivarium on a reverse 12 h/12 h light/dark cycle with ad libitum access to food and water. All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs
Cocaine (COC; National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile physiological saline. Cocaine or saline vehicle was infused intravenously (IV) in a volume of 0.1 ml over 4 s. SB334867 (N-[2-methyl-6-benzoxazolyl]-N′-1,5-n-aphthyridin-4-yl urea; Eli Lilly Research Laboratories, Indianapolis, IN) was dissolved in 10% dimethyl sulfoxide (v/v) and 1% hydroxypropyl-β-cyclodextrin (w/v) in sterile water and administered intraperitoneally (IP) in a volume of 5 ml/kg, 30 min before the behavioral testing.
Apparatus
The animals were trained and tested in standard 29 × 24 × 19.5 cm operant conditioning chambers (Med Associates, St. Albans, VT) located inside ventilated sound-attenuating cubicles. All of the chambers were equipped with two retractable levers (6 cm above the grid floor), a white cue light above each lever, and a house light located at the top of the chamber’s front panel. Auditory stimuli consisted of a 70 dB white noise produced by a white noise generator (Med Associates, St. Albans, VT) presented via an 80 Ω speaker located in the center of the chamber’s front panel just below the house light and an intermittent tone (7 kHz, 70 dB) generated by a tone source (Sonalert with volume control, Med Associates, St. Albans, VT) also positioned in the center of the chamber’s front panel just above the speaker. Intravenous COC infusions were administered by a syringe pump (Razel Scientific Instruments, Stamford, CT) located outside the sound-attenuating cubicles. Sweetened condensed milk (SCM) was delivered by a syringe pump into drinking reservoirs located on the front panel centered between the active and inactive levers, 4 cm above the grid floor. A computer controlled the delivery of fluids, presentation of auditory stimuli, and recording of behavioral data.
Self-Administration and Conditioning
Behavioral training and testing were conducted as previously described [7–9]. Briefly, rats designated for COC reinstatement were surgically prepared with jugular catheters and given 7 days of recovery before commencing self-administration training. Rats designated for testing with SCM were not subjected to surgical procedures. Self-administration of COC (0.25 mg/0.1 ml, intravenous [IV], delivered over 4 s) or SCM (2:1 [v/v] in distilled water; 0.1 ml delivered into a 0.2-ml receptacle) began on a fixed-ratio 1 (FR1) schedule of reinforcement in daily 120-min (cocaine) or 40-min (SCM) sessions, 5 days per week. Responses at the right, active lever were reinforced, followed by a 20-s time-out (TO) period signaled by illumination of a cue light above the active lever. During this time, the lever remained inactive to prevent accidental overdosing with cocaine. To maintain identical training and experimental conditions, the signaled TO period was also in effect during SCM self-administration. Responses at the left, inactive lever had no scheduled consequences.
Following 2 weeks of COC or SCM self-administration training, a contingency was introduced whereby responses at the active lever were differentially reinforced in the presence of distinct discriminative stimuli (SD) that signaled reinforcer availability vs. non-availability. A constant 70 dB white noise served as a discriminative stimulus (S+) for availability of the reinforcer (COC or SCM), whereas illumination of a 2.8 W house light located at the top of the chamber’s front panel served as a discriminative stimulus (S−) that signaled non-availability of the reinforcer (i.e., saline solution instead of COC or no consequence instead of SCM). Sessions were initiated by extension of the levers into the chambers and concurrent onset of the respective SD that remained present until termination of the session by retraction of the levers. In the presence of the S+, responses at the right, active lever were reinforced by COC or SCM on an FR1 schedule and, similar to training, followed by a 20-s TO period signaled by illumination of a cue light above the lever. In the presence of the S−, depression of the right active lever was followed by an intermittent tone, during which the lever remained inactive for 20 s. Three daily sessions (each lasting 1 h for the cocaine group and 20 min for the SCM group) separated by 30-min intervals were conducted, with two “reward” sessions and one “non-reward” session sequenced in random order. SCM conditioning sessions were restricted to 20 min to avoid satiety by excessive ingestion of SCM and ensure that the levels of responding during the first and second SCM sessions were comparable [7–9]. Sessions were initiated by presentation of the respective SD and extension of the levers. The SD remained present until termination of the session by retraction of the levers. After 8 training days (i.e., a total of 16 “reward” and eight “non-reward” sessions), both the COC and SCM groups were placed on extinction (EXT) conditions in both the cocaine and SCM groups in daily 1-h sessions, during which the reinforcers and SD were withheld until a criterion of ≤ 5 responses/session for 3 consecutive days was reached. Reinstatement tests then began under extinction conditions but with reintroduction of the SD, similar to the conditioning phase. To verify that reinstatement was selectively controlled by the reward-paired SD (i.e., S+), the rats were tested in the presence of the SD paired with non-reward (i.e., S−) on the first day of the reinstatement phase. Two days later, tests of the effects of SB334867 on S+-induced reinstatement began. SB334867 was administered 30 min before the onset of the sessions at doses of 0, 1, 3, 10, or 20 mg/kg (IP). Each animal was tested only once with one dose of SB334867 according to a between-subjects design (COC: 0 mg/kg [n = 11], 1 mg/kg [n = 9], 3 mg/kg [n = 9], and 10 mg/kg [n = 10]; SCM: 0 mg/kg [n = 10], 1 mg/kg [n = 9], 3 mg/kg [n = 9], and 10 mg/kg [n = 9]).
Statistical Analysis
Differences in responding at the active lever between the respective reward and non-reward conditions during the last day of the training/conditioning phase were analyzed using paired t-tests, and comparisons of COC S+- or SCM S+-induced reinstatement (under vehicle conditions) were analyzed using unpaired t-tests. Differences in the number of responses between the extinction and reinstatement phases in the COC and SCM groups were analyzed separately using one-way within-subjects analysis of variance (ANOVA). The effects of SB334867 on reinstatement responses induced by the COC S+ or SCM S+ were first analyzed using a two-way between-subjects ANOVA, followed by separate one-way between-subjects ANOVAs for the COC and SCM groups. Significant results in the ANOVAs were followed by Protected Least Significant Difference post hoc tests or pairwise comparisons.
Results
After conditioning, the rats showed stable COC and SCM self-administration and a significant reduction of responding during non-reward sessions (COC, t38 = 15.2, p < 0.001, Fig. 1a; SCM, t36 = 44.3, p < 0.001, Fig. 1a). Responding was then extinguished in sessions during which the reinforcers and stimuli were absent (Fig. 1b), and the effects of SB334867 on reinstatement elicited by the COC or SCM S+ were tested. Both stimuli elicited a similar (t19 = 1.79, p = 0.09, Fig. 1b) recovery of responding (p < 0.01 vs. EXT and S−, pairwise comparison after ANOVA: COC: F2,20 = 20.6, p < 0.001; SCM: F2,16 = 24.8, p < 0.001). SB334867 dose-dependently diminished COC S+-induced reinstatement (two-way between-subjects ANOVA overall dose effect F3,67 = 3.2, p < 0.05) with significant reductions at 3 and 10 mg/kg (p < 0.001 vs. 0 mg/kg, PLSD following one-way ANOVA: F3,34 = 4.3, p < 0.01, Fig. 1b). In contrast, SB334867 did not prevent the reinstating effect of the SCM S+ at any doses tested (one-way ANOVA: F3,33 = 0.09, p > 0.05, Fig. 1b). Inactive lever responses remained low (≤ 4) throughout testing (not shown).
Figure 1.
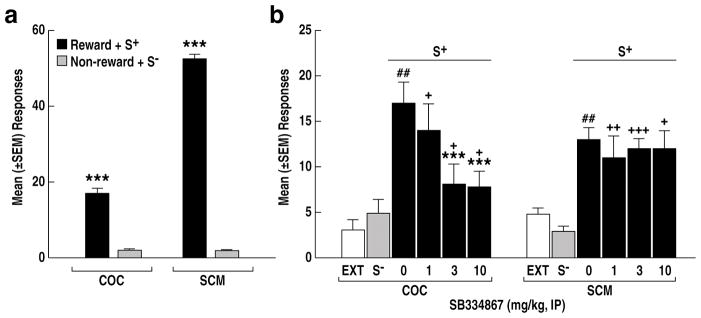
(a) Active lever responses during conditioning sessions in the presence of stimuli paired with COC (reward + S+) vs. non-availability of COC (non-reward + S−) or active lever responses during conditioning sessions in the presence of stimuli paired with SCM (reward + S+) vs. non-availability of SCM (non- reward + S−). ***p < 0.001, vs. non-reward + S−. (b) Following extinction (EXT), presentation of the COC S+ or SCM S+ elicited strong recovery of responding in vehicle-treated rats (0 mg/kg, ##p < 0.01, vs. EXT and S−) and modification of conditioned reinstatement across doses of SB334867 (***p < 0.001, vs. 0 mg/kg; +p < 0.05, ++p < 0.01, vs. EXT and S−).
Discussion
The present findings demonstrate that Hcrt-r1 blockade prevents cocaine-seeking behavior without interfering with the motivating effects of a stimulus conditioned to a highly palatable conventional reinforcer. These findings further support a differential role for the Orx/Hcrt system in drug-seeking behavior vs. normal motivated behavior and are consistent with the hypothesis that the hypothalamic Orx/Hcrt system is a substrate for cocaine seeking and that Hcrt-r1 plays an important role in the modulation of cocaine-seeking behavior.
Before discussing the implication of the findings, it is necessary to examine the differences in baseline levels of responding during conditioning, that were considerably higher with SCM than cocaine, although the level of reinstatement induced by the cocaine- and SCM- associated stimulus was similar. It is well established, however, that reinforcing efficacy and rate of responding on fixed-ratio schedules are not necessarily correlated [10,11]. Indeed, the concentration of SCM used in the present study has been shown to maintain breaking points (an index of reinforcing efficacy) under a progressive-ratio schedule comparable to those measured with cocaine at the present dose [12–14]. Moreover, the magnitude of the response reinstatement induced by the cocaine- and SCM S+ during the reinstatement tests were statistically identical, which was the goal of the present paradigm, suggesting that under the present conditions reliable and comparable conditioning effects occurred for the cocaine and SCM reinforcers, consistent with previous reports [7–9,12].
One hypothesis concerning the control of drug-seeking behavior is that the neural circuits that mediate these effects are common motivational circuits that are more robustly activated by drug-related stimuli and not specific to addiction-related events. This activation that normally governs responding for natural rewards may have created new motivational states or tilted processes that normally govern responding for natural rewards toward drug-directed behavior [15]. Pharmacological manipulation of the Orx/Hcrt system is particularly effective in modifying the conditioned effects of drug cues in conditioned place preference and reinstatement studies [3,16,17]. In the present study, SB334867 exerted a clear preferential effect on cocaine vs. SCM seeking. One possible explanation for this specific effect on cocaine seeking is that, during conditioning, cocaine has neuroadaptively altered neural systems that regulate motivation normally directed at natural rewards, which is revealed by pharmacological (e.g., SB334867) manipulations. This hypothesis is consistent with earlier findings that described maladaptive recruitment of the Orx/Hcrt system by drugs of abuse, reflected by neuroadaptative changes that occur in the VTA [18]. Self-administration of both cocaine and natural reward induces common, short-lasting, increased glutamatergic function in VTA dopaminergic neurons [18]. This enhanced synaptic strength is persistent and resistant to extinction only in rats that self-administer cocaine [18]. The participation of the VTA in cocaine-induced neuronal and behavioral changes requires Orx/Hcrt inputs. Hcrt-r1 activation in the VTA is necessary for the development of cocaine-induced locomotor sensitization [4], and Orx-A/Hcrt-1-mediated N-methyl-D-aspartate receptor plasticity in the VTA is increased in rats that self-administer cocaine [19]. Short-lasting neuroadaptations in VTA dopaminergic neurons are induced by high-fat chocolate food pellets [19], suggesting that the Hcrt/Orx-VTA system initially regulates the motivation to obtain reinforcers in general (e.g., drug or highly palatable food). In contrast, drug-induced neuroadaptation of the Hcrt/Orx-VTA system is long-lasting, an effect that may be linked to the tilting of this system toward controlling drug-directed behavior.
SB338467 (10–30 mg/kg) decreases discrete cue-induced reinstatement of cocaine seeking [6]. In the present study, SB334867 prevented the conditioned reinstatement of cocaine seeking at a much lower dose (3 mg/kg), with a trend already observed at 1 mg/kg, and was specific to cocaine seeking up to 10 mg/kg. The inhibitory effects of SB334867 on natural reward seeking were previously described [20], but still unclear is why SB334867 suppressed both cocaine and natural reward seeking in earlier studies. One hypothesis is that because of the use of higher doses in earlier work (the lowest dose tested in the previous studies was the highest dose tested in the present study [6,20]), SB334867 might have antagonized Hcrt-r2, which is known to suppress locomotion [6]. Further studies are warranted to clarify the nonspecific effect of SB334867 at higher doses.
Overall, the present data show that antagonizing Hcrt-r1 preferentially reverses the conditioned reinstatement of cocaine vs. SCM seeking, suggesting that targeting Hcrt-r1 offers an approach to prevent cocaine seeking without nonspecific side effects that interfere with normal motivated behavior.
Acknowledgments
This is publication number 24058 from The Scripps Research Institute. Research was supported by NIH/NIDA DA033344 (R.M-F.), DA08467, and DA07348 (F.W.). We thank Dr. D. McKinzie, Eli Lilly (Indianapolis, IN), for providing SB3384867 and M. Arends for editorial assistance.
Abbreviations
- SB334867
N-(2-Methyl-6-benzoxazolyl)-N′-1,5-n aphthyridin-4-yl urea
- Orx/Hcrt
orexin/hypocretin
- Hcrt-r1
hypocretin receptor-1
Footnotes
Conflict of Interest: None declared
References
- 1.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 2.Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 3.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 4.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement motivated by cocaine vs natural reward. Neuropsychopharmacology. 2007;32:1967–1973. doi: 10.1038/sj.npp.1301323. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- 11.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 12.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 13.Roberts DC. Self-administration of GBR 12909 on a fixed ratio and progressive ratio schedule in rats. Psychopharmacology (Berl) 1993;111:202–206. doi: 10.1007/BF02245524. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Fardon R, Weiss F. BTCP is a potent reinforcer in rats: Comparison of behavior maintained on fixed- and progressive-ratio schedules. Pharmacol Biochem Behav. 2002;72:343–353. doi: 10.1016/s0091-3057(01)00764-x. [DOI] [PubMed] [Google Scholar]
- 15.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 2013;226:155–165. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


