Abstract
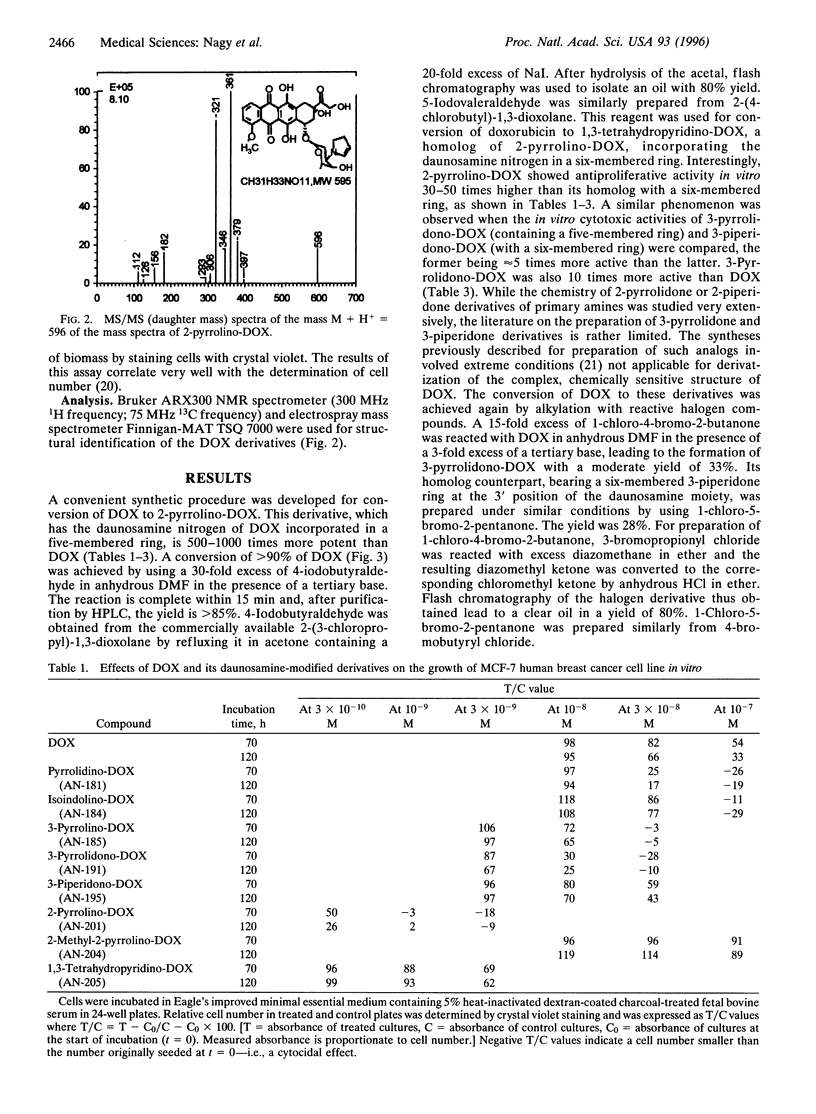
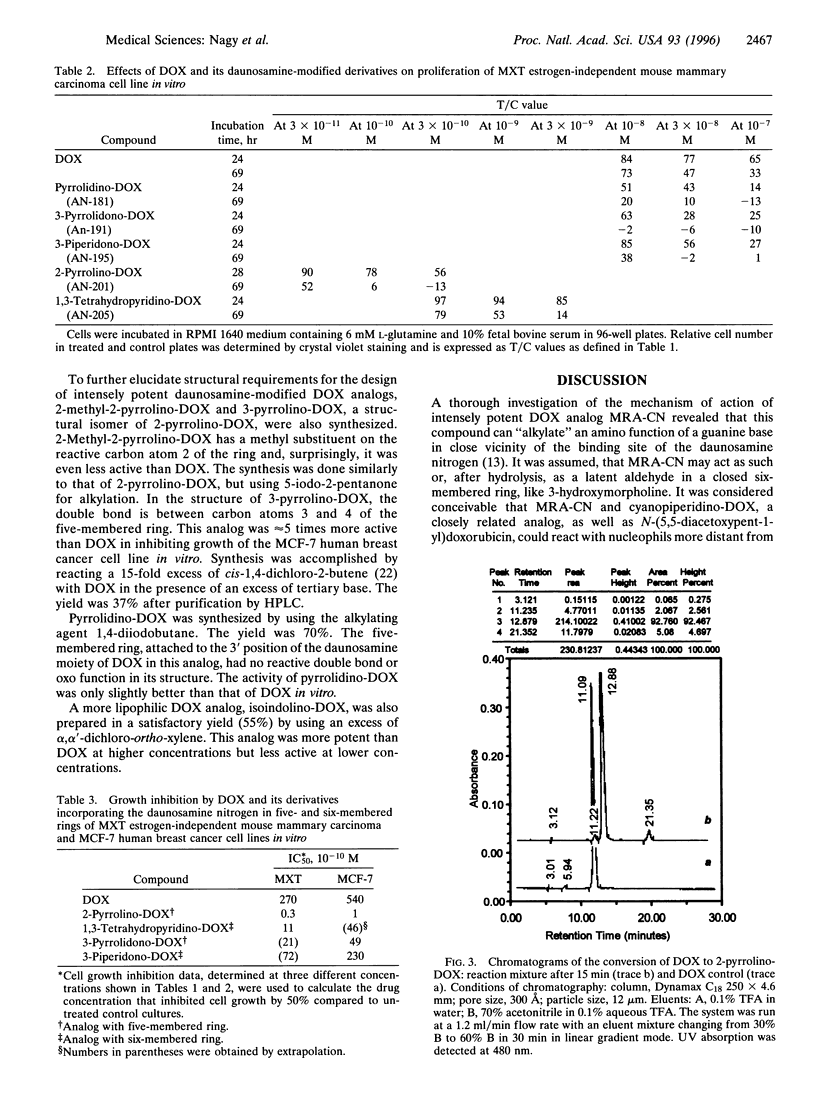
A convenient, high yield conversion of doxorubicin to 3'-deamino-3'-(2''-pyrroline-1''-yl)doxorubicin is described. This daunosamine-modified analog of doxorubicin is 500-1000 times more active in vitro than doxorubicin. The conversion is effected by using a 30-fold excess of 4-iodobutyraldehyde in anhydrous dimethylformamide. The yield is higher than 85%. A homolog of this compound, 3'-deamino-3'-(1'',3''-tetrahydropyridine-1''-yl)doxorubicin, was also synthesized by using 5-iodovaleraldehyde. In this homolog, the daunosamine nitrogen is incorporated into a six- instead of a five-membered ring. This analog was 30-50 times less active than its counterpart with a five-membered ring. A similar structure-activity relationship was found when 3'-deamino-3'-(3''-pyrrolidone-1''-yl)doxorubicin (containing a five-membered ring) and 3'-deamino-3'-(3''-piperidone-1''-yl)doxorubicin (with a six-membered ring) were tested in vitro, the former being 5 times more potent than the latter. To further elucidate structure-activity relationships, 3'-deamino-3'-(pyrrolidine-1''-yl)doxorubicin, 3'-deamino-3'-(isoindoline-2''-yl)doxorubicin, 3'-deamino-3'-(2''-methyl-2''-pyrroline-1''-yl)doxorubicin, and 3'-deamino-3'-(3''-pyrroline-1''-yl)doxorubicin were also synthesized and tested. All the analogs were prepared by using reactive halogen compounds for incorporating the daunosamine nitrogen of doxorubicin into a five- or six-membered ring. These highly active antineoplastic agents can be used for incorporation into targeted cytotoxic analogs of luteinizing hormone-releasing hormone intended for cancer therapy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton E. M., Tong G. L., Mosher C. W., Wolgemuth R. L. Intensely potent morpholinyl anthracyclines. J Med Chem. 1984 May;27(5):638–645. doi: 10.1021/jm00371a014. [DOI] [PubMed] [Google Scholar]
- Arcamone F., Cassinelli G., Fantini G., Grein A., Orezzi P., Pol C., Spalla C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng. 1969 Nov;11(6):1101–1110. doi: 10.1002/bit.260110607. [DOI] [PubMed] [Google Scholar]
- Bristow M. R., Thompson P. D., Martin R. P., Mason J. W., Billingham M. E., Harrison D. C. Early anthracycline cardiotoxicity. Am J Med. 1978 Nov;65(5):823–832. doi: 10.1016/0002-9343(78)90802-1. [DOI] [PubMed] [Google Scholar]
- Cherif A., Farquhar D. N-(5,5-diacetoxypent-1-yl)doxorubicin: a new intensely potent doxorubicin analogue. J Med Chem. 1992 Aug 21;35(17):3208–3214. doi: 10.1021/jm00095a017. [DOI] [PubMed] [Google Scholar]
- Cullinane C., Phillips D. R. Thermal stability of DNA adducts induced by cyanomorpholinoadriamycin in vitro. Nucleic Acids Res. 1993 Apr 25;21(8):1857–1862. doi: 10.1093/nar/21.8.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J., Anderson L., Willmott N., Smyth J. F. The molecular pharmacology of doxorubicin in vivo. Eur J Cancer. 1991;27(5):532–535. doi: 10.1016/0277-5379(91)90209-v. [DOI] [PubMed] [Google Scholar]
- DIMARCO A., GAETANI M., OREZZI P., SCARPINATO B. M., SILVESTRINI R., SOLDATI M., DASDIA T., VALENTINI L. 'DAUNOMYCIN', A NEW ANTIBIOTIC OF THE RHODOMYCIN GROUP. Nature. 1964 Feb 15;201:706–707. doi: 10.1038/201706a0. [DOI] [PubMed] [Google Scholar]
- Gao Y. G., Liaw Y. C., Li Y. K., van der Marel G. A., van Boom J. H., Wang A. H. Facile formation of a crosslinked adduct between DNA and the daunorubicin derivative MAR70 mediated by formaldehyde: molecular structure of the MAR70-d(CGTnACG) covalent adduct. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4845–4849. doi: 10.1073/pnas.88.11.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesson M. I., Johnston J. B., Anhalt C. D., Begleiter A. Effects of 3'-(3-cyano-4-morpholinyl)-3'-deaminoadriamycin and structural analogues on DNA in HT-29 human colon carcinoma cells. Cancer Res. 1987 Nov 15;47(22):5935–5938. [PubMed] [Google Scholar]
- Jesson M. I., Johnston J. B., Robotham E., Begleiter A. Characterization of the DNA-DNA cross-linking activity of 3'-(3-cyano-4-morpholinyl)-3'-deaminoadriamycin. Cancer Res. 1989 Dec 15;49(24 Pt 1):7031–7036. [PubMed] [Google Scholar]
- Johnston J. B., Habernicht B., Acton E. M., Glazer R. I. 3'-(3-Cyano-4-morpholinyl)-3'-deaminoadriamycin: a new anthracycline with intense potency. Biochem Pharmacol. 1983 Nov 1;32(21):3255–3258. doi: 10.1016/0006-2952(83)90214-9. [DOI] [PubMed] [Google Scholar]
- Moscow J. A., Cowan K. H. Multidrug resistance. J Natl Cancer Inst. 1988 Mar 2;80(1):14–20. doi: 10.1093/jnci/80.1.14. [DOI] [PubMed] [Google Scholar]
- Reile H., Birnböck H., Bernhardt G., Spruss T., Schönenberger H. Computerized determination of growth kinetic curves and doubling times from cells in microculture. Anal Biochem. 1990 Jun;187(2):262–267. doi: 10.1016/0003-2697(90)90454-h. [DOI] [PubMed] [Google Scholar]
- Sikic B. I., Ehsan M. N., Harker W. G., Friend N. F., Brown B. W., Newman R. A., Hacker M. P., Acton E. M. Dissociation of antitumor potency from anthracycline cardiotoxicity in a doxorubicin analog. Science. 1985 Jun 28;228(4707):1544–1546. doi: 10.1126/science.4012308. [DOI] [PubMed] [Google Scholar]
- Streeter D. G., Taylor D. L., Acton E. M., Peters J. H. Comparative cytotoxicities of various morpholinyl anthracyclines. Cancer Chemother Pharmacol. 1985;14(2):160–164. doi: 10.1007/BF00434357. [DOI] [PubMed] [Google Scholar]
- Weiss R. B. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992 Dec;19(6):670–686. [PubMed] [Google Scholar]
- Zwelling L. A., Altschuler E., Cherif A., Farquhar D. N-(5,5-diacetoxypentyl)doxorubicin: a novel anthracycline producing DNA interstrand cross-linking and rapid endonucleolytic cleavage in human leukemia cells. Cancer Res. 1991 Dec 15;51(24):6704–6707. [PubMed] [Google Scholar]


