Abstract
Neovascularization is an innate physiologic response by which tissues respond to various stimuli through collateral remodeling (arteriogenesis) and new vessel formation from existing vessels (angiogenesis) or from endothelial progenitor cells (vasculogenesis). Diabetes has a major impact on the neovascularization process but the response varies between different organ systems. While excessive angiogenesis complicates diabetic retinopathy, impaired neovascularization contributes to coronary and peripheral complications of diabetes. How diabetes influences cerebral neovascularization remained unresolved until recently. Diabetes is also a major risk factor for stroke and poor recovery after stroke. In this review, we discuss the impact of diabetes, stroke, and diabetic stroke on cerebral neovascularization, explore potential mechanisms involved in diabetes-mediated neovascularization as well as the effects of the diabetic milieu on poststroke neovascularization and recovery, and finally discuss the clinical implications of these effects.
Keywords: acute stroke, diabetes, arteriogenesis, angiogenesis, brain recovery
Introduction
The simple fact that the mammalian brain, which accounts for only 2% of the body mass, actually receives 20% of the cardiac output makes one realize the complexity and the importance of the cerebrovascular network in proper brain function.1, 2 It is estimated that the capillary network of the brain runs ∼400 miles long and that there are up to 100 billion vessels in the brain.2, 3, 4 This elaborate vascular system, especially the cerebral microvasculature, quickly adapts and responds to physiologic, pathologic, and microenvironmental stimuli in a very dynamic manner. For instance, under hypoxic conditions, blood vessel networks expand to meet the growing oxygen demands and brain capillary density can double in 3 weeks.5 This neovascularization response requires new vessel formation through sprouting angiogenesis as well as remodeling of the existing vasculature to form new collaterals. Both these processes are tightly modulated by environmental cues and in this context, it is highly likely that the neovascularization response of the brain may differ under physiologic and disease conditions.
Diabetes increases the risk of a number of neurologic disorders including stroke, vascular cognitive impairment, and Alzheimer's disease, in all of which the cerebrovasculature has an important role in disease onset, progression, and treatment.6 On the basis of the fact that diabetes is the most rapidly increasing risk factor for stroke, stroke is the leading cause of disability, and reparative angiogenesis is being pursued as a therapeutic strategy, the purpose of this review is to take a closer look at cerebral neovascularization in diabetes and stroke.
I. Neovascularization Processes: Vasculogenesis, Angiogenesis, and Arteriogenesis and Vascular Remodeling
Neovascularization (new blood vessel formation) occurs through vasculogenesis, angiogenesis, and/or arteriogenesis. Although all three can occur in response to tissue hypoxia and injury, they differ in the molecular triggers and underlying mechanisms. Because excellent recent reviews describe these concepts in detail,2, 7, 8, 9, 10, 11 we will briefly describe these processes and focus on the key players that are involved in cerebral neovascularization in diabetes and stroke described in next sections.
Vasculogenesis is the formation of a primitive endothelial network from mesenchymal stem cells or endothelial progenitor cells in response to local cues. This unorganized and undifferentiated plexus is further developed by angiogenesis not only mediating embryonic blood vessel formation, but also contributing to neovascularization in the adult.12, 13
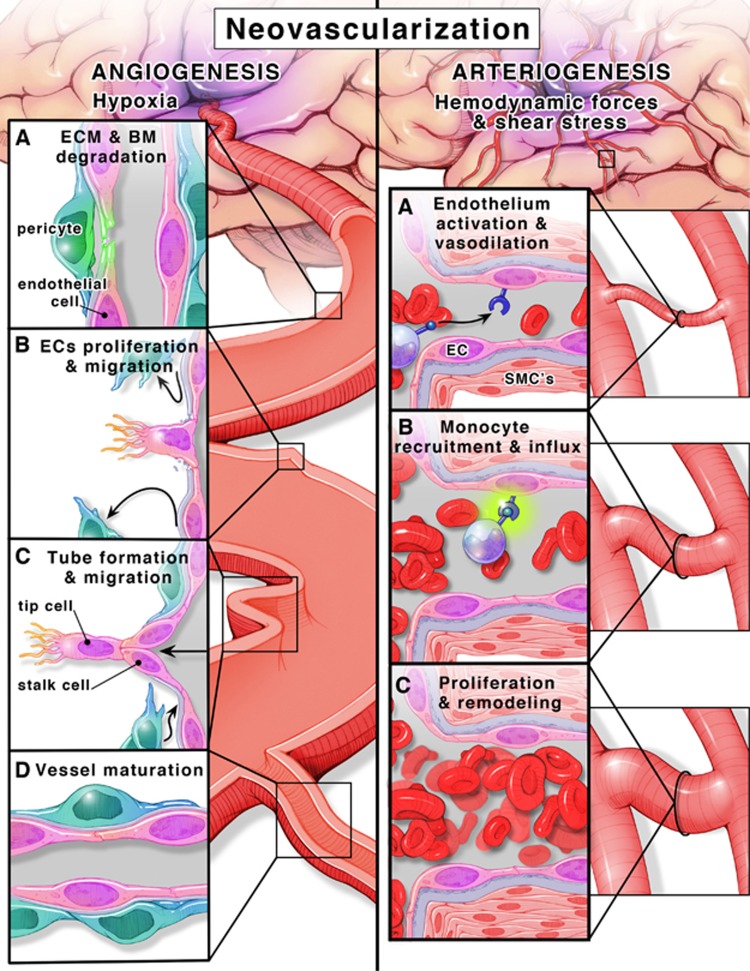
Angiogenesis is defined as the formation of new capillaries from preexisting vessels in a multistep process (Figure 1). Hypoxia is a key stimulus for angiogenesis and through the activation of hypoxia inducible factor-1α, pro-angiogenic molecules such as vascular endothelial growth factor-A (VEGF-A) and VEGF receptor 2 (VEGFR-2) (flt-1), angiopoietins (Ang-1 and -2) and cognate receptor Tie-2, neuropilin-1, and basic fibroblast growth factor are stimulated. These growth factors activate otherwise quiescent endothelial cells to start the angiogenic cascade. When there is VEGF-A and the Ang-2/Ang-1 ratio is high, sprouting angiogenesis occurs.14, 15 Specialized endothelial cells (the so-called ‘tip' cells) lead the process along the VEGF-A gradient.7, 8, 16 It was recently shown that endothelial cells are in a constant competition to assume the tip cell role and there is a dynamic exchange from a stalk cell to a tip cell or vice versa to ensure that the sprout is guided in the correct direction toward highest VEGF-A levels.17, 18 In this regard, vessel sprouting is similar to axonal sprouting where guidance signals regulate sprout direction and elongation.19, 20 Roundabout-4 (Robo4), an endothelial cell-specific member of the neuronal guidance molecules,19, 21, 22 has been shown to inhibit endothelial cell migration and has a role in angiogenesis and vascular patterning, vascular stability, and directional endothelial cell migration.23, 24, 25 Tip cells also inhibit stalk cells from becoming tip cells via the Notch-Delta like ligand (deltall) system and again this is dynamically regulated. These tube-like structures are stabilized and become capillaries by Ang-1-mediated recruitment of pericytes and basement membrane deposition.8, 16, 26
Figure 1.
Angiogenesis, formation of new vessels from existing ones (left panel). (A) ECM and BM degradation. Microenvironment hypoxia is a key stimulus for angiogenesis. Hypoxia activates the transcription factor HIF-1α that stimulates the transcription of pro-angiogenic molecules, growth factors (VEGF-A, VEGFR-2, FGF, and MMPs) and switches the environment balance toward angiogenic milieu (Ang-1/Ang-2). MMPs mediate ECM and BM degradation. (B) ECs proliferation and migration. Pericytes (blue) detach away from ECs (pink). The nearest EC to the highest gradient for VEGF transforms to tip cell that guide the following ECs (stalk cells) toward hypoxic tissue. (C) Tube formation and migration. Stalk cells proliferate and migrate forming a tube-like structure. (D) Vessel maturation. In the final stages, recruitment of pericytes promotes maturation and stabilization. Arteriogenesis, transformation of existing vessels into larger vessels in the normoxic tissue surrounding the ischemic area (right panel). (A) Endothelium activation and vasodilation. Hemodynamic forces and increased shear stress in collaterals activate vascular endothelium to proliferate and induce vasodilation. (B) Monocyte recruitment and influx. Upregulation of VCAM-1 and ICAM-1 and increased expression of MCP-1 and GM-CSF result in recruitment of monocytes. (C) Proliferation and remodeling. Monocytes transformed into macrophages secrete TNF-α and FGF that induce SMC (pink cells with pink nuclei) proliferation. Eventually, SMCs proliferation promotes outward remodeling and vessel maturation. Ang-1, angiopoietin-1; Ang-2, angiopoietin-2;, ECM, extracellular matrix; BM, basement membrane; ECs, endothelial cells; FGF, fibroblast growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HIF-1α, hypoxia inducible factor-1α; ICAM-1, intercellular adhesion molecule-1; MCP-1, monocyte chemotactic protein-1; MMPs, matrix metalloproteases; VEGF-A, vascular endothelial growth factor-A; VCAM-1, vascular cell adhesion molecule-1; SMCs, smooth muscle cells; TNF-α, tumor necrosis factor-α; VEGFR-2, VEGF receptor 2.
Arteriogenesis, however, is a further development of these capillaries into larger vessels like arterioles and venules or remodeling and maturation of already existing collateral arterioles that are subjected to low flow conditions under normal conditions. Unlike angiogenesis, arteriogenesis occurs in the normoxic tissue surrounding the ischemic area, triggered by increased fluid shear stress as a result of blood flow redistribution after vessel occlusion. Physical forces and increased shear stress result in endothelium activation (Figure 1).27 Together with vasodilation and increased permeability, upregulation of adhesion molecules and monocyte chemoattractant protein-1 results in monocyte recruitment and migration to the endothelium. These events trigger a proliferative phenotype in all layers of the developing vessel.28, 29 Smooth muscle cells (SMCs) switch from the contractile to the proliferative phenotype and start to divide and increase the wall thickness to accommodate the increase in lumen diameter then redifferentiate into the contractile phenotype in the later steps of the arteriogenesis cascade resulting in overall collateral vessel maturation and perfusion.28 A visual comparison between angiogenesis and arteriogenesis is depicted in Figure 1.
II. Diabetes and Neovascularization
The impact of diabetes on neovascularization, in particular on angiogenesis, is most widely studied in the eye and peripheral vasculature. Thus, we will first review the effect of diabetes on cerebral neovascularization and then compare with other vascular beds to provide a perspective.
A. Cerebral neovascularization
In the cerebral circulation, the impact of diabetes on neovascularization was not explored until recently. We have shown that diabetes causes increased, yet dysfunctional, neovascularization in the cerebrovasculature.30, 31, 32 There is increased arteriogenesis (greater number of collaterals and increased vascular tortuosity) in the pial vasculature of type 2 diabetic Goto-Kakizaki (GK) rats.30 A follow-up study provided evidence for increased cerebral angiogenesis and arteriogenesis.31 Vascular density, volume, and surface area in the brain parenchyma were greater in diabetic animals. These indices of neovascularization were greater in the cortex and progressively increased from front to the back of the brain. However, this augmented angiogenesis was associated with poor vessel wall maturity as indicated by reduced pericytes and increased nonperfused vessels and permeability.31
Glycemic control prevents this dysfunctional cerebral neovascularization in diabetes, suggesting that hyperglycemia is a major player driving the angiogenic response.32 Comparative studies with the db/db mouse model of type 2 diabetes showed that augmented cerebral neovascularization is not unique to the GK model of diabetes. Goto-Kakizaki rats had an increase in both microvessel and macrovessel densities suggestive of angiogenesis and arteriogenesis, whereas db/db mice had an increase only in the microvasculature. While branch density and tortuosity of penetrating arterioles were increased in both models of diabetes, lumen diameter of penetrating arterioles was increased only in GK rats. The fact that these models of type 2 diabetes are different species with different disease severity strongly suggests that diabetes has a profound effect on brain microvasculature. Using the same GK model, Beauquis et al33 reported decreased vascularization and capillary branching in the dentate gyrus of the hippocampus, an area associated with memory and learning processes. It is also possible that there are differences in the angiogenic response in very specialized areas of the brain and needs further evaluation especially with respect to disease severity and duration.
This cerebral neovascularization is similar to pathologic angiogenesis that occurs in diabetic retinopathy.16 The retina is like the brain in the sense that it has its own neurovascular unit. Retinal ischemia is a complex event and diabetes-mediated neurodegeneration and glial inflammation contribute to increased apoptosis in pericytes and endothelial cells in retinal microvessels leading to acellular capillary formation and vascular regression.34 Collectively, these changes lead to upregulation of angiogenic molecules VEGF-A, erythropoietin (EPO), and other vascular growth factors34, 35, 36, 37 and result in pathologic angiogenesis, increased vascular leakage, and bleeding.38, 39, 40
Diabetes-induced dysfunctional cerebral neovascularization response is vastly different from the neovascularization observed in other vascular beds. In the coronary circulation, diabetes alters the balance between pro- and antiangiogenic growth factors, impairs endothelial function, and mediates an imbalance in microenvironment redox state of the coronary circulation41 resulting in impaired coronary collateral growth and cardiac angiogenesis.42, 43, 44, 45, 46 In the peripheral circulation, there is again impaired neovascularization in experimental models.31, 47, 48, 49 In the renal circulation, there is increased renal angiogenesis in early stages of diabetes in both clinical and experimental studies.50, 51, 52, 53, 54 These early vascular changes were attributed to increased VEGF expression and mild inflammation.51, 55 At later stages of diabetes, vascular regression occurs, where chronic inflammation leads to increases in vascular permeability, thickening of the glomerular basement membrane, endothelial cell apoptosis, and loss of peritubular capillaries.
B. Potential mechanisms for diabetes-induced dysfunctional neovascularization
1. Cerebrovascular dysfunction and decreased cerebral blood flow. Constant cerebral blood flow is critical for neuronal function and the brain quickly responds to hypoxia by increasing capillary density.9, 56 Numerous studies have reported cerebrovascular dysfunction in various diabetes models at the large artery or small penetrating arterioles levels as we recently reviewed.6 We have also shown that in the GK model, there is cerebrovascular dysfunction and decreased cerebral blood flow,57 which develops shortly after the onset of diabetes. This is accompanied by upregulation of hypoxia inducible factor-1 (unpublished data), suggesting that early vascular dysfunction and decreased blood flow create a hypoxic environment that may be the initial trigger for increased cerebral neovascularization.58 As discussed above, in the retina, hyperglycemia-induced changes in the neurovascular unit and capillary drop-out contribute to pathologic angiogenesis. In the brain, the initial cause of hypoxia seems to be different and improvement of vascular function may be a good therapeutic target to prevent dysfunctional angiogenesis (Figure 2).
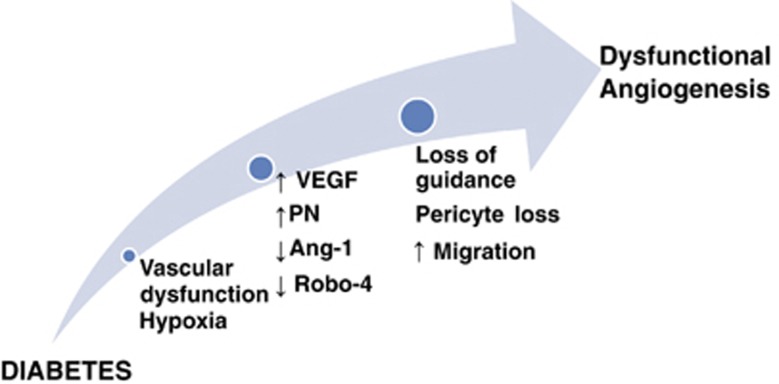
Figure 2.
Cerebral angiogenesis in diabetes. A schematic illustrating the mechanisms by which diabetes induces cerebral dysfunctional angiogenesis. Diabetic hyperglycemia induces vascular dysfunction that creates a state of cerebral hypoxia. Diabetes-induced hypoxia triggers a series of events including (1) increased production of vascular endothelial growth factor (VEGF), (2) increased oxidative and nitrative stress (increased peroxynitrite (PN) formation), (3) decreased angiopoietin-1 (Ang-1), and (4) decreased expression of guidance molecule, Roundabout-4 (Robo-4). These events lead to pericyte loss, increase endothelial migration with loss of guidance that cumulatively increases dysfunctional cerebral angiogenesis.
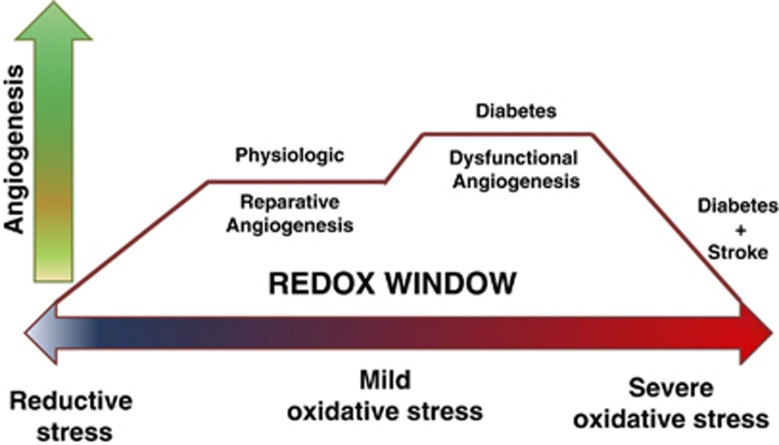
2. Augmented vascular endothelial growth factor-A signaling: involvement of oxidative and nitrative stress. While physiologic angiogenesis represents a fine balance between numerous anti- and pro-angiogenic growth factors, it is widely accepted that VEGF-A has a central role in the regulation of neovascularization. Vascular endothelial growth factor-A is important for endothelial cell proliferation, survival, migration, and tube formation, as well as matrix degradation and vessel permeability.59 Vascular endothelial growth factor-A primarily binds to VEGFR-2,60 then activating extracellular signal-regulated kinases 1 and 2, Src, and phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt) to stimulate cell survival and migration.61 The neovascularization process is affected not only by VEGF and VEGFR-2 levels but also by the redox state of the microenvironment. Mild, but not severe, increases in reactive oxygen and nitrogen species are needed to transduce VEGF's angiogenic signal. This concept is now known as the redox window42 (Figure 3) and might be one explanation for the angiogenic paradox (increased versus impaired angiogenesis) in different vascular beds in diabetes as well as the reason why ‘therapeutic angiogenesis trials' failed.42, 62
Figure 3.
Redox window and angiogenesis. A diagram representing the redox window concept: the tissue redox state ranging from reductive to oxidative levels is depicted on the X axis. Y axis is the angiogenesis process. Physiologic/reparative angiogenesis requires the tissue microenvironment to express mild levels of oxidative stress. Extreme levels of reductive or oxidative stress impair angiogenesis. Oxidative stress levels in diabetes depend on disease severity and promote dysfunctional angiogenesis. Adding stroke to diabetes greatly increases oxidative stress and corrupts the reparative angiogenic process after stroke.
Several studies provided evidence as to how the redox window modulates VEGF-A signaling in diabetic retinopathy.63, 64, 65, 66 Low levels of peroxynitrite, which is rapidly generated from the interaction between superoxide and nitric oxide, sustain and amplify VEGFR-2 signaling leading to pathologic angiogenesis. However, high levels of peroxynitrite nitrate the p85 regulatory subunit of the PI3 kinase and divert the pro-survival effects of VEGF-A to apoptosis in retinal endothelial cells, again emphasizing the redox window concept in angiogenesis.63 We have extended these findings to the brain microvasculature. Endothelial cells isolated from brain microvessels of diabetic GK animals interestingly retain their angiogenic properties in culture and exhibit augmented cell migration and tube formation as compared with cells isolated from control animals. These cells have increased basal VEGF-A and phosphorylated VEGFR-2 levels. The angiogenic properties of endothelial cells that are isolated from diabetic animals can be blocked when cells are treated with a VEGF-A neutralizing antibody or the peroxynitrite scavenger FeTPPs [5,10,15,20-tetrakis (4-sulfonatophenyl) porphyrinato iron (III)].31 These cells also exhibit a defect in their barrier function as measured by transendothelial resistance. They take a longer period of time to establish barrier function as compared with control cells and they are more susceptible to peroxynitrite-mediated loss of barrier function. As will be discussed below, when ischemia/reperfusion is overlaid on this pathology, the redox window is shifted to the right toward excessive oxidative stress and endothelial cell death.
3. Impaired vessel guidance and maturation. As discussed under the general description of angiogenesis, the robo is a family of proteins that act as guidance receptors and were originally identified in the nervous system. Activation of robo1 to 3 by slit ligands (Slit1 to 3) provides repulsive signals for axons.8 Robo4 is uniquely expressed in endothelial cells and its ligand is Slit2.23, 25 The Robo4/Slit2 signaling pathway has recently been identified as a regulator of microvascular maturation, endothelial permeability, and angiogenesis.67, 68, 69 Our ongoing studies suggest that Robo4 protein is significantly decreased in the cerebral microvasculature of diabetic GK rats that develop erratic and dysfunctional angiogenesis (unpublished data). Interestingly, crosstalk between VEGF receptor tyrosine kinases and integrin signaling has been reported. There is a protein–protein interaction between Robo4 and β3 integrin that is associated with a reduction in Robo4/Slit2 signaling leading to vascular hyperpermeability.70 A better understanding of how diabetes impacts Robo4 regulation, especially by VEGF-A, may provide novel targets to prevent and/or treat dysfunctional cerebral angiogenesis in diabetes.
Another important step in angiogenesis is vessel maturation. Angiopoietin-1 promotes migration, sprouting, and survival of endothelial cells through activation of Tie-2 tyrosine kinase receptor14, 15 and it is critical for vessel stabilization. Angiopoietin-2 acts as an antagonist for Ang-1 and inhibits Ang-1-promoted Tie-2 signaling and vessel maturation and stabilization. An Ang-1 peptide mimetic treatment was reported to accelerate wound healing in diabetic animals71 as well as preserve the renal microvasculature.72 An increase in Ang-2/Ang-1 ratio was found to be associated with angiogenic activity in patients with diabetic retinopathy.73 Whether this system is altered in the brain microvasculature in diabetes needs to be established.
Pericytes, located at the periphery of the microvessel wall, communicate with endothelial cells and other cells of the neurovascular unit and are very important for neovascularization and vessel maturation.74 At early stages of angiogenesis, pericytes migrate away as an initial step to allow endothelial cell proliferation and migration.74 At later stages of vessel formation, pericytes increase the stability of newly formed vessels via the prevention of angiogenesis and promote vessel stability via Ang-1 and platelet-derived growth factor-B.75, 76, 77 In the brain, Wnt/β-catenin signaling, a critical pathway in developmental angiogenesis and vascular differentiation, promotes vessel maturation by increasing endothelial platelet derived growth factor-B expression and recruiting pericytes.78 Hyperglycemia-induced dysfunction causes loss of pericytes, which is a hallmark of diabetic retinopathy and other diabetes-induced vascular disease.79 Diabetic rats have less pericytes along microvessels of the brain and increased cerebral angiogenesis.31 However, whether the loss of pericytes gives way to angiogenesis or newly formed vessels are unable to recruit pericytes for maturation is yet to be determined. In this context, similar to the Ang-1/Tie-2 system, the regulation of the platelet derived growth factor-B/platelet derived growth factor receptor-β system in the brain microvasculature in diabetes is still unknown and warrants further research.
4. Chronic inflammation. Diabetes causes a state of chronic inflammation80 and increases VEGF production and other inflammatory cytokines that activate NF-κB, inducing the secretion of several factors including interleukin-1, interleukin-6, tumor necrosis factor-α, chemokine C–C motif ligand-5, and transforming growth factor-β, all of which stimulate angiogenesis.81 Oxidative stress seems to be the link between inflammation and angiogenesis.
In response to inflammation, not only adaptive but also the innate immunity is activated. In this regard, toll-like receptors have a critical role in regulation of the innate immune response. Toll-like receptors may be involved in the regulation of endothelial cell survival and the angiogenic response as well. Lipopolysaccharide, a well-established ligand for TLR4, induces endothelial sprouting.82 In addition, both TLR2 and TLR3 have been reported to promote angiogenesis.83 While there is strong evidence for the key role of inflammation and inflammation-associated oxidative stress plays in angiogenesis, most, if not all, of this evidence comes from pathologic angiogenesis associated with tumor growth and our understanding of the role of this pathway in dysfunctional angiogenesis of the brain is yet to be shown.
5. Uncharted mechanisms and remaining questions. As discussed above, VEGF-A and Ang-1 are the main pro-angiogenic factors in the brain and the eye but undoubtedly other factors are involved. There are two newly identified molecules that seem to be uniquely involved in pathologic angiogenesis. While searching the retinal microvessel transcriptome for factors contributing to the erratic neovascularization that occurs in diabetic retinopathy, Wang et al84 discovered a protein called leucine-rich alpha-2-glycoprotein 1 of previously unknown function. Leucine-rich alpha-2-glycoprotein 1 mediates angiogenesis through the regulation of endothelial transforming growth factor-β signaling and this novel protein may be predominantly involved in uncontrolled angiogenesis. The other intriguing protein is ataxia telangiectasia mutated kinase or simply ATM kinase, which is involved in DNA repair and damage. Activation of ATM by oxidative stress suppresses the p38MAP kinase pathway and leads to excessive neovascularization in the retina.85 Further research is needed to determine how diabetes impacts the expression and action of these proteins across vascular beds and especially in the brain.
The net result of angiogenesis depends on the balance of pro- and antiangiogenic factors. Angiopoietin-2, angiostatin, endostatin, thrombospondin-1, and soluble VEGF receptor (sFlt-1) are among antiangiogenic factors that have been shown to impact the neovascularization process in other tissues.86, 87 However, the regulation and the impact of these antiangiogenic factors in the brain in diabetes are not fully understood.
In summary, diabetes stimulates dysfunctional neovascularization of the brain. Potential underlying mechanisms that are briefly discussed above are summarized in Figure 2. Unstable, leaky and dysfunctional vessels cause increased blood–brain barrier permeability and cannot meet the demands of the brain for proper blood flow and nutrient delivery. Dysfunctional angiogenesis of the brain in diabetes is a new concept. Our knowledge of pathologic angiogenesis comes from tumor angiogenesis and diabetic retinopathy. Given that diabetes is an exponentially growing risk factor for stroke and neurodegenerative disorders with cognitive impairment including dementia and Alzheimer's disease, there is an urgent need for further studies involving the effect of the type of diabetes and the degree/duration of hyperglycemia on spatial and temporal regulation of cerebral angiogenesis and maturation.
III. Stroke and Neovascularization
Angiogenesis genes are upregulated within minutes of the onset of cerebral ischemia in rodents.88, 89 There is now mounting evidence that angiogenesis occurs in concert with neurogenesis and synaptogenesis in experimental models of recovery after ischemic brain injury.90 These processes take place in response to a disparate range of interventions, from cortical stimulation91 to antidepressant therapy.92 Although the time course of angiogenesis and neurogenesis overlaps, many investigators have now concluded that the angiogenesis occurs first and leads to axonal remodeling93 and neuroblast migration along new blood vessels.94 A recent study showed that endothelial cells transplanted into the brain promote vasculogenesis and enhance neurogenesis further providing support for this concept.95 This neuroplasticity is important for meaningful functional recovery, but may be diminished by aging and other comorbidities.93
There is emerging evidence that neuroplasticity and recovery after brain injury involve areas remote from the injury itself.96, 97 In an investigation of the beneficial effects of EPO on motor recovery, EPO was administered after temporary middle cerebral artery occlusion (MCAO) in a rodent model and was shown to induce improved perilesional remodeling that was accompanied by increased axonal sprouting from the contralesional hemisphere.96 This concept was supported by an investigation showing an early increase in brain-derived neurotrophic factor expression in both hemispheres after experimental ischemia in rats, and this was followed by a rise in synaptophysin (a marker of synaptogenesis) ipsilaterally.97 Since it is clear that neuroblasts migrate along blood vessels in areas of angiogenesis after stroke,94 it is logical that the plastic changes in the contralesional hemisphere are accompanied by contralesional angiogenesis. In fact, it has been shown that blockade of VEGFR-2 can prevent postischemic neurovascular remodeling by preventing neuroblast migration along blood vessels.98 Most investigators have focused their efforts on quantification of angiogenesis after stroke in the peri-infarct areas, however.90
Recently, we showed that the angiotensin II type 1 receptor antagonist, candesartan, when administered at reperfusion in a rat model of temporary MCAO, promoted recovery and angiogenesis in the contralesional striatum at 7 days after the stroke.99 This was confirmed in a more recent investigation where normoglycemic rats showed increased angiogenesis and recovery after stroke compared with diabetic animals, where vascular regression after stroke accompanied a much poorer functional outcome.100 In summary, angiogenesis occurs after stroke and is closely linked to recovery. A lack of consensus on the contribution of angiogenesis to stroke recovery may be due to a failure to look beyond the peri-infarct area and the use of the contralesional hemisphere as a convenient control. The impact of premorbid vascular diseases on cerebral angiogenesis after stroke needs to be further investigated.
IV. Neovascularization after Stroke in Diabetes
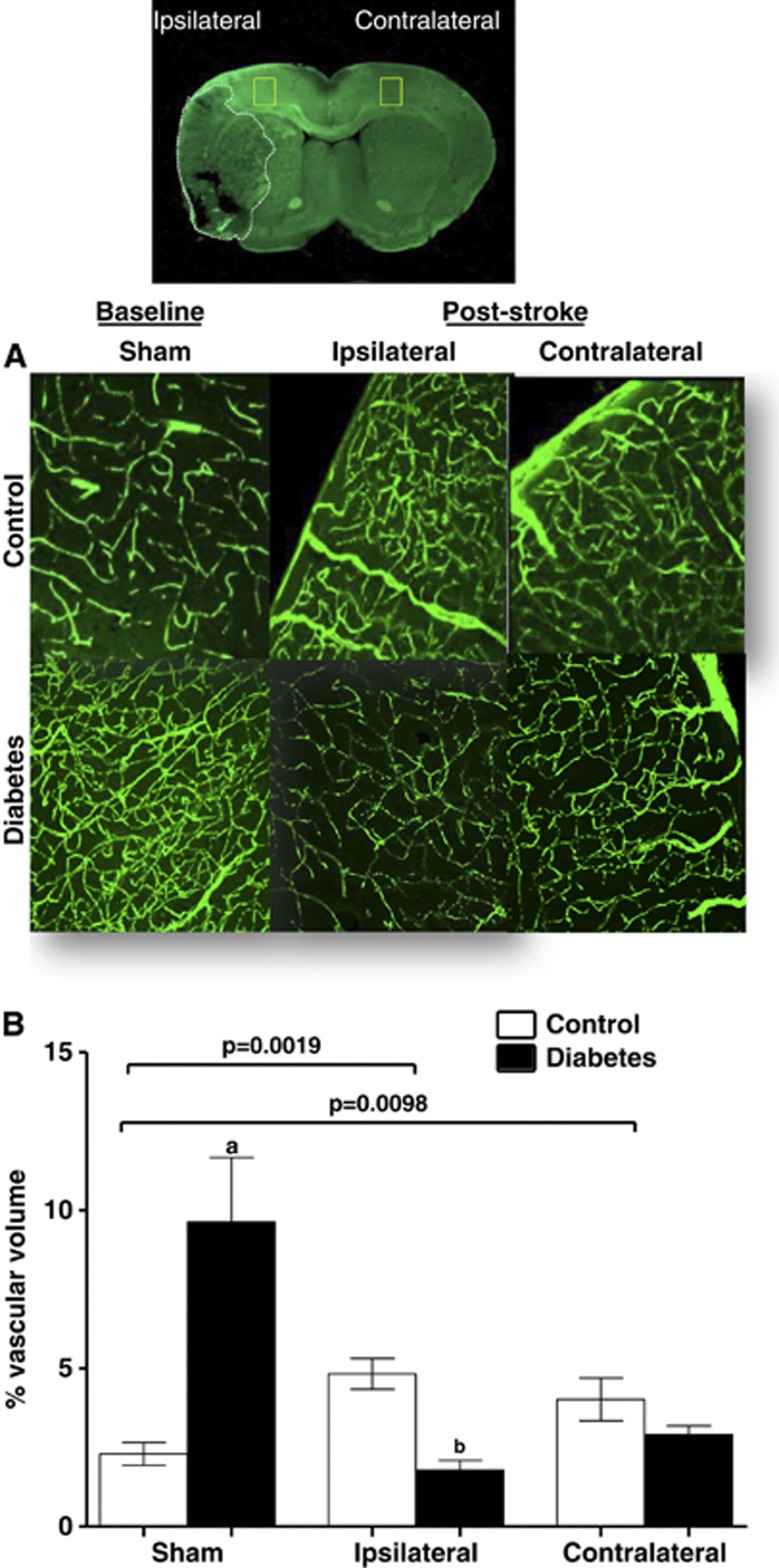
Angiogenesis can improve functional recovery from stroke as discussed above. However, it has to be recognized that most experimental studies used young and healthy animals without confounding factors that are commonly found in patients. As discussed above, diabetes stimulates dysfunctional and uncontrolled angiogenesis in the cerebral vasculature. If these animals are subjected to ischemic stroke, then they develop greater vascular injury including hemorrhagic transformation, especially around the infarcted area, and edema. Ultimately, animals exhibit poor functional outcome.101, 102, 103 To determine the impact of diabetes on cerebral neovascularization after an ischemic event, we compared and contrasted various indices of cerebral neovascularization in the ipsilateral ischemic and contralateral hemispheres of control and diabetic rats subjected to sham or stroke surgery. Several important observations were made. While there was reparative neovascularization in control animals in both ischemic and nonischemic hemispheres as compared with the sham group, diabetic animals developed a significant vasoregression in both hemispheres (Figure 4).100 This was associated with increased astrocytic swelling and poor functional recovery. Glycemic control during the recovery phase after stroke partially prevented the robust decline in vascularization and improved outcome. Other studies also showed that type 1 and type 2 diabetes impair angiogenesis after stroke.104, 105 Vessel density in the ipsilateral hemisphere 14 days after stroke is increased but these vessels are not mature as indicated by reduced diameter, arteriolar density, and SMCs.104, 105 These studies were conducted in stroked animals and there were no sham control animals thus it is not possible to comment on how stroke injury affected the neovascularization response in the nonischemic hemisphere.
Figure 4.
Diabetes impairs poststroke neovascularization in the ipsilateral and contralateral hemispheres. Vascularization was assessed in the ipsilateral and contralateral hemispheres in control and diabetic animals 14 days after 90-minute occlusion of the middle cerebral artery. Sham animals were exposed to anesthesia and neck dissection was performed and sutured without occluding middle cerebral artery. Three-dimensional reconstruction of the fluorescein isothiocyanate (FITC)-stained vasculature was achieved analysis of the z-stack confocal images by the Volocity program. (A) Representative cortical images contrasting ipsilateral and contralateral zones in control and diabetic animals. (B) Plot depicting vascular volume across groups. aP<0.05 versus sham control or ipsilateral control and diabetes, bP<0.05 versus control. Data were analyzed with a 2 × 2 design for disease (control versus diabetes) and intervention (sham versus stroke) in the ipsilateral or contralateral hemispheres. There was a significant interaction indicating important differences in vascularization at baseline and after stroke in the diabetes group. n=6 to 9. (Modified from100 with permission from Lippincott, Williams, and Wilkins.)
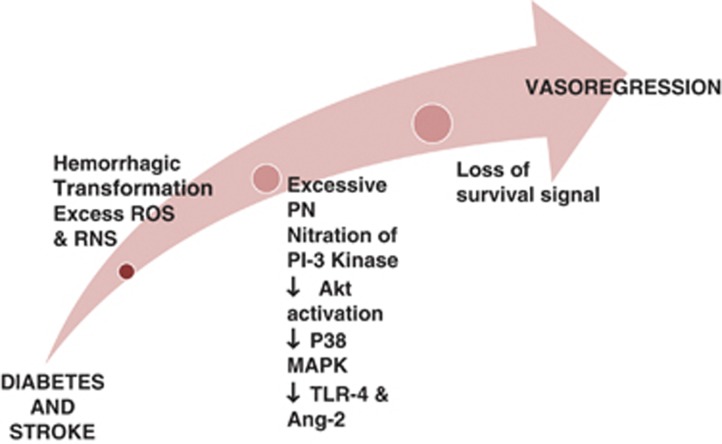
The mechanisms by which diabetes impairs the repair process and causes this dramatic decline in the cerebrovascular network after stroke are unknown and likely to be multifactorial (Figure 5). One potential mechanism may be the redox microenvironment as discussed above. It appears that in diabetic stroke, there is a nitrative switch. In diabetic animals, there is even greater peroxynitrite formation in the cerebrovasculature after stroke and this is associated with greater endothelial apoptosis (unpublished data). There is a significant decrease in Akt signaling and a concomitant increase in p38 signaling in brain microvascular endothelial cells isolated from diabetic animals when exposed to hypoxia and reoxygenation which can be prevented by peroxynitrite scavenging.
Figure 5.
Cerebral neovascularization in diabetes after stroke. A representative diagram illustrating the mechanisms by which vasoregression of cerebral vessels occurs in diabetes after stroke. When stroke is overlayed on diabetes pathology, there is greater hemorrhagic transformation and free iron accumulation that induces cell death. In addition, adding stroke injury to diabetes dramatically increases peroxynitrite (PN) formation and nitration of p85 regulatory subunit of PI3 kinase that downregulates the downstream pro-survival Akt pathway and activates the pro-apoptic p38MAP kinase pathway. Finally, increases in angiopoietin-2 (Ang-2) and activation of toll-like receptor 4 (TLR4) increase endothelial death and promote vasoregression in diabetes after stroke. ROS, reactive oxygen species; RNS, reactive nitrogen species.
It is also of great interest that in both type 1 and type 2 diabetes models, in which repair and recovery are impaired, there is increased bleeding into the brain after ischemic stroke.101, 102, 103, 104, 105 However, the impact of bleeding on vascular repair has not been fully studied. Evidence from intracranial hemorrhage models suggests that hemoglobin and heme released from red blood cells enter the brain parenchyma and free iron from further degradation of the heme molecule, disrupts cellular integrity and function via increased oxidative stress.106, 107, 108, 109 It is also intriguing that heme upregulates TLR4,110 a gate keeper of the innate immune system and TLR4 mediates disruption of endothelial barrier function.111 These observations collectively raise the possibility of bleeding and TLR4 being additional mechanisms involved in the vasoregression and impaired repair process after diabetic stroke.
The possibility of increased antiangiogenic molecules mediating vascular regression cannot be overlooked. An interesting study showed that angiogenesis is impaired in the GK diabetic model after stroke and this is associated with decreased VEGF and increased angiostatin signaling.112 In other studies where immature vessel formation was observed,104, 105 authors reported increased Ang-2 and decreased Ang-1 expression in the brain sections of diabetic rats.103, 104
V. Clinical Relevance
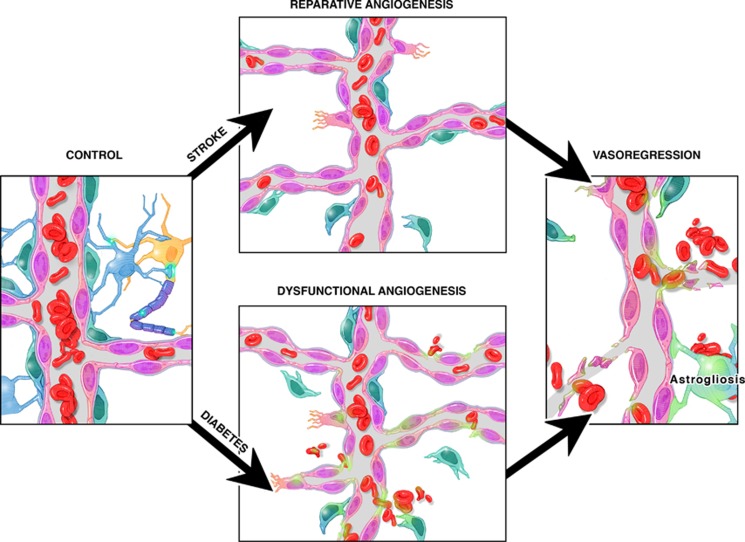
On the basis of experimental evidence, cerebral neovascularization response differs in diabetes, stroke, and diabetic stroke (Figure 6). Important questions remain unresolved with respect to the clinical relevance of these studies: (1) How can we promote adaptive brain neovascularization in health and disease? and (2) Is cerebral angiogenesis always good or attainable?
Figure 6.
Cerebral neovascularization in diabetes and stroke. Diabetes causes dysfunctional angiogenesis. Stroke stimulates reparative angiogenesis in the nondiabetic state. However, when stroke occurs in diabetes, survival signals are lost leading to vasoregression.
From a diabetes standpoint, the first strategy is to evaluate the impact of glycemic control. We have shown that regulation of blood glucose is an effective strategy to prevent pathologic neovascularization of the brain and improves vessel maturity.32 We now have evidence that glycemic control with metformin can also reverse established remodeling (Abdelsaid et al, Life Sciences, in press). When one looks at the clinical trials including DCCT (Diabetes Control and Complications Trial) and UKPD (United Kingdom Prospective Diabetes), glycemic control does not decrease stroke incidence that is considered to be a macrovascular complication of diabetes. Given that tight glucose control prevents microvascular complications such as retinopathy and nephropathy, the impact of glycemic control on microvascular disease of the brain such as cognitive impairment and the recovery phase after stroke remains to be determined.
From a stroke perspective, examples of different therapeutic interventions to promote arteriogenesis and angiogenesis are listed in Tables 1 and 2. Three of these agents (candesartan, EPO, and granulocyte colony stimulating factor) showed extremely promising results in experimental studies and made their way to humans, but findings from these clinical trials were disappointing. While they passed initial small-scale phase I clinical safety trials, they failed to show improvement or further worsened stroke outcome in larger multiphase II/III trials.113, 114, 115 These failures are not only due to the vast genetic differences between human and rodent brains, but could also be attributed to the lack of full characterization of these agents experimentally to allow for rigorous design of clinical trials with appropriate patient population, dosing regimen, and end points. A more complete understanding of the mechanisms of actions of these drugs is imperative for translating them from bench to bedside.
Table 1. Arteriogenic interventions in experimental stroke research.
| Treatment | Author | Species | Ischemia model | Assessment techniques | End points |
|---|---|---|---|---|---|
| GM-CSF | Buschmann et al119 | Sprague-Dawley rats | Brain hypoperfusion: left common carotid and bilateral vertebral artery occlusion. | Angiogram using latex perfusion and histochemical analysis for monocytes (CD 68) and proliferation marker, Ki-67 | Posterior cerebral artery arteriogenesis together with increased cell proliferation and monocyte infiltration |
| Niacin | Chen et al120 | Wistar rats | tMCAO for 2 hours | Latex perfusion and double histochemical staining for cell proliferation marker, BrdU and VSMCs (α-SMA) | Increased diameter of the Circle of Willis arteries together with increased arterial diameter and BrdU-positive VSMCs in the ischemic border zone |
| Simvastatin | Zacharek et al121 | Wistar rats | tMCAO for 2 hours | Double histochemical staining for cell proliferation marker, BrdU and VSMCs (α-SMA) | Increased arterial density, diameter, and perimeter in the ischemic border zone |
| G-CSF | Sugiyama et al122 | C57BL/6 mice | Left common carotid artery occlusion | Angiogram using latex perfusion and histochemical analysis for monocytes (Mac-2) | Leptomeningeal collateral growth and monocyte infiltration in the dorsal surface of the brain |
| Tocotrienol (Vitamin E) | Rink et al123 | Mongrel canines | tMCAO for 1 hour | Digital subtraction angiography | Improved leptomeningeal collateral circulation |
| Simvastatin and Human umblical cord blood cells combination | Cui et al124 | Wistar rats | tMCAO for 2 hours | Double immunofluorescent staining for BrdU (marker of proliferating cells) and α-SMA (VSMCs marker) | The diameter and the density of α-SMA arteries were significantly increased in the ipsilateral hemisphere. In addition, the percentage of BrdU-VSMC in the artery walls was also significantly increased at 14 days after stroke |
| GW3965 (synthetic liver X receptor agonist) | Cui et al125 | C57BL/6J mice | tMCAO for 2.5 hours | Double immunofluorescent staining for BrdU (marker of proliferating cells) and α-SMA (VSMCs marker) | GW3965 treatment significantly increased the arterial density and the diameter of α-SMA arteries and the percentage of BrdU-SMCs in arteries |
CD 68, cluster of differentiation 68; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; BrdU, bromodeoxyuridine; α-SMA, α-smooth muscle actin; tMCAO, temporary middle cerebral artery occlusion; SMC, smooth muscle cell; VSMCs, vascular SMCs.
Table 2. Angiogenic interventions in experimental stroke research.
| Treatment | Author | Species | Ischemia model | Assessment techniques | End points |
|---|---|---|---|---|---|
| EPO | Wang et al126 | Wistar rats | Embolic MCAO | Immunohistochemical analysis of FITC-dextran perfused vessels | Increased number of capillary segments at the ischemic boundary |
| Atorvastatin | Chen et al127 | C57BL/6J mice | pMCAO | Immunohistochemical analysis for BrdU and vWF (marker of ECs) double staining | Increased vessel density at 14 days |
| Physical activity and exercise | Gertz et al128 | C57BL/6J mice | tMCAO for 30 minutes | Double histochemical staining for cell proliferation marker, BrdU, and vWF (marker of ECs) | Increased vessel density in the ischemic striatum at 4 weeks |
| Niaspan (Niacin) | Chen et al129 | Wistar rats | tMCAO for 2 hours | Double immunostaining for cell proliferation marker, BrdU, and endothelial cells (vWF) | BrdU-positive endothelial cells were significantly increased in the ipsilateral hemisphere together with increased vascular perimeter and density |
| Sildenafil | Ding et al130 | Wistar rats | Embolic MCAO | Magnetic resonance imaging | Increased angiogenesis at 6 weeks |
| Niaspan (Niacin) | Ye et al105 | Streptozotocin induced type 1 diabetes in Wistar rats | tMCAO for 2 hours | Immunostaining for vWF (marker of ECs) and α-SMA (marker of VSMCs) in addition to costaining with BrdU (proliferation marker) | Increased vascular and arterial density as well as increased vessel perimeter and arterial diameter in the ischemic brain, in addition to a significant increase in vascular endothelial cells proliferation |
| Candesartan | Guan et al99 | Wistar rats | tMCAO for 3 hours | Immunohistochemical analysis for laminin | Increased cerebrovascular density in both hemispheres at 7 days |
| Valproate | Wang et al131 | Sprague-Dawley rats | tMCAO for 60 minutes | Double staining for the proliferation marker, Ki67, and endothelial cells (RECA-1) | Increased microvessel density in the ipsilateral cortex at 14 days |
| Simvastatin and human umbilical cord blood cells combination | Cui et al124 | Wistar rats | tMCAO for 2 hours | Double immunofluorescent staining for BrdU (marker of proliferating cells) and vWF (marker of ECs) | Vascular density and perimeter were significantly increased together with a significant increase in the percentage of BrdU-ECs in the ischemic hemisphere |
| GW3965 (synthetic liver X receptor agonist) | Cui et al125 | C57BL/6J mice | tMCAO for 2.5 hours | Double immunofluorescent staining for BrdU (marker of proliferating cells) and vWF | GW3965 treatment significantly increased the vascular density and the perimeter of vWF vessels and the percentage of BrdU-ECs in vessels |
| GM6001 pan-MMP inhibitor | Yang et al132 | Spontaneously hypertensive rats (SHRs) | tMCAO for 90 minutes | Counting microvessels labeled with RECA-1 (marker of ECs) | Increased number of vessels in the peri-infarct area at 3 weeks |
Epo, erythropoietin; BrdU, bromodeoxyuridine; ECs, endothelial cells; α-SMA, α-smooth muscle actin; tMCAO, temporary middle cerebral artery occlusion; pMCAO, permanent middle cerebral artery occlusion; VSMCs, vascular smooth muscle cells; MMP, matrix metalloproteinase; FITC, fluorescein isothiocyanate; vWF, von Willebrand factor; RECA-1, rat endothelial cell antigen.
From a diabetic stroke perspective, stimulation of angiogenesis does not seem to be a good strategy, at least for now. Chen et al116 showed that cell therapy with bone marrow stromal cells improved repair and functional recovery in control but not in diabetic animals. On the contrary, this approach worsened blood–brain barrier integrity. As elegantly reviewed, therapeutic revascularization and vascular repair strategies face many challenges in other tissues involved in diabetic complications.117, 118 Studies focusing on the impact of these strategies in the brain to prevent and treat cerebral complications of diabetes are only beginning.
Acknowledgments
AE is a research pharmacologist at the Charlie Norwood Veterans Affairs Medical Center in Augusta, Georgia. Authors would like to thank Ms Colby Polonsky for her assistance with Figures 1 and 6.
The authors declare no conflict of interest.
Footnotes
References
- Mink JW, Blumenschine RJ, Adams DB. Ratio of central nervous system to body metabolism in vertebrates: its constancy and functional basis. Am J Physiol. 1981;241:R203–R212. doi: 10.1152/ajpregu.1981.241.3.R203. [DOI] [PubMed] [Google Scholar]
- Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Begley DJ, Brightman MW. Structural and functional aspects of the blood-brain barrier. Prog Drug Res. 2003;61:39–78. doi: 10.1007/978-3-0348-8049-7_2. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ.The cerebral circulationIn: Granger NGaJ, (ed). Integrated systems physiology: from molecule to function Morgan & Claypool Life Sciences: San Rafael (CA)2009 [PubMed] [Google Scholar]
- Xu K, Lamanna JC. Chronic hypoxia and the cerebral circulation. J Appl Physiol. 2006;100:725–730. doi: 10.1152/japplphysiol.00940.2005. [DOI] [PubMed] [Google Scholar]
- Ergul A, Kelly-Cobbs A, Abdalla M, Fagan SC. Cerebrovascular complications of diabetes: focus on stroke. Endocr Metab Immune Disord Drug Targets. 2012;12:148–158. doi: 10.2174/187153012800493477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxid Redox Signal. 2007;9:1363–1371. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- Ward NL, Moore E, Noon K, Spassil N, Keenan E, Ivanco TL, et al. Cerebral angiogenic factors, angiogenesis, and physiological response to chronic hypoxia differ among four commonly used mouse strains. J Appl Physiol. 2007;102:1927–1935. doi: 10.1152/japplphysiol.00909.2006. [DOI] [PubMed] [Google Scholar]
- Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W. Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med. 2006;10:45–55. doi: 10.1111/j.1582-4934.2006.tb00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ. Embryonic and adult vasculogenesis. Birth Defects Res C Embryo Today. 2003;69:73–82. doi: 10.1002/bdrc.10003. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Smadja DM, Levy BI. Postischemic revascularization: from cellular and molecular mechanisms to clinical applications. Physiol Rev. 2013;93:1743–1802. doi: 10.1152/physrev.00006.2013. [DOI] [PubMed] [Google Scholar]
- Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3:a006569. doi: 10.1101/cshperspect.a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arese M, Serini G, Bussolino F. Nervous vascular parallels: axon guidance and beyond. Int J Dev Biol. 2011;55:439–445. doi: 10.1387/ijdb.103242ma. [DOI] [PubMed] [Google Scholar]
- Lee CY, Bautch VL. Ups and downs of guided vessel sprouting: the role of polarity. Physiology (Bethesda) 2011;26:326–333. doi: 10.1152/physiol.00018.2011. [DOI] [PubMed] [Google Scholar]
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Adams RH, Eichmann A. Axon guidance molecules in vascular patterning. Cold Spring Harb Perspect Biol. 2010;2:a001875. doi: 10.1101/cshperspect.a001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, et al. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Samant GV, Pramanik K, Loscombe PW, Pendrak ML, Roberts DD, et al. Silencing of directional migration in roundabout4 knockdown endothelial cells. BMC Cell Biol. 2008;9:61. doi: 10.1186/1471-2121-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M. Angiogenesis: where do we stand now. Circulation. 2005;111:1556–1566. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]
- Cai W, Schaper W. Mechanisms of arteriogenesis. Acta Biochim Biophys Sin. 2008;40:681–692. [PubMed] [Google Scholar]
- van Oostrom MC, van Oostrom O, Quax PH, Verhaar MC, Hoefer IE. Insights into mechanisms behind arteriogenesis: what does the future hold. J Leukoc Biol. 2008;84:1379–1391. doi: 10.1189/jlb.0508281. [DOI] [PubMed] [Google Scholar]
- Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol. 2012;3:353. doi: 10.3389/fphys.2012.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, et al. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes. 2010;59:228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Somanath PR, El-Remessy AB, Kelly-Cobbs A, Stern JE, Dore-Duffy P, et al. Enhanced cerebral but not peripheral angiogenesis in the Goto-Kakizaki model of type 2 diabetes involves VEGF and peroxynitrite signaling. Diabetes. 2012;61:1533–1542. doi: 10.2337/db11-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Johnson M, Fagan SC, Ergul A. Cerebral neovascularization and remodeling patterns in two different models of type 2 diabetes. PLoS ONE. 2013;8:e56264. doi: 10.1371/journal.pone.0056264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauquis J, Homo-Delarche F, Giroix MH, Ehses J, Coulaud J, Roig P, et al. Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats. Exp Neurol. 2010;222:125–134. doi: 10.1016/j.expneurol.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- Whitmire W, Al-Gayyar MM, Abdelsaid M, Yousufzai BK, El-Remessy AB. Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far. Mol Vis. 2011;17:300–308. [PMC free article] [PubMed] [Google Scholar]
- Loukovaara S, Robciuc A, Holopainen JM, Lehti K, Pessi T, Liinamaa J, et al. Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFbeta1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol. 2013;91:531–539. doi: 10.1111/j.1755-3768.2012.02473.x. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Nakama T, Ishikawa K, Arima M, Tachibana T, Nakao S, et al. Antiangiogenic shift in vitreous after vitrectomy in patients with proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53:6997–7003. doi: 10.1167/iovs.12-9671. [DOI] [PubMed] [Google Scholar]
- Rodrigues M, Xin X, Jee K, Babapoor-Farrokhran S, Kashiwabuchi F, Ma T, et al. VEGF secreted by hypoxic muller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes. 2013;62:3863–3873. doi: 10.2337/db13-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorey NJ, Shen W, Chung SH, Zhu L, Gillies MC. The role of glia in retinal vascular disease. Clin Exp Optom. 2012;95:266–281. doi: 10.1111/j.1444-0938.2012.00741.x. [DOI] [PubMed] [Google Scholar]
- Ali TK, Al-Gayyar MM, Matragoon S, Pillai BA, Abdelsaid MA, Nussbaum JJ, et al. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 2011;54:657–668. doi: 10.1007/s00125-010-1935-1. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Hoareau-Aveilla C, Oltean S, Harper SJ, Bates DO. The anti-angiogenic isoforms of VEGF in health and disease. Biochem Soc Trans. 2009;37 (Pt 6:1207–1213. doi: 10.1042/BST0371207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, et al. Redox-dependent mechanisms in coronary collateral growth: the "redox window" hypothesis. Antioxid Redox Signal. 2009;11:1961–1974. doi: 10.1089/ars.2009.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocic P. Why is coronary collateral growth impaired in type II diabetes and the metabolic syndrome. Vascul Pharmacol. 2012;57:179–186. doi: 10.1016/j.vph.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask AJ, Delbin MA, Katz PS, Zanesco A, Lucchesi PA. Differential coronary resistance microvessel remodeling between type 1 and type 2 diabetic mice: impact of exercise training. Vascul Pharmacol. 2012;57:187–193. doi: 10.1016/j.vph.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota E, Matsunaga T, Chilian WM. Myocardial angiogenesis. Mol Cell Biochem. 2004;264:35–44. doi: 10.1023/b:mcbi.0000044372.65864.18. [DOI] [PubMed] [Google Scholar]
- Chilian WM, Penn MS, Pung YF, Dong F, Mayorga M, Ohanyan V, et al. Coronary collateral growth—back to the future. J Mol Cell Cardiol. 2012;52:905–911. doi: 10.1016/j.yjmcc.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Azab MF, Hazem RM, Moustafa YM. Role of simvastatin and/or antioxidant vitamins in therapeutic angiogenesis in experimental diabetic hindlimb ischemia: effects on capillary density, angiogenesis markers, and oxidative stress. Eur J Pharmacol. 2012;690:31–41. doi: 10.1016/j.ejphar.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Altavilla D, Bitto A, Polito F, Marini H, Minutoli L, Di Stefano V, et al. Polydeoxyribonucleotide (PDRN): a safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc Hematol Agents Med Chem. 2009;7:313–321. doi: 10.2174/187152509789541909. [DOI] [PubMed] [Google Scholar]
- Emanueli C, Monopoli A, Kraenkel N, Meloni M, Gadau S, Campesi I, et al. Nitropravastatin stimulates reparative neovascularisation and improves recovery from limb Ischaemia in type-1 diabetic mice. Br J Pharmacol. 2007;150:873–882. doi: 10.1038/sj.bjp.0707142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min W, Yamanaka N. Three-dimensional analysis of increased vasculature around the glomerular vascular pole in diabetic nephropathy. Virchows Arch A Pathol Anat Histopathol. 1993;423:201–207. doi: 10.1007/BF01614771. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009;58:1471–1478. doi: 10.2337/db09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose K, Maeshima Y, Yamamoto Y, Kitayama H, Takazawa Y, Hirokoshi K, et al. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes. 2005;54:2891–2903. doi: 10.2337/diabetes.54.10.2891. [DOI] [PubMed] [Google Scholar]
- Osterby R, Hartmann A, Bangstad HJ. Structural changes in renal arterioles in Type I diabetic patients. Diabetologia. 2002;45:542–549. doi: 10.1007/s00125-002-0780-2. [DOI] [PubMed] [Google Scholar]
- Guo M, Ricardo SD, Deane JA, Shi M, Cullen-McEwen L, Bertram JF. A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. J Anat. 2005;207:813–821. doi: 10.1111/j.1469-7580.2005.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani A, Gilbert RE. The endothelium in diabetic nephropathy. Semin Nephrol. 2012;32:199–207. doi: 10.1016/j.semnephrol.2012.02.006. [DOI] [PubMed] [Google Scholar]
- LaManna JC. Hypoxia in the central nervous system. Essays Biochem. 2007;43:139–151. doi: 10.1042/BSE0430139. [DOI] [PubMed] [Google Scholar]
- Kelly-Cobbs AI, Prakash R, Coucha M, Knight RA, Li W, Ogbi SN, et al. Cerebral myogenic reactivity and blood flow in type 2 diabetic rats: role of peroxynitrite in hypoxia-mediated loss of myogenic tone. J Pharmacol Exp Ther. 2012;342:407–415. doi: 10.1124/jpet.111.191296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Li W, Elgebaly MM, Bruno A, Fagan SC. Hyperglycemia, diabetes and stroke: focus on the cerebrovasculature. Vascul Pharmacol. 2009;51:44–49. doi: 10.1016/j.vph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci. 2013;70:1753–1761. doi: 10.1007/s00018-013-1282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- Graupera M, Potente M. Regulation of angiogenesis by PI3K signaling networks. Exp Cell Res. 2013;319:1348–1355. doi: 10.1016/j.yexcr.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92:1037–1045. doi: 10.1016/j.lfs.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Abdelsaid MA, Pillai BA, Matragoon S, Prakash R, Al-Shabrawey M, El-Remessy AB. Early intervention of tyrosine nitration prevents vaso-obliteration and neovascularization in ischemic retinopathy. J Pharmacol Exp Ther. 2010;332:125–134. doi: 10.1124/jpet.109.157941. [DOI] [PubMed] [Google Scholar]
- Ali TK, El-Remessy AB. Diabetic retinopathy: current management and experimental therapeutic targets. Pharmacotherapy. 2009;29:182–192. doi: 10.1592/phco.29.2.182. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Al-Shabrawey M, Platt DH, Bartoli M, Behzadian MA, Ghaly N, et al. Peroxynitrite mediates VEGF's angiogenic signal and function via a nitration-independent mechanism in endothelial cells. FASEB J. 2007;21:2528–2539. doi: 10.1096/fj.06-7854com. [DOI] [PubMed] [Google Scholar]
- El-Remessy AB, Bartoli M, Platt DH, Fulton D, Caldwell RB. Oxidative stress inactivates VEGF survival signaling in retinal endothelial cells via PI 3-kinase tyrosine nitration. J Cell Sci. 2005;118 (Pt 1:243–252. doi: 10.1242/jcs.01612. [DOI] [PubMed] [Google Scholar]
- Jones CA, Nishiya N, London NR, Zhu W, Sorensen LK, Chan AC, et al. Slit2-Robo4 signalling promotes vascular stability by blocking Arf6 activity. Nat Cell Biol. 2009;11:1325–1331. doi: 10.1038/ncb1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London NR, Li DY. Robo4-dependent Slit signaling stabilizes the vasculature during pathologic angiogenesis and cytokine storm. Curr Opin Hematol. 2011;18:186–190. doi: 10.1097/MOH.0b013e328345a4b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AW, Mathivet T, Larrivee B, Tong RK, Kowalski J, Pibouin-Fragner L, et al. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with UNC5B. Dev Cell. 2011;20:33–46. doi: 10.1016/j.devcel.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yu J, Kuzontkoski PM, Zhu W, Li DY, Groopman JE. Slit2/Robo4 signaling modulates HIV-1 gp120-induced lymphatic hyperpermeability. PLoS Pathog. 2012;8:e1002461. doi: 10.1371/journal.ppat.1002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, et al. Age-dependent impairment of HIF-1alpha expression in diabetic mice: Correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008;217:319–327. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Kim DH, Lee AS, Lee S, Kang KP, Lee SY, et al. Peritubular capillary preservation with COMP-angiopoietin-1 decreases ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol. 2009;297:F952–F960. doi: 10.1152/ajprenal.00064.2009. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2005;139:476–481. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- Reis M, Czupalla CJ, Ziegler N, Devraj K, Zinke J, Seidel S, et al. Endothelial Wnt/beta-catenin signaling inhibits glioma angiogenesis and normalizes tumor blood vessels by inducing PDGF-B expression. J Exp Med. 2012;209:1611–1627. doi: 10.1084/jem.20111580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [DOI] [PubMed] [Google Scholar]
- Ahmad FK, He Z, King GL. Molecular targets of diabetic cardiovascular complications. Curr Drug Targets. 2005;6:487–494. doi: 10.2174/1389450054021990. [DOI] [PubMed] [Google Scholar]
- Kota SK, Meher LK, Jammula S, Kota SK, Krishna SV, Modi KD. Aberrant angiogenesis: The gateway to diabetic complications. Indian J Endocrinol Metab. 2012;16:918–930. doi: 10.4103/2230-8210.102992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollet I, Opina CJ, Zimmerman C, Leong KG, Wong F, Karsan A. Bacterial lipopolysaccharide directly induces angiogenesis through TRAF6-mediated activation of NF-kappaB and c-Jun N-terminal kinase. Blood. 2003;102:1740–1742. doi: 10.1182/blood-2003-01-0288. [DOI] [PubMed] [Google Scholar]
- Kim YW, West XZ, Byzova TV. Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med (Berl) 2013;91:323–328. doi: 10.1007/s00109-013-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Abraham S, McKenzie JA, Jeffs N, Swire M, Tripathi VB, et al. LRG1 promotes angiogenesis by modulating endothelial TGF-beta signalling. Nature. 2013;499:306–311. doi: 10.1038/nature12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno Y, Nakamura-Ishizu A, Otsu K, Suda T, Kubota Y. Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nat Med. 2012;18:1208–1216. doi: 10.1038/nm.2846. [DOI] [PubMed] [Google Scholar]
- Kida Y, Tchao BN, Yamaguchi I.Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease Pediatr Nephrol 2013. doi: 10.1007/500467-013-2430-4(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Fligny C, Duffield JS. Activation of pericytes: recent insights into kidney fibrosis and microvascular rarefaction. Curr Opin Rheumatol. 2013;25:78–86. doi: 10.1097/BOR.0b013e32835b656b. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab. 2003;23:166–180. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- Krupinski J, Issa R, Bujny T, Slevin M, Kumar P, Kumar S, et al. A putative role for platelet-derived growth factor in angiogenesis and neuroprotection after ischemic stroke in humans. Stroke. 1997;28:564–573. doi: 10.1161/01.str.28.3.564. [DOI] [PubMed] [Google Scholar]
- Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke. 2012;43:2270–2274. doi: 10.1161/STROKEAHA.111.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Kameda M, Yasuhara T, Morimoto T, Kondo A, Shingo T, et al. Electrical stimulation of the cerebral cortex exerts antiapoptotic, angiogenic, and anti-inflammatory effects in ischemic stroke rats through phosphoinositide 3-kinase/Akt signaling pathway. Stroke. 2009;40:e598–e605. doi: 10.1161/STROKEAHA.109.563627. [DOI] [PubMed] [Google Scholar]
- Espinera AR, Ogle ME, Gu X, Wei L. Citalopram enhances neurovascular regeneration and sensorimotor functional recovery after ischemic stroke in mice. Neuroscience. 2013;247:1–11. doi: 10.1016/j.neuroscience.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Li L, Zhang L, Zhang Z, Lu M, et al. Longitudinal magnetic resonance imaging of sildenafil treatment of embolic stroke in aged rats. Stroke. 2011;42:3537–3541. doi: 10.1161/STROKEAHA.111.622092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Tajiri N, Shinozuka K, Vasconcellos J, Kaneko Y, Lee HJ, et al. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke. 2013;44:3473–3481. doi: 10.1161/STROKEAHA.113.001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitmeir R, Kilic E, Kilic U, Bacigaluppi M, ElAli A, Salani G, et al. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain. 2011;134 (Pt 1:84–99. doi: 10.1093/brain/awq344. [DOI] [PubMed] [Google Scholar]
- Madinier A, Bertrand N, Rodier M, Quirie A, Mossiat C, Prigent-Tessier A, et al. Ipsilateral versus contralateral spontaneous post-stroke neuroplastic changes: involvement of BDNF. Neuroscience. 2013;231:169–181. doi: 10.1016/j.neuroscience.2012.11.054. [DOI] [PubMed] [Google Scholar]
- Li WL, Fraser JL, Yu SP, Zhu J, Jiang YJ, Wei L. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp Brain Res. 2011;214:503–513. doi: 10.1007/s00221-011-2849-y. [DOI] [PubMed] [Google Scholar]
- Guan W, Somanath PR, Kozak A, Goc A, El-Remessy AB, Ergul A, et al. Vascular protection by angiotensin receptor antagonism involves differential VEGF expression in both hemispheres after experimental stroke. PLoS ONE. 2011;6:e24551. doi: 10.1371/journal.pone.0024551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Li W, Qu Z, Johnson MA, Fagan SC, Ergul A. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke. 2013;44:2875–2882. doi: 10.1161/STROKEAHA.113.001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, et al. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewa HF, Kozak A, El-Remessy AB, Frye RF, Johnson MH, Ergul A, et al. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther. 2009;330:532–540. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgebaly MM, Prakash R, Li W, Ogbi S, Johnson MH, Mezzetti EM, et al. Vascular protection in diabetic stroke: role of matrix metalloprotease-dependent vascular remodeling. J Cereb Blood Flow Metab. 2010;30:1928–1938. doi: 10.1038/jcbfm.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis. 2011;43:285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Chopp M, Cui X, Zacharek A, Cui Y, Yan T, et al. Niaspan enhances vascular remodeling after stroke in type 1 diabetic rats. Exp Neurol. 2011;232:299–308. doi: 10.1016/j.expneurol.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. Role of iron in brain injury after intraventricular hemorrhage. Stroke. 2010;42:465–470. doi: 10.1161/STROKEAHA.110.602755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Keep RF, He Y, Sagher O, Hua Y, Xi G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow Metab. 2011;30:1793–1803. doi: 10.1038/jcbfm.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Hua Y, He Y, Wang L, Hu H, Keep RF, et al. Iron accumulation and DNA damage in a pig model of intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:123–128. doi: 10.1007/978-3-7091-0693-8_20. [DOI] [PubMed] [Google Scholar]
- Mehta SH, Webb RC, Ergul A, Tawak A, Dorrance AM. Neuroprotection by tempol in a model of iron-induced oxidative stress in acute ischemic stroke. Am J Physiol Regul Integr Comp Physiol. 2004;286:R283–R288. doi: 10.1152/ajpregu.00446.2002. [DOI] [PubMed] [Google Scholar]
- Lin S, Zhong Q, Lv FL, Zhou Y, Li JQ, Wang JZ, et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation. 2012;9:46. doi: 10.1186/1742-2094-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson RK, Chiang ET, Garcia JG. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc Res. 2011;81:189–197. doi: 10.1016/j.mvr.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Bi X, Jia Q, Shangguan S. The possible mechanism for impaired angiogenesis after transient focal ischemia in type 2 diabetic GK rats: different expressions of angiostatin and vascular endothelial growth factor. Biomed Pharmacother. 2010;64:208–213. doi: 10.1016/j.biopha.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Schrader J, Luders S, Kulschewski A, Berger J, Zidek W, Treib J, et al. The ACCESS study: evaluation of acute candesartan cilexetil therapy in stroke survivors. Stroke. 2003;34:1699–1703. doi: 10.1161/01.STR.0000075777.18006.89. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Laage R, Vogt G, Koch W, Kollmar R, Schwab S, et al. AXIS: a trial of intravenous granulocyte colony-stimulating factor in acute ischemic stroke. Stroke. 2010;41:2545–2551. doi: 10.1161/STROKEAHA.110.579508. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ye X, Yan T, Zhang C, Yang XP, Cui X, et al. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42:3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Calzi S, Neu MB, Shaw LC, Kielczewski JL, Moldovan NI, Grant MB. EPCs and pathological angiogenesis: when good cells go bad. Microvasc Res. 2010;79:207–216. doi: 10.1016/j.mvr.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarajapu YP, Grant MB. The promise of cell-based therapies for diabetic complications: challenges and solutions. Circ Res. 2010;106:854–869. doi: 10.1161/CIRCRESAHA.109.213140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann IR, Busch HJ, Mies G, Hossmann KA. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation. 2003;108:610–615. doi: 10.1161/01.CIR.0000074209.17561.99. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Ding GL, Shehadah A, Jiang Q, et al. Niaspan treatment increases tumor necrosis factor-alpha-converting enzyme and promotes arteriogenesis after stroke. J Cereb Blood Flow Metab. 2009;29:911–920. doi: 10.1038/jcbfm.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Cui X, Yang Y, Chopp M. Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke. 2009;40:254–260. doi: 10.1161/STROKEAHA.108.524116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Yagita Y, Oyama N, Terasaki Y, Omura-Matsuoka E, Sasaki T, et al. Granulocyte colony-stimulating factor enhances arteriogenesis and ameliorates cerebral damage in a mouse model of ischemic stroke. Stroke. 2011;42:770–775. doi: 10.1161/STROKEAHA.110.597799. [DOI] [PubMed] [Google Scholar]
- Rink C, Christoforidis G, Khanna S, Peterson L, Patel Y, Khanna S, et al. Tocotrienol vitamin E protects against preclinical canine ischemic stroke by inducing arteriogenesis. J Cereb Blood Flow Metab. 2011;31:2218–2230. doi: 10.1038/jcbfm.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Dai J, Zhang C, Yan T, et al. Combination treatment of stroke with sub-therapeutic doses of Simvastatin and human umbilical cord blood cells enhances vascular remodeling and improves functional outcome. Neuroscience. 2012;227:223–231. doi: 10.1016/j.neuroscience.2012.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Cui Y, Roberts C, Chen J. The neurorestorative benefit of GW3965 treatment of stroke in mice. Stroke. 2013;44:153–161. doi: 10.1161/STROKEAHA.112.677682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz K, Priller J, Kronenberg G, Fink KB, Winter B, Schrock H, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res. 2006;99:1132–1140. doi: 10.1161/01.RES.0000250175.14861.77. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Jiang H, Roberts C, Zhang C, et al. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, et al. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J Cereb Blood Flow Metab. 2008;28:1440–1448. doi: 10.1038/jcbfm.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Tsai LK, Munasinghe J, Leng Y, Fessler EB, Chibane F, et al. Chronic valproate treatment enhances postischemic angiogenesis and promotes functional recovery in a rat model of ischemic stroke. Stroke. 2012;43:2430–2436. doi: 10.1161/STROKEAHA.112.652545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Thompson JF, Taheri S, Salayandia VM, McAvoy TA, Hill JW, et al. Early inhibition of MMP activity in ischemic rat brain promotes expression of tight junction proteins and angiogenesis during recovery. J Cereb Blood Flow Metab. 2013;33:1104–1114. doi: 10.1038/jcbfm.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]