Abstract
The Saccharomyces cerevisiae FPS1 gene, which encodes a channel protein belonging to the MIP family, has been isolated previously as a multicopy suppressor of the growth defect of the fdp1 mutant (allelic to GGS1/TPS1) on fermentable sugars. Here we show that overexpression of FPS1 enhances glycerol production. Enhanced glycerol production caused by overexpression of GPD1 encoding glycerol-3-phosphate dehydrogenase also suppressed the growth defect of ggs1/tps1 delta mutants, suggesting a novel role for glycerol production in the control of glycolysis. The suppression of ggs1/tps1 delta mutants by GPD1 depends on the presence of Fps1. Mutants lacking Fps1 accumulate a greater part of the glycerol intracellularly, indicating that Fps1 is involved in glycerol efflux. Glycerol-uptake experiments showed that the permeability of the yeast plasma membrane for glycerol consists of an Fps1-independent component probably due to simple diffusion and of an Fps1-dependent component representing facilitated diffusion. The Escherichia coli glycerol facilitator expressed in a yeast fps1 delta mutant can restore the characteristics of glycerol uptake, production and distribution fully, but restores only partially growth of a ggs1/tps1 delta fps1 delta double mutant on glucose. Fps1 appears to be closed under hyperosmotic stress when survival depends on intracellular accumulation of glycerol and apparently opens rapidly when osmostress is lifted. The osmostress-induced High Osmolarity Glycerol (HOG) response pathway is not required for inactivation of Fps1. We conclude that Fps1 is a regulated yeast glycerol facilitator controlling glycerol production and cytosolic concentration, and might have additional functions.
Full text
PDF

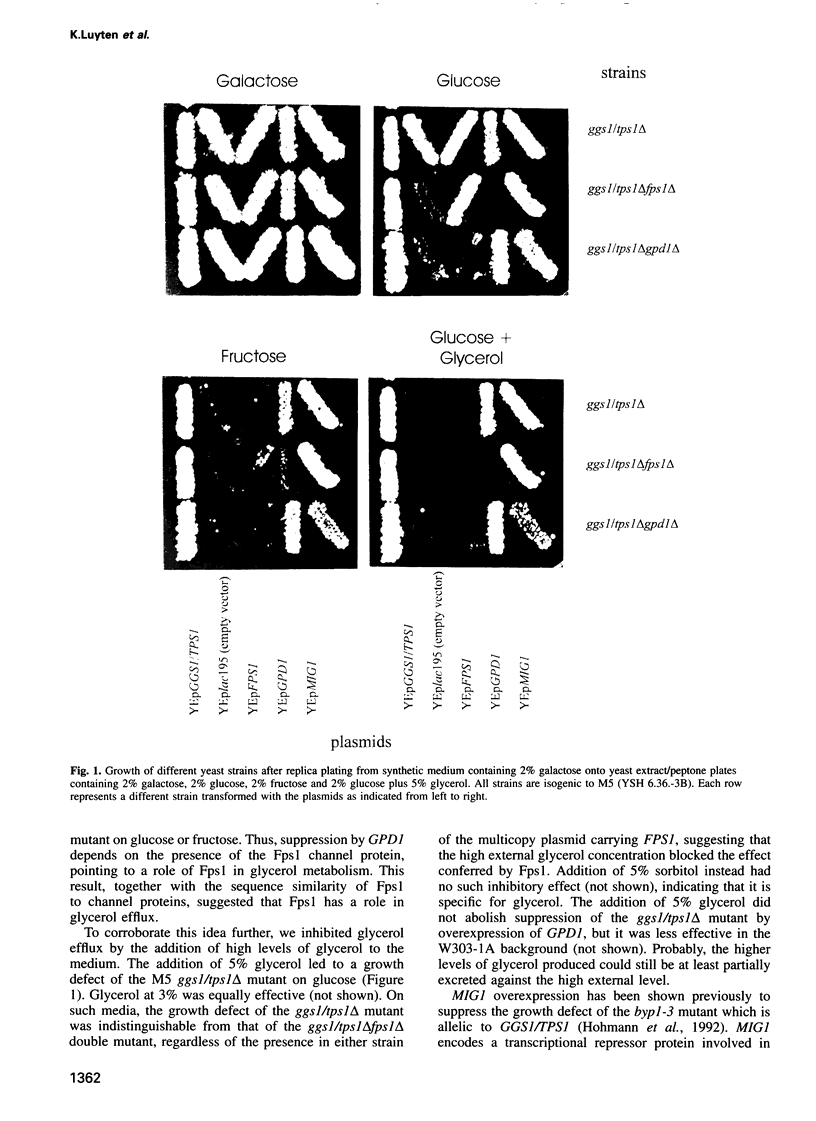
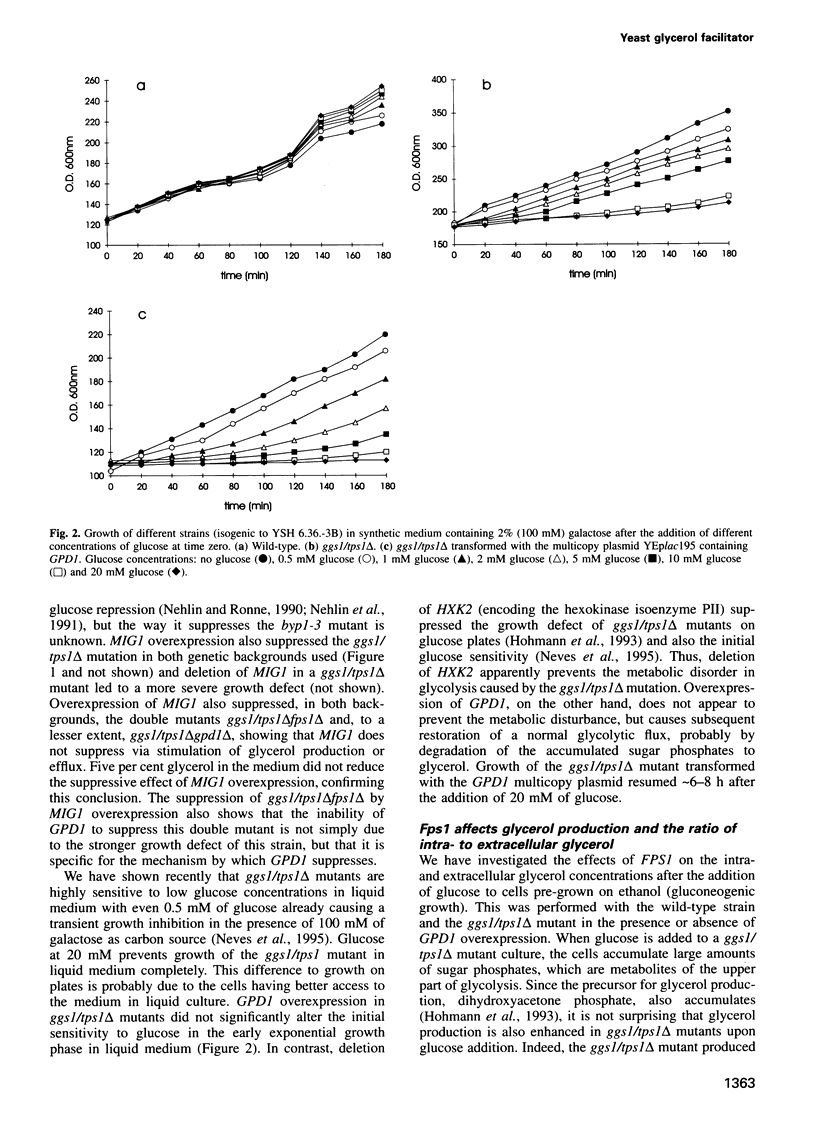




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertyn J., Hohmann S., Prior B. A. Characterization of the osmotic-stress response in Saccharomyces cerevisiae: osmotic stress and glucose repression regulate glycerol-3-phosphate dehydrogenase independently. Curr Genet. 1994 Jan;25(1):12–18. doi: 10.1007/BF00712960. [DOI] [PubMed] [Google Scholar]
- Albertyn J., Hohmann S., Thevelein J. M., Prior B. A. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol Cell Biol. 1994 Jun;14(6):4135–4144. doi: 10.1128/mcb.14.6.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André L., Hemming A., Adler L. Osmoregulation in Saccharomyces cerevisiae. Studies on the osmotic induction of glycerol production and glycerol-3-phosphate dehydrogenase (NAD+) FEBS Lett. 1991 Jul 29;286(1-2):13–17. doi: 10.1016/0014-5793(91)80930-2. [DOI] [PubMed] [Google Scholar]
- Bell W., Klaassen P., Ohnacker M., Boller T., Herweijer M., Schoppink P., Van der Zee P., Wiemken A. Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. Eur J Biochem. 1992 Nov 1;209(3):951–959. doi: 10.1111/j.1432-1033.1992.tb17368.x. [DOI] [PubMed] [Google Scholar]
- Blomberg A., Adler L. Physiology of osmotolerance in fungi. Adv Microb Physiol. 1992;33:145–212. doi: 10.1016/s0065-2911(08)60217-9. [DOI] [PubMed] [Google Scholar]
- Blázquez M. A., Lagunas R., Gancedo C., Gancedo J. M. Trehalose-6-phosphate, a new regulator of yeast glycolysis that inhibits hexokinases. FEBS Lett. 1993 Aug 23;329(1-2):51–54. doi: 10.1016/0014-5793(93)80191-v. [DOI] [PubMed] [Google Scholar]
- Brewster J. L., Gustin M. C. Positioning of cell growth and division after osmotic stress requires a MAP kinase pathway. Yeast. 1994 Apr;10(4):425–439. doi: 10.1002/yea.320100402. [DOI] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Cannon J. F., Pringle J. R., Fiechter A., Khalil M. Characterization of glycogen-deficient glc mutants of Saccharomyces cerevisiae. Genetics. 1994 Feb;136(2):485–503. doi: 10.1093/genetics/136.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro I. M., Loureiro-Dias M. C. Glycerol utilization in Fusarium oxysporum var. lini: regulation of transport and metabolism. J Gen Microbiol. 1991 Jul;137(7):1497–1502. doi: 10.1099/00221287-137-7-1497. [DOI] [PubMed] [Google Scholar]
- Charlab R., Oliveira D. E., Panek A. D. Investigation of the relationship between sst1 and fdp mutations in yeast and their effect on trehalose synthesis. Braz J Med Biol Res. 1985;18(4):447–454. [PubMed] [Google Scholar]
- Chrispeels M. J., Agre P. Aquaporins: water channel proteins of plant and animal cells. Trends Biochem Sci. 1994 Oct;19(10):421–425. doi: 10.1016/0968-0004(94)90091-4. [DOI] [PubMed] [Google Scholar]
- De Virgilio C., Hottiger T., Dominguez J., Boller T., Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. I. Genetic evidence that trehalose is a thermoprotectant. Eur J Biochem. 1994 Jan 15;219(1-2):179–186. doi: 10.1111/j.1432-1033.1994.tb19928.x. [DOI] [PubMed] [Google Scholar]
- Denor P. F., Courtright J. B. Genetic and enzymatic characterization of the inducible glycerol dissimilatory system of Neurospora crassa. J Bacteriol. 1982 Aug;151(2):912–917. doi: 10.1128/jb.151.2.912-917.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushimi K., Uchida S., Hara Y., Hirata Y., Marumo F., Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993 Feb 11;361(6412):549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- Gancedo C., Gancedo J. M., Sols A. Glycerol metabolism in yeasts. Pathways of utilization and production. Eur J Biochem. 1968 Jul;5(2):165–172. doi: 10.1111/j.1432-1033.1968.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Gietz R. D., Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988 Dec 30;74(2):527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- González M. I., Stucka R., Blázquez M. A., Feldmann H., Gancedo C. Molecular cloning of CIF1, a yeast gene necessary for growth on glucose. Yeast. 1992 Mar;8(3):183–192. doi: 10.1002/yea.320080304. [DOI] [PubMed] [Google Scholar]
- Guerrero F. D., Jones J. T., Mullet J. E. Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted. Sequence and expression of three inducible genes. Plant Mol Biol. 1990 Jul;15(1):11–26. doi: 10.1007/BF00017720. [DOI] [PubMed] [Google Scholar]
- Heller K. B., Lin E. C., Wilson T. H. Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol. 1980 Oct;144(1):274–278. doi: 10.1128/jb.144.1.274-278.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann S., Huse K., Valentin E., Mbonyi K., Thevelein J. M., Zimmermann F. K. Glucose-induced regulatory defects in the Saccharomyces cerevisiae byp1 growth initiation mutant and identification of MIG1 as a partial suppressor. J Bacteriol. 1992 Jun;174(12):4183–4188. doi: 10.1128/jb.174.12.4183-4188.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann S., Neves M. J., de Koning W., Alijo R., Ramos J., Thevelein J. M. The growth and signalling defects of the ggs1 (fdp1/byp1) deletion mutant on glucose are suppressed by a deletion of the gene encoding hexokinase PII. Curr Genet. 1993;23(4):281–289. doi: 10.1007/BF00310888. [DOI] [PubMed] [Google Scholar]
- Hohmann S., Van Dijck P., Luyten K., Thevelein J. M. The byp1-3 allele of the Saccharomyces cerevisiae GGS1/TPS1 gene and its multi-copy suppressor tRNA(GLN) (CAG): Ggs1/Tps1 protein levels restraining growth on fermentable sugars and trehalose accumulation. Curr Genet. 1994 Oct;26(4):295–301. doi: 10.1007/BF00310492. [DOI] [PubMed] [Google Scholar]
- Holmberg C., Beijer L., Rutberg B., Rutberg L. Glycerol catabolism in Bacillus subtilis: nucleotide sequence of the genes encoding glycerol kinase (glpK) and glycerol-3-phosphate dehydrogenase (glpD). J Gen Microbiol. 1990 Dec;136(12):2367–2375. doi: 10.1099/00221287-136-12-2367. [DOI] [PubMed] [Google Scholar]
- Hottiger T., Boller T., Wiemken A. Rapid changes of heat and desiccation tolerance correlated with changes of trehalose content in Saccharomyces cerevisiae cells subjected to temperature shifts. FEBS Lett. 1987 Aug 10;220(1):113–115. doi: 10.1016/0014-5793(87)80886-4. [DOI] [PubMed] [Google Scholar]
- Hottiger T., De Virgilio C., Hall M. N., Boller T., Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur J Biochem. 1994 Jan 15;219(1-2):187–193. doi: 10.1111/j.1432-1033.1994.tb19929.x. [DOI] [PubMed] [Google Scholar]
- Kotyk A., Alonso A. Transport of ethanol in baker's yeast. Folia Microbiol (Praha) 1985;30(1):90–91. doi: 10.1007/BF02922503. [DOI] [PubMed] [Google Scholar]
- Larsson K., Ansell R., Eriksson P., Adler L. A gene encoding sn-glycerol 3-phosphate dehydrogenase (NAD+) complements an osmosensitive mutant of Saccharomyces cerevisiae. Mol Microbiol. 1993 Dec;10(5):1101–1111. doi: 10.1111/j.1365-2958.1993.tb00980.x. [DOI] [PubMed] [Google Scholar]
- Ma H. Protein phosphorylation in plants: enzymes, substrates and regulators. Trends Genet. 1993 Jul;9(7):228–230. doi: 10.1016/0168-9525(93)90075-s. [DOI] [PubMed] [Google Scholar]
- Maurel C., Reizer J., Schroeder J. I., Chrispeels M. J., Saier M. H., Jr Functional characterization of the Escherichia coli glycerol facilitator, GlpF, in Xenopus oocytes. J Biol Chem. 1994 Apr 22;269(16):11869–11872. [PubMed] [Google Scholar]
- Maurel C., Reizer J., Schroeder J. I., Chrispeels M. J. The vacuolar membrane protein gamma-TIP creates water specific channels in Xenopus oocytes. EMBO J. 1993 Jun;12(6):2241–2247. doi: 10.1002/j.1460-2075.1993.tb05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall J., Kaasen I., Strøm A. R. A yeast gene for trehalose-6-phosphate synthase and its complementation of an Escherichia coli otsA mutant. FEMS Microbiol Lett. 1993 Feb 15;107(1):25–30. doi: 10.1016/0378-1097(93)90348-6. [DOI] [PubMed] [Google Scholar]
- Mellor J., Dobson M. J., Roberts N. A., Tuite M. F., Emtage J. S., White S., Lowe P. A., Patel T., Kingsman A. J., Kingsman S. M. Efficient synthesis of enzymatically active calf chymosin in Saccharomyces cerevisiae. Gene. 1983 Sep;24(1):1–14. doi: 10.1016/0378-1119(83)90126-9. [DOI] [PubMed] [Google Scholar]
- Mizuno T. Random cloning of bent DNA segments from Escherichia coli chromosome and primary characterization of their structures. Nucleic Acids Res. 1987 Sep 11;15(17):6827–6841. doi: 10.1093/nar/15.17.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S., Mizuno T. Nucleotide sequence of the region encompassing the glpKF operon and its upstream region containing a bent DNA sequence of Escherichia coli. Nucleic Acids Res. 1989 Jun 12;17(11):4378–4378. [PMC free article] [PubMed] [Google Scholar]
- Nehlin J. O., Carlberg M., Ronne H. Control of yeast GAL genes by MIG1 repressor: a transcriptional cascade in the glucose response. EMBO J. 1991 Nov;10(11):3373–3377. doi: 10.1002/j.1460-2075.1991.tb04901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlin J. O., Ronne H. Yeast MIG1 repressor is related to the mammalian early growth response and Wilms' tumour finger proteins. EMBO J. 1990 Sep;9(9):2891–2898. doi: 10.1002/j.1460-2075.1990.tb07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves M. J., Hohmann S., Bell W., Dumortier F., Luyten K., Ramos J., Cobbaert P., de Koning W., Kaneva Z., Thevelein J. M. Control of glucose influx into glycolysis and pleiotropic effects studied in different isogenic sets of Saccharomyces cerevisiae mutants in trehalose biosynthesis. Curr Genet. 1995 Jan;27(2):110–122. doi: 10.1007/BF00313424. [DOI] [PubMed] [Google Scholar]
- Ouyang L. J., Whelan J., Weaver C. D., Roberts D. M., Day D. A. Protein phosphorylation stimulates the rate of malate uptake across the peribacteroid membrane of soybean nodules. FEBS Lett. 1991 Nov 18;293(1-2):188–190. doi: 10.1016/0014-5793(91)81183-9. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Rao Y., Jan L. Y., Jan Y. N. Similarity of the product of the Drosophila neurogenic gene big brain to transmembrane channel proteins. Nature. 1990 May 10;345(6271):163–167. doi: 10.1038/345163a0. [DOI] [PubMed] [Google Scholar]
- Reizer J., Reizer A., Saier M. H., Jr The MIP family of integral membrane channel proteins: sequence comparisons, evolutionary relationships, reconstructed pathway of evolution, and proposed functional differentiation of the two repeated halves of the proteins. Crit Rev Biochem Mol Biol. 1993;28(3):235–257. doi: 10.3109/10409239309086796. [DOI] [PubMed] [Google Scholar]
- Schaaff I., Green J. B., Gozalbo D., Hohmann S. A deletion of the PDC1 gene for pyruvate decarboxylase of yeast causes a different phenotype than previously isolated point mutations. Curr Genet. 1989 Feb;15(2):75–81. doi: 10.1007/BF00435452. [DOI] [PubMed] [Google Scholar]
- Sweet G., Gandor C., Voegele R., Wittekindt N., Beuerle J., Truniger V., Lin E. C., Boos W. Glycerol facilitator of Escherichia coli: cloning of glpF and identification of the glpF product. J Bacteriol. 1990 Jan;172(1):424–430. doi: 10.1128/jb.172.1.424-430.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M., Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem Sci. 1995 Jan;20(1):3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- Thevelein J. M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984 Mar;48(1):42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevelein J. M. The RAS-adenylate cyclase pathway and cell cycle control in Saccharomyces cerevisiae. Antonie Van Leeuwenhoek. 1992 Aug;62(1-2):109–130. doi: 10.1007/BF00584466. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989 Feb 24;56(4):619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Van Aelst L., Hohmann S., Bulaya B., de Koning W., Sierkstra L., Neves M. J., Luyten K., Alijo R., Ramos J., Coccetti P. Molecular cloning of a gene involved in glucose sensing in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1993 May;8(5):927–943. doi: 10.1111/j.1365-2958.1993.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Van Aelst L., Hohmann S., Zimmermann F. K., Jans A. W., Thevelein J. M. A yeast homologue of the bovine lens fibre MIP gene family complements the growth defect of a Saccharomyces cerevisiae mutant on fermentable sugars but not its defect in glucose-induced RAS-mediated cAMP signalling. EMBO J. 1991 Aug;10(8):2095–2104. doi: 10.1002/j.1460-2075.1991.tb07742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J., Van Rooijen R., Dijkema C., Swart K., Sealy-Lewis H. M. Glycerol uptake mutants of the hyphal fungus Aspergillus nidulans. J Gen Microbiol. 1988 Mar;134(3):655–659. doi: 10.1099/00221287-134-3-655. [DOI] [PubMed] [Google Scholar]
- Voegele R. T., Sweet G. D., Boos W. Glycerol kinase of Escherichia coli is activated by interaction with the glycerol facilitator. J Bacteriol. 1993 Feb;175(4):1087–1094. doi: 10.1128/jb.175.4.1087-1094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuorio O. E., Kalkkinen N., Londesborough J. Cloning of two related genes encoding the 56-kDa and 123-kDa subunits of trehalose synthase from the yeast Saccharomyces cerevisiae. Eur J Biochem. 1993 Sep 15;216(3):849–861. doi: 10.1111/j.1432-1033.1993.tb18207.x. [DOI] [PubMed] [Google Scholar]
- Wiemken A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Van Leeuwenhoek. 1990 Oct;58(3):209–217. doi: 10.1007/BF00548935. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Zampighi G. A., Hall J. E., Kreman M. Purified lens junctional protein forms channels in planar lipid films. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8468–8472. doi: 10.1073/pnas.82.24.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]