Abstract
In the central nervous system, deficits in cholinergic neurotransmission correlate with decreased attention and cognitive impairment, while stimulation of neuronal nicotinic acetylcholine receptors improves attention, cognitive performance and neuronal resistance to injury as well as produces robust analgesic and anti-inflammatory effects. The rational basis for the therapeutic use of orthosteric agonists and positive allosteric modulators (PAMs) of nicotinic receptors arises from the finding that functional nicotinic receptors are ubiquitously expressed in neuronal and non-neuronal tissues including brain regions highly vulnerable to traumatic and ischemic types of injury (e.g., cortex and hippocampus). Moreover, functional nicotinic receptors do not vanish in age-, disease- and trauma-related neuropathologies, but their expression and/or activation levels decline in a subunit- and brain region-specific manner. Therefore, augmenting the endogenous cholinergic tone by nicotinic agents is possible and may offset neurological impairments associated with cholinergic hypofunction. Importantly, because neuronal damage elevates extracellular levels of choline (a selective agonist of α7 nicotinic acetylcholine receptors) near the site of injury, α7-PAM-based treatments may augment pathology-activated α7-dependent auto-therapies where and when they are most needed (i.e., in the penumbra, post-injury). Thus, the nicotinic-PAM-based treatments are expected to be highly efficacious with fewer side effects as compared to a more indiscriminate action of exogenous orthosteric agonists. In this review, I will summarize the existing trends in therapeutic applications of nicotinic PAMs.
Keywords: positive allosteric modulator, nicotinic acetylcholine receptor, PNU-120596, choline, cerebral ischemia, analgesia
1. Positive Allosteric Modulation of Nicotinic Acetylcholine Receptors
Neuronal nicotinic acetylcholine receptors play critical roles in maintaining cognitive, autonomic and immune homeostasis as summarized in recent comprehensive reviews (Bencherif et al., 2011; Lendvai et al., 2013; Olincy et al., 2006; Wallace and Porter, 2011). In the central nervous system, deficits in cholinergic neurotransmission correlate with reduced attention and cognitive impairment (Bartus et al., 1982; Cabrera et al., 2006), while cholinergic hyperactivity correlates with depression (Janowsky et al., 1974). Although the muscarinic acetylcholine receptor component of cholinergic activity is essential for healthy cognition (Petersen, 1977), pro-cognitive effects of nicotinic agonists are evident and treatments that support activation of central nicotinic acetylcholine receptors can offset neurological impairments associated with cholinergic hypofunction (Kitagawa et al., 2003; Levin et al., 2006; Newhouse et al., 2004). In addition, nicotinic receptors modulate nociceptive neurotransmission and have been viewed as promising targets of non-opioid analgesics (Damaj et al., 2000; Lee et al., 2011; Rode et al., 2012; Umana et al., 2013; Zhu et al., 2011).
As an alternative to somewhat indiscriminate action of exogenous orthosteric nicotinic agonists, positive allosteric modulators (PAMs) of nicotinic receptors have been proposed as a novel promising approach to counteracting neurocognitive deficits (Callahan et al., 2013; Hurst et al., 2005; McLean et al., 2012; Thomsen et al., 2011), acute and chronic nociception (Freitas et al., 2012; Freitas et al., 2013; Lee et al., 2011; Munro et al., 2012; Zhu et al., 2011) and cerebral ischemia (Kalappa et al., 2013; Sun et al., 2013). Nicotinic PAMs offer at least three important advantages over exogenous orthosteric nicotinic agonists. First, PAMs alone do not activate nicotinic receptors, but they increase the activation efficacy/potency of endogenous nicotinic agonists (i.e., choline and acetylcholine) which are released naturally as needed. Thus, the native spatiotemporal patterns of nicotinic receptor activation may remain largely intact. One possible exception is α7-PAMs that inhibit α7 desensitization, e.g., 1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)urea (i.e., PNU-120596). These PAMs (known as Type-II) can recruit endogenous choline (a selective agonist of α7 nicotinic acetylcholine receptors) and produce persistent α7 receptor activation (Freitas et al., 2013; Gusev and Uteshev, 2010; Kalappa et al., 2010; Munro et al., 2012). Therefore, certain spatiotemporal patterns of α7 activation mediated by endogenous choline may exist only in the presence, but not absence of α7-PAMs. Secondly, allosteric binding sites are evolutionary less conserved than orthosteric sites (Yang et al., 2012) and thus, allosteric sites exhibit greater structural diversity than orthosteric sites. Therefore, allosteric sites are more likely to allow selective targeting by synthetic compounds than orthosteric sites. This property may have arisen from an apparent delay in the evolutionary development of allostery relative to orthosteric activity of ligand-gated receptors (Yang et al., 2012). Finally, activation of nicotinic receptors by nicotinic agonists is reduced by desensitization. As a result, neurocognitive, behavioral and analgesic effects of nicotinic agonists are expected to develop tolerance (Harris et al., 2004; Lendvai et al., 2013; Umana et al., 2013). Some α7-PAMs inhibit α7 receptor desensitization (Dinklo et al., 2011; Faghih et al., 2009; Hurst et al., 2005) and thus, may reduce tolerance to make nicotinic treatments more efficacious (Freitas et al., 2012).
Desensitization also serves as a cytoprotective mechanism against potentially toxic receptor overstimulation and theoretically, PAMs that inhibit α7 desensitization may cause cytotoxicity. However, the existing experimental data argue that PAMs that inhibit α7 desensitization are therapeutically efficacious (Freitas et al., 2012; Freitas et al., 2013; Hu et al., 2009; Hurst et al., 2005; Kalappa et al., 2013; McLean et al., 2012; Munro et al., 2012; Sun et al., 2013). Moreover, in addition to potentiating α7 activation, α7-PAMs may also potentiate α7 open channel block (Kalappa and Uteshev, 2013) further raising the threshold for cytotoxicity.
The rational basis for the therapeutic use of orthosteric agonists and PAMs of nicotinic receptors arises from the finding that functional nicotinic receptors are ubiquitously expressed in neuronal and non-neuronal tissues including brain regions highly vulnerable to traumatic and ischemic types of injury (e.g., cortex and hippocampus). Moreover, functional nicotinic receptors do not vanish in age-, disease- and trauma-related neuropathologies, but their expression and/or activation levels decline in a subunit- and brain region-specific manner (Kelso and Oestreich, 2012; Leonard et al., 2000; Nordberg and Winblad, 1986). Therefore, augmenting the endogenous cholinergic tone by PAMs is possible and may offset neurological impairments associated with cholinergic hypofunction.
2. Exploring nicotinic-PAMs as therapeutic alternatives to orthosteric nicotinic agonists
Neurodegenerative, sensorimotor and psychiatric disorders associated with cognitive decline and decreased attention have been linked to deficits in the expression and function of nicotinic acetylcholine receptors (Freedman et al., 1995; Guan et al., 2000; Leonard et al., 2000; Nordberg and Winblad, 1986; Perry et al., 1995). By contrast, activation of nicotinic receptors by endogenous and exogenous nicotinic agents is generally pro-cognitive and can be therapeutic in patients and animal models of age-, disease- and trauma-related neurocognitive dysfunctions (Arendash et al., 1995; Bencherif et al., 2011; Guseva et al., 2008; Kelso and Oestreich, 2012; Lendvai et al., 2013; Olincy et al., 2006; Thomsen et al., 2011; Verbois et al., 2003; Wallace and Porter, 2011). Therefore, there is a clear rationale for exploring nicotinic-PAM-based treatments as alternatives to orthosteric nicotinic agonists. In fact, PNU-120596, the first α7-PAM with an in vivo efficacy, improved the auditory gating deficits in rats (Hurst et al., 2005), a major functional biomarker in schizophrenia research. Other α7-PAMs have since been synthesized and showed similar efficacies in restoration of auditory gating in rodents (Dinklo et al., 2011; Faghih et al., 2009; Ng et al., 2007).
Functional α7 nicotinic receptors expression is beneficial to the nervous system, as moderate activation of these receptors enhances cellular resistance to brain injury, which has been demonstrated in both in vivo and ex vivo experimental models of dementias, cerebral ischemic stroke and traumatic brain injury (Akaike et al., 2010; Del Barrio et al., 2011; Egea et al., 2007; Guseva et al., 2008; Kaneko et al., 1997; Li et al., 1999; Parada et al., 2013; Roncarati et al., 2009; Shimohama et al., 1998; Takeuchi et al., 2009). For example, neuroprotection by nicotine was lost in α7 knock-out mice exposed to oxygen-glucose deprivation (Egea et al., 2007); while activation of α7 nicotinic receptors by low concentrations of a partial selective agonist protected pheochromocytoma-12 (PC12) cells from death in a nerve growth factor (NGF)/serum deprivation toxicity model (Li et al., 1999). The mechanisms underlying α7-mediated neuroprotection may involve activation of the serine/threonine-specific protein kinase and B-cell lymphoma protein (i.e., AKT/Bcl-2)-dependent pathways (Akaike et al., 2010; Shimohama, 2009). These likely mechanisms would allow neurons meet the energy demand of ischemic/hypoglycemic conditions and delay the ultimate failure of the Na+/K+-ATPase pumps by delaying mitochondrial dysfunction. Such a failure would cause a rapid loss of the neuronal trans-membrane electrochemical gradient leading to terminal anoxic depolarization and spreading depression (Kalappa et al., 2013; White et al., 2012).
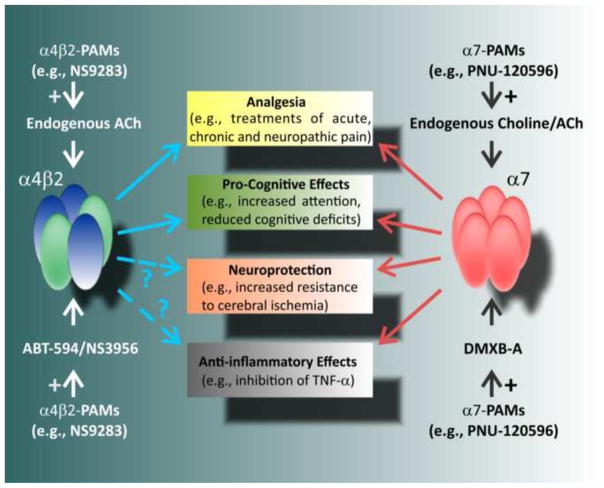
Choline, a ubiquitous cell membrane building material and a precursor/metabolite of acetylcholine, is a selective endogenous agonist of α7 nicotinic receptors (Alkondon et al., 1997; Papke et al., 1996). The endogenous levels of extracellular choline (<10 μM) are sub-threshold for α7 activation (Uteshev et al., 2003) due to choline’s low potency for α7 activation (EC50~0.5 mM) (Papke and Papke, 2002) and tendency to induce α7 desensitization (IC50~40 μM) (Uteshev et al., 2003). However, under conditions of energy deprivation, cellular dysfunction and injury/death, the extracellular concentration of choline can be considerably elevated (Djuricic et al., 1991; Gasull et al., 2000; Kiewert et al., 2010; Rao et al., 2000) providing a large source of this endogenous α7 agonist. Significantly elevated levels of choline have been recently demonstrated by direct measurements in the ischemic core and penumbra in the middle cerebral artery occlusion (MCAO) model of ischemic stroke in rats (Kiewert et al., 2010). In this regard, the hypothesis that MCAO-induced focal elevations in the extracellular levels of choline near the site of injury act as a form of ischemia-activated penumbral auto-therapy is very intriguing. While these elevated levels of choline may be neuroprotective even in the absence of α7-PAMs, it would be expected that α7-PAM-based treatments will significantly augment the injury-induced α7-dependent neuroprotection by endogenous choline where and when it is most needed (i.e., in the ischemic penumbra, post-injury) (Kalappa et al., 2013; Sun et al., 2013). Furthermore, α7-PAMs would be expected to expand the range of ischemic penumbra that falls under significant injury-induced α7-dependent neuroprotection by endogenous choline, because α7-PAMs increase the α7 activation efficacy/potency of choline and appear to convert sub-neuroprotective levels of choline into neuroprotective. Conversely, because of the elevated levels of choline in ischemic penumbra, α7-PAMs may not require co-application of exogenous α7 agonists to produce significant neuroprotection in focal cerebral ischemia (Kalappa et al., 2013; Sun et al., 2013). While not necessary in ischemic injury, co-administration of nicotinic agonists and nicotinic-PAMs appears to be critical in treatments of acute, chronic and neuropathic pain (Umana et al., 2013) (Fig. 1), conditions that are not expected to elevate the levels of extracellular choline and acetylcholine.
Fig. 1. A schematic representation of potential therapeutic contributions of α4β2- and α7-PAMs.
α4β2 and α7 nicotinic-PAMs enhance the activation efficacy/potency of endogenous (i.e., choline and acetylcholine) and exogenous (e.g., DMXB-A, the code name GTS-21 (Kem, 2000)) orthosteric nicotinic receptor agonists. However, α7-PAMs appear to have a broader spectrum of therapeutic activity as compared to α4β2-PAMs, possibly, because they appear to be able to convert endogenous choline into a potent therapeutic agent. DMXB-A is a selective α7 nicotinic receptor agonist that has been extensively tested at various phases of pre-clinical and clinical trials as a potential therapy for a number of neurological conditions, including Alzheimer’s disease (Kem, 2000) and most recently, schizophrenia (Freedman et al., 2008; Olincy et al., 2006) (see http://clinicaltrials.gov/). As a novel therapeutic opportunity, nicotinic-PAMs that could recruit endogenous cholinergic pathways and enhance pathology-activated auto-therapies would hold significant translational potential. Because of the ubiquitous expression of nicotinic receptors in neuronal and non-neuronal tissues, developing highly selective nicotinic-PAMs which, ideally, could target only a single player, i.e., the nicotinic receptors, would allow nicotinic-PAM-based interventions to target and recruit multiple endogenous neuronal and non-neuronal therapeutic cholinergic pathways.
In the MCAO model of cerebral ischemic stroke in rats, PNU-120596 has significantly reduced cerebral infarct volume and neurological deficits when the drug was administered up to 6 hours post-MCAO (Kalappa et al., 2013; Sun et al., 2013). Such a remarkable post-MCAO effectiveness of PNU-120596 invites additional pre-clinical studies of α7-PAMs as a conceptually novel family of treatments that are based on a substantively different mechanism, i.e., recruiting and enhancing injury-induced α7-dependent auto-therapy by endogenous cholinergic pathways. Treatments that incorporate endogenous mechanisms are expected to be highly efficacious and cause fewer adverse reactions as compared to treatments utilizing exogenous orthosteric agonists. Moreover, as functional α7 nicotinic receptors are ubiquitously expressed in both neuronal and non-neuronal tissues, the presence of neurovascular and/or immune components in the therapeutic effects of α7-PAMs would not be surprising, as we have recently discussed (Sun et al., 2013). A possible contribution of the immune system in the therapeutic effects of α7-PAMs would be consistent with the ubiquitous expression of functional α7 nicotinic receptors in many immune cells (Wang et al., 2003) and the anti-inflammatory efficacy of PNU-120596 (Munro et al., 2012; Parada et al., 2013). Potential non-neuronal sources of nicotinic-PAM-mediated brain protection are not well understood and present a great interest.
3. Analgesic effects of nicotinic-PAMs
Serious adverse reactions and a high potential for addiction reduce clinical enthusiasm for the use of opioids as analgesics (Umana et al., 2013). Moreover, opioids may not always be effective against neuropathic pain. Therefore, there is a critical need in developing non-opioid compounds with analgesic potency comparable to that produced by opioids. Nicotinic receptors have been viewed as promising targets of non-opioid analgesics (Badio and Daly, 1994; Nirogi et al., 2013; Qian et al., 1993; Umana et al., 2013). For example, epibatidine, a compound isolated from the skin of South African frogs Epipedobates tricolor, is a highly potent non-opioid analgesic with a potency for neuropathic pain (Badio and Daly, 1994). As an analgesic, epibatidine is ~100-fold more potent than morphine and its analgesic action is derived from direct binding and activation of α4β2 and α7 nicotinic receptors (Gerzanich et al., 1995). However, the initial optimism for the use of epibatidine and (R)-5-(2-azetidinylmethoxy)-2-chloropyridine (i.e., ABT-594), an epibatidine-based selective agonist of α4β2 nicotinic receptors) as potent analgesics has declined because of serious autonomic adverse reactions (Rowbotham et al., 2009). The recent development of α4β2-PAMs (e.g., 3-(3-(pyridine-3-yl)-1,2,4-oxadiazol-5-yl)benzonitrile; (i.e., NS9283) revived the interest in developing nicotinic receptor-based analgesic tools because of the distinct therapeutic qualities of PAMs discussed above. For example, while NS9283 alone does not produce significant analgesic effects in animal models of acute, persistent, neuropathic and inflammatory pain, a combination of NS9283 with ABT-594 (Lee et al., 2011; Zhu et al., 2011) or 1-(5-chloropyridin-3-yl)-[1,4]diazepane fumarate (i.e., NS3956; another potent orthosteric α4β2 agonists) (Rode et al., 2012), significantly enhances the analgesic efficacy/potency of these treatments. Importantly, the autonomic adverse reactions of ABT-594 (e.g., emesis, heart rate, body temperature likely caused by activation of α3β4 nicotinic receptors) and sensorimotor performance (e.g., locomotion, rotarod performance, exploratory behavior) are not potentiated by NS9283 indicating that PAM-based approaches can increase the therapeutic index of α4β2 agonists in treating pain (Lee et al., 2011; Zhu et al., 2011).
In addition to α4β2 nicotinic receptors, α7 nicotinic receptors act as another potential target of analgesic drugs (Damaj et al., 2000; Feuerbach et al., 2009; Medhurst et al., 2008). Not surprisingly, α7-PAMs have been demonstrated to produce robust anti-nociceptive effects by enhancing the efficacy/potency of endogenous choline (Freitas et al., 2012; Freitas et al., 2013; Munro et al., 2012). The analgesic effects of nicotinic-PAMs can be further enhanced by combining PAMs with exogenous orthosteric nicotinic agonists as discussed previously (Freitas et al., 2013; Umana et al., 2013). Together these results demonstrate that nicotinic-PAMs hold significant promise as potent analgesics targeting α4β2 and α7 nicotinic receptors.
4. Conclusions
Activation of nicotinic receptors produces robust pro-cognitive, neuroprotective, analgesic and anti-inflammatory effects (Fig. 1). Nicotinic PAMs may act as a powerful alternative to exogenous orthosteric nicotinic receptor agonists, such as 3-(2,4-dimethoxybenzylidene)-anabaseine (i.e., DMXB-A, the code name, GTS-21 (Kem, 2000)) to help counteract neurocognitive deficits, nociception and brain injury. As a novel therapeutic opportunity, nicotinic-PAMs that could recruit endogenous cholinergic pathways and enhance pathology-activated auto-therapies would hold significant translational potential. Because of the ubiquitous expression of nicotinic receptors in neuronal and non-neuronal tissues, developing highly selective nicotinic-PAMs which, ideally, could target only a single player, i.e., the nicotinic receptors, would allow nicotinic-PAM-based interventions to target and recruit multiple endogenous neuronal and non-neuronal therapeutic cholinergic pathways. Although a lot remains to be learned about nicotinic-PAMs, recent pre-clinical findings create a strong sense of optimism and further extend the therapeutic promise of this novel class of compounds.
Acknowledgments
I thank Dr. John Dani and Dr. Daniel McGehee for productive discussions and criticism. I thank Dr. Imad Damaj for helpful suggestions. This study was supported by the NIH grant DK082625 and a grant from the Rainwater Charitable Foundation to VU.
Footnotes
Chemical compounds discussed in this article: 1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)urea; i.e., PNU-120596 (PubChem CID: 311434); choline chloride (PubChem CID: 6209); epibatidine (PubChem CID: 854023); (R)-5-(2-azetidinylmethoxy)-2-chloropyridine; i.e., ABT-594 (PubChem CID: 3075702); 3-(2,4-dimethoxybenzylidene)-anabaseine); i.e., DMXB-A (PubChem CID: 6438361).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike A, Takada-Takatori Y, Kume T, Izumi Y. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of alpha4 and alpha7 receptors in neuroprotection. J Mol Neurosci. 2010;40:211–216. doi: 10.1007/s12031-009-9236-1. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Sengstock GJ, Sanberg PR, Kem WR. Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res. 1995;674:252–259. doi: 10.1016/0006-8993(94)01449-r. [DOI] [PubMed] [Google Scholar]
- Badio B, Daly JW. Epibatidine, a potent analgetic and nicotinic agonist. Mol Pharmacol. 1994;45:563–569. [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Lippiello PM, Lucas R, Marrero MB. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci. 2011;68:931–949. doi: 10.1007/s00018-010-0525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera SM, Chavez CM, Corley SR, Kitto MR, Butt AE. Selective lesions of the nucleus basalis magnocellularis impair cognitive flexibility. Behav Neurosci. 2006;120:298–306. doi: 10.1037/0735-7044.120.2.298. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Hutchings EJ, Kille NJ, Chapman JM, Terry AV., Jr Positive allosteric modulator of alpha 7 nicotinic-acetylcholine receptors, PNU-120596 augments the effects of donepezil on learning and memory in aged rodents and non-human primates. Neuropharmacology. 2013;67:201–212. doi: 10.1016/j.neuropharm.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of alpha7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- Del Barrio L, Martin-de-Saavedra MD, Romero A, Parada E, Egea J, Avila J, McIntosh JM, Wonnacott S, Lopez MG. Neurotoxicity induced by okadaic acid in the human neuroblastoma SH-SY5Y line can be differentially prevented by alpha7 and beta2* nicotinic stimulation. Toxicol Sci. 2011;123:193–205. doi: 10.1093/toxsci/kfr163. [DOI] [PubMed] [Google Scholar]
- Dinklo T, Shaban H, Thuring JW, Lavreysen H, Stevens KE, Zheng L, Mackie C, Grantham C, Vandenberk I, Meulders G, Peeters L, Verachtert H, De Prins E, Lesage AS. Characterization of 2-[[4-fluoro-3-(trifluoromethyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazoleme thanol (JNJ-1930942), a novel positive allosteric modulator of the {alpha}7 nicotinic acetylcholine receptor. J Pharmacol Exp Ther. 2011;336:560–574. doi: 10.1124/jpet.110.173245. [DOI] [PubMed] [Google Scholar]
- Djuricic B, Olson SR, Assaf HM, Whittingham TS, Lust WD, Drewes LR. Formation of free choline in brain tissue during in vitro energy deprivation. J Cereb Blood Flow Metab. 1991;11:308–313. doi: 10.1038/jcbfm.1991.63. [DOI] [PubMed] [Google Scholar]
- Egea J, Rosa AO, Sobrado M, Gandia L, Lopez MG, Garcia AG. Neuroprotection afforded by nicotine against oxygen and glucose deprivation in hippocampal slices is lost in alpha7 nicotinic receptor knockout mice. Neuroscience. 2007;145:866–872. doi: 10.1016/j.neuroscience.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Faghih R, Gopalakrishnan SM, Gronlien JH, Malysz J, Briggs CA, Wetterstrand C, Ween H, Curtis MP, Sarris KA, Gfesser GA, El-Kouhen R, Robb HM, Radek RJ, Marsh KC, Bunnelle WH, Gopalakrishnan M. Discovery of 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonami de (A-867744) as a novel positive allosteric modulator of the alpha7 nicotinic acetylcholine receptor. J Med Chem. 2009;52:3377–3384. doi: 10.1021/jm9003818. [DOI] [PubMed] [Google Scholar]
- Feuerbach D, Lingenhoehl K, Olpe HR, Vassout A, Gentsch C, Chaperon F, Nozulak J, Enz A, Bilbe G, McAllister K, Hoyer D. The selective nicotinic acetylcholine receptor alpha7 agonist JN403 is active in animal models of cognition, sensory gating, epilepsy and pain. Neuropharmacology. 2009;56:254–263. doi: 10.1016/j.neuropharm.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biological psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Carroll FI, Damaj MI. The Antinociceptive Effects of Nicotinic Receptors alpha7-Positive Allosteric Modulators in Murine Acute and Tonic Pain Models. J Pharmacol Exp Ther. 2012;344:264–275. doi: 10.1124/jpet.112.197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas K, Negus SS, Carroll FI, Damaj MI. In vivo pharmacological interactions between a type II positive allosteric modulator of alpha7 nicotinic ACh receptors and nicotinic agonists in a murine tonic pain model. Br J Pharmacol. 2013;169:567–579. doi: 10.1111/j.1476-5381.2012.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasull T, DeGregorio-Rocasolano N, Zapata A, Trullas R. Choline release and inhibition of phosphatidylcholine synthesis precede excitotoxic neuronal death but not neurotoxicity induced by serum deprivation. J Biol Chem. 2000;275:18350–18357. doi: 10.1074/jbc.M910468199. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, Lindstrom J. Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol Pharm. 1995;48:774–782. [PubMed] [Google Scholar]
- Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer’s disease. J Neurochem. 2000;74:237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- Gusev AG, Uteshev VV. Physiological concentrations of choline activate native alpha7-containing nicotinic acetylcholine receptors in the presence of PNU-120596 [1-(5-chloro-2,4-dimethoxyphenyl)-3-(5-methylisoxazol-3-yl)-urea] J Pharmacol Exp Ther. 2010;332:588–598. doi: 10.1124/jpet.109.162099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseva MV, Hopkins DM, Scheff SW, Pauly JR. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J Neurotrauma. 2008;25:975–983. doi: 10.1089/neu.2008.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2004;29:1378–1385. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Hu M, Gopalakrishnan M, Li J. Positive allosteric modulation of alpha7 neuronal nicotinic acetylcholine receptors: lack of cytotoxicity in PC12 cells and rat primary cortical neurons. Br J Pharmacol. 2009;158:1857–1864. doi: 10.1111/j.1476-5381.2009.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, Groppi VE, Allaman G, Ogier R, Bertrand S, Bertrand D, Arneric SP. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- Kalappa BI, Gusev AG, Uteshev VV. Activation of functional alpha7-containing nAChRs in hippocampal CA1 pyramidal neurons by physiological levels of choline in the presence of PNU-120596. PloS one. 2010;5:e13964. doi: 10.1371/journal.pone.0013964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalappa BI, Sun F, Johnson SR, Jin K, Uteshev VV. A positive allosteric modulator of alpha7 nAChRs augments neuroprotective effects of endogenous nicotinic agonists in cerebral ischaemia. Br J Pharmacol. 2013;169:1862–1878. doi: 10.1111/bph.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalappa BI, Uteshev VV. The dual effect of PNU-120596 on alpha7 nicotinic acetylcholine receptor channels. Eur J Pharmacol. 2013;718:226–234. doi: 10.1016/j.ejphar.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Maeda T, Kume T, Kochiyama H, Akaike A, Shimohama S, Kimura J. Nicotine protects cultured cortical neurons against glutamate-induced cytotoxicity via alpha7-neuronal receptors and neuronal CNS receptors. Brain Res. 1997;765:135–140. doi: 10.1016/s0006-8993(97)00556-8. [DOI] [PubMed] [Google Scholar]
- Kelso ML, Oestreich JH. Traumatic brain injury: central and peripheral role of alpha7 nicotinic acetylcholine receptors. Curr Drug Targets. 2012;13:631–636. doi: 10.2174/138945012800398964. [DOI] [PubMed] [Google Scholar]
- Kem WR. The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: studies with DMXBA (GTS-21) Behav Brain Res. 2000;113:169–181. doi: 10.1016/s0166-4328(00)00211-4. [DOI] [PubMed] [Google Scholar]
- Kiewert C, Mdzinarishvili A, Hartmann J, Bickel U, Klein J. Metabolic and transmitter changes in core and penumbra after middle cerebral artery occlusion in mice. Brain Res. 2010;1312:101–107. doi: 10.1016/j.brainres.2009.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE, Burnett AL. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2003;28:542–551. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- Lee CH, Zhu C, Malysz J, Campbell T, Shaughnessy T, Honore P, Polakowski J, Gopalakrishnan M. alpha4beta2 neuronal nicotinic receptor positive allosteric modulation: an approach for improving the therapeutic index of alpha4beta2 nAChR agonists in pain. Biochem Pharmacol. 2011;82:959–966. doi: 10.1016/j.bcp.2011.06.044. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Kassai F, Szajli A, Nemethy Z. alpha7 nicotinic acetylcholine receptors and their role in cognition. Brain Res Bull. 2013;93:86–96. doi: 10.1016/j.brainresbull.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, Lee M, Adler L, Olincy A, Ross R, Freedman R. Smoking and schizophrenia: abnormal nicotinic receptor expression. Eur J Pharmacol. 2000;393:237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Li Y, Papke RL, He YJ, Millard WJ, Meyer EM. Characterization of the neuroprotective and toxic effects of alpha7 nicotinic receptor activation in PC12 cells. Brain Res. 1999;830:218–225. doi: 10.1016/s0006-8993(99)01372-4. [DOI] [PubMed] [Google Scholar]
- McLean SL, Idris NF, Grayson B, Gendle DF, Mackie C, Lesage AS, Pemberton DJ, Neill JC. PNU-120596, a positive allosteric modulator of alpha7 nicotinic acetylcholine receptors, reverses a sub-chronic phencyclidine-induced cognitive deficit in the attentional set-shifting task in female rats. J Psychopharmacol. 2012;26:1265–1270. doi: 10.1177/0269881111431747. [DOI] [PubMed] [Google Scholar]
- Medhurst SJ, Hatcher JP, Hille CJ, Bingham S, Clayton NM, Billinton A, Chessell IP. Activation of the alpha7-nicotinic acetylcholine receptor reverses complete freund adjuvant-induced mechanical hyperalgesia in the rat via a central site of action. The journal of pain: official journal of the American Pain Society. 2008;9:580–587. doi: 10.1016/j.jpain.2008.01.336. [DOI] [PubMed] [Google Scholar]
- Munro G, Hansen R, Erichsen H, Timmermann D, Christensen J, Hansen H. The alpha7 nicotinic ACh receptor agonist compound B and positive allosteric modulator PNU-120596 both alleviate inflammatory hyperalgesia and cytokine release in the rat. Br J Pharmacol. 2012;167:421–435. doi: 10.1111/j.1476-5381.2012.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, Zheng L, Stevens KE, Gee KW. Nootropic alpha7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8059–8064. doi: 10.1073/pnas.0701321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirogi R, Goura V, Abraham R, Jayarajan P. alpha4beta2* neuronal nicotinic receptor ligands (agonist, partial agonist and positive allosteric modulators) as therapeutic prospects for pain. Eur J Pharmacol. 2013;712:22–29. doi: 10.1016/j.ejphar.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Winblad B. Reduced number of [3H] nicotine and [3H] acetylcholine binding sites in the frontal cortex of Alzheimer brains. Neurosci Lett. 1986;72:115–119. doi: 10.1016/0304-3940(86)90629-4. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, Stevens JO, Martin L, Adler LE, Soti F, Kem WR, Freedman R. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Archives of general psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci Lett. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- Papke RL, Papke JKP. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J of Pharmacol. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada E, Egea J, Buendia I, Negredo P, Cunha AC, Cardoso S, Soares MP, Lopez MG. The Microglial alpha7-Acetylcholine Nicotinic Receptor Is a Key Element in Promoting Neuroprotection by Inducing Heme Oxygenase-1 via Nuclear Factor Erythroid-2-Related Factor 2. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2012.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry EK, Morris CM, Court JA, Cheng A, Fairbairn AF, McKeith IG, Irving D, Brown A, Perry RH. Alteration in nicotine binding sites in Parkinson’s disease, Lewy body dementia and Alzheimer’s disease: possible index of early neuropathology. Neuroscience. 1995;64:385–395. doi: 10.1016/0306-4522(94)00410-7. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Scopolamine induced learning failures in man. Psychopharmacology (Berl) 1977;52:283–289. doi: 10.1007/BF00426713. [DOI] [PubMed] [Google Scholar]
- Qian C, Li T, Shen TY, Garahan LL, Eckman J, Biftu T, Ip S. Epibatidine is a nicotinic analgesic. Eur J Pharmacol. 1993;250:13–14. doi: 10.1016/0014-2999(93)90043-h. [DOI] [PubMed] [Google Scholar]
- Rao AM, Hatcher JF, Dempsey RJ. Lipid alterations in transient forebrain ischemia: possible new mechanisms of CDP-choline neuroprotection. J Neurochem. 2000;75:2528–2535. doi: 10.1046/j.1471-4159.2000.0752528.x. [DOI] [PubMed] [Google Scholar]
- Rode F, Munro G, Holst D, Nielsen EO, Troelsen KB, Timmermann DB, Ronn LC, Grunnet M. Positive allosteric modulation of alpha4beta2 nAChR agonist induced behaviour. Brain Res. 2012;1458:67–75. doi: 10.1016/j.brainres.2012.03.064. [DOI] [PubMed] [Google Scholar]
- Roncarati R, Scali C, Comery TA, Grauer SM, Aschmi S, Bothmann H, Jow B, Kowal D, Gianfriddo M, Kelley C, Zanelli U, Ghiron C, Haydar S, Dunlop J, Terstappen GC. Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders. J Pharmacol Exp Ther. 2009;329:459–468. doi: 10.1124/jpet.108.150094. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Duan WR, Thomas J, Nothaft W, Backonja MM. A randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of ABT-594 in patients with diabetic peripheral neuropathic pain. Pain. 2009;146:245–252. doi: 10.1016/j.pain.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Shimohama S. Nicotinic receptor-mediated neuroprotection in neurodegenerative disease models. Biol Pharm Bull. 2009;32:332–336. doi: 10.1248/bpb.32.332. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Greenwald DL, Shafron DH, Akaike A, Maeda T, Kaneko S, Kimura J, Simpkins CE, Day AL, Meyer EM. Nicotinic α7 receptors protect against glutamate neurotoxicity and neuronal ischemic damage. Brain Res. 1998;779:359–363. doi: 10.1016/s0006-8993(97)00194-7. [DOI] [PubMed] [Google Scholar]
- Sun F, Jin K, Uteshev VV. A Type-II Positive Allosteric Modulator of alpha7 nAChRs Reduces Brain Injury and Improves Neurological Function after Focal Cerebral Ischemia in Rats. PloS one. 2013;8:e73581. doi: 10.1371/journal.pone.0073581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Yanagida T, Inden M, Takata K, Kitamura Y, Yamakawa K, Sawada H, Izumi Y, Yamamoto N, Kihara T, Uemura K, Inoue H, Taniguchi T, Akaike A, Takahashi R, Shimohama S. Nicotinic receptor stimulation protects nigral dopaminergic neurons in rotenone-induced Parkinson’s disease models. J Neurosci Res. 2009;87:576–585. doi: 10.1002/jnr.21869. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, El-Sayed M, Mikkelsen JD. Differential immediate and sustained memory enhancing effects of alpha7 nicotinic receptor agonists and allosteric modulators in rats. PloS one. 2011;6:e27014. doi: 10.1371/journal.pone.0027014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umana IC, Daniele CA, McGehee DS. Neuronal nicotinic receptors as analgesic targets: It’s a winding road. Biochem Pharmacol. 2013 doi: 10.1016/j.bcp.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Regulation of neuronal function by choline and 40H-GTS-21 through alpha 7 nicotinic receptors. J Neurophysiol. 2003;89:1797–1806. doi: 10.1152/jn.00943.2002. [DOI] [PubMed] [Google Scholar]
- Verbois SL, Hopkins DM, Scheff SW, Pauly JR. Chronic intermittent nicotine administration attenuates traumatic brain injury-induced cognitive dysfunction. Neuroscience. 2003;119:1199–1208. doi: 10.1016/s0306-4522(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Porter RH. Targeting the nicotinic alpha7 acetylcholine receptor to enhance cognition in disease. Biochem Pharmacol. 2011;82:891–903. doi: 10.1016/j.bcp.2011.06.034. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- White SH, Brisson CD, Andrew RD. Examining protection from anoxic depolarization by the drugs dibucaine and carbetapentane using whole cell recording from CA1 neurons. J Neurophysiol. 2012;107:2083–2095. doi: 10.1152/jn.00701.2011. [DOI] [PubMed] [Google Scholar]
- Yang JS, Seo SW, Jang S, Jung GY, Kim S. Rational engineering of enzyme allosteric regulation through sequence evolution analysis. PLoS Comput Biol. 2012;8:e1002612. doi: 10.1371/journal.pcbi.1002612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CZ, Chin CL, Rustay NR, Zhong C, Mikusa J, Chandran P, Salyers A, Gomez E, Simler G, Lewis LG, Gauvin D, Baker S, Pai M, Tovcimak A, Brown J, Komater V, Fox GB, Decker MW, Jacobson PB, Gopalakrishnan M, Lee CH, Honore P. Potentiation of analgesic efficacy but not side effects: co-administration of an alpha4beta2 neuronal nicotinic acetylcholine receptor agonist and its positive allosteric modulator in experimental models of pain in rats. Biochem Pharmacol. 2011;82:967–976. doi: 10.1016/j.bcp.2011.05.007. [DOI] [PubMed] [Google Scholar]