Abstract
Epilepsy is a prevalent neurological disorder associated with significant morbidity and mortality, but the only available drug therapies target its symptoms rather than the underlying cause. The process that links brain injury or other predisposing factors to the subsequent emergence of epilepsy is termed epileptogenesis. Substantial research has focused on elucidating the mechanisms of epileptogenesis so as to identify more specific targets for intervention, with the hope of preventing epilepsy before seizures emerge. Recent work has yielded important conceptual advances in this field. We suggest that such insights into the mechanisms of epileptogenesis converge at the level of cortical circuit dysfunction.
Epilepsy (BOX 1) is a common end point of many forms of acquired brain pathology (such as tumours, infection, stroke and traumatic brain injury). Epilepsy can also be the result of the mutation of a single gene (genetic epilepsy), and it can be one component of a neurodevelopmental disorder. The most common epilepsy syndrome in adults — mesial temporal lobe epilepsy (TLE; see BOX 2) — is typically considered to be an acquired disorder, resulting from injury to a previously normal brain. However, there are also familial (presumed genetic) forms of TLE. Although usually applied to the acquired epilepsies, the concept of epileptogenesis might also be relevant to the genetic epilepsies. An anti-epileptogenic intervention could be used in the context of a broader neurodevelopmental abnormality that includes epilepsy if an underlying defect could be identified and modulated before seizures began. Conversely, there could be potentially modifiable genetic contributions to what are classically considered to be acquired epilepsies, including TLE1–4. For example, an individual might have a genetic predisposition to risk factors for epilepsy, such as status epilepticus or hippocampal sclerosis, which in turn drive epileptogenesis.
Box 1. Definitions: from seizure to epilepsy.
Seizures
Seizures are abnormal, paroxysmal changes in the electrical activity of the brain. Seizures emerge from and are an inherent property of cerebral cortical structures. However, a (single) seizure can occur in an otherwise healthy individual; such individuals are generally not considered to have epilepsy.
Epilepsy
Epilepsy, by contrast, is a neurological disorder defined as a brain state that supports recurrent, unprovoked seizures.
Epileptogenesis
Epileptogenesis is the process by which the previously normal brain is functionally altered and biased towards the generation of the abnormal electrical activity that subserves chronic seizures. The concept of a ‘mechanism of epilepsy’ refers to any biological feature of the brain that drives or supports recurrent, unprovoked seizures. Examples of these biological features include molecular, anatomical or circuit level alterations, such as cell death or dysregulation of an ion channel or neurotransmitter receptor. By this definition, epilepsy mechanisms may be the downstream effectors of, but are distinct from, a prior epileptogenic process.
The process of epileptogenesis is classically thought to occur in three phases: first, the occurrence of a precipitating injury or event; second, a ‘latent’ period during which changes set in motion by the preceding injury act to transform the previously normal brain into an epileptic brain; and third, chronic, established epilepsy. It is during the latent period that the process of acquired epileptogenesis is thought to coalesce, and it is at this point in the process that interventions might be used to prevent the subsequent development of epilepsy.
The distinction between seizures, epilepsy and epileptogenesis is well illustrated by the accumulating data indicating that classical anticonvulsants, which can terminate an ongoing seizure and decrease or prevent the occurrence of future seizures in epileptic individuals, are wholly ineffective in preventing the process of epileptogenesis169.
Box 2. Temporal lobe epilepsy.
Mesial temporal lobe epilepsy (TLE; also known as limbic epilepsy), refers to an electroclinical syndrome in which seizures emanate from the temporal lobe. TLE is a prominent and prevalent epilepsy syndrome in children, adolescents and adults. This syndrome is often associated with mesial temporal sclerosis — a pattern of hippocampal damage, including atrophy and gliosis, seen in humans with TLE and in animal models of chronic TLE. TLE is typically considered to be an acquired epilepsy syndrome that is triggered by a precipitating factor, such as head trauma, infection or tumour, or an episode of prolonged, uncontrolled seizure (status epilepticus), although there are known familial forms of mesial TLE1–4,170,171. Lateral TLE172 is a distinct syndrome that will not be discussed in this Review. From a basic science perspective, TLE has generally been considered as an acquired condition; likewise, temporal lobe epileptogenesis has been considered relevant to the acquired epilepsies only.
Animal models of acquired TLE have been a particularly useful tool in the study of epileptogenesis, because the animal model resembles the human condition in many key respects — chronic TLE can be induced in rodents using various types of brain injury that also cause TLE in humans. Previous studies have identified changes in molecular signalling pathways in animal models of acquired TLE, including alterations in immediate-early gene expression and the expression of various ion channels and neurotransmitter receptors. Proposed circuit-level mechanisms include synaptic reorganization, axonal sprouting and the generation of new, pathological, recurrent excitatory synaptic connections (for example, mossy fibre sprouting). Cellular-level mechanisms include inflammation, neuronal cell death (including the specific loss of mossy cells and vulnerable populations of inhibitory GABAergic interneurons), reactive astrocytosis and dispersion of existing and the generation of new dentate granule cells in ectopic locations. Some of these changes are likely to be involved in and perhaps required for the pathogenesis of epilepsy, whereas others might be reparative of or compensatory for the initial injury and ongoing chronic epileptic condition, and still other changes might simply be epiphenomenal.
In this Review, rather than attempting to provide a broad overview of epileptogenesis, we restrict our discussion to a set of emerging themes. We review the role of specific large-scale molecular cell signalling pathways in epileptogenesis, highlighting the mammalian target of rapamycin (mTOR) and repressor element 1 (RE1)-silencing transcription factor (REST; also known as neuron-restrictive silencer factor (NRSF)) pathways in acquired and genetic epilepsies. We discuss how dysfunction of specific subtypes of neurons might lead to epilepsy, with a focus on the theory of cortical GABAergic interneuron dysfunction as the basis of the infantile-onset epileptic encephalopathy of Dravet syndrome. Finally, we consider the pathology of discrete elements of neuronal circuits in mesial TLE. For a broader overview, see recent general reviews of the mechanisms of acquired epileptogenesis5,6.
Seizures are network events that require re-entrant activation of populations of neurons embedded within brain circuits; hence, epilepsy is inextricably a circuit-level phenomenon and cannot be understood outside this context. It is likely that multiple and diverse mechanisms of epileptogenesis overlap to various extents among different epilepsy syndromes, both acquired and genetic. However, for a discrete genetic mutation or dysfunction of a molecular signalling pathway or specific cell type to produce epilepsy, it must do so by altering the function of circuits. We suggest that a greater mechanistic understanding of circuit function and circuit-level dysfunction in epilepsy will lead to the development of successful and broadly applicable interventions in epileptogenic processes, which remain a primary unmet need in epilepsy therapy.
Molecular signalling pathways in epileptogenesis
The possibility that a brain injury or genetic abnormality might lead to the development of epilepsy through maladaptive activity of one or more large-scale molecular signalling pathways is inherently attractive, as this would suggest that directed manipulation of these pathways might be able to modify the epileptogenic process before the appearance of seizures. Research is motivated by an attempt to identify molecular signalling processes that might be involved in driving the formation of the aberrant neuronal circuitry seen in the epileptic brain, including features such as axonal sprouting and cell death that develop following a delay after an initial brain insult. Important potential master regulators of epileptogenesis have emerged in recent years, including brain-derived neurotrophic factor (BDNF)–tropomyosin-related kinase B (TRKB; also known as NTRK2) signalling7–9, the mTOR pathway10–12, the REST pathway13 and other transcriptional regulators14. Here, we focus on the roles of mTOR and REST given the recent and rapid accumulation of evidence implicating these pathways in temporal lobe epileptogenesis.
Mammalian target of rapamycin
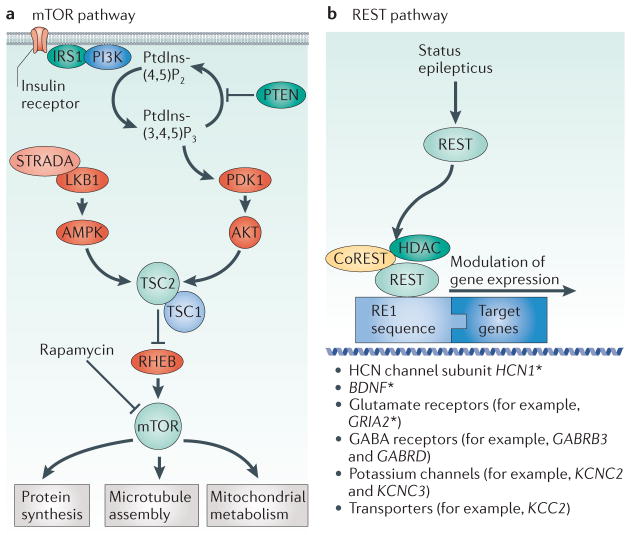
mTOR is a serine/ threonine protein kinase that is involved in multiple basic cellular functions, including cell growth, proliferation and survival. mTOR is regulated by pathways that respond to glutamatergic transmission and the energy state of the cell (FIG. 1). In turn, mTOR regulates synaptic plasticity15, neuronal structure16 and the expression of various ion channels and neurotransmitter receptors17. The mTOR pathway is strongly associated with epilepsy. Genetic mutations in many components of the mTOR pathway, including tuberous sclerosis complex 1 (TSC1) and TSC2, STE20-related kinase adaptor-α (STRADA), phosphatase and tensin homologue (PTEN), the gene encoding the serine/threonine kinase AKT isoform that is predominant in the brain (AKT3), phosphatidylinosi-tol-4,5-bisphosphate 3-kinase-α (PIK3CA) and MTOR itself, produce syndromes that include epilepsy10,11,18–20 (FIG. 1). In addition, manipulation of the mTOR pathway can modulate acquired epilepsy in animal models.
Figure 1. Mechanistic roles of the mTOR pathway and REST in epileptogenesis.
a | A simplified version of the mammalian target of rapamycin (mTOR) pathway, highlighting epilepsy-related molecules. For a more complete illustration of the mTOR pathway, see REF. 12. b | A schematic depicting repressor element 1 (RE1)-silencing transcription factor (REST)-mediated gene silencing triggered by status epilepticus. REST co-repressor 1 (CoREST), a component of the histone deacetylase (HDAC) complex, interacts with REST to mediate transcriptional repression. The asterisk indicates genes for which there is specific experimental evidence suggesting regulation by REST or HDAC inhibiton after status epilepticus49,56. AMPK, AMP-activated protein kinase; BDNF, brain-derived neurotrophic factor; GABRB3; type A GABA receptor β3 subunit; GABRD; type A GABA receptor δ-subunit; GRIA2, ionotropic AMPA 2 glutamate receptor; HCN1, hyperpolarization-activated cyclic nucleotide-gated channel subunit 1; IRS1, insulin receptor substrate 1; KCC2; potassium/chloride co-transporter 2; KCNC, potassium voltage-gated channel subfamily C; LKB1, liver kinase B1 (also known as serine/threonine kinase STK11); mTORC1, mTOR complex 1; PDK1, 3-phosphoinositide-dependent kinase 1; PI3K, phosphoinositide 3-kinase; PtdIns(4,5)P2, phosphatidylinositol-4,5-biphosphate; PtdIns(3,4,5)P3, phosphatidylinositol-3,4,5-triphosphate; PTEN, phosphatase and tensin homologue; RHEB, RAS homologue enriched in brain; STRADA, STE20-related adaptor protein-α; TSC, tuberous sclerosis complex. Part a is modified, with permission, from REF. 11 © (2011) Elsevier.
The role of the mTOR pathway in genetically determined epilepsy11,18 is typified by tuberous sclerosis, which results from mutations in TSC1 and TSC2 (FIG. 1). The mechanisms of seizure generation in tuberous sclerosis are debated21, but seizures are thought to originate around or within cortical tubers22, which are foci of abnormal neuronal migration and cellular growth. Intracranial electroencephalographic monitoring in patients with tuberous sclerosis has provided evidence that at least some tubers are intrinsically epileptogenic and that they might in some cases be more epileptogenic than the perituberal cortex23. Tsc1GFAP conditional knockout mice, in which Tsc1 is deleted from glia, show astrogliosis and dislamination, with pyramidal cell dispersion in the hippocampus24. Glia in these mice show altered expression of gap junction proteins, potassium channels and astrocytic glutamate transporters25. Treatment of these mice with the mTOR inhibitor rapamycin suppresses seizures; however, seizures return if rapamycin is withdrawn26.
Interestingly, selective deletion of the mTOR pathway gene Pten from a small subset of dentate granule cells in mice produces TLE27. The mechanism by which deletion of Pten in a subset of dentate granule cells might lead to epilepsy is unclear, but it caused cellular-level structural abnormalities including increased spine density and granule cell hypertrophy, and this abnormal cell growth might itself be epileptogenic. Mossy fibre sprouting in these mice was the same from wild-type granule cells as from those in which Pten had been deleted, which suggests that mossy fibre sprouting might be a consequence rather than a cause of epilepsy, at least in this mouse model.
Somatic mosaicism for gain-of-function mutations in the mTOR pathway components AKT3, PIK3CA and MTOR are responsible for some cases of the dramatic focal cortical brain malformation that occurs in hemimegalencephaly28,29. This condition is almost invariably associated with medically intractable epilepsy. Mice harbouring an activating mutation in Akt3 have epilepsy and increased brain size30.
The mTOR pathway might also be involved in acquired epilepsy. This has been investigated using rodent models of chronic acquired TLE after status epilepticus induced by the chemoconvulsants kainate31 or pilocarpine32–34 (kainate- or pilocarpine-induced status epilepticus), TLE induced by electrical stimulation of the angular bundle35 or the amyg-dala36, post-traumatic epilepsy after brain injury37,38 and models of infantile spasms39 and early hypoxic-ischaemic injury. Rapamycin treatment before kainate-induced status epilepticus prevented the development of spontaneous recurrent seizures in a subset of rats31; in those rats that did develop epilepsy, rapamycin increased the latency to seizure development and decreased the frequency of spontaneous seizures. When applied after the induction of status epilepticus, rapamycin decreased cell death, mossy fibre sprouting and aberrant dentate granule cell neurogenesis, all of which are histopathological features associated with TLE. However, in mice, seizure frequency was unchanged by rapamycin treatment given after pilocarpine-induced status epilepticus, although the degree of mossy fibre sprouting was decreased34.
Mossy fibre sprouting was inhibited by rapamycin given before or after status epilepticus in the kainate- or pilocarpine-induced model of TLE in rats31–34 and after status epilepticus induced by electrical stimulation of the angular bundle35 but not in a model of TLE induced by electrical stimulation of the amygdala36. However, upon treatment withdrawal, the degree of sprouting returned to the levels seen in untreated epileptic animals32, and seizures recurred after treatment removal33. Hence, whereas manipulation of mTOR signalling in most rodent models of acquired epilepsy appears to modulate seizure frequency and decrease the degree of various measures of TLE neuropathology, such as mossy fibre sprouting and hilar cell loss, mTOR pathway modulation in such models has not been shown to prevent the development of epilepsy permanently. It seems that continued rapamycin administration is required to maintain any anti-epileptogenic effects. Some authors have referred to the effects of rapamycin as ‘antiseizure’ or ‘epileptostatic’, given that rapamycin has not been shown to enact a prevention of epilepsy development that persists after rapamycin withdrawal40,41. Despite these exciting recent developments and the clear role of mTOR pathway molecules in genetic epilepsy syndromes, the mechanism (or mechanisms) by which manipulation of mTOR signalling after brain injury (such as that due to status epilepticus) might modulate epileptogenesis remain unclear. These mechanisms might include inhibition of mossy fibre sprouting, neuroprotection and maintenance of the integrity of the blood–brain barrier35.
Repressor element 1-silencing transcription factor
REST is a transcription factor that binds to DNA-binding motifs called neuron-restrictive silencer elements (NRSEs; also known as RE1 elements). REST negatively regulates the expression of many neuronal genes in non-neuronal cells and neuronal precursor cells. It also regulates neuronal gene expression in mature neurons42,43. REST binds to various co-repressors, which recruit his-tone deacetylase 1 (HDAC1) and HDAC2 (FIG. 1); these deacetylases regulate chromatin structure and repress the expression of hundreds of neuronal genes44. Almost 2,000 genes have REST-binding motifs44 (although this number could be much greater45), including ~10% of all neuronally expressed genes46, some of which encode proteins that are key regulators of neuronal excitability and have been independently proposed to be involved in epilepsy mechanisms47. Such genes include type A GABA (GABAA) receptor β3 subunit (GABRB3), GABAA receptor δ-subunit (GABRD) and the ionotropic AMPA2 glutamate receptor (GRIA2), BDNF and genes encoding hyperpolarization-activated cyclic nucleotide-gated (HCN) channel subunits 1–4 (HCN1–HCN4).
More direct evidence that alterations in REST signalling might be involved in epileptogenesis continues to accumulate48. Status epilepticus in animal models increases the expression of REST in the hippocampus and alters the histone acetylation of prominent neuronally expressed genes42,49. Status epilepticus induces promoter-specific acetylation of BDNF that is inhibited by HDAC inhibitors49,50. In the kindling model, inhibition of glycolysis induces the binding of REST to the BDNF promoter, which prevents BDNF upregulation and modulates epileptogenesis, providing an interesting mechanistic link between glycolysis, BDNF–TRKB signalling (see above) and the REST system51. The ultimate effector (or effectors) of this signalling cascade remain unknown.
The role of HCN channels in epileptogenesis has been reviewed in REF. 52. Dendritic HCN channels in hippocampal pyramidal cells are dysregulated after prolonged febrile seizures or after kainate- or pilocarpine-induced status epilepticus in rodents, leading to what has been termed an ‘acquired HCN channelopathy’ (to differentiate this from a genetic channelopathy)53–55. This phenomenon might be partly due to REST-mediated transcriptional repression of Hcn156. Binding of REST to Hcn1 is increased after the induction of status epilepticus in a rodent model of TLE, resulting in decreased HCN1-mediated ionic currents (Ih) and altered function of CA1 pyramidal cells. Interference with REST binding to target genes (including Hcn1) using an anti-REST decoy oligodeoxynucleotide was associated with decreased seizure frequency in rats after kainate-induced status epilepticus. These examples show that status epilepticus might activate the REST pathway to suppress important regulators of neuronal excitability that have been independently proposed to be involved in pathogenic epilepsy mechanisms. It will be important to determine which gene targets of REST are up- or downregulated by this pathway after status epilepticus or other brain injury. This will prove challenging given the broad activity of this pathway across the genome. If a certain REST target was identified as particularly important in driving epileptogenesis, would it be possible to selectively interrupt status epilepticus-induced, REST-mediated suppression of specific targets? Can the REST system be safely modulated in humans after brain injury (for example, with HDAC inhibitors)? Although long-term treatment with such agents might be accompanied by unacceptable toxicity, perhaps a single treatment or a short course of treatment given immediately after injury would prove safe.
The mTOR and REST pathways are exciting new potential targets for intervention in the epileptogenic process, but specific questions remain. To what extent is activation of the mTOR or REST pathways involved in driving epileptogenesis rather than being the result of this process? What are the circuit-level effects of the manipulation of key molecular signalling cascades such as the mTOR pathway and REST after status epilepticus or other brain injury? What are the relevant molecular effectors of mTOR pathway blockade or HDAC inhibition in the prevention of epileptogenesis in animal models of acquired TLE? And how widespread is the relevance of these signalling cascades across various forms of epilepsy beyond TLE?
Extensive research in animal models and in humans after brain injury using a range of prophylactic anticonvulsants, anti-inflammatory agents and neuroprotective agents has failed to demonstrate anti-epileptogenic effects5,6. What hope is there that targeting the mTOR and REST pathways will meet with greater success? As opposed to classical anticonvulsants, for example, which act on one or a few discrete targets, modulation of mTOR or REST would have broad action. As there are likely to be multiple divergent mechanisms of epilepsy development, perhaps polytherapy cocktails that target multiple, parallel pathways will prove effective. Using epileptogenic circuit-level dysfunction (as discussed below) will allow us to place in context the downstream effects of mTOR and/or REST system blockade on epilepsy development and facilitate translational efforts towards therapy development.
Cell type-specific dysfunction and epilepsy
Previous work studying the basic mechanisms of limbic epilepsy has identified dysfunction at the cellular level that might suggest potential targeted anti-epileptogenic interventions. Here, we discuss specific subtypes of cortical inhibitory GABAergic interneurons for which a mechanistic role in epilepsy is increasingly being recognized. A larger discussion of cortical interneuron subtypes is beyond the scope of this Review and has been addressed recently57–60. More general roles of GABAergic inhibition in epilepsy mechanisms have been reviewed in REFS 61–64, including the emerging potential use of GABAergic interneuron transplantation as a therapeutic treatment modality for epilepsy65–67.
Somatostatin-positive hilar interneurons
Immuno-reactivity for the neuropeptide somatostatin defines various overlapping subtypes of GABAergic interneurons in the cerebral cortex. In the dentate gyrus, most GABAergic interneuron somata that are immunopositive for somatostatin are located in the hilus68; approximately 50% of all hilar GABAergic interneurons are somatostatin-positive69. The synaptic terminal fields of somatostatin-positive hilar interneurons are most prominent in the outer molecular layer of the dentate gyrus, where the fibres of the perforant path terminate70,71; in addition, the dendrites of these cells are targeted by recurrent collaterals of the granule cell-derived mossy fibres72. In turn, somatostatin-positive terminals ramify primarily with granule cell dendrites. Thus, hilar somatostatin interneurons are ideally positioned to modulate the influence of the perforant path on dentate granule cells (FIG. 2).
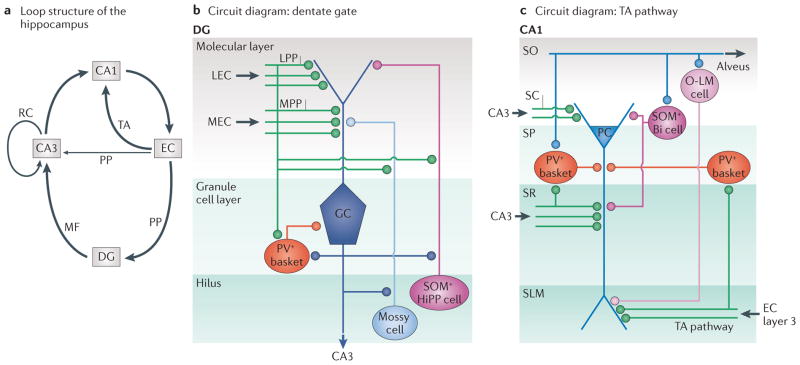
Figure 2. Embedded loop structures of the temporal lobe.
a | The hippocampus receives two inputs, the perforant path (PP) and the temporoammonic (TA) pathway. The entorhinal cortex (EC) is in turn the target of hippocampal output, creating multiple excitatory loops. There is a long loop that originates from the EC and projects to the dentate gyrus (DG) via the PP, from the DG to area CA3 via mossy fibres (MFs), from area CA3 to area CA1 and from area CA1 back to the subiculum (not shown) and/or EC. An intermediate-length loop originates from the EC and projects to area CA3 (via the PP pathway), then to area CA1 and from there to the subiculum and/or EC. A short loop projects from the EC to area CA1 (via the TA pathway) and back to the subiculum and/or EC. Recurrent collaterals (RCs) between CA3 pyramidal cells (PCs) are also shown. b | Simplified connectivity within the DG. Many connections and various inhibitory interneuron subtypes are omitted for clarity58,173,174. PCs and stellate cells within layer 2 of the medial EC (MEC) and lateral EC (LEC) form the PP to the middle and outer molecular layer, respectively, of the hippocampal DG, as well as to the distal dendrites of PCs in area CA3 (not shown)173. In the dentate, lateral PP (LPP) and medial PP (MPP) axons synapse onto dentate granule cells (GCs) and onto GABAergic interneurons (including parvalbumin-positive (PV+) basket cells and somatostatin-positive (SOM+) hilar PP-associated (HiPP) cells) as well as mossy cells in the dentate hilus. c | Selected connections of the TA pathway, which proceeds from layer 3 EC pyramidal and spiny stellate neurons (not shown) to the distal dendrites and distal apical tuft dendrites of CA1 neurons in stratum lacunosum-moleculare (SLM) in the short loop mentioned above as well as to the distal dendrites of CA2 (the intermediate-length loop, not shown). Extrinsic excitatory connections are shown in green; excitatory cells and intrinsic excitatory connections are shown in blue; and inhibitory cells and intrinsic inhibitory connections are shown in red and purple. Bi, bistratified cell; O-LM, oriens lacunosum-moleculare. SC, Schaffer collateral; SO, stratum oriens; SR, stratum radiatum.
Selective loss of somatostatin-positive GABAergic hilar interneurons is a well-documented and consistent finding in animal models of TLE73,74 and is found in the sclerotic hippocampus from humans with TLE75, indicating that these neurons are highly and selectively vulnerable to excitotoxic and ischaemic damage. In the kainate model of chronic TLE in mice, surviving somatostatin-positive hilar interneurons show axonal sprouting to form new synapses with dentate granule cells76, which is consistent with increased somatostatin immunoreactivity in the dentate molecular layer in human TLE77. This might be a way to restore the compromised levels of inhibition that are produced in part by the loss of inhibitory interneurons. Interestingly, axonal sprouting by somatostatin-positive hilar interneurons is inhibited by rapamycin78. The consequences of the loss of somatostatin-positive hilar interneurons and the changes that surviving neurons undergo in the context of hippocampal circuit function are subjects of ongoing investigation.
Fast-spiking cells and epilepsy
Parvalbumin-positive fast-spiking basket cells are key regulators of inhibition in the cerebral cortex. Fast-spiking cells are the most numerous subtype of cortical interneuron79,80. These cells form powerful, proximally localized synapses on the soma and axon initial segment (AIS) of postsynaptic cells, exerting fine control over the timing and probability of target cell discharge. Fast-spiking cells mediate feedforward and feedback inhibition that is recruited by specific spatio-temporal patterns of excitatory activity81. In addition, fast-spiking cells form dense interconnected networks through specific gap junctional coupling, which contributes to the function of these cells in the generation of fast oscillations within cortical structures. Modelling studies predict that perisomatically targeted inhibitory cell activity produces gamma oscillations82. Optogenetic activation of parvalbumin-positive fast-spiking cells augments gamma-band oscillatory activity in the neocortex83,84. In turn, dysfunction of interneuron-mediated gamma-band synchronization has been proposed to contribute to TLE mechanisms85. Specific optogenetic activation of parvalbumin-positive interneurons in the hippocampus terminated seizures in a mouse model of TLE86.
Genetic manipulation of interneuron progenitors in the medial ganglionic eminence (MGE) that are destined to become parvalbumin-positive fast-spiking cells causes epilepsy in mice87–89. In addition, genetic mutation or experimental manipulation of various ion channel genes that are prominently or exclusively expressed in fast-spiking cells (including the voltage-gated potassium channel genes KCNA1 (REFS 90–93) and KCNC2 (REF. 93)) causes epilepsy in humans and in mouse models.
Mutations of SCN1A (which encodes the voltage-gated sodium channel subunit NaV1.1) causes a spectrum of related epilepsy syndromes, including generalized epilepsy with febrile seizures plus (GEFS+) and Dravet syndrome94–96. Initial functional studies of SCN1A mutations associated with GEFS+ yielded inconsistent results; however, similar studies of the more severe Dravet syndrome phenotype showed a loss of function caused by haploinsufficiency97. The loss of function caused by haploinsufficiency was explained by evidence showing that mutation of SCN1A generates epilepsy by a selective reduction in the sodium current in cortical inhibitory GABAergic interneurons relative to pyramidal cells, which leads to decreased action potential firing frequency in interneurons98–103. This was a very attractive hypothesis as to the mechanism of Dravet syndrome, given the important role of fast-spiking cells in the regulation of cortical inhibition. The sodium current might be particularly reduced in cerebral cortical parvalbumin-positive fast-spiking interneurons in which NaV1.1 appears to be prominently and perhaps differentially expressed relative to pyramidal cells and parvalbumin-negative interneurons104. Further supporting this theory, interneuron-specific deletion of Scn1a in the forebrain using Dlx1/2 Cre transgenic mice causes an epilepsy phenotype that is similar to global heterozygous deletion of Scn1a105. In addition, pathology in these mice begins to appear at the same time that fast-spiking interneurons acquire a mature electrophysiological phenotype106,107, which might be the developmental time point at which cortical structures begin to rely on fast-spiking cells as a major source of inhibition. However, a published voltage-clamp recording of sodium currents from interneurons and principal cells in mouse models of Dravet syndrome exists only for dissociated hippocampal neurons in culture98 and neurons from the visual cortex104.
Previous data showed that NaV1.1 is expressed at the AIS, soma and dendrites of pyramidal cells108,109, but more recent findings in adult rats suggest that NaV1.1 expression in the neocortex and hippocampus is restricted to the AIS of parvalbumin-positive interneurons110. This unresolved issue complicates the interpretation of the proposed cell type-specific dysfunction in mouse models of Dravet syndrome. Such dysfunction might reflect true cell type-specific differences in NaV1.1 expression or differential compensation by other sodium channel genes in pyramidal cells as compared with interneurons. This compensation might occur differently in the various mouse models of Dravet syndrome (such as Scn1a knockout mice, knock-in mice harbouring a nonsense mutation in Scn1a or bacterial artificial chromosome (BAC) transgenic mice expressing a mutation from a human patient with Dravet syndrome), although haploinsufficiency seems to be a common theme. Genetic background strain and developmental effects might also affect the phenotype of mouse models of Dravet syndrome. That interneuron-specific Scn1a knockout recapitulates the phenotype of the global null mutation shows that Scn1a deletion in cortical interneurons is sufficient to produce a Dravet-like phenotype in mice; however, this does not prove that forebrain interneurons are the necessary and exclusive locus of cellular dysfunction in Dravet syndrome or that the absence of NaV1.1 in fast-spiking cells is the mechanism of Dravet syndrome. As mentioned above, many perturbations directed towards fast-spiking cells produce severe epilepsy, at least in mouse models, so it is not surprising that deletion of a major sodium channel that underlies action potential generation in these cells will cause hyperexcitability in cortical circuits with resultant epilepsy. Furthermore, although the SCN1A mutation is the most common cause of Dravet syndrome, accounting for approximately 70% of cases95,96, mutations in other genes can cause a Dravet or Dravet-like phenotype. These genes include GABRG2 (REF. 111), the gene encoding sodium channel-β1 subunit (SCN1B)112,113, the gene encoding NaV1.2 (SCN2A)114,115 and protocadherin 19 (PCDH19)116, suggesting that multiple genetic mechanisms can produce Dravet or Dravet-like syndrome. Whether these genetic mutations converge on fast-spiking cell dysfunction is unknown.
Dravet syndrome spectrum disorders might represent a point of convergence between acquired and genetic mechanisms of epilepsy. Mutations of SCN1A (or other Dravet syndrome spectrum genes) generate a strong genetic predisposition to febrile and afebrile seizures in humans. Patients with Dravet syndrome are not born with epilepsy, just as mice with Scn1a (or Scn1b117) mutations do not exhibit spontaneous seizures until the late second or early third postnatal week. Like patients with Dravet syndrome spectrum disease, mouse models initially show temperature-sensitive seizures before the emergence of spontaneous seizures98,100,104. As described above, prolonged febrile and afebrile seizures (particularly status epilepticus) can trigger a secondary cascade of events that might compound the pathology, leading to neuronal plasticity and epileptogenesis118,119. Interestingly, focal TLE has been reported in patients with the SCN1A mutation and GEFS+120,121. TLE has also been reported in a small group of patients with GEFS+ that is due to the SCN1B mutation, including one patient without known history of prolonged febrile seizure or status epilepticus who had TLE with mesial temporal sclerosis (BOX 2) and who underwent successful temporal lobectomy122. Of further interest, transgenic mice expressing a gain-of-function Scn2a mutation have epilepsy and mesial temporal sclerosis123. Although MRI scans of patients with Dravet syndrome are typically normal initially, hippocampal sclerosis develops over time in some patients124–126. This concept of epileptogenesis, linking sodium channel mutations, febrile seizures, status epilepticus and TLE, blurs the distinction between the classical mechanisms of epilepsy that are driven either by acquired brain injury or genetic mutation. Hence, it might be possible to intervene medically before the emergence of seizures in at-risk patients, in whom risk is defined by vulnerable genetic background in addition to exposure to brain injury. Possible interventions might include dietary therapies (for example, the ketogenic diet127), stem cell-based therapies to replace a dysfunctional cell type or targeted pharmacological or optogenetic agents tailored to a specific neuronal cell type.
The mechanisms by which the SCN1A mutation leads to epilepsy are highly controversial and are the subject of intense ongoing investigation. Recent data on Scn1b null mice might further complicate the picture. These mice show abnormal neuronal proliferation, neuronal migration and axonal targeting at early developmental time points, before the appearance of seizures128, suggesting that neurodevelopmental abnormalities might contribute to epilepsy in these mice. As Scn1b regulates the physiology, surface expression and subcellular targeting of Scn1a, perhaps Dravet syndrome represents a neurodevelopmental disorder of which epilepsy is but one prominent component. That said, the delayed appearance of epilepsy does not necessarily imply an underlying epileptogenic process; it could be that the circuit elements that underlie seizure generation are simply not mature until a particular age (see above). This is clear from the fact that seizures appear days, months or years after birth in Dravet syndrome and in other genetic epilepsies. We suggest that a more complete understanding of these issues will require further investigation of the roles of fast-spiking cells in the normal function of cortical circuits and the mechanisms and consequences of fast-spiking cell dysfunction in circuit operations in Scn1a mutant mice.
Circuit dysfunction in epileptogenesis
Seizures are network-level phenomena, and the incomplete knowledge of neuronal circuit function has left a gap in our understanding of how disruption at a molecular or cellular level generates epilepsy in the intact organism. We argue that bridging this gap will require large-scale, dynamic, circuit-level analysis. Ongoing research in animal models of TLE has been facilitated by recent advances in network-level functional imaging techniques such as voltage-sensitive dye (VSD) and calcium imaging, which allow simultaneous monitoring of the activity of large numbers of neurons at high temporal resolution. The additional use of optogenetic techniques allows researchers to manipulate defined subsets of neurons within these circuits with millisecond precision86,129,130. For example, in an animal model of post-stroke epilepsy, HCN channel dysregulation renders thalamic relay neurons hyperexcitable, and optogenetic silencing of these cells interrupts seizures131. These techniques are ideally suited for the study of circuit-level phenomena such as seizures and the circuit-level dysfunctions that underlie epilepsy.
Within the temporal lobe, there is an intimate relationship between the hippocampus and entorhinal cortex — two potentially epileptogenic brain regions — formed by multiple re-entrant circuit loops (FIG. 2). These synaptic loops are important for normal hippocampal network operations but also render the temporal lobe particularly vulnerable to the generation of abnormal electrical activity and underlie the predisposition of temporal lobe structures to epileptogenesis132,133. Here, we focus our discussion on two important discrete circuit elements in the temporal lobe, both of which have been proposed to be involved in the pathology of TLE: the dentate gate between the entorhinal cortex and area CA3, and the temporoammonic pathway between the entorhinal cortex and area CA1. Large-scale dynamic imaging has provided insights into the normal operation and dysfunction of these circuit elements in epilepsy.
The dentate gate in limbic epileptogenesis
The dentate gyrus has been a particular focus in the study of TLE for various reasons. The dentate gyrus is the locus for the classical temporal lobe circuit rearrangement of mossy fibre sprouting, which is seen in human TLE and in animal models134,135; sprouting also occurs from CA3 pyramidal cells and mossy cells in the dentate hilus in animal models of TLE136. The time course of the appearance of mossy fibre sprouting, which occurs following a delay after brain injury, is concordant with the latent period of temporal lobe epileptogenesis. However, the role of mossy fibre sprouting in epileptogenesis remains controversial135. There are additional anatomical alterations in this region in TLE, including dispersion of dentate granule cells, abnormal granule cell neurogenesis, the appearance of granule cells in ectopic locations and the loss of mossy cells and vulnerable populations of inhibitory interneurons in the dentate gyrus and hilar region.
Under normal conditions, the dentate gyrus strongly filters afferent input, relaying only a small component of incoming, synchronous cortical synaptic inputs to its downstream target, hippocampal area CA3. This phenomenon has been termed the ‘dentate gate’132,133,137. Dentate gating is thought to allow the dentate gyrus to filter entorhinal cortex input through the perforant path to the hippocampus, selecting inputs and enacting a ‘sparse coding’ scheme that is thought to be important for the computational functions of the dentate gyrus in pattern separation138. Dentate granule cells also have roles in the modulation of CA3 place cell behaviour138 and in cognitive processes such as learning and memory139,140. In addition, the gate is thought to protect downstream area CA3, which is vulnerable to metabolic challenges, from excitotoxic damage that could be induced by aberrant hypersynchronous entorhinal cortical activity. A significant contributor to this emergent network phenomenon is the small percentage of dentate gyrus granule cells that are activated by perforant path stimulation (2–5% in vitro141). A similarly small percentage (2%) of dentate granule cells are active during spatial navigation in animals in vivo142. For these reasons, the assessment of gating is important as a network-level output measure of dentate gyrus function under normal conditions and of circuit dysfunction in epilepsy. This phenomenon can readily be shown electrophysiologically in vitro, where epileptiform activity in the entorhinal cortex induced by pharmacological manipulation does not propagate through the dentate gyrus to area CA3 under control conditions (FIG. 3) but does so, for example, after blockade of GABAergic inhibition (FIG. 3) or in the kindled hippocampus143. Dentate gating has also been demonstrated using large-scale VSD imaging of the dentate: stimulation of the perforant path produced an excitatory response in the inner molecular layer of the dentate gyrus that subsequently spread to the granule cell layer of the dentate gyrus as well as the proximal hilus but did not activate area CA3 (REF. 144). Understanding the mechanisms that underlie the breakdown of dentate filter function is a goal of ongoing research, as protecting or bolstering the gate after brain injury or the induction of status epilepticus could theoretically modulate epileptogenesis.
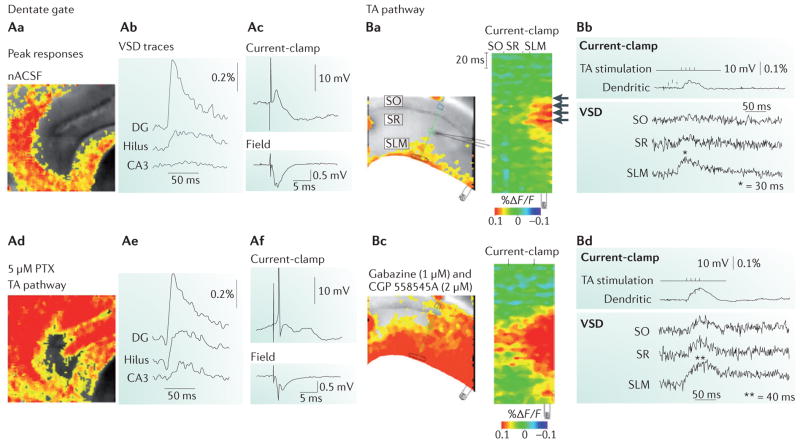
Figure 3. Temporal lobe circuit elements under normal conditions.
A | Dentate gating in the hippocampus under normal conditions (parts Aa–Ac) and after blockade of GABAergic inhibition (parts Ad–Af). Aa | A voltage-sensitive dye (VSD) image of depolarization that is restricted to the dentate gyrus granule cell layer in response to stimulation of the perforant path in an acute brain slice from the rat hippocampus. Ab | The VSD response is quantified in the dentate gyrus, hilus and area CA3. Ac | The top panel shows a current-clamp recording from a dentate gyrus granule cell and the botttom panel shows the field potential in the dentate gyrus. Ad | The collapse of the dentate after blockade of GABAergic inhibition using 5 μM picrotoxin (PTX; a non-competitive type A (GABAA) receptor antagonist) is illustrated using VSD imaging. Ae | The quantification of the VSD response shows collapse of the dentate gate, with activation of upstream area CA3 after GABAergic blockade. Af | Blockade of inhibition elicits hyperactivation of dentate granule cells in response to perforant path activation, as shown by the current-clamp recording (top panel) and the field potential (bottom panel). B | Temporoammonic (TA) pathway function in area CA1 under normal conditions (parts Ba, Bb) and following GABAergic blockade (parts Bc, Bd). Ba | Activation within area CA1 in response to stimulation of the TA pathway is spatially restricted to the distal dendrites of CA1 pyramidal cells (indicated by the arrows), which is shown using VSD imaging. Bb | The top panel shows a current-clamp recording from a CA1 pyramidal cell dendrite in response to TA pathway activation, and the bottom panel shows the quantification of the VSD response in stratum oriens (SO), stratum radiatum (SR) and stratum lacunosum-moleculare (SLM) 30 ms after extracellular stimulation of the TA pathway, with evoked excitatory responses (indicated by the asterisk) in SLM. Bc | A VSD image shows that GABAergic feedforward inhibition after application of the GABAA antagonist gabazine (1 μm) and the GABAB antagonist CGP 55845A (2 μm) spatially restricts TA pathway activation to the distal dendrites of CA1 pyramidal cells, with loss of spatial segregation within area CA1. Bd | The top panel shows a current-clamp recording from a CA1 pyramidal cell dendrite, and the the bottom panel shows the quantification of the VSD response, with propagation of the response to SR and SO. F, fluorescence; nACSF, normal artificial cerebrospinal fluid. Part A is reproduced, with permission, from REF. 137 © (2007) Elsevier. Part B is reproduced, with permission, from REF. 154 © (2005) Society for Neuroscience.
The mechanisms of dentate gating are probably diverse but are likely to include the intrinsic electrophysiological properties of dentate granule cells, unique integrative features of granule cell dendrites145 and multiple forms of inhibition that operate within the dentate gyrus. There is potent feedforward and feedback inhibition of perforant path-evoked dentate gyrus granule cell activity (FIG. 2), and granule cells are also subjected to powerful tonic inhibition. Although the granule cells themselves rarely fire action potentials in response to perforant path activation, resident GABAergic interneurons are also targets of perforant path input (for example, parvalbumin-positive fast-spiking basket cells in the dentate gyrus granule cell layer146 and, perhaps, somatostatin-positive cells in the hilus147) and have been shown to activate readily, thereby constraining granule cell activity through feedforward and feedback inhibition.
There is accumulating evidence that transient dentate gate dysfunction develops during the latent phase of TLE in rodent models. Such data suggest that this dysfunction involves GABAA receptor alterations, dysregulation of the maintenance of chloride gradients and acute loss of GABAergic interneurons in the dentate hilus. The generation of acute brain slices prepared from epileptic rodents after the induction of status epilepticus combined with large-scale, high-speed dynamic optical imaging of population activity has proven to be a powerful approach to the elucidation of circuit-level mechanisms of epileptogenesis.
VSD imaging in rodent brain slices showed network-level dysfunction of the dentate gate during the latent period of chronic TLE148. Dentate-mediated filtering of perforant path activation, as measured by the depolarization of upstream area CA3, was compromised during the latent period but recovered during the chronic phase of TLE (FIG. 4). Hence, the dentate gate is ‘open’ for a brief period of time corresponding to the latent period. One mechanism underlying the breakdown of the dentate gate is a depolarizing shift of the reversal potential for GABA-mediated postsynaptic potentials (EGABA) in dentate gyrus granule neurons148: EGABA is typically very hyperpolarized in granule cells under baseline conditions but shifts to markedly less hyperpolarized levels 1 week after the induction of status epilepticus, returning again to baseline levels in the chronic phase of TLE. The basis of this transient depolarizing shift in EGABA is decreased expression of potassium/chloride co-transporter 2 (KCC2; also known as SLC12A5), which normally extrudes chloride from cells and sets EGABA at very hyperpolarized levels in granule cells; the result is altered synaptic integration in dentate granule cells and transient pathological hyper-excitability in response to perforant path activation. The developmental ‘GABA switch’ that renders GABA hyperpolarizing involves the upregulation of KCC2 and the down-regulation of sodium/potassium/chloride co-transporter 1 (NKCC1; also known as SLC12A2)149,150. The above observation is interesting in the context of the finding that KCC2 expression can be suppressed by REST151 (as well as by neurotrophins152), possibly linking a molecular signalling cascade with the transient dentate gate dysfunction that is seen during the latent period.
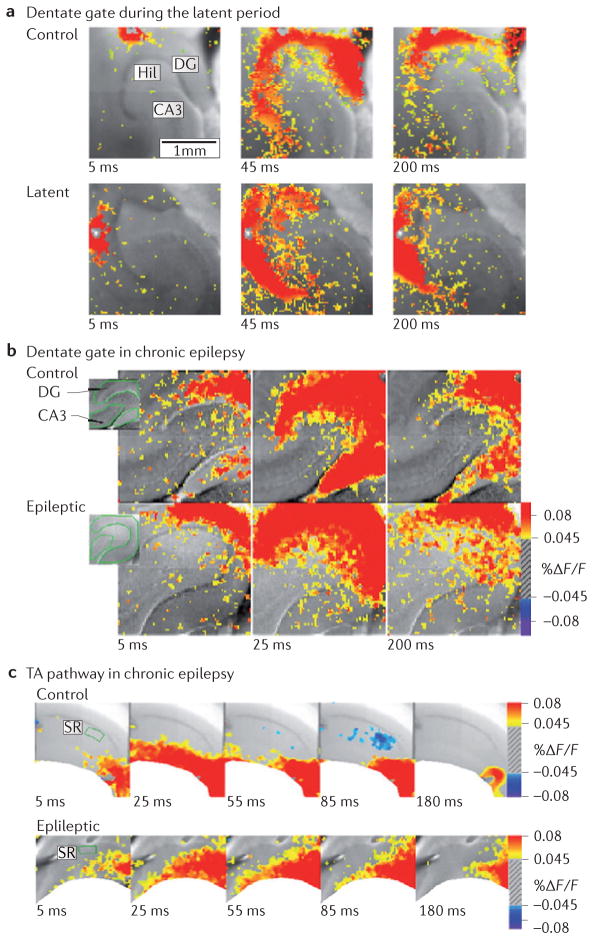
Figure 4. Circuit dysfunction in temporal lobe epilepsy.
a | Dentate gating is impaired during the latent period (1 week after status epilepticus) in an animal model of chronic temporal lobe epilepsy (TLE). The top panel shows voltage-sensitive dye (VSD) recordings of perforant path stimulation under control conditions 5 ms after stimulation (left), at peak (middle) and later (200 ms; right). The top right panel shows restriction of the perforant path-evoked depolarization to the molecular layer of the dentate gyrus (DG). Note that there is minimal area CA3 activation. During the latent period (bottom panel), identical stimulation reveals a delayed peak response as well as >200% increase in activation (as measured by areal pixel activation) in area CA3. b | Gating function is retained in the chronic phase of acquired TLE in the rodent model. The two panels show VSD images of the DG 5 ms (left), 25 ms (middle) and 200 ms (right) after stimulation of the perforant path in control rats (top panel) and in chronically epileptic rats (bottom panel). Under both conditions, there is a strong response in the DG that fails to propagate to area CA3. c | Temporoammonic (TA) pathway dysfunction in the chronic phase of acquired TLE. VSD imaging of TA pathway function in control animals (top panel) and epileptic animals (bottom panel) at 5 ms, 25 ms, 55 ms, 85 ms and 180 ms after stimulation of the TA pathway, producing spatially restricted activity in control animals that aberrantly propagates to the strata radiatum and pyramidale in the epileptic condition. Hil, hilus; F, fluorescence. Part a is reproduced, with permission, from REF. 148 © (2007) Society for Neuroscience. Parts b and c are reproduced, with permission, from REF. 144 © (2006) Society for Neuroscience.
The potential anti-epileptogenic properties of the NKCC1 antagonist bumetanide have been tested in the pilocarpine-induced status epilepticus model of TLE in rats. Bumetanide, when administered alone after status epilepticus, had no effect on the subsequent development and progression of epilepsy, and the addition of bumetanide to a cocktail of diazepam and phenobarbital administered after status epilepticus did not produce any additional effect on seizure frequency beyond that of diazepam and phenobarbital alone153. The development of other strategies to protect the dentate gate after status epilepticus are needed to test the hypothesis that maintenance of dentate gate function during the latent period could be anti-epileptogenic.
Temporoammonic pathway dysregulation in limbic epileptogenesis
Under normal conditions, the excitatory postsynaptic potential (EPSP) generated by temporo-ammonic input to area CA1 is restricted to the apical tuft of CA1 pyramidal cells by GABAergic inhibition154, does not propagate to the cell soma154,155 and hence does not typically elicit action potential firing154,155. Instead, the soma primarily responds to temporoammonic pathway activation with an inhibitory postsynaptic potential, which reflects proximally targeted disynaptic inhibition. This response of area CA1 to temporoammonic pathway stimulation is another circuit-level output measure of a component of the hippocampal circuit that can be used to assay large-scale network function (or dysfunction). The mechanisms that underlie this phenomenon include a high density of HCN channels in distal dendrites of CA1 hippocampal pyramidal cells156,157 and disynaptic feedforward inhibition that might originate from oriens lacunosum-moleculare (O-LM) interneurons. Pharmacologic blockade of GABAergic inhibition results in the loss of this spatial segregation of temporoammonic excitatory input in the stratum lacunosum-moleculare, with subsequent propagation of temporoammonic EPSPs to the stratum radiatum and stratum oriens154. Correspondingly, feedback inhibition driven by stimulation of GABAergic interneurons in the stratum oriens further constrains temporoammonic pathway-evoked EPSPs in CA1 hippocampal pyramidal cells.
Temporoammonic pathway dysfunction in rodent models of acquired chronic TLE results from disinhibition owing to acute loss of interneurons after status epilepticus, upregulation of T-type calcium currents (ICa,T) in CA1 hippocampal pyramidal cells and downregulation of dendritic 158,159. Overall, dendritic inhibition Ih is decreased in CA1 hippocampal pyramidal cells in rodent models of TLE160. The A-type potassium current (IA) is downregulated in CA1 hippocampal pyramidal cell dendrites in the pilocarpine model of chronic TLE in rats53. In the same model, ICa,T appears to be upregulated in dendrites of CA1 hippocampal pyramidal cells, which leads to a change in firing mode from tonic to bursting159,161–163. It is thought that CA1 hippocampal pyramidal neurons probably possess an intrinsic burst mechanism mediated by ICa,T that is normally suppressed by IA; however, downregulation of dendritic IA in combination with upregulation of ICa,T unmasks and amplifies aberrant bursting in these cells159.
Among temporal lobe structures, the dentate gyrus is the least prone to the generation of spontaneous epileptiform discharges in vitro164. Inhibition in the dentate gyrus is enhanced rather than impaired in the chronic phase in the kainate165 and pilocarpine166,167 rat models of TLE165, which is consistent with the results described above. Surprisingly, although dentate gate function is impaired during the latent period, it recovers in the chronic phase of TLE in a rodent model144 (FIG. 4). In addition, activation of area CA1 is actually decreased in the chronic phase of TLE in response to the stimulation of the Schaffer collateral pathway. However, it is the processing of entorhinal cortex input by area CA1 that is markedly impaired in chronic TLE. Excitation in area CA1 that occurred in response to the stimulation of the temporoammonic pathway was increased tenfold in chronic TLE144. Combined, these dynamic changes create a transient breakdown of the dentate gate, which is followed by long-standing pathological hyperexcitability within the temporoammonic pathway that allows aberrant entorhinal cortical input to enter the hippocampus through the disinhibited temporoammonic pathway. Thus, circuit-level analysis allows network excitability to be compared between control and epileptic brains at multiple time points in the epileptogenic process, integrating the multitude of changes that occur during limbic epileptogenesis and generating a large-scale physiological output measure that correlates with changes in the intact organism.
Conclusions
Here, we have presented a selected review of recent work on the mechanisms of epileptogenesis, citing specific examples chosen from a set of emerging themes in the field, including dysregulation of molecular signalling pathways, cell type-specific dysfunction and pathology of discrete circuit elements. Some of the issues that have been discussed remain quite controversial. Although we have largely restricted our discussion to mechanisms of TLE and Dravet syndrome, this focus can and should be expanded to other brain areas and models of other epilepsy syndromes, both acquired and genetic. We argue that the roles of individual molecules, particular cell types or a large-scale molecular signalling cascade in epileptogenesis will be best understood in the context of a circuit analyses. This can be seen in the overlap between the themes that we have discussed.
Epilepsy prevention was defined as Benchmark Area I of the 2007 National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Research Benchmarks168. The data highlighted here illustrate new avenues that hold promise of achieving this goal. Theorized mechanisms of epileptogenesis have moved beyond alterations of ion channels and neurotransmitter receptors to include cell type-specific dysfunction such as ‘interneuronopathies’ as well as dysregulation of master regulatory pathways such as REST and mTOR. We suggest that these various themes all converge at the level of brain circuits, the defining neurophysiological mediators of epilepsy. However, significant questions face the field as we move forward, and we have raised many more questions than we have answered. This is perhaps necessarily the case as we are still without anti-epileptogenic therapies, although we do have new and qualitatively different treatment targets. Future research directions should explore the effects of genetic manipulations, dysfunction of defined cell types and alterations in key cell signalling pathways on circuit function. Such analyses establish a framework within which we can analyse potential molecular and cellular targets for pharmacological and genetic manipulation and therapeutic intervention in the epileptogenic process. This concept provides support for the idea that epileptogenesis is a clinical problem that is amenable to medical intervention and hope for a future in which epilepsy can be prevented.
Acknowledgments
We thank L. Isom, E. Marsh and three anonymous referees for assistance in the preparation of this manuscript.
Glossary
- Dravet syndrome
Previously referred to as severe myoclonic epilepsy of infancy, Dravet syndrome is a spectrum of severe infantile-onset epileptic encephalopathy that is due to an underlying genetic cause, most commonly mutation of the voltage-gated sodium channel subunit NaV1.1. Typically, infants are initially normal, then develop febrile and afebrile seizures, progressing to myoclonus and multiple seizure types, and cognitive impairment
- Circuits
Brain elements that are composed of a collection of neurons of various types and are connected to one another to form a network or ensemble, the operation of which is directed towards a particular function or domain of information processing. Circuits receive inputs, process information and produce outputs
- Tuberous sclerosis
An autosomal dominant multisystem disorder that is caused by a mutation of the tumour suppressor genes tuberous sclerosis complex 1 (TSC1; encoding hamartin) or TSC2 (encoding tuberin). It is accompanied by the characteristic brain lesions of cerebral cortical tubers and subependymal nodules, and by the neurological sequelae of epilepsy, variable intellectual disability and autism
- Mossy fibre sprouting
A process thought to be triggered by cell death of hilar mossy cells, leading to de-innervation of dentate granule cell dendrites, with subsequent ‘sprouting’ of mossy fibre collaterals and pathological granule cell autoinnervation. Whether this is a cause or consequence of temporal lobe pathology in temporal lobe epilepsy is controversial
- Hemimegalencephaly
A malformation of cortical development that is characterized by the pathological enlargement of one cerebral hemisphere and is highly associated with developmental delay and intractable epilepsy
- Kainate- or pilocarpine-induced status epilepticus
Standard and widely used models of acquired chronic focal epilepsy in rodents in which status epilepticus is elicited via the administration of a chemoconvulsant, either the glutamate agonist kainic acid (kainate) or the muscarinic acetylcholine receptor agonist pilocarpine
- Hyperpolarization-activated cyclic nucleotide-gated (HCN) channel
An ion channel that is activated by hyperpolarization and mediates a mixed cationic current known as Ih. It has multiple important functions in the regulation of neuronal excitability. HCN channels are encoded by four genes (HCN1–HCN4)
- Kindling
A phenomenon whereby seizures themselves contribute to epilepsy progression. The kindling model in experimental animals involves the generation of repeated, brief, focal seizures using electrical or chemical stimulation, which subsequently leads to permanently enhanced sensitivity to such stimuli and longer, more severe seizures, and ultimately to chronic epilepsy
- GABA switch
In the developing brain, GABA is an excitatory neurotransmitter and depolarizes neurons. As the brain matures, a shift in the chloride potential of neurons causes the effects of GABA to become hyperpolarizing and hence inhibitory
- Stratum lacunosum- moleculare
A hippocampal layer that is located superficially relative to the stratum radiatum beneath the pial surface and contains the perforant path input onto distal dendritic tufts of CA1 pyramidal cells
- Stratum radiatum
A hippocampal layer located just superficially relative to the stratum lucidum in area CA3 and to the pyramidal cell layer in areas CA2 and CA1 that contains the Schaffer collateral axons from areas CA3 to CA1 and the dendrites of CA1 pyramidal cells
- Stratum oriens
A relatively cell-free layer in the CA subfields of the hippocampus that is located below the pyramidal cell layer that contains the axons of hippocampal pyramidal cells, along with various types of GABAergic interneurons including oriens-lacunosum moleculare interneurons
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Douglas A. Coulter’s homepage: http://stokes.chop.edu/web/coulter/ The Children’s Hospital of Philadelphia Division of Neurology:
http://www.chop.edu/service/neurology/home.html ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Helbig I, Scheffer IE, Mulley JC, Berkovic SF. Navigating the channels and beyond: unravelling the genetics of the epilepsies. Lancet Neurol. 2008;7:231–245. doi: 10.1016/S1474-4422(08)70039-5. [DOI] [PubMed] [Google Scholar]
- 2.Berkovic SF, et al. Familial temporal lobe epilepsy: a common disorder identified in twins. Ann Neurol. 1996;40:227–235. doi: 10.1002/ana.410400214. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi E, Li LM, Lopes-Cendes I, Cendes F. Magnetic resonance imaging evidence of hippocampal sclerosis in asymptomatic, first-degree relatives of patients with familial mesial temporal lobe epilepsy. Arch Neurol. 2002;59:1891–1894. doi: 10.1001/archneur.59.12.1891. [DOI] [PubMed] [Google Scholar]
- 4.Cendes F, Lopes-Cendes I, Andermann E, Andermann F. Familial temporal lobe epilepsy: a clinically heterogeneous syndrome. Neurology. 1998;50:554–557. doi: 10.1212/wnl.50.2.554. [DOI] [PubMed] [Google Scholar]
- 5.Loscher W, Brandt C. Prevention or modification of epileptogenesis after brain insults: experimental approaches and translational research. Pharmacol Rev. 2010;62:668–700. doi: 10.1124/pr.110.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitkänen A, Lukasiuk K. Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol. 2011;10:173–186. doi: 10.1016/S1474-4422(10)70310-0. [DOI] [PubMed] [Google Scholar]
- 7.McNamara JO, Huang YZ, Leonard AS. Molecular signaling mechanisms underlying epileptogenesis. Sci STKE. 2006;2006:re12. doi: 10.1126/stke.3562006re12. [DOI] [PubMed] [Google Scholar]
- 8.Binder DK. Neurotrophins in the dentate gyrus. Prog Brain Res. 2007;163:371–397. doi: 10.1016/S0079-6123(07)63022-2. [DOI] [PubMed] [Google Scholar]
- 9.McNamara JO, Scharfman HE. In: Jasper’s Basic Mechanisms of the Epilepsies. 4. Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Oxford Univ. Press; 2012. pp. 514–531. The classic textbook on basic mechanisms of epilepsy and epileptogenesis, which is now in its recently-released fourth edition. [PubMed] [Google Scholar]
- 10.Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:27–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crino PB. mTOR: a pathogenic signaling pathway in developmental brain malformations. Trends Mol Med. 2011;17:734–742. doi: 10.1016/j.molmed.2011.07.008. A prescient review of diverse types of focal brain malformation, suggesting that a spectrum of disorders are due to aberrant mTOR pathway signalling (‘TORopathies’) [DOI] [PubMed] [Google Scholar]
- 12.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qureshi IA, Mehler MF. Epigenetic mechanisms underlying human epileptic disorders and the process of epileptogenesis. Neurobiol Dis. 2010;39:53–60. doi: 10.1016/j.nbd.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks-Kayal AR, Raol YH, Russek SJ. Alteration of epileptogenesis genes. Neurotherapeutics. 2009;6:312–318. doi: 10.1016/j.nurt.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang SJ, et al. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Nat Acad Sci USA. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M. Control of dendritic arborization by the phosphoinositide-3′-kinase–Akt–mammalian target of rapamycin pathway. J Neurosci. 2005;25:11300–11312. doi: 10.1523/JNEUROSCI.2270-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raab-graham KF, Haddick PCG, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–148. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 18.Crino PB. Focal brain malformations: seizures, signaling, sequencing. Epilepsia. 2009;50:3–8. doi: 10.1111/j.1528-1167.2009.02289.x. [DOI] [PubMed] [Google Scholar]
- 19.Striano P, Zara F. Genetics: mutations in mTOR pathway linked to megalencephaly syndromes. Nature Rev Neurol. 2012;8:542–544. doi: 10.1038/nrneurol.2012.178. [DOI] [PubMed] [Google Scholar]
- 20.McDaniel SS, Wong M. Therapeutic role of mammalian target of rapamycin (mTOR) inhibition in preventing epileptogenesis. Neurosci Lett. 2011;497:231–239. doi: 10.1016/j.neulet.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong M. Mechanisms of epileptogenesis in tuberous sclerosis complex and related malformations of cortical development with abnormal glioneuronal proliferation. Epilepsia. 2008;49:8–21. doi: 10.1111/j.1528-1167.2007.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes GL, Stafstrom CE. Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia. 2007;48:617–630. doi: 10.1111/j.1528-1167.2007.01035.x. [DOI] [PubMed] [Google Scholar]
- 23.Mohamed AR, et al. Intrinsic epileptogenicity of cortical tubers revealed by intracranial EEG monitoring. Neurology. 2012;79:2249–2257. doi: 10.1212/WNL.0b013e3182768923. [DOI] [PubMed] [Google Scholar]
- 24.Uhlmann EJ, et al. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 25.Zeng LH, et al. Abnormal glutamate homeostasis and impaired synaptic plasticity and learning in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2007;28:184–196. doi: 10.1016/j.nbd.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pun RYK, et al. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012;75:1022–1034. doi: 10.1016/j.neuron.2012.08.002. Selective deletion of PTEN from a small subset of dentate gyrus granule cells in adult mice is sufficient to generate TLE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JH, et al. De novo somatic mutations in components of the PI3K–AKT3–mTOR pathway cause hemimegalencephaly. Nature Genet. 2012;44:941–945. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poduri A, et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron. 2012;74:41–48. doi: 10.1016/j.neuron.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokuda S, et al. A novel Akt3 mutation associated with enhanced kinase activity and seizure susceptibility in mice. Hum Mol Genet. 2011;20:988–999. doi: 10.1093/hmg/ddq544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, et al. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. Rapamycin given to mice after pilocarpine-induced status epilepticus markedly decreased the extent of mossy fibre sprouting, with no effect on the development of epilepsy or on seizure frequency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Vliet Ea, et al. Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood–brain barrier leakage but not microglia activation. Epilepsia. 2012;53:1254–1263. doi: 10.1111/j.1528-1167.2012.03513.x. [DOI] [PubMed] [Google Scholar]
- 36.Sliwa A, Plucinska G, Bednarczyk J, Lukasiuk K. Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci Lett. 2012;509:105–109. doi: 10.1016/j.neulet.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 37.Erlich S, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Rapamycin is a neuroprotective treatment for traumatic brain injury. Neurobiol Dis. 2007;26:86–93. doi: 10.1016/j.nbd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, et al. Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J Cereb Blood Flow Metab. 2007;27:939–949. doi: 10.1038/sj.jcbfm.9600393. [DOI] [PubMed] [Google Scholar]
- 39.Raffo E, Coppola A, Ono T, Briggs SW, Galanopoulou AS. A pulse rapamycin therapy for infantile spasms and associated cognitive decline. Neurobiol Dis. 2011;43:322–329. doi: 10.1016/j.nbd.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galanopoulou AS, et al. Identification of new epilepsy treatments: issues in preclinical methodology. Epilepsia. 2012;53:571–582. doi: 10.1111/j.1528-1167.2011.03391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galanopoulou AS, Gorter Ja, Cepeda C. Finding a better drug for epilepsy: the mTOR pathway as an antiepileptogenic target. Epilepsia. 2012;53:1119–1130. doi: 10.1111/j.1528-1167.2012.03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palm K, Belluardo N, Metsis M, Timmusk T. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J Neurosci. 1998;18:1280–1296. doi: 10.1523/JNEUROSCI.18-04-01280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Johnson DS, Mortazavi A, Myers RM, Wold B. Genome-wide mapping of in vivo protein–DNA interactions. Science. 2007;316:1497–1502. doi: 10.1126/science.1141319. [DOI] [PubMed] [Google Scholar]
- 45.Otto SJ, et al. A new binding motif for the transcriptional repressor REST uncovers large gene networks devoted to neuronal functions. J Neurosci. 2007;27:6729–6739. doi: 10.1523/JNEUROSCI.0091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortazavi A, Leeper Thompson EC, Garcia ST, Myers RM, Wold B. Comparative genomics modeling of the NRSF/REST repressor network: from single conserved sites to genome-wide repertoire. Genome Res. 2006;16:1208–1221. doi: 10.1101/gr.4997306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roopra A, Huang Y, Dingledine R. Neurological disease: listening to gene silencers. Mol Interv. 2001;1:219–228. [PubMed] [Google Scholar]
- 48.Roopra A, Dingledine R, Hsieh J. Epigenetics and epilepsy. Epilepsia. 2012;53:2–10. doi: 10.1111/epi.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y, Doherty JJ, Dingledine R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci. 2002;22:8422–8428. doi: 10.1523/JNEUROSCI.22-19-08422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang Y, Myers SJ, Dingledine R. Transcriptional repression by REST: recruitment of Sin3A and histone deacetylase to neuronal genes. Nature Neurosci. 1999;2:867–872. doi: 10.1038/13165. [DOI] [PubMed] [Google Scholar]
- 51.Garriga-Canut M, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nature Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. Pharmacologic inhibition of glycolysis during kindling epileptogenesis recruits REST to target genes including BDNF, leading to suppression of BDNF expression, reduced neuronal excitability and attenuated progression of kindling. [DOI] [PubMed] [Google Scholar]
- 52.Noam Y, Bernard C, Baram TZ. Towards an integrated view of HCN channel role in epilepsy. Curr Opin Neurobiol. 2011;21:873–879. doi: 10.1016/j.conb.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernard C, et al. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–535. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- 54.Jung S, Warner LN, Pitsch J, Becker AJ, Poolos NP. Rapid loss of dendritic HCN channel expression in hippocampal pyramidal neurons following status epilepticus. J Neurosci. 2011;31:14291–14295. doi: 10.1523/JNEUROSCI.1148-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen K, et al. Persistently modified h-channels after complex febrile seizures convert the seizure-induced enhancement of inhibition to hyperexcitability. Nature Med. 2001;7:331–337. doi: 10.1038/85480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McClelland S, et al. Neuron-restrictive silencer factor-mediated hyperpolarization-activated cyclic nucleotide gated channelopathy in experimental temporal lobe epilepsy. Ann Neurol. 2011;70:454–464. doi: 10.1002/ana.22479. In the kainate model of acquired chronic TLE in rodents, interference with the interaction between REST and target genes using antisense oligodeoxy-nucleotides prevented status epilepticus-induced REST-mediated repression of HCN1 and led to a decrease in spontaneous seizure frequency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 58.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ascoli GA, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nature Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Defelipe J, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nature Rev Neurosci. 2013:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meldrum B. Physiological changes during prolonged seizures and epileptic brian damage. Neuropadiatrie. 1978;9:213–212. doi: 10.1055/s-0028-1091481. [DOI] [PubMed] [Google Scholar]
- 62.Bernard C, Cossart R, Hirsch JC, Esclapez M, Ben-Ari Y. What is GABAergic inhibition? How is it modified in epilepsy? Epilepsia. 2000;41:S90–S95. doi: 10.1111/j.1528-1157.2000.tb01564.x. [DOI] [PubMed] [Google Scholar]
- 63.Mann EO, Mody I. The multifaceted role of inhibition in epilepsy: seizure-genesis through excessive GABAergic inhibition in autosomal dominant nocturnal frontal lobe epilepsy. Curr Opin Neurol. 2008;21:155–160. doi: 10.1097/WCO.0b013e3282f52f5f. [DOI] [PubMed] [Google Scholar]
- 64.Galanopoulou AS. Mutations affecting GABAergic signaling in seizures and epilepsy. Pflügers Arch. 2010;460:505–523. doi: 10.1007/s00424-010-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sebe JY, Baraban SC. The promise of an interneuron-based cell therapy for epilepsy. Dev Neurobiol. 2011;71:107–117. doi: 10.1002/dneu.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson SA, Baraban SC. In: Jasper’s Basic Mechanisms of the Epilepsies. 4. Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Oxford Univ. Press; 2012. pp. 1122–1126. [PubMed] [Google Scholar]
- 67.Maisano X, et al. Differentiation and functional incorporation of embryonic stem cell-derived GABAergic interneurons in the dentate gyrus of mice with temporal lobe epilepsy. J Neurosci. 2012;32:46–61. doi: 10.1523/JNEUROSCI.2683-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Houser CR. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res. 2007;163:217–232. doi: 10.1016/S0079-6123(07)63013-1. [DOI] [PubMed] [Google Scholar]
- 69.Jinno S, Kosaka T. Patterns of expression of neuropeptides in GABAergic nonprincipal neurons in the mouse hippocampus: quantitative analysis with optical disector. J Comp Neurol. 2003;461:333–349. doi: 10.1002/cne.10700. [DOI] [PubMed] [Google Scholar]
- 70.Bakst I, Avendano C, Morrison JH, Amaral DG. An experimental analysis of the origins of somatostatin-like immunoreactivity in the dentate gyrus of the rat. J Neurosci. 1986;6:1452–1462. doi: 10.1523/JNEUROSCI.06-05-01452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Katona I, Acsády L, Freund TF. Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience. 1999;88:37–55. doi: 10.1016/s0306-4522(98)00302-9. [DOI] [PubMed] [Google Scholar]
- 72.Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- 74.Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- 75.De Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- 76.Zhang W, et al. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci. 2009;29:14247–14256. doi: 10.1523/JNEUROSCI.3842-09.2009. In a mouse model of chronic TLE, surviving somatostatin- positive hilar interneurons exhibit axonal sprouting and form functional inhibitory connections with dentate gyrus granule cells at increased frequency as demonstrated anatomically and physiologically. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mathern GW, Babb TL, Pretorius JK, Leite JP. Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci. 1995;15:3990–4004. doi: 10.1523/JNEUROSCI.15-05-03990.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buckmaster PS, Wen X. Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy. Epilepsia. 2011;52:2057–2064. doi: 10.1111/j.1528-1167.2011.03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat. 2007;1:3. doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 82.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 83.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Avoli M, De Curtis M. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 2011;95:104–132. doi: 10.1016/j.pneurobio.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nature Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cobos I, et al. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nature Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- 88.Butt SJB, et al. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Batista-Brito R, et al. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron. 2009;63:466–481. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smart SL, et al. Deletion of the KV1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 91.Eunson LH, et al. Clinical, genetic, and expression studies of mutations in the potassium channel gene KCNA1 reveal new phenotypic variability. Ann Neurol. 2000;48:647–656. [PubMed] [Google Scholar]
- 92.Goldberg EM, et al. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lau D, et al. Impaired fast-spiking, suppressed cortical inhibition, and increased susceptibility to seizures in mice lacking Kv3.2 K+ channel proteins. J Neurosci. 2000;20:9071–9085. doi: 10.1523/JNEUROSCI.20-24-09071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Escayg A, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nature Genet. 2000;24:343–345. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- 95.Claes L, et al. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–1332. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harkin LA, et al. The spectrum of SCN1A-related infantile epileptic encephalopathies. Brain. 2007;130:843–852. doi: 10.1093/brain/awm002. [DOI] [PubMed] [Google Scholar]
- 97.Ohmori I, Kahlig KM, Rhodes TH, Wang DW, George AL. Nonfunctional SCN1A is common in severe myoclonic epilepsy of infancy. Epilepsia. 2006;47:1636–1642. doi: 10.1111/j.1528-1167.2006.00643.x. [DOI] [PubMed] [Google Scholar]
- 98.Yu FH, et al. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nature Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- 99.Ragsdale DS. How do mutant Nav1.1 sodium channels cause epilepsy? Brain Res Rev. 2008;58:149–159. doi: 10.1016/j.brainresrev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 100.Oakley JC, Kalume F, Yu FH, Scheuer T, Catterall WA. Temperature- and age-dependent seizures in a mouse model of severe myoclonic epilepsy in infancy. Proc Nat Acad Sci USA. 2009;106:3994–3999. doi: 10.1073/pnas.0813330106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mullen SA, Scheffer IE. Translational research in epilepsy genetics: sodium channels in man to interneuronopathy in mouse. Arch Neurol. 2009;66:21–26. doi: 10.1001/archneurol.2008.559. [DOI] [PubMed] [Google Scholar]
- 102.Martin MS, et al. Altered function of the SCN1A voltage-gated sodium channel leads to γ-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J Biol Chem. 2010;285:9823–9834. doi: 10.1074/jbc.M109.078568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia. 2010;51:1650–1658. doi: 10.1111/j.1528-1167.2010.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ogiwara I, et al. Nav1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. Knock-in mice harbouring an Scn1a nonsense mutation that is also found in patients with Dravet syndrome develop temperature-sensitive and spontaneous seizures resembling the human condition. Immunohistochemistry indicates restricted expression of NaV1.1 protein at the soma and AIS of parvalbumin-positive basket cells; neocortical fast-spiking cells exhibit profoundly abnormal physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheah CS, et al. Specific deletion of NaV1.1 sodium channels in inhibitory interneurons causes seizures and premature death in a mouse model of Dravet syndrome. Proc Nat Acad Sci USA. 2012;109:14646–14651. doi: 10.1073/pnas.1211591109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron diversity series: fast in, fast out--temporal and spatial signal processing in hippocampal interneurons. Trend Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 107.Goldberg EM, et al. Rapid developmental maturation of neocortical FS cell intrinsic excitability. Cererb Cortex. 2011;21:666–682. doi: 10.1093/cercor/bhq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- 109.Gong B, Rhodes KJ, Bekele-Arcuri Z, Trimmer JS. Type I and type II Na+ channel α-subunit polypeptides exhibit distinct spatial and temporal patterning, and association with auxiliary subunits in rat brain. J Comp Neurol. 1999;412:342–352. [PubMed] [Google Scholar]
- 110.Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harkin LA, et al. Truncation of the GABAA-receptor γ2 subunit in a family with generalized epilepsy with febrile seizures plus. Am J Hum Genet. 2002;70:530–536. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Patino Ga, et al. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci. 2009;29:10764–10778. doi: 10.1523/JNEUROSCI.2475-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ogiwara I, et al. A homozygous mutation of voltage-gated sodium channel βI gene SCN1B in a patient with Dravet syndrome. Epilepsia. 2012;53:e200–e203. doi: 10.1111/epi.12040. [DOI] [PubMed] [Google Scholar]
- 114.Kamiya K, et al. A nonsense mutation of the sodium channel gene SCN2A in a patient with intractable epilepsy and mental decline. J Neurosci. 2004;24:2690–2698. doi: 10.1523/JNEUROSCI.3089-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shi X, et al. Missense mutation of the sodium channel gene SCN2A causes Dravet syndrome. Brain Dev. 2009;31:758–762. doi: 10.1016/j.braindev.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 116.Depienne C, et al. Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females. PLoS Genet. 2009;5:e1000381. doi: 10.1371/journal.pgen.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen C, et al. Mice lacking sodium channel β1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci. 2004;24:4030–4042. doi: 10.1523/JNEUROSCI.4139-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McClelland S, Dubé CM, Yang J, Baram TZ. Epileptogenesis after prolonged febrile seizures: mechanisms, biomarkers and therapeutic opportunities. Neurosci Lett. 2011;497:155–162. doi: 10.1016/j.neulet.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shinnar S, et al. MRI abnormalities following febrile status epilepticus in children: the FEBSTAT study. Neurology. 2012;79:871–877. doi: 10.1212/WNL.0b013e318266fcc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abou-Khalil B, et al. Partial and generalized epilepsy with febrile seizures plus and a novel SCN1A mutation. Neurology. 2001;57:2265–2272. doi: 10.1212/wnl.57.12.2265. [DOI] [PubMed] [Google Scholar]
- 121.Sugawara T, et al. Nav1.1 mutations cause febrile seizures associated with afebrile partial seizures. Neurology. 2001;57:703–705. doi: 10.1212/wnl.57.4.703. [DOI] [PubMed] [Google Scholar]
- 122.Scheffer IE, et al. Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain. 2007;130:100–109. doi: 10.1093/brain/awl272. An interesting case series including a patient with GEFS+ due to the SCN1B mutation but no history of febrile or afebrile status epilepticus who developed TLE with classic mesial temporal sclerosis and become seizure-free after unilateral temporal lobectomy. [DOI] [PubMed] [Google Scholar]
- 123.Kearney JA, et al. A gain-of-function mutation in the sodium channel gene Scn2a results in seizures and behavioral abnormalities. Neuroscience. 2001;102:307–317. doi: 10.1016/s0306-4522(00)00479-6. [DOI] [PubMed] [Google Scholar]
- 124.Striano P, et al. Brain MRI findings in severe myoclonic epilepsy in infancy and genotype–phenotype correlations. Epilepsia. 2007;48:1092–1096. doi: 10.1111/j.1528-1167.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- 125.Siegler Z, et al. Hippocampal sclerosis in severe myoclonic epilepsy in infancy: a retrospective MRI study. Epilepsia. 2005;46:704–708. doi: 10.1111/j.1528-1167.2005.41604.x. [DOI] [PubMed] [Google Scholar]
- 126.Guerrini R, Striano P, Catarino C, Sisodiya SM. Neuroimaging and neuropathology of Dravet syndrome. Epilepsia. 2011;52:30–34. doi: 10.1111/j.1528-1167.2011.02998.x. [DOI] [PubMed] [Google Scholar]
- 127.Nangia S, Caraballo RH, Kang HC, Nordli DR, Scheffer IE. Is the ketogenic diet effective in specific epilepsy syndromes? Epilepsy Res. 2012;100:252–257. doi: 10.1016/j.eplepsyres.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 128.Brackenbury WJ, Yuan Y, Malley HAO, Parent JM, Isom LL. Abnormal neuronal patterning occurs during early postnatal brain development of Scn1b-null mice and precedes hyperexcitability. Proc Nat Acad Sci USA. 2013;110:1089–1094. doi: 10.1073/pnas.1208767110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nature Rev Neurosci. 2012;13:251–266. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trend Cog Sci. 2011;15:592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paz JT, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nature Neurosci. 2013;16:64–70. doi: 10.1038/nn.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heinemann U, et al. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res Suppl. 1992;7:273–280. [PubMed] [Google Scholar]
- 133.Lothman EW, Stringer JL, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res Suppl. 1992;7:301–313. [PubMed] [Google Scholar]
- 134.Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res. 2007;163:755–773. doi: 10.1016/S0079-6123(07)63041-6. [DOI] [PubMed] [Google Scholar]
- 135.Buckmaster PS. In: Jasper’s Basic Mechanisms of the Epilepsies. 4. Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Oxford Univ. Press; 2012. pp. 416–431. [PubMed] [Google Scholar]
- 136.Zhang W, Huguenard JR, Buckmaster PS. Increased excitatory synaptic input to granule cells from hilar and CA3 regions in a rat model of temporal lobe epilepsy. J Neurosci. 2012;32:1183–1196. doi: 10.1523/JNEUROSCI.5342-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Coulter DA, Carlson GC. Functional regulation of the dentate gyrus by GABA-mediated inhibition. Prog Brain Res. 2007;163:235–243. doi: 10.1016/S0079-6123(07)63014-3. [DOI] [PubMed] [Google Scholar]
- 138.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 139.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 141.Yu EP, et al. Protracted postnatal development of sparse, specific dentate granule cell activation in the mouse hippocampus. J Neurosci. 2013;33:2947–2960. doi: 10.1523/JNEUROSCI.1868-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chawla MK, et al. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- 143.Behr J, Lyson KJ, Mody I. Enhanced propagation of epileptiform activity through the kindled dentate gyrus. J Neurophysiol. 1998;79:1726–1732. doi: 10.1152/jn.1998.79.4.1726. [DOI] [PubMed] [Google Scholar]
- 144.Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Krueppel R, Remy S, Beck H. Dendritic integration in hippocampal dentate granule cells. Neuron. 2011;71:512–528. doi: 10.1016/j.neuron.2011.05.043. Unique synaptic integration by dentate gyrus granule cell dendrites relative to other principal cells in the cerebral cortex may contribute to dentate gating. [DOI] [PubMed] [Google Scholar]
- 146.Ewell LA, Jones MV. Frequency-tuned distribution of inhibition in the dentate gyrus. J Neurosci. 2010;30:12597–12607. doi: 10.1523/JNEUROSCI.1854-10.2010. This paper shows that the dentate gate is subserved at least in part by feedforward inhibition by fast-spiking interneurons onto dentate gyrus granule cells. However, granule cell activity in response to stimulation of the perforant path is frequency-dependent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leranth C, Malcolm AJ, Frotscher M. Afferent and efferent synaptic connections of somatostatin-immunoreactive neurons in the rat fascia dentata. J Comp Neurol. 1990;295:111–122. doi: 10.1002/cne.902950110. [DOI] [PubMed] [Google Scholar]
- 148.Pathak HR, et al. Disrupted dentate granule cell chloride regulation enhances synaptic excitability during development of temporal lobe epilepsy. J Neurosci. 2007;27:14012–14022. doi: 10.1523/JNEUROSCI.4390-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 150.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nature Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 151.Yeo M, Berglund K, Augustine G, Liedtke W. Novel repression of Kcc2 transcription by REST–RE-1 controls developmental switch in neuronal chloride. J Neurosci. 2009;29:14652–14662. doi: 10.1523/JNEUROSCI.2934-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rivera C, et al. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brandt C, Nozadze M, Heuchert N, Rattka M, Löscher W. Disease-modifying effects of phenobarbital and the NKCC1 inhibitor bumetanide in the pilocarpine model of temporal lobe epilepsy. J Neurosci. 2010;30:8602–8612. doi: 10.1523/JNEUROSCI.0633-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ang CW, Carlson GC, Coulter DA. Hippocampal CA1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies. J Neurosci. 2005;25:9567–9580. doi: 10.1523/JNEUROSCI.2992-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Soltesz I. Brief history of cortico-hippocampal time with a special reference to the direct entorhinal input to CA1. Hippocampus. 1995;5:120–124. doi: 10.1002/hipo.450050206. [DOI] [PubMed] [Google Scholar]
- 156.Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron. 2007;56:1076–1089. doi: 10.1016/j.neuron.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nature Neurosci. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- 158.Coulter DA, et al. Hippocampal microcircuit dynamics probed using optical imaging approaches. J Physiol. 2011;589:1893–1903. doi: 10.1113/jphysiol.2010.202184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nature Rev Neurosci. 2008;9:357–369. doi: 10.1038/nrn2371. [DOI] [PubMed] [Google Scholar]
- 160.Cossart R, et al. Dendritic but not somatic GABAergic inhibition is decreased in experimental epilepsy. Nature Neurosci. 2001;4:52–62. doi: 10.1038/82900. [DOI] [PubMed] [Google Scholar]
- 161.Sanabria ER, Su H, Yaari Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J Physiol. 2001;532:205–216. doi: 10.1111/j.1469-7793.2001.0205g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Su H, et al. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J Neurosci. 2002;22:3645–3655. doi: 10.1523/JNEUROSCI.22-09-03645.2002. A thorough review of ion channel alterations in epilepsy and other neurological disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yaari Y, Yue C, Su H. Recruitment of apical dendritic T-type Ca2+ channels by backpropagating spikes underlies de novo intrinsic bursting in hippocampal epileptogenesis. J Physiol. 2007;580:435–450. doi: 10.1113/jphysiol.2007.127670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bear J, Lothman EW. An in vitro study of focal epileptogenesis in combined hippocampalparahippocampal slices. Epilepsy Res. 1993;14:183–193. doi: 10.1016/0920-1211(93)90043-7. [DOI] [PubMed] [Google Scholar]
- 165.Wu K, Leung LS. Increased dendritic excitability in hippocampal ca1 in vivo in the kainic acid model of temporal lobe epilepsy: a study using current source density analysis. Neuroscience. 2003;116:599–616. doi: 10.1016/s0306-4522(02)00567-5. [DOI] [PubMed] [Google Scholar]
- 166.Gibbs JW, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- 167.Cohen AS, Lin DD, Quirk GL, Coulter DA. Dentate granule cell GABAA receptors in epileptic hippocampus: enhanced synaptic efficacy and altered pharmacology. Eur J Neurosci. 2003;17:1607–1616. doi: 10.1046/j.1460-9568.2003.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kelley MS, Jacobs MP, Lowenstein DH. The NINDS epilepsy research benchmarks. Epilepsia. 2009;50:579–582. doi: 10.1111/j.1528-1167.2008.01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50:10–13. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 170.Vadlamudi L, Scheffer IE, Berkovic SF. Genetics of temporal lobe epilepsy. J Neurol Neurosurg Psych. 2003;74:1359–1361. doi: 10.1136/jnnp.74.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ribeiro P, et al. Expression profile of Lgi1 gene in mouse brain during development. J Mol Neurosci. 2008;35:323–329. doi: 10.1007/s12031-008-9096-0. [DOI] [PubMed] [Google Scholar]
- 172.Michelucci R, Pasini E, Nobile C. Lateral temporal lobe epilepsies: clinical and genetic features. Epilepsia. 2009;50:52–54. doi: 10.1111/j.1528-1167.2009.02122.x. [DOI] [PubMed] [Google Scholar]
- 173.Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Morgan RJ, Santhakumar V, Soltesz I. Modeling the dentate gyrus. Prog Brain Res. 2007;163:639–658. doi: 10.1016/S0079-6123(07)63035-0. [DOI] [PubMed] [Google Scholar]