Abstract
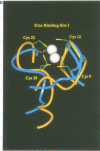
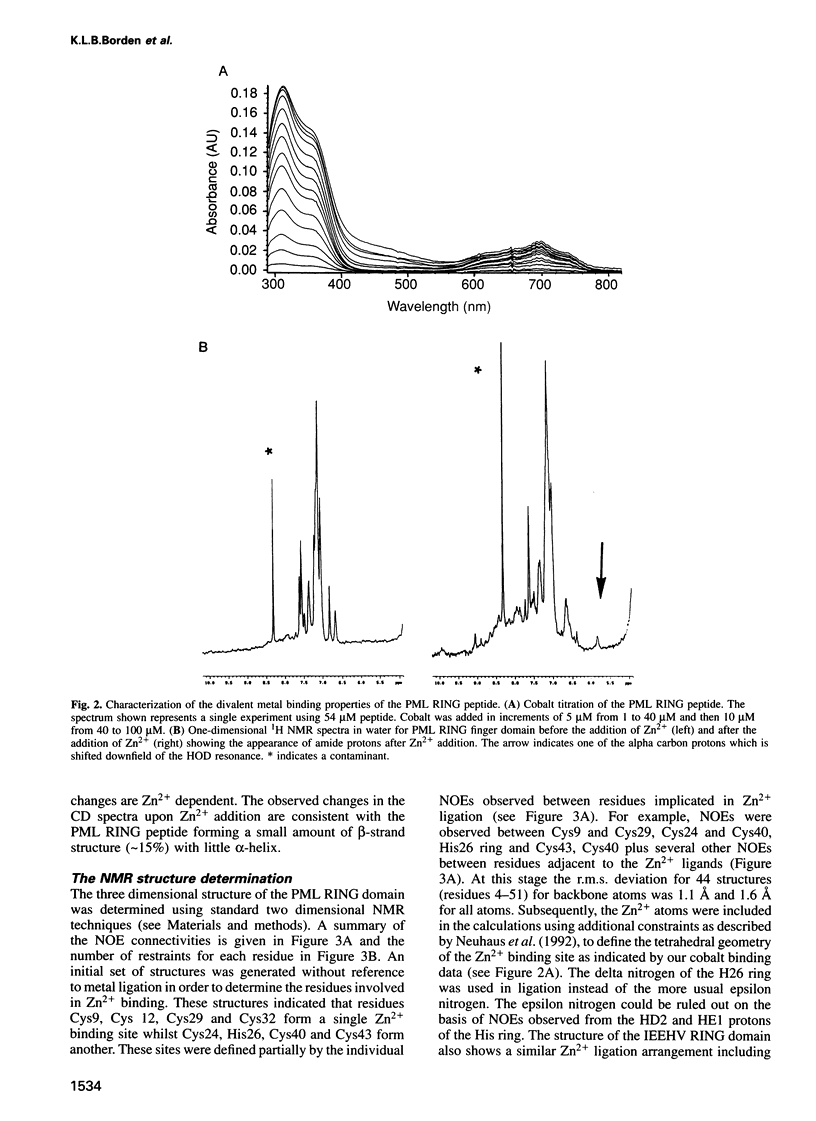
Acute promyelocytic leukaemia (APL) has been ascribed to a chromosomal translocation event which results in a fusion protein comprising the PML protein and the retinoic acid receptor alpha. PML is normally a component of a nuclear multiprotein complex (termed ND10, Kr bodies, nuclear bodies, PML oncogenic domains or PODs) which is disrupted in the APL disease state. PML contains a number of characterized motifs including a Zn2+ binding domain called the RING or C3HC4 finger. Here we describe the solution structure of the PML RING finger as solved by 1H NMR methods at physiological pH with r.m.s. deviations for backbone atoms of 0.88 and 1.39 A for all atoms. Additional biophysical studies including CD and optical spectroscopy, show that the PML RING finger requires Zn2+ for autonomous folding and that cysteines are used in metal ligation. A comparison of the structure with the previously solved equine herpes virus IE110 RING finger, shows significant differences suggesting that the RING motif is structurally diverse. The role of the RING domain in PML nuclear body formation was tested in vivo, by using site-directed mutagenesis and immunofluorescence on transiently transfected NIH 3T3 cells. Independently mutating two pairs of cysteines in each of the Zn2+ binding sites prevents PML nuclear body formation, suggesting that a fully folded RING domain is necessary for this process. These results suggest that the PML RING domain is probably involved in protein-protein interactions, a feature which may be common to other RING finger domains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascoli C. A., Maul G. G. Identification of a novel nuclear domain. J Cell Biol. 1991 Mar;112(5):785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P. N., Luisi B., Milner A., Elliott M., Everett R. Structure of the C3HC4 domain by 1H-nuclear magnetic resonance spectroscopy. A new structural class of zinc-finger. J Mol Biol. 1994 Mar 25;237(2):201–211. doi: 10.1006/jmbi.1994.1222. [DOI] [PubMed] [Google Scholar]
- Borden K. L., Martin S. R., O'Reilly N. J., Lally J. M., Reddy B. A., Etkin L. D., Freemont P. S. Characterisation of a novel cysteine/histidine-rich metal binding domain from Xenopus nuclear factor XNF7. FEBS Lett. 1993 Dec 6;335(2):255–260. doi: 10.1016/0014-5793(93)80741-c. [DOI] [PubMed] [Google Scholar]
- Campbell I. D., Spitzfaden C. Building proteins with fibronectin type III modules. Structure. 1994 May 15;2(5):333–337. doi: 10.1016/s0969-2126(00)00034-4. [DOI] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Dyck J. A., Maul G. G., Miller W. H., Jr, Chen J. D., Kakizuka A., Evans R. M. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994 Jan 28;76(2):333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Fagioli M., Alcalay M., Pandolfi P. P., Venturini L., Mencarelli A., Simeone A., Acampora D., Grignani F., Pelicci P. G. Alternative splicing of PML transcripts predicts coexpression of several carboxy-terminally different protein isoforms. Oncogene. 1992 Jun;7(6):1083–1091. [PubMed] [Google Scholar]
- Freemont P. S., Hanson I. M., Trowsdale J. A novel cysteine-rich sequence motif. Cell. 1991 Feb 8;64(3):483–484. doi: 10.1016/0092-8674(91)90229-r. [DOI] [PubMed] [Google Scholar]
- Freemont P. S. The RING finger. A novel protein sequence motif related to the zinc finger. Ann N Y Acad Sci. 1993 Jun 11;684:174–192. doi: 10.1111/j.1749-6632.1993.tb32280.x. [DOI] [PubMed] [Google Scholar]
- Futreal P. A., Liu Q., Shattuck-Eidens D., Cochran C., Harshman K., Tavtigian S., Bennett L. M., Haugen-Strano A., Swensen J., Miki Y. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994 Oct 7;266(5182):120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- Goddard A. D., Borrow J., Freemont P. S., Solomon E. Characterization of a zinc finger gene disrupted by the t(15;17) in acute promyelocytic leukemia. Science. 1991 Nov 29;254(5036):1371–1374. doi: 10.1126/science.1720570. [DOI] [PubMed] [Google Scholar]
- Grignani F., Fagioli M., Alcalay M., Longo L., Pandolfi P. P., Donti E., Biondi A., Lo Coco F., Grignani F., Pelicci P. G. Acute promyelocytic leukemia: from genetics to treatment. Blood. 1994 Jan 1;83(1):10–25. [PubMed] [Google Scholar]
- Grignani F., Ferrucci P. F., Testa U., Talamo G., Fagioli M., Alcalay M., Mencarelli A., Grignani F., Peschle C., Nicoletti I. The acute promyelocytic leukemia-specific PML-RAR alpha fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell. 1993 Aug 13;74(3):423–431. doi: 10.1016/0092-8674(93)80044-f. [DOI] [PubMed] [Google Scholar]
- Kakizuka A., Miller W. H., Jr, Umesono K., Warrell R. P., Jr, Frankel S. R., Murty V. V., Dmitrovsky E., Evans R. M. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991 Aug 23;66(4):663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Karas M., Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal Chem. 1988 Oct 15;60(20):2299–2301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- Kastner P., Perez A., Lutz Y., Rochette-Egly C., Gaub M. P., Durand B., Lanotte M., Berger R., Chambon P. Structure, localization and transcriptional properties of two classes of retinoic acid receptor alpha fusion proteins in acute promyelocytic leukemia (APL): structural similarities with a new family of oncoproteins. EMBO J. 1992 Feb;11(2):629–642. doi: 10.1002/j.1460-2075.1992.tb05095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken M. H., Puvion-Dutilleul F., Guillemin M. C., Viron A., Linares-Cruz G., Stuurman N., de Jong L., Szostecki C., Calvo F., Chomienne C. The t(15;17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO J. 1994 Mar 1;13(5):1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering R., Hanson I. M., Borden K. L., Martin S., O'Reilly N. J., Evan G. I., Rahman D., Pappin D. J., Trowsdale J., Freemont P. S. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2112–2116. doi: 10.1073/pnas.90.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Fleming T. P., Crescenzi M., Molloy C. J., Blam S. B., Reynolds S. H., Aaronson S. A. Development of a highly efficient expression cDNA cloning system: application to oncogene isolation. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5167–5171. doi: 10.1073/pnas.88.12.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Mu Z. M., Chin K. V., Liu J. H., Lozano G., Chang K. S. PML, a growth suppressor disrupted in acute promyelocytic leukemia. Mol Cell Biol. 1994 Oct;14(10):6858–6867. doi: 10.1128/mcb.14.10.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., Erlich H. Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):263–273. doi: 10.1101/sqb.1986.051.01.032. [DOI] [PubMed] [Google Scholar]
- Neuhaus D., Nakaseko Y., Schwabe J. W., Klug A. Solution structures of two zinc-finger domains from SWI5 obtained using two-dimensional 1H nuclear magnetic resonance spectroscopy. A zinc-finger structure with a third strand of beta-sheet. J Mol Biol. 1992 Nov 20;228(2):637–651. doi: 10.1016/0022-2836(92)90846-c. [DOI] [PubMed] [Google Scholar]
- Nilges M., Clore G. M., Gronenborn A. M. Determination of three-dimensional structures of proteins from interproton distance data by hybrid distance geometry-dynamical simulated annealing calculations. FEBS Lett. 1988 Mar 14;229(2):317–324. doi: 10.1016/0014-5793(88)81148-7. [DOI] [PubMed] [Google Scholar]
- Perez A., Kastner P., Sethi S., Lutz Y., Reibel C., Chambon P. PMLRAR homodimers: distinct DNA binding properties and heteromeric interactions with RXR. EMBO J. 1993 Aug;12(8):3171–3182. doi: 10.1002/j.1460-2075.1993.tb05986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. A., Etkin L. D. A unique bipartite cysteine-histidine motif defines a subfamily of potential zinc-finger proteins. Nucleic Acids Res. 1991 Nov 25;19(22):6330–6330. doi: 10.1093/nar/19.22.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy B. A., Etkin L. D., Freemont P. S. A novel zinc finger coiled-coil domain in a family of nuclear proteins. Trends Biochem Sci. 1992 Sep;17(9):344–345. doi: 10.1016/0968-0004(92)90308-v. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Inaguma Y., Hiai H., Hirose F. Developmentally regulated expression of a human "finger"-containing gene encoded by the 5' half of the ret transforming gene. Mol Cell Biol. 1988 Apr;8(4):1853–1856. doi: 10.1128/mcb.8.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Pagano J. S. Infectious poliovirus RNA: a sensitive method of assay. Virology. 1965 Nov;27(3):434–436. doi: 10.1016/0042-6822(65)90126-1. [DOI] [PubMed] [Google Scholar]
- Wagner G., Braun W., Havel T. F., Schaumann T., Go N., Wüthrich K. Protein structures in solution by nuclear magnetic resonance and distance geometry. The polypeptide fold of the basic pancreatic trypsin inhibitor determined using two different algorithms, DISGEO and DISMAN. J Mol Biol. 1987 Aug 5;196(3):611–639. doi: 10.1016/0022-2836(87)90037-4. [DOI] [PubMed] [Google Scholar]
- Weis K., Rambaud S., Lavau C., Jansen J., Carvalho T., Carmo-Fonseca M., Lamond A., Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RAR alpha in acute promyelocytic leukemia cells. Cell. 1994 Jan 28;76(2):345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Cai W., Schaffer P. A. Cooperativity among herpes simplex virus type 1 immediate-early regulatory proteins: ICP4 and ICP27 affect the intracellular localization of ICP0. J Virol. 1994 May;68(5):3027–3040. doi: 10.1128/jvi.68.5.3027-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991 Aug 23;66(4):675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]