Abstract
Nanobiomechanics of living cells is very important to understand cell–materials interactions. This would potentially help to optimize the surface design of the implanted materials and scaffold materials for tissue engineering. The nanoindentation techniques enable quantifying nanobiomechanics of living cells, with flexibility of using indenters of different geometries. However, the data interpretation for nanoindentation of living cells is often difficult. Despite abundant experimental data reported on nanobiomechanics of living cells, there is a lack of comprehensive discussion on testing with different tip geometries, and the associated mechanical models that enable extracting the mechanical properties of living cells. Therefore, this paper discusses the strategy of selecting the right type of indenter tips and the corresponding mechanical models at given test conditions.
Keywords: cell mechanics, nanobiomechanics, nanoindentation, modelling
1. Introduction
The mechanical properties of living cells can affect their physical interactions with their surrounding extracellular matrix [1,2], potentially influencing the process of mechanical signal transduction in living tissues [3–7]. Alterations in cell properties are of fundamental importance for a wide range of processes, and changes in cell mechanics are associated with conditions such as osteoarthritis [8], asthma [9], cancer [10], inflammation [11] and malaria [12]. The mechanical properties of living cells have been quantified using various testing methods, such as micropipette aspiration [8,13], magnetic twisting cytometry [14], optical tweezers [15–17] and nanoindentation [18–20]. Although there are some good review papers on cell mechanics [21,22], they mainly focus on using micropipette aspiration techniques. From the perspective of cell mechanics, one should be aware of what is measured with respect to particular techniques. For example, it is often observed that the cell appears softer [23] during micropipette aspirations compared with cytocompression [24] or indentation with a large spherical tip [25]. During micropipette aspirations, it was observed that the cytoskeleton can be disrupted [26,27]. In such a case, there is no (or very limited) tensile stress in the actin fibres, which significantly contributes to cell stiffness. Therefore, cell mechanics can be approximated, as cytosol reinforced with bundles of actin fibres (with diameter of 9–10 nm). The weight concentration of actin fibres is 1–10% for non-muscle cells and 10–20% for muscle cells, and the elastic modulus of these actin fibres is 1.3–2.5 GPa [28].
This paper will shed light on the nanoindentation techniques, because investigation of mechanical properties of living cells at the nanometre (or submicrometre) scale is essential for understanding how cells interact with the surrounding materials. Cells would sense and respond to the nanoscale (or microscale) features on the materials surface. For example, when in contact with implanted devices or scaffold materials, cells interact with nanoscale (or submicroscale) surface features in topography [29,30] and surface chemistry [31,32]. Therefore, the nanobiomechanics of the living cells is very important for surface design of the implanted materials and the scaffold materials for tissue engineering. In addition, it also helps us to improve the understanding of cell interaction with nanoparticles, which is important for nanotoxicology [33] and nanomedicine [34]. Compared with other measurement techniques, nanoindentation has the advantage of in situ imaging of the indented cells with a high resolution, a very good control of the probe position and loading (or unloading) speed, and the flexibility of using different probe geometries (e.g. flat punch spherical, pyramidal and conical). It also has the unique feature of mapping the measured mechanical properties over the investigated surface of the sample [35]. However, data interpretation for nanoindentation of living cells is often difficult.
Despite abundant experimental data reporting nanobiomechanics of living cells, there is a lack of comprehensive discussion on testing with different tip geometries and mechanical models.
Therefore, the goals of this study were (i) to present the strategy of selecting the right type of indenter tips; (ii) to illustrate cell mechanics at different test conditions; (iii) to discuss the mechanical models that enable extracting the mechanical properties of living cells during nanoindentation.
2. Experimental aspects
2.1. Nanoindenter apparatus and atomic force microscope
Nanoindentation is also known as depth sensing indentation, in which the indentation load–depth–time (P – δ – t) profile is recorded. It enables probing the mechanical properties at the nanoscale or microscale. For such a small-scale indentation, there are different approaches to take with respect to the testing instrumentation. In general, we could divide them into nanoindenter apparatus and atomic force microscope (AFM). The key difference between the commercial nanoindentation apparatus and the AFM is on the different transducer operation mechanisms: the former uses electrical capacitance gages (figure 1a) or magnetic coils to directly drive the indenter into the sample. When a voltage is applied, an electrostatic force is generated between the pick-up electrode and drive plates (as depicted in figure 1a), resulting in the movement of the pick-up electrode between the drive plates. While the AFM actuates the tip indirectly via the bending of a cantilever, the AFM operates by measuring attractive or repulsive forces between a tip and the sample, which causes vertical deflection of the cantilever. To detect the displacement of the cantilever, a laser is reflected at the back of the cantilever and collected in a photodiode (figure 1b).
Figure 1.
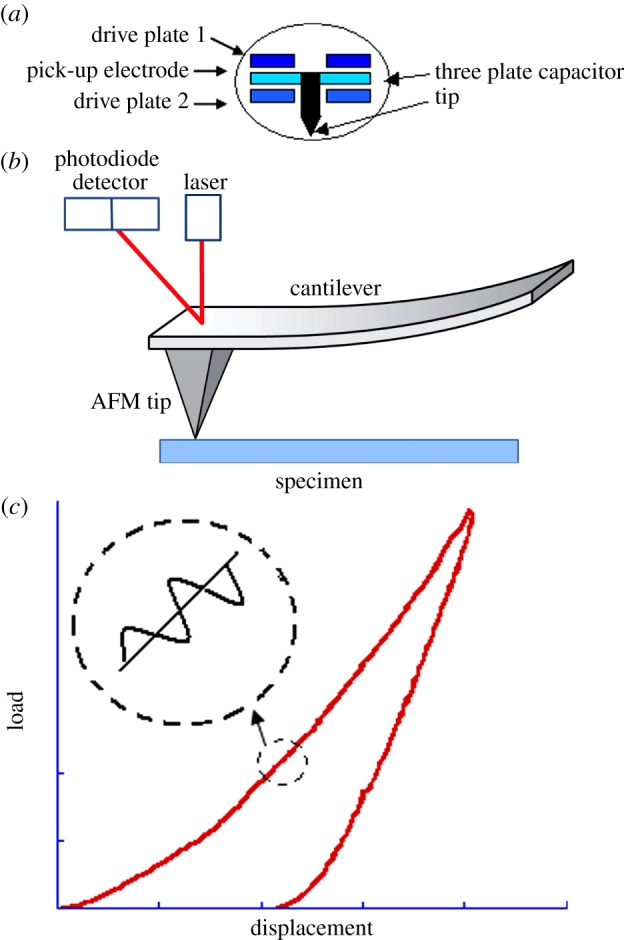
(a) Schematic of electrical capacitance gages that drives nanoindenter, (b) the bending of a cantilever that actuates the AFM tip and (c) dynamic drive signal superposed with the force curve which enables dynamic mechanical measurement during nanoindentation. (Online version in colour.)
In addition to quasi-static loading, a dynamic drive signal can be superposed with the force curve (figure 1c) in both the nanoindenter apparatus [36] and the AFM [37]. This enables measurement of the storage modulus, loss modulus and phase angle, which can be converted to the instantaneous modulus, equilibrium modulus and viscosity [38].
The nanoindenter apparatus allows better control of the indentation force and displacement. The AFM offers the unique advantages of applying very small indentation forces (below 100 pN), but accurate calibration is not easy [39]. Owing to ultra-high resolutions in force and displacement, AFM nanoindentation is particularly useful for probing living cells and subcellular components such as the cell membrane and cytoskeleton.
2.2. Choice of appropriate atomic force microscope tips
There are various tip geometries that can be fitted with the AFM cantilever for nanoindentation tests. The advantages and disadvantages of these tips are discussed as follows. The shape of cells can be spherical or spreading in morphology, depending on the physiological conditions and microenvironment of the living cells and cell types. The choice of appropriate AFM tips depends on cell morphology, cell type and what is of interest (cellular mechanics or subcellular mechanics). This would provide useful guidelines for designing experimental protocols.
2.2.1. Flat punch
Indentation of cells with a flat-ended cylindrical punch (figure 2) is also known as cytoindentation [19]. In this case, the size of the flat punch is much smaller than the cell. This type of indenter is preferred for a very soft and fragile cellular or subcellular structure. The advantage is that data interpretation is relatively straightforward because it avoids the complication of determining the contact area. The contact area is less likely to be affected by thermal drift or creep. The drawback is the spatial resolution is relatively limited compared with the pointed indenter (e.g. pyramid and conical tip); therefore, it is not suitable to characterize fine features. In addition, there are also other practical concerns for using flat punches, such as alignment, detection of contact point and force concentration around edges. The tips are usually made of silicon or glass.
Figure 2.
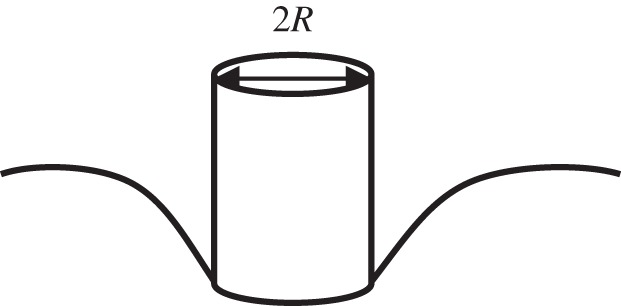
Schematic of nanoindentation by a flat-ended cylindrical punch.
2.2.2. Spherical tip
This type of indenter (figure 3) is also ideal for very soft and fragile cellular or subcellular structures. This type of tip would be particularly useful if the elastic properties of the materials were to change with strain. As the effective strain is related to the ratio of the contact radius and the tip radius, it enables determining the stress–strain curves of the indented materials. The typical spherical tip is made of glass which is easy to manufacture. Similar to the flat punch probe, the spherical glass probe is less likely to cause damage to cells [40]. Again, this may not be good for probing fine features. The typical radius of the probe for indentation of cells is 2.5–10 μm. The representative strain for the spherical tip is the ratio of the contact radius over the effective tip radius [41].
Figure 3.
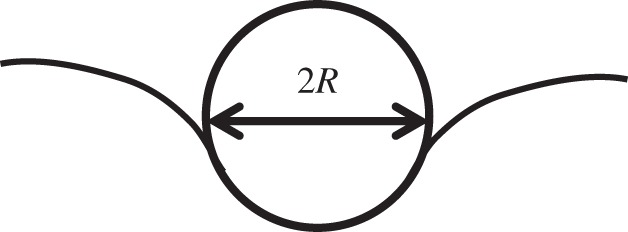
Schematic of nanoindentation by a spherical tip.
2.2.3. Pyramid tip
At a given penetration, the pyramid indenter (figure 4) yields a much smaller contact area compared with the spherical and flat punch tips. It is particularly useful to probe fine features such as the cytoskeleton. Owing to the crystalline structure of silicon, it can be easily etched at certain plane directions, enabling massive production of the probes. The drawback is that the sharp edges may damage the fragile cell membrane or nuclear membrane; therefore, it is not recommended for indentation of living cells.
Figure 4.
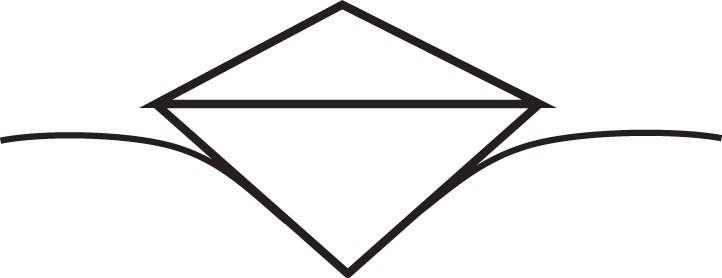
Schematic of nanoindentation by a pyramid tip.
2.2.4. Conical tip
Similar to the pyramid tip, the conical tip (figure 5) yields a much smaller contact area compared with the spherical and flat punch tip. Compared with the pyramid tip, it is less likely to cause damage in lateral directions because it does not have sharp edges. It also circumvents complicated data interpretation owing to coupling of anisotropic soft materials and orientation of the pyramid tip. In principle, the semi-included angle of the probe will not affect the measured elastic or plastic properties, if the appropriate models are used. But it affects the relationship between the yield strength and hardness. At a given penetration, the deformation-affected volume is related to the semi-included angle of the probe [42,43]. Therefore, to eliminate the effect of the substrate or the surrounding matrix, one may need to choose a tip with smaller semi-included angle although the increased stress intensity underneath the very sharp tip might cause puncture of the cell membrane. The tip radius also contributes to the effective deformation zone as discussed in [42,44]. But this influence is not that significant if the tip radius is much smaller than the penetration. When using this probe to do indentation at shallow penetration, it would only sense localized properties mainly resulting from the cell membrane with the underlying cortex or individual cytoskeleton. Sometimes, it may simply measure the bending stiffness of the cell membrane. In such a case, it is unlikely to obtain the mechanical properties of the whole cell. For similar geometries such as a cone (a cylindrical punch can be treated as cone with a semi-apical angle of 90°) and a pyramid, the effective strain is a constant and related to the semi-apical angle (θ) [41].
Figure 5.
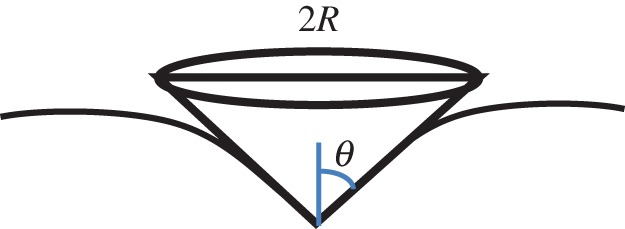
Schematic of nanoindentation by a conical tip.
2.2.5. Extended atomic force microscope testing rigs
In recent years, another type of indentation, cytocompression [45,46], has been widely used to assess the mechanical properties of single cells. In principle, this is an extended AFM indentation on top of cytoindentation. The primary difference in the deformation mechanisms for cytoindentation and cytocompression is in the relative size between the flat punch and the cell. The former has a flat punch diameter well below that of cell. The latter (figure 6) has a flat punch diameter exceeding that of the cell. In such a case, data interpretation is the same as in normal compression tests. The indenter (i.e. the flat plate) is often made of glass, which enables in situ observation of cell deformation [20,47].
Figure 6.
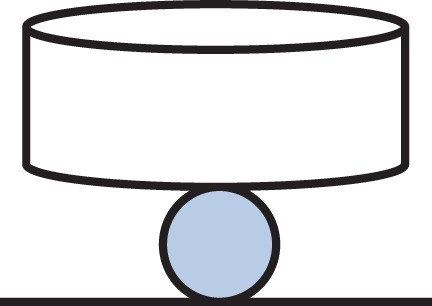
Schematic of cytocompression of a spherical cell.
2.2.6. Summary
Tables 1 and 2 summarize the effective contact radius, effective contact stress and representative strain for various tip geometries. Where, δc is the contact depth.
Table 1.
Terms and explanations.
| terms | description |
|---|---|
| P | force |
| δ | displacement |
| t | time |
| T | normalized time constant |
| a | contact radius |
| R | tip radius |
| θ | semi-included angle of indenter |
| D | diffusivity |
| V | Poisson's ratio |
| K | permeability |
| η | viscosity |
| G | shear modulus |
| G∞ | equilibrium shear modulus |
| G0 | instantaneous shear modulus |
| E | Young's modulus |
| E∞ | equilibrium elastic modulus |
| E0 | instantaneous elastic modulus |
| HA | aggregate modulus |
| γ | surface energy |
Table 2.
The summary of the effective contact radius, effective contact stress and representative strain for various tip geometries.
| tip geometry |
effective contact radius (ac) | effective contact stress | representative strain | |
|---|---|---|---|---|
| sphere | 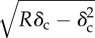 |
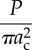 |
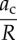 |
|
| cylindrical punch | R | 0.1433cos2θ + 0.205cosθ + 0.0191a | ||
| cone | 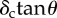 |
|||
| pyramid | triangle-base | 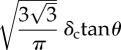 |
||
| square-base | 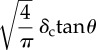 |
|||
aThis empirical expression is based on best fitting of the raw data in [41], see appendix A.
Table 3 summarizes brief guidelines of recommended tip geometries of cells with varied morphologies.
Table 3.
The summary of a brief guideline of recommended tip geometries for cells with varied morphologies.
| flat punch | spherical tip | pyramid tip | conical tip | |
|---|---|---|---|---|
| rounded cell | ✓ | ✓ | ||
| spreading cell | ||||
| soft cell | ✓ | ✓ | ||
| stiff cell | ✓ | ✓ | ✓ | |
| subcellular structure | ✓ | ✓ |
3. Mechanical modelling
Estimation of cell mechanics requires the use of analytical models (or empirical models) of which there are two principal types, namely structure-based models and continuum models. The former include tensegrity [48,49] and percolation models [50], which consider cell mechanics to be dominant by the collective discrete loading bearing element. The latter include linear elastic [51–53], hyperelastic [54–56], poroelastic (also known as biphasic model) [57] and viscoelastic models [8,58]. The continuum model may be interpreted as load-bearing elements that are infinitesimally small relative to the size of the cell.
3.1. Structure-based models
3.1.1. Tensegrity model
The tensegrity model is based on the use of isolated components under compression inside a net of continuous tension, in such a way that the compressed members (usually bars or struts) do not touch each other and the pre-stressed, tensioned cables [59], as shown in (figure 7). This model was coined by Buckminster Fuller in the 1960s. Such a concept was then introduced by Ingber [48] to cell mechanics. It assumes that a cell stabilizes its structure by incorporating compression-resistant elements to resist the global pull of the contractile cytoskeleton [48]. This simple stick and string tensegrity model predicts that a cell appears round when unattached (owing to the internal tension) or attached with a very soft substrate, and spreads out when attached to a stiff substrate. All these agree well with experimental observations [48,61].
Figure 7.
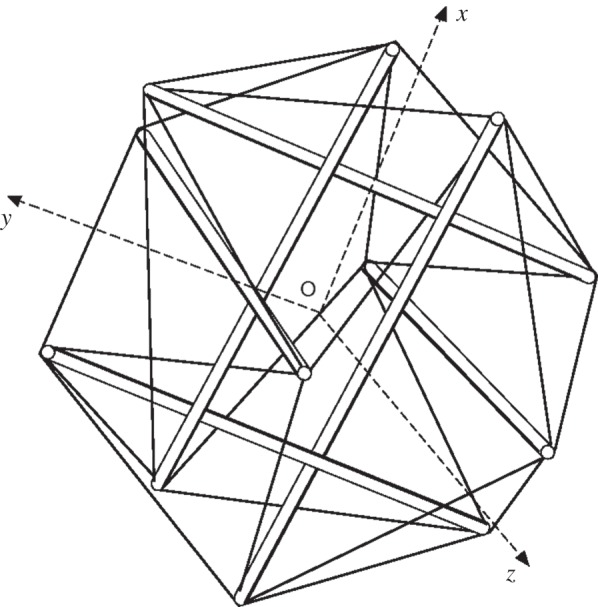
A schematic tensegrity model of cell structure for a spherical cell [60].
3.1.2. Percolation models
The percolation theory describes the behaviour of connected clusters in a random manner. It was introduced in mathematics and then it was applied to materials science. Recently, such a theory was introduced to describe the cell structure and its mechanics [50]. The percolation cluster shown in figure 8 contains substructures of tensegrity on a small scale. These tensegrity substructures are likely to contribute to inherent tension in the cytoskeleton. Owing to the random nature of their interconnection, percolation networks are so flexible that they can easily adapt to the dynamic conditions that affect cells [50].
Figure 8.
A schematic percolation model of cell structure. The smaller interior cube representing the nucleus is supported by the pre-stressed cytoskeletal network [50].
3.1.3. Summary of structure-based models
The percolation and tensegrity models of the cytoskeleton are not mutually exclusive, but complementary. As commented in [50], it is possible that during evolution certain locally ordered tensegrity-type structures may have emerged from more randomly interconnected percolation structures. These structure-based models are very successful at explaining a range of physical observations on cell mechanics such as cell spreading [62], cell migration [63], cell detachment [64] and mechanosensation [65], etc. But it is difficult to quantify the mechanical properties of living cells or subcellular structures.
3.2. Continuum models
Despite neglecting microstructural features, continuum models enable quantifying the mechanical properties of cells under various conditions that could provide essential information of cellular subpopulations [25], disease [18], malignant transformation and cell–materials interactions [2]. However, it is worth pointing out that there is no universal mechanical model available to quantify the mechanical properties of living cells at various physiological and microenvironmental conditions, because living cells can dynamically adapt to their environment. The feasible methodology is to choose appropriate models according to testing conditions (or microenvironment) for a given cell type. Therefore, this paper will focus on the discussion of the feasibilities of various continuum models at given test conditions.
3.2.1. Elastic model
If the tests were performed slowly such that the cell reaches equilibrium, then it is reasonable to use elastic models. At relatively small deformation, a simple linear elastic model may be used to find the Young modulus of the cell (E). Evidence has been shown that this simple elastic model can still reveal useful insights of cell mechanics such as the stiffness ratio of the nucleus over the cytoplasm [51].
If the cell undergoes large deformation, then it may reach the nonlinear elastic region. The neo-Hookean (NH) model, also known as the Gaussian model, is one of the most widely used nonlinear elastic (or hyperelastic) models owing to its simplicity. For example, it has been successfully used to model the deformation of single cells in cytocompression tests [66]. For incompressible materials, the NH model has the following energy function [67]
| 3.1 |
where W is the strain energy density, lj(j = 1,2,3) are the three principal stretch ratios. The Young modulus E is given by
| 3.2 |
Other more sophisticated hyperealstic models have also been developed, such as the polynominal and Ogden models. The strain energy function for the former is given by the flowing equation [68]:
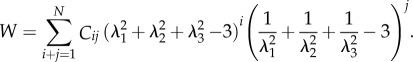 |
3.3 |
The Young modulus E is given by
| 3.4 |
The Ogden model can describe a wide range of strain-hardening characteristics, and it takes the following form as described in previous studies [67–69]:
| 3.5 |
where αi is strain-hardening (or stiffening) exponent. The constants μi are related to the initial Young modulus E, by
| 3.6 |
This model has been adopted to describe cell mechanics when the chondrocyte is embedded in various hydrogel scaffolds [2].
3.2.2. Poroelastic model
The poroelastic model attributes the time-dependence to the flow of a fluid through an elastic (or viscoelastic) porous solid. Such a model was first proposed by Biot [70] and was based on the assumptions of linearity between the stress (sij, p) and the strain (εij, ρ) and reversibility of the deformation process. With the respective addition of the scalar quantities p and ρ to the stress and strain group, the linear constitutive relations can be obtained by extending the known elastic expressions. The most general form for isotropic material constitutive behaviour response is described as follows [70]:
| 3.7 |
and
| 3.8 |
where K and G are bulk and shear modulus of the drained elastic solid. The parameters H and M characterize the coupling between solid and fluid stress and strain.
This model was originally developed for soil mechanics and it was then applied to describe the mechanics of hydrogels [71] and tissues such as bone [72] and cartilage [73]. Very recently, it has also been applied to cell mechanics [24]. When it comes down to cell mechanics, the material properties of interest include the shear modulus G (or aggregate modulus HA), and Poisson's ratio of the solid matrix and Darcy permeability k. The permeability could be analogous to the diffusion coefficient, but driven by the mechanical gradient instead of the chemical gradient.
During stress relaxation, it gives [74]
| 3.9 |
where P(∞) and P(0) signify the force at infinite time (t = ∞) and t = 0, respectively.
Where the normalized time τ is given by
| 3.10 |
where a is the contact radius and t is time.
The diffusivity D is given by
| 3.11 |
where v, G, k and η are Poisson's ratio, shear modulus and permeability of the solid matrix and viscosity of the solvent, respectively.
The equilibrium Young modulus E is given by
| 3.12 |
The relation between the aggregate modulus and the Young modulus is given by
| 3.13 |
This poroelastic model is quite similar to the biphasic theory of the mixture of an incompressible solid and incompressible fluid which was independently developed by Mow et al. [75] and Bowen [76]. In addition, by replacing the elastic media with viscoelastic media to account for the intrinsic viscoelastic properties of the actin filaments, a more complex model (poro-viscoelastic) [77] can be used to describe soft tissue or cell mechanics. When the cell is exposed to an ionic solution, the ionic charge also contributes to the mechanical responses of cells. In such a case, a third phase (i.e. the ionic phase) can be included in the constitutive equation, which is called the triphasic model [78].
3.2.3. Spring-dashpot viscoelastic models
The basic premise of viscoelasticity of this type is that it replaces some elastic springs with a time-dependent dashpot as shown in figure 9. These spring-dashpot models usually refer to viscoelastic models. In a general manner, the empirical Prony series [79] has been used to describe the material's time-dependent constitutive response in the experimental time domain.
Figure 9.
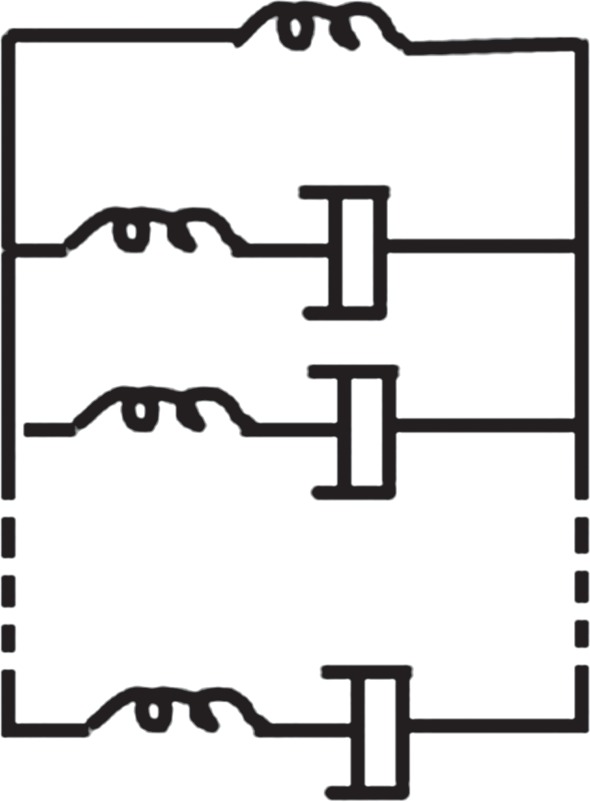
Schematic of the generalized Maxwell model.
For stress relaxation, the relaxation shear modulus G(t) in an empirical Prony series is given by
| 3.14 |
where G∞ is the equilibrium shear modulus and the instantaneous shear modulus 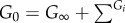 or creep, the creep compliance J(t) in an empirical Prony series is given by
or creep, the creep compliance J(t) in an empirical Prony series is given by
| 3.15 |
where the parameters Ci are associated with compliance values (inverse shear modulus).
The stress relaxation modulus and the creep compliance are not explicitly inverse in the time domain but are in the Laplace domain (i.e. 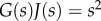 ).
).
The equilibrium shear modulus (G∞) and the instantaneous shear modulus (G0) can be determined by the following equations:
| 3.16 |
and
| 3.17 |
3.2.4. Power-law rheology
Power-law rheology (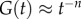 , where n is a positive constant) has also been adopted to describe the mechanics of certain cells. For example, it is found that n = 0.2 for lung epithelial cells during stress relaxation [80]. The physical basis of this power law may be related to molecular adjustment of the cytoskeleton matrix, which is similar to soft glassy materials close to the glass transition [80]. Such a model may work better for those cells that do not have a strong cytoskeleton structure. For example, such a power-law rheology model has also been applied to neutrophils and macrophages [81].
, where n is a positive constant) has also been adopted to describe the mechanics of certain cells. For example, it is found that n = 0.2 for lung epithelial cells during stress relaxation [80]. The physical basis of this power law may be related to molecular adjustment of the cytoskeleton matrix, which is similar to soft glassy materials close to the glass transition [80]. Such a model may work better for those cells that do not have a strong cytoskeleton structure. For example, such a power-law rheology model has also been applied to neutrophils and macrophages [81].
3.2.5. Summary
The advantage of the elastic models is that they eliminate the number of elastic parameters, which are easy for empirical curve fitting and numerical simulations. It was argued that poroelastic models provide physical constants related to the material microstructure, compared with the empirical spring-dashpot viscoelastic models. However, it must be pointed out that poroelastic models homogenize the whole structure and may not be able to predict structure reorganization of the cell during external stimuli (e.g. cell migration and cell spreading) compared with those structure-based models (e.g. tensegrity model). Therefore, one should also treat structure-based models and the continuum models as complementary to each other.
4. Nanoindentation models
As mentioned earlier, only the nanoindentation models (continuum based) for the tips (i.e. flat punch, spherical and conical tip) suitable for testing living cells will be discussed in detail. Once the AFM deflection–displacement curves are converted to force–displacement curves, indentation theories would be applicable. The models below are based on assumptions of small deformation and negligible tip–cell adhesions. More detailed discussion is provided in §5, when these assumptions are invalid.
4.1. Elastic nanoindentation models
Flat punch: the force–displacement (P − δ) relation is given by [82]
| 4.1 |
where R and ν are the radius of the flat punch and Poisson's ratio, respectively.
Spherical tip: the force–displacement relation is given by the Hertz elastic model [83]
| 4.2 |
where R are the radius of the spherical indenter.
Conical tip: the force–displacement relation is given by [84]
| 4.3 |
where θ is the semi-included angle of the conical indenter.
4.2. Poroelastic–nanoindentation models
Flat punch: the force–time (P − t) relation is given by [74]
| 4.4 |
and
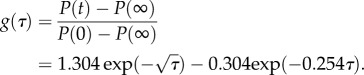 |
4.5 |
Spherical tip: the force–time relation is given by [74]
| 4.6 |
and
| 4.7 |
Conical tip: the force–time relation is given by [74]
| 4.8 |
and
| 4.9 |
where a is the contact radius, and τ is the normalized time constant as defined earlier.
A simpler poroelastic model to describe cell mechanics has been presented in [85,86], which considers fluid propagates through a cell owing to a local pressure increase in a two-dimensional manner. This model requires other techniques to determine the pore size and viscosity, rather than relying on the analysis of the force–time–displacement curve alone. For pore size, it can be estimated by hindered tracer particle diffusion experiments, and for viscosity it can be estimated by a nanoparticle diffusion experiment [85,86].
4.3. Viscoelastic-nanoindentation models
When viscoelastic models were adopted, experimentalists used either the stress relaxation (for tests performed under displacement control) or creep (for tests performed under force control) period to determine viscoelastic parameters. It is advantageous to use stress relaxation or creep for data analysis because they circumvent the possible complexity in nonlinear changes during ramping.
Flat punch: the following force–time and the displacement–time relations can be obtained by incorporating the viscoelastic model into equation (3.16)
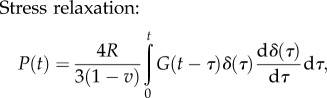 |
4.10 |
and
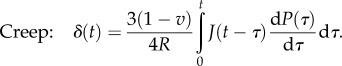 |
4.11 |
More rigorous analytical solutions were given by [87], which consider that the elastic components may have different Poisson's ratios.
Spherical tip: the force–time and the displacement–time relations are given by [88,89]
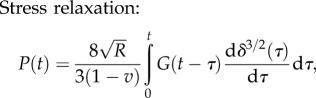 |
4.12 |
and
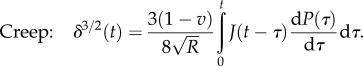 |
4.13 |
Conical tip: the force–time and the displacement–time relations are given by [90]
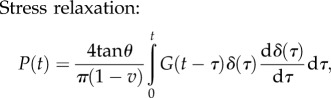 |
4.14 |
and
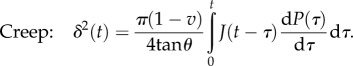 |
4.15 |
For complicated load–time or displacement–time histories, Boltzmann hereditary integrals can be used to find full P – δ – t profiles [58,91].
5. Discussion
It must be pointed out that most researchers compared measured cell mechanical properties across published results without analysing the experimental techniques related to controlling factors on cell mechanics. These factors include tip geometry, indentation penetration, cell morphology, cell type and mechanical models. Therefore, it is essential to discuss how these factors affect the measured cell mechanical properties.
5.1. Cell mechanics determined by different models
Depending on the models to be adopted, it may affect the values of determined mechanical properties of cells. Table 4 summarizes the elastic properties of bovine articular chondrocytes determined by the biphasic and spring-dashpot viscoelastic models. It can be seen that these two models give similar results. However, the cell actually experiences large deformation, which was not considered in both models.
Table 4.
Elastic properties of bovine articular chondrocytes determined by the biphasic and spring-dashpot models [24].
| continuum models | elastic modulus (kPa) |
|---|---|
| biphasic model | 1.38 ± 0.46a |
| spring-dashpot model | 1.48 ± 0.35 |
aNote that the original aggregate modulus value of HA (2.58 kPa) for biphasic model is converted to the equilibrium modulus based on equation (3.13), with Poisson's ratio of 0.38 [25].
Although many researchers adopted viscoelastic models to describe the time-dependent behaviour of cell mechanics [8,25,26,92], other researchers found that poroelastic models are more appropriate in their experimental test protocols [86,93]. However, it has been shown that neither the poroelastic model nor the viscoelastic model is capable of capturing the complete mechanical responses of cells during creep or stress relaxation [94,95]. A recent paper published in Nature Materials [95] has demonstrated that the poroelastic model captures the time-dependent mechanics of cells at a short-time scale (less than 0.5 s), but the viscoelastic model appears to work better at a long-time scale [95]. This may suggest that, at a short-time scale, it is fluid diffusion that governs the time-dependent behaviour of cells. At a long-time scale, it is likely that the collective viscoelastic behaviour of the polymer-like materials inside cell and cytoskeleton dynamics governs the time-dependent behaviour of the cell. Therefore, a further improved model, poro-viscoelastic model presented in [77,96–98] may be more appropriate. If the cell is exposed to an osmotic environment, then it passively swells or shrinks. The poroelastic (biphasic) model is limited to describe the effective materials parameters that vary with extracellular osmolality [99]. The triphasic model [78] has the capability to better describe the mechanochemical coupling by introducing both mechanical and chemical parameters in the governing equations, which could better describe the mechanochemical equilibrium of the cells [99].
It must be pointed out that, in the poroelastic (biphasic) model and triphasic model, small deformation and incompressible solid matrix are assumed. In a real test or physiological loading, cells may experience large deformation. In addition, the solid matrix in cells may not be incompressible. It would be very complex to include all these in the constitutive equations of poroelastic models and triphasic models. However, for viscoelastic models, it is straightforward to account for the large deformation and compressibility of the solids via the hyperviscoelastic model.
The above-mentioned models deal with static loading. Indeed, oscillatory measurement associated with the AFM can be used to determine the viscoelastic properties of the living cell. More details can be found in [37,100].
5.2. Cell mechanics determined by different indenters
As discussed in §2, at a given force, the effective deformation zone varies significantly with tip geometry. Consequently, this can affect the measured mechanical properties of cells. In general, when using a pyramid tip with low penetration, the results are more likely to be affected by spatial heterogeneity, as observed in [101]. Table 5 summarizes the comparison of elastic moduli (determined by elastic contact model) for osteoblastic cells (spread morphology) measured by a pyramidal and spherical tip. It has been shown that elastic moduli measured by a pyramidal tip are about four times higher compared with those measured by a spherical tip. When using a sharp conical or pyramid tip for spreading cells, it senses the region near an actin filament, as observed by Hoh et al. [104]. In such a case, the probed elastic response may be significantly affected by this stiff filament (with elastic modulus on the order of GPa).
Table 5.
The comparison of elastic moduli (determined by elastic contact model) for osteoblastic cells (spread morphology) measured by pyramidal and spherical AFM tip.
Table 6 summarizes the comparison of elastic moduli (determined by the elastic contact model) for lung epithelial cells measured by a pyramidal and a flat punch tip. In this test, large penetration of 3 µm was applied via a pyramid tip, and the indentations were not far from the nucleus. In that case, it probes the overall cell mechanics. That is why it yields a similar result to that determined by a flat punch tip. However, it should be pointed out that the thin-layer effect was not considered in the original paper. Table 7 summarizes the comparison of viscoelastic properties for a single bovine chondrocyte using cytoindentation and cytocompression. The indenter diameter for cytoindentation is 5 μm [106], which is almost half of the cell diameter. The penetration for cytoindentation is similar to the cell radius; therefore, it is expected that it senses the properties of the whole cell instead of local cellular properties. In such a case, it would be expected that the elastic properties determined by cytoindentation are similar to those by cytocompression. As shown in table 7, the equilibrium moduli and viscosity determined by these two indenters are quite similar. However, the instantaneous modulus determined by cytoindentation is over three times higher than cytocompression. This may suggest that indenter size might affect the instantaneous mechanical responses of cells.
Table 6.
The comparison of elastic moduli (determined by elastic contact model) for lung epithelial cells measured by a pyramidal and a flat punch tip.
Table 7.
The comparison of viscoelastic properties for single bovine chondrocyte (spherical morphology) using cytoindentation and cytocompression.
5.3. Cell morphology on cell mechanics
As cells are living materials, they respond to mechanical, physical and chemical stimuli. Cells will change their morphology after being removed from their native environment. Depending on the physical properties or surface chemistry of the substrate that they are seeded on, cells may spread along the substrate or retain a spherical shape. Figure 10 displays a typical example that shows the substrate stiffness regulates the shape of fibroblasts. Table 8 summarizes the mechanical properties of the different cell types and the change in their morphology. In that case, a borosilicate glass sphere (5 µm diameter) probe was used for the AFM nanoindentation [107].
Figure 10.
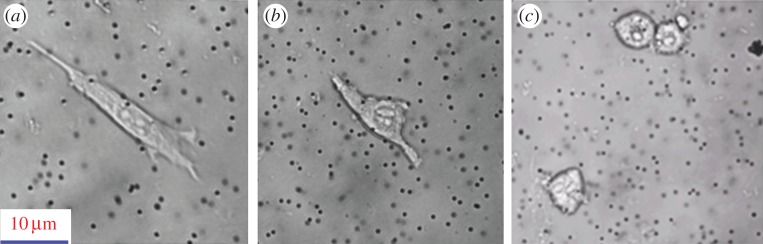
The morphology of fibroblast cultured on polyacrylamide gels with varied stiffness: (a) 10.4 kPa, (b) 3.31 kPa and (c) 0.56 kPa.
Table 8.
Comparison of viscoelastic properties for AFM indentation (with a spherical tip) of osteoblasts, chondrocyte, adipocytes, adipose-derived adult stem cells (ADAS) and mesenchymal stem cells (MSCs) in spread and spherical morphologies [107].
| cell morphology | cell type | E∞ (kPa) | E0 (kPa)a | η (kPa s−1)a |
|---|---|---|---|---|
| spread | Osteoblast | 4.5 ± 2.3 | 11.6 ± 33.9 | 112.2 ± 450 |
| chondrocyte | 1.0 ± 1.6 | 1.70 ± 3.13 | 5.80 ± 14 | |
| ADAS cell | 1.7 ± 1.1 | 3.81 ± 15.4 | 20.2 ± 47.8 | |
| MSC | 2.3 ± 2.1 | 4.23 ± 14.4 | 20.5 ± 128 | |
| spherical | osteoblast | 0.60 ± 0.78 | 2.1 ± 3.7 | 10.3 ± 21.4 |
| chondrocyte | 0.45 ± 0.42 | 0.91 ± 1.3 | 4.5 ± 8.9 | |
| ADAS cell | 0.37 ± 0.31 | 1.6 ± 2.6 | 8.8 ± 16.6 | |
| MSC | 0.52 ± 0.60 | 5.3 ± 16.1 | 46.3 ± 140 |
aNote that the values of E0 and viscosity η were recalculated based on the raw data in [107].
For all the above-presented models, they assume a sample with infinite thickness and width compared with indentation depth. When probing the spreading cells, if the indentation size is comparable to the local thickness, then it invalidates these assumptions. Therefore, the estimation of cell mechanical properties will be affected by the stiffness of the underlying substrate. In such a case, the thin-layer model proposed by Darling et al. [108] could be applicable. This model introduces a geometry factor to describe the force–displacement relation. For example, for a spherical tip, the original elastic model can be modified as follows:
| 5.1 |
where f1(χ) is geometrical correction factor which is given by
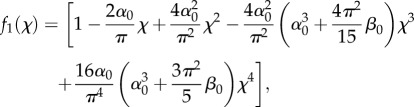 |
5.2 |
and
| 5.3 |
where h is the cell thickness,
| 5.4 |
and
| 5.5 |
This geometry factor f1(χ) can be easily incorporated into the above-mentioned viscoelastic models.
For the thin-layer-based poroelastic model, empirical correction parameters can be obtained based on finite-element simulation [109]. For example, the following empirical model is for spherical indenter [109]:
| 5.6 |
| 5.7 |
and
| 5.8 |
It needs to be pointed out that depending on where the indentation is located, one needs to use different thin-layer models (i.e. non-adhered thin-layer model [110] or adhered thin-layer model [111,112]). For example, it was found that when indentation was made at a thin region relatively near the edge of the fibroblast, the adhered thin-layer model is more appropriate [37]. Although when indenting a thin region further from the edge of the fibroblast, the non-adhered thin-layer model may be more suitable [37]. One needs to be aware that all these thin-layer models are valid for cells with approximately uniform thickness (i.e. well-spread). Otherwise, computational models are required, which will be discussed in §5.7. To minimize the thin-layer effect, a tip with a smaller half-angle is preferred [113].
When indenting a specimen with finite thickness and finite width, there is complex coupling between the stiffening effect, caused by the underlying substrate, and the softening effect, caused by a free edge in the lateral direction. A typical example is to use a spherical tip indenting into a spherical cell at a large deformation. Thus, another model has been presented to deal with this coupling effect [58].
The following empirical geometry factor f2(χ) can then be used [58]:
| 5.9 |
and
| 5.10 |
This geometrical factor can be incorporated into the viscoelastic models [58], and it has been successfully applied to extract viscoelastic properties of spherical chondrocytes adherent to a glass plate.
5.4. A special case: cell trapped inside a well
Microfabricated well arrays have been proposed to trap cells before nanoindentation [113–115]. This would enable automatic indentation for high-throughput screening methods that may prove useful for cells sorting. All the existing contact mechanics-based models only deal with indented materials placed on a flat surface (i.e. the bottom surface is constrained vertically), free from lateral constraint. Little work has been carried out for indented materials placed within a well (figure 11); in that case, the assumptions in the classic Hertz model and thin-layer models have been violated. This problem has recently been dealt with by Chen [116]. Interestingly, it introduces a similar correction parameter to f2(χ).
Figure 11.
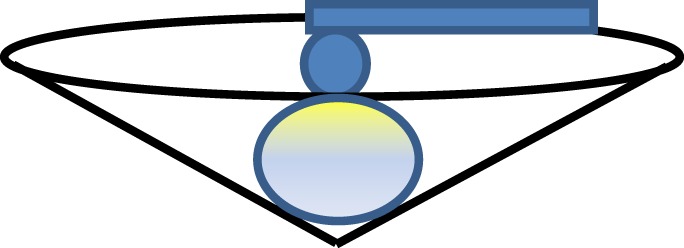
The schematic of AFM indentation of an isolated chondrocyte by a spherical probe.
5.5. Substrate modulation
The substrate physics may affect the cell mechanical properties, even if the cell maintains the same morphology. Table 9 summarizes the comparison of elastic moduli (determined by the elastic contact model) for the same murine NIH3T3 fibrobalst cell line (spread morphology) cultured on different substrates indented by a spherical tip. Table 10 summarizes the comparison of elastic moduli (determined by elastic contact model) for adult bovine chondrocytes (spherical morphology) placed on tissue culture plastic and on uncoated glass, probed by cytocompression. In both cases, it is evident that the substrates can significantly regulate cell mechanical properties even if the cell shape is retained.
Table 9.
The comparison of elastic moduli (determined by elastic contact model) for the same murine NIH3T3 fibrobalst cell line (spread morphology) cultured on different substrates indented by a spherical tip.
Table 10.
The comparison of elastic moduli (determined by elastic contact model) for adult bovine chondrocytes (spherical morphology) are placed on a tissue culture plastic and on an uncoated glass, determined by cytocompression.
Similar behaviour of the matrix modulating cell mechanics was also found in a three-dimensional scaffold. For example, when a bovine chondrocyte cell line were seeded in different scaffolds, they maintained a spherical morphology, but different mechanical properties with regard to the three-dimensional scaffold [2].
5.6. Cell–tip adhesion
Some degree of tip–cell adhesion may be unavoidable in nanoindentation. This would be affected by the composition and surface chemistry of the tip. There are different models that incorporate tip–sample adhesion, such as the Johnson–Kendall–Roberts (JKR) [118], Derjaguin–Muller–Toporov [119] and Maugis–Dugdale [120] models. Among which, the JKR model is appropriate for the indentation of relatively compliant materials with probes of relatively large radii and strong adhesive forces. This would be preferred to compliant materials such as cells, in particular for a spherical indenter in contact with spherical cells [121].
During unloading, one may observe the negative force resulting from the surface adhesion, as shown in figure 12. Such a pull-out force is related to the surface energy (γ) at the cell–tip interface and is given by [83]
| 5.11 |
Figure 12.
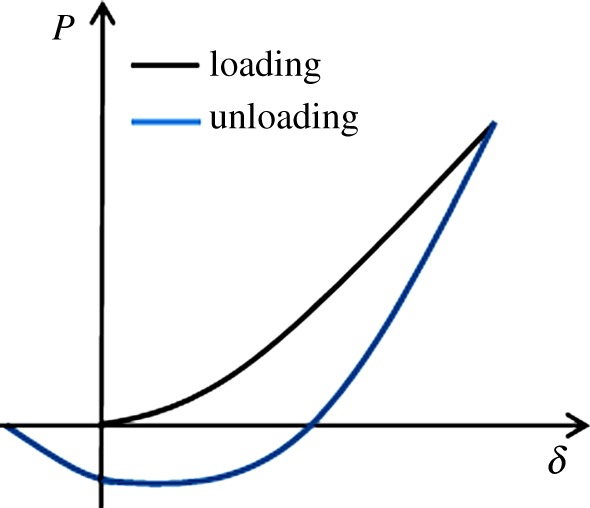
Schematic of force–displacement curve for a typical adhesive indentation. (Online version in colour.)
In such a case, one needs to replace the force (P) in all the equations above by P + Ppull. More detailed discussions about the adhesive contact during AFM indentation has been summarized by Lin & Horkay [122].
5.7. Inverse finite-element analysis
All the previous mechanical models work properly when the irregularity of cell shape is negligible. For example, when indenting a spread cell (with non-uniform thickness) with a conical tip at small depth, the effective-deformed region is mainly confined within the dotted region, as shown in figure 13. In that case, the cell can be assumed a regular shape with a single radius, so that the previous models in §4 are applicable. However, at greater penetration, the effective deformation zone spreads out, and the irregularity of cell shape becomes important. In that case, one should treat the previous thin-layer model with caution, because the non-uniform thickness and the change of curvature becomes important. Therefore, three-dimensional finite-element analysis (FEA) with the true cell shape is necessary. Figure 14 displays a flowchart of inverse FEA to determine the mechanical properties of cells during nanoindentation. In that case, the three-dimensional model a of cell and its nucleus model can be reconstructed from the sliced images obtained by confocal microscopy [123].
Figure 13.
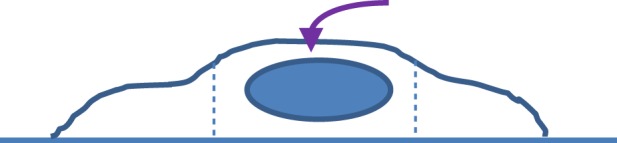
Schematic of indenting a spread cell (with non-uniform thickness) with a conical tip at small depth; in that case, the cell can be assumed as regular shape.
Figure 14.
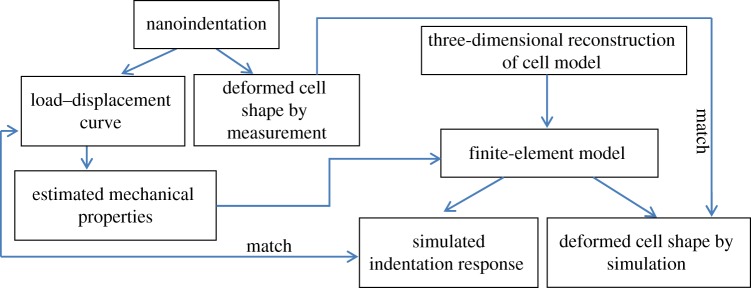
Flowchart of finite-element analysis to determine the mechanical properties of cells during nanoindentation.
In addition, in order to obtain the mechanical properties of a subcellular structure of living cells without mechanical or chemically separating them from living cells, FEA is also essential. For example, finite-element simulations have been used to do inverse analysis to obtain the mechanical properties of cytoplasm [2,51,54], nucleus elastic properties [2,51,54] or the pericellular matrix [114]. As living materials, the external force may trigger passive stress generation in the cell. FEA can be used to investigate how the passive stress may affect the elastic modulus by treating it as residual stress [124].
5.8. Summary of strategy of selecting tip geometry and mechanical models
Prior to discussing the strategy to select tip geometry and size, it is good to recall the cellular structure. A eukaryotic cell (excluding the red blood cell) is composed of cell membrane, cytoplasm (including cytosol, cytoskeleton and other suspended organelles) and nucleus (including nucleus membrane, nucleoplasm, a structure layer nuclear lamina, nucleus and other suspended organelles). In this paper, we discuss the design indentation protocols and the associated indentation models with regard to the cell morphology, because cell morphology will affect the boundary conditions of the indentation models. In addition, cell morphology can be regulated by the substrate as shown in figure 10. Cell type is also important, and we will generally classify the cell as a cell with a strong cytoskeleton (such as osteoblast, chondrocyte and muscle cells), medium strong cytoskeleton (such as fibroblast cell, epithelial cell and endothelial cell) and weak cytoskeleton (such as fat cell, neutrophils, blebbing cell and mesenchymal stem cell).
If one is interested in the mechanics of the cell membrane (with thickness of approx. 7 nm), then a very small indentation (blow 1 nm) should be made with a conical or pyramid tip with a small semi-included angle. This is due to the fact that the tip with a smaller semi-included angle could restrict the effective deformation zone. A simple mechanical model such as an elastic model is acceptable.
If one is interested in the mechanics of cytoplasm, a small indentation, less than 10% of the vertical distance from the nucleus (i.e. less than 0.1d as shown in figure 15) should be made with a conical tip. The suitable penetration depth varies with the semi-included angle. Alternatively, one can locate the indentation near the edge, away from the nucleus. The poroelastic model is more appropriate. It is also recommended to use a cone with a big angle to prevent high strain-induced nonlinear elasticity. For a typical commercial AFM probe, the tip radius is 4–10 nm. One may need to use the modified models for a blunt cone or truncated pyramid as discussed by Lin & Horkay [122], when penetration is comparable to the tip radius.
Figure 15.
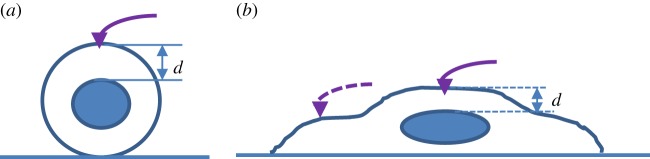
Schematic of indentation of (a) a spherical and (b) spread cell.
If one is interested in the mechanics of the whole cell (mixed responses of cytoplasm and nucleus), then a bigger indentation (more than 0.1d) should be made with a flat punch or a spherical tip. For a well-spread cell (i.e. thickness is small), a conical tip with a big semi-apical angle is appropriate. In this case, poroelastic, viscoelastic and more complex poro-viscoelastic models are appropriate for the cells with a strong or medium strong cytoskeleton. If the cell undergoes large deformation, then the hyperviscoelastic model is more appropriate [66], particularly for cells with a strong cytoskeleton. The power-law rheology model may be more suitable for cells with a weak cytoskeleton.
The guidelines about the relative penetration above are a rough estimation based on a coating/substrate system [44]; some dedicated computational modelling is required to further reveal this rule of thumb to better guide the experimentalists to design test protocols.
If cells are exposed to osmotic pressure, then the poroelastic model (or poro-viscoelastic models) should be used. If the ionic charge is important, then a triphasic model is more appropriate [78,125]. If penetration is comparable to the cell thickness (typically for a spread cell), then a thin-layer model in combination with a viscoelastic/poroelastic model should be used. When the contact radius is comparable to cell width and thickness, the model proposed by Chen & Lu [58] should be used to account for coupling of the free edge effect and the thin-layer effect.
6. Conclusion and perspectives
The exploration of nanobiomechanics of living cells helps us to understand a range of processes such as disease progression and cell–materials interactions. This provides essential information for cellular therapy and tissue engineering. A range of mechanical models has been discussed. The structural models have the advantage in predicting how cells rearrange their structure (e.g. cell spreading and cell migration) according to external stimuli. The continuum models enable quantifying the mechanical properties in cells under various conditions that could provide essential information of cellular subpopulations, disease, malignant transformation and cell–materials interactions.
It is almost impossible to get an accurate universal physical model to quantify the mechanical properties of cells during indentation, owing to the complex cell structure and the complicated mechanotransduction mechanisms (e.g. via cytoskeleton and nuclear lamina). Therefore, it is more realistic to use the averaged continuum model accompanied by appropriate testing protocols if the overall cell mechanics is of interest. One should also treat structure-based models and the continuum models as complementary to each other. Various factors (e.g. cell morphology, substrate, tip geometry and relative indentation penetration with regard to cell size) that affect quantifying the mechanical properties of cells have been evaluated. When researchers compare the measured cell mechanical properties across the literature, they should be aware that interpretation of the results can be significantly affected by the mechanical models adopted at given test conditions. Accordingly, this paper presents strategies to select tip geometries and the associated mechanical models at given test conditions. When the irregular shape of a cell plays an important role in cell mechanics, it violates the boundary conditions of all the analytical and semi-analytical mechanical models. In that case, FEA incorporating the true cell shape is essential.
The way that cells sense and respond to the substrate is expected to be temporal in nature [126]. In addition, passive stresses may be generated in the cell during indentation. Therefore, the outlook for modelling cell mechanics should be to incorporate these dynamic effects to the mechanical modelling and FEA.
Acknowledgements
The author acknowledges Dr Bill Chaudhry for his contribution of figure 10. The author also acknowledges Prof. Van. C. Mow and Prof. Farshid Guilak for useful discussion on viscoelastic, poroelastic (biphasic) and triphasic models. In addition, the author acknowledges the anonymous reviewers for very constructive comments.
Appendix A
Figure 16 presents how the effective strain changes with the semi-apical angle of the conical tip, with the raw data taken from [41].
Figure 16.
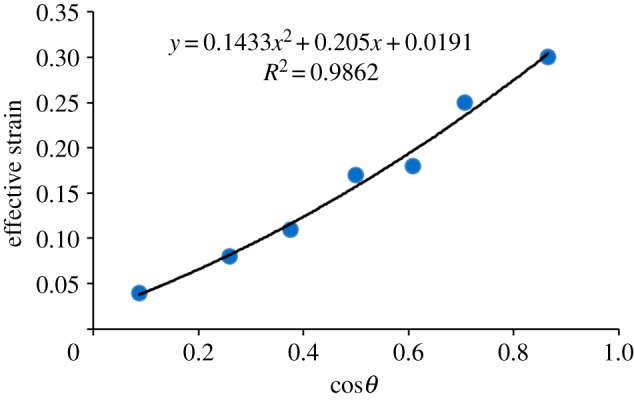
The effective strain changes with the semi-apical angle of the conical tip, with the raw data taken from [41].
Funding statement
This work is partially supported by ‘A new frontier in design: the simulation of open engineered biological systems’, EP/K039083/1 and EPSRC-Newcastle University sandpit workshop award.
References
- 1.Lee DA, Knight MM, Campbell JJ, Bader DL. 2011. Stem cell mechanobiology. J. Cell. Biochem. 112, 1–9. ( 10.1002/jcb.22758) [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Irianto J, Inamdar S, Pravincumar P, Lee DA, Bader DL, Knight MM. 2012. Cell mechanics, structure, and function are regulated by the stiffness of the three-dimensional microenvironment. Biophys. J. 103, 1188–1197. ( 10.1016/j.bpj.2012.07.054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingber DE. 1994. Cellular tensegrity and mechanochemical transduction In Cell mechanics and cellular engineering (Mow VC, Guilak F, Tran-Son-Tay R. eds), pp. 329–342. New York, NY, Springer.
- 4.Ingber DE. 2006. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20, 811–827. ( 10.1096/fj.05-5424rev) [DOI] [PubMed] [Google Scholar]
- 5.Liedert A, Kaspar D, Claes L, Ignatius A. 2006. Signal transduction pathways involved in mechanical regulation of HB-GAM expression in osteoblastic cells. Biochem. Biophys. Res. Commun. 342, 1070–1076. ( 10.1016/j.bbrc.2006.02.063) [DOI] [PubMed] [Google Scholar]
- 6.Robling AG, Bellido T, Turner CH. 2006. Mechanical stimulation in vivo reduces osteocyte expression of selerostin. J. Musculoskelet. Neuronal Interact. 6, 354–354. [PubMed] [Google Scholar]
- 7.Setton LA, Chen J. 2006. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J. Bone Joint Surg. Am. A 88, 52–57. ( 10.2106/jbjs.f.00001) [DOI] [PubMed] [Google Scholar]
- 8.Trickey WR, Baaijens FPT, Laursen TA, Alexopoulos LG, Guilak F. 2006. Determination of the Poisson's ratio of the cell: recovery properties of chondrocytes after release from complete micropipette aspiration. J. Biomech. 39, 78–87. ( 10.1016/j.jbiomech.2004.11.006) [DOI] [PubMed] [Google Scholar]
- 9.Xu J, et al. 2011. Effects of micropatterned curvature on the motility and mechanical properties of airway smooth muscle cells. Biochem. Biophys. Res. Commun. 415, 591–596. ( 10.1016/j.bbrc.2011.10.111) [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Hong JW, Kim J, Shin JH. 2013. Comparative study on the differential mechanical properties of human liver cancer and normal cells. Anim. Cells Syst. 17, 170–178. ( 10.1080/19768354.2013.789452) [DOI] [Google Scholar]
- 11.Ekpenyong AE, et al. 2012. Viscoelastic properties of differentiating blood cells are fate- and function-dependent. PLoS ONE 7, 0045237 ( 10.1371/journal.pone.0045237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee GYH, Lim CT. 2007. Biomechanics approaches to studying human diseases. Trends Biotechnol. 25, 111–118. ( 10.1016/j.tibtech.2007.01.005) [DOI] [PubMed] [Google Scholar]
- 13.Tan SJ, Li Q, Lim CT. 2010. Manipulation and isolation of single cells and nuclei. Methods Cell Biol 98, pp. 79–96. ( 10.1016/S0091-679X(10)98004-7) [DOI] [PubMed] [Google Scholar]
- 14.Puig-De-Morales-Marinkovic M, Turner KT, Butler JP, Fredberg JJ, Suresh S. 2007. Viscoelasticity of the human red blood cell. Am. J. Physiol. Cell Physiol. 293, C597–C605. ( 10.1152/ajpcell.00562.2006) [DOI] [PubMed] [Google Scholar]
- 15.Suresh S, Spatz J, Mills JP, Micoulet A, Dao M, Lim CT, Beil M, Seufferlein T. 2005. Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomater. 1, 15–30. ( 10.1016/j.actbio.2004.09.001) [DOI] [PubMed] [Google Scholar]
- 16.Dao M, Lim CT, Suresh S. 2003. Mechanics of the human red blood cell deformed by optical tweezers. J. Mech. Phys. Solids 51, 2259–2280. ( 10.1016/j.jmps.2003.09.019) [DOI] [Google Scholar]
- 17.Liu Y-P, Li C, Liu K-K, Lai ACK. 2006. The deformation of an erythrocyte under the radiation pressure by optical stretch. J. Biomech. Eng. Trans. ASME 128, 830–836. ( 10.1115/1.2354204) [DOI] [PubMed] [Google Scholar]
- 18.Li QS, Lee GYH, Ong CN, Lim CT. 2008. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun. 374, 609–613. ( 10.1016/j.bbrc.2008.07.078) [DOI] [PubMed] [Google Scholar]
- 19.Shin D, Athanasiou K. 1999. Cytoindentation for obtaining cell biomechanical properties. J. Orthop. Res. 17, 880–890. ( 10.1002/jor.1100170613) [DOI] [PubMed] [Google Scholar]
- 20.Han S-K, Colarusso P, Herzog W. 2009. Confocal microscopy indentation system for studying in situ chondrocyte mechanics. Med. Eng. Phys. 31, 1038–1042. ( 10.1016/j.medengphy.2009.05.013) [DOI] [PubMed] [Google Scholar]
- 21.Lim CT, Zhou EH, Quek ST. 2006. Mechanical models for living cells: a review. J. Biomech. 39, 195–216. ( 10.1016/j.jbiomech.2004.12.008) [DOI] [PubMed] [Google Scholar]
- 22.Hochmuth RM. 2000. Micropipette aspiration of living cells. J. Biomech. 33, 15–22. ( 10.1016/s0021-9290(99)00175-x) [DOI] [PubMed] [Google Scholar]
- 23.Trickey WR, Lee GM, Guilak F. 2000. Viscoelastic properties of chondrocytes from normal and osteoarthritic human cartilage. J. Orthop. Res. 18, 891–898. ( 10.1002/jor.1100180607) [DOI] [PubMed] [Google Scholar]
- 24.Leipzig ND, Athanasiou KA. 2005. Unconfined creep compression of chondrocytes. J. Biomech. 38, 77–85. ( 10.1016/j.jbiomech.2004.03.013) [DOI] [PubMed] [Google Scholar]
- 25.Darling EM, Zauscher S, Guilak F. 2006. Viscoelastic properties of zonal articular chondrocytes measured by atomic force microscopy. Osteoarthritis Cartilage 14, 571–579. ( 10.1016/j.joca.2005.12.003) [DOI] [PubMed] [Google Scholar]
- 26.Pravincumar P, Bader DL, Knight MM. 2012. Viscoelastic cell mechanics and actin remodelling are dependent on the rate of applied pressure. PLoS ONE 7, e43938 ( 10.1371/journal.pone.0043938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohashi T, Hagiwara M, Bader DL, Knight MM. 2006. Intracellular mechanics and mechanotransduction associated with chondrocyte deformation during pipette aspiration. Biorheology 43, 201–214. [PubMed] [Google Scholar]
- 28.Kamm R, Lammerding J, Mofrad M. 2010. Cellular nanomechanics. New York, NY: Springer. [Google Scholar]
- 29.Dalby MJ, Gadegaard N, Curtis ASG, Oreffo ROC. 2007. Nanotopographical control of human osteoprogenitor differentiation. Curr. Stem Cell Res. Ther. 2, 129–138. ( 10.2174/157488807780599220) [DOI] [PubMed] [Google Scholar]
- 30.Bidan CM, Kommareddy KP, Rumpler M, Kollmannsberger P, Fratzl P, Dunlop JWC. 2013. Geometry as a factor for tissue growth: towards shape optimization of tissue engineering scaffolds. Adv. Healthcare Mater. 2, 186–194. ( 10.1002/adhm.201200159) [DOI] [PubMed] [Google Scholar]
- 31.Salinas CN, Anseth KS. 2008. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 29, 2370–2377. ( 10.1016/j.biomaterials.2008.01.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J, Bauer S, von der Mark K, Schmuki P. 2007. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 7, 1686–1691. ( 10.1021/nl070678d) [DOI] [PubMed] [Google Scholar]
- 33.Babin K, Antoine F, Goncalves DM, Girard D. 2013. TiO2, CeO2 and ZnO nanoparticles and modulation of the degranulation process in human neutrophils. Toxicol. Lett. 221, 57–63. ( 10.1016/j.toxlet.2013.05.010) [DOI] [PubMed] [Google Scholar]
- 34.Lo Schiavo V, Robert P, Mishal Z, Puech P-H, Gentile F, Decuzzi P, Bongrand P, Limozin L. 2013. Transient adhesion mediated by ligand-receptor interaction on surfaces of variable nanotopography. Int. J. Nanotechnol. 10, 404–418. ( 10.1504/ijnt.2013.053512) [DOI] [Google Scholar]
- 35.Thomas G, Burnham NA, Camesano TA, Wen Q. 2013. Measuring the mechanical properties of living cells using atomic force microscopy. J. Visual. Exp. 76, e50497 ( 10.3791/50497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, Birch MA, Bull SJ. 2010. Nanomechanical characterization of tissue engineered bone grown on titanium alloy in vitro. J. Mater. Sci. Mater. Med. 21, 277–282. ( 10.1007/s10856-009-3843-9) [DOI] [PubMed] [Google Scholar]
- 37.Mahaffy RE, Park S, Gerde E, Kas J, Shih CK. 2004. Quantitative analysis of the viscoelastic properties of thin regions of fibroblasts using atomic force microscopy. Biophys. J. 86, 1777–1793. ( 10.1016/s0006-3495(04)74245-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young R, Lovell P. 1991. Introduction to polymers, 2nd edn Cambridge, UK: Cambridge University Press. [Google Scholar]
- 39.Portoles JF, Cumpson PJ. 2013. A compact torsional reference device for easy, accurate and traceable AFM piconewton calibration. Nanotechnology 24, 335706 ( 10.1088/0957-4484/24/33/335706) [DOI] [PubMed] [Google Scholar]
- 40.Hansma HG, Hoh JH. 1994. Biomolecular imaging with the atomic-force microscope. Annu. Rev. Biophys. Biomol. Struct. 23, 115–139. ( 10.1146/annurev.bb.23.060194.000555) [DOI] [PubMed] [Google Scholar]
- 41.Atkins A, Tabor D. 1965. Plastic indentation in metals with cones. J. Mech. Phys. Solids 13, 149–164. ( 10.1016/0022-5096(65)90018-9) [DOI] [Google Scholar]
- 42.Chen J, Bull SJ. 2006. On the relationship between plastic zone radius and maximum depth during nanoindentation. Surface Coatings Technol. 201, 4289–4293. ( 10.1016/j.surfcoat.2006.08.099) [DOI] [Google Scholar]
- 43.Chen J, Bull SJ. 2006. A critical examination of the relationship between plastic deformation zone size and Young's modulus to hardness ratio in indentation testing. J. Mater. Res. 21, 2617–2627. ( 10.1557/jmr.2006.0323) [DOI] [Google Scholar]
- 44.Chen J, Bull SJ. 2009. On the factors affecting the critical indenter penetration for measurement of coating hardness. Vacuum 83, 911–920. ( 10.1016/j.vacuum.2008.11.007) [DOI] [Google Scholar]
- 45.Ofek G, Willard VP, Koay EJ, Hu JC, Lin P, Athanasiou KA. 2009. Mechanical characterization of differentiated human embryonic stem cells. J. Biomech Eng. Trans. ASME 131, 061011 ( 10.1115/1.3127262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shieh AC, Athanasiou KA. 2006. Biomechanics of single zonal chondrocytes. J. Biomech. 39, 1595–1602. ( 10.1016/j.jbiomech.2005.05.002) [DOI] [PubMed] [Google Scholar]
- 47.Turunen SM, Han SK, Herzog W, Korhonen RK. 2013. Cell deformation behavior in mechanically loaded rabbit articular cartilage 4 weeks after anterior cruciate ligament transection. Osteoarthritis Cartilage 21, 505–513. ( 10.1016/j.joca.2012.12.001) [DOI] [PubMed] [Google Scholar]
- 48.Ingber DE. 1993. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J. Clin. Ultrasound 21, 613–627. [DOI] [PubMed] [Google Scholar]
- 49.Ingber DE. 1997. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 59, 575–599. ( 10.1146/annurev.physiol.59.1.575) [DOI] [PubMed] [Google Scholar]
- 50.Forgacs G. 1995. On the possible role of cytoskeletal filamentous networks in intracellular signaling: an approach based on percolation. J. Cell Sci. 108, 2131–2143. [DOI] [PubMed] [Google Scholar]
- 51.Ofek G, Natoli RM, Athanasiou KA. 2009. In situ mechanical properties of the chondrocyte cytoplasm and nucleus. J. Biomech. 42, 873–877. ( 10.1016/j.jbiomech.2009.01.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freeman PM, Natarajan RN, Kimura JH, Andriacchi TP. 1994. Chondrocyte cells respond mechanically to compressive loads. J. Orthop. Res. 12, 311–320. ( 10.1002/jor.1100120303) [DOI] [PubMed] [Google Scholar]
- 53.Rao CS, Reddy CE. 2008. An FEM approach into nanoindentation on linear elastic and viscoelastic characterization of soft living cells. Int. J. Nanotechnol. Appl. 2, 55–68. [Google Scholar]
- 54.Caille N, Thoumine O, Tardy Y, Meister JJ. 2002. Contribution of the nucleus to the mechanical properties of endothelial cells. J. Biomech. 35, 177–187. ( 10.1016/s0021-9290(01)00201-9) [DOI] [PubMed] [Google Scholar]
- 55.Nair K, Yan K, Sun W. 2007. A multi level numerical model for quantifying cell deformation in encapsulated alginate structures. J. Mech. Mater. Struct. 2, 1121–1139. ( 10.2140/jomms.2007.2.1121) [DOI] [Google Scholar]
- 56.Kang I, Panneerselvam D, Panoskaltsis VP, Eppell SJ, Marchant RE, Doerschuk CM. 2008. Changes in the hyperelastic properties of endothelial cells induced by tumor necrosis factor-alpha. Biophys. J. 94, 3273–3285. ( 10.1529/biophysj.106.099333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guilak F, Mow VC. 2000. The mechanical environment of the chondrocyte: a biphasic finite element model of cell-matrix interactions in articular cartilage. J. Biomech. 33, 1663–1673. ( 10.1016/s0021-9290(00)00105-6) [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Lu G. 2012. Finite element modelling of nanoindentation based methods for mechanical properties of cells. J. Biomech. 45, 2810–2816. ( 10.1016/j.jbiomech.2012.08.037) [DOI] [PubMed] [Google Scholar]
- 59.Calladine CR. 1978. Fuller, Buckminster tensegrity structures and Maxwell, Clerk rules for construction of stiff frames. Int. J. Solids Struct. 14, 161–172. ( 10.1016/0020-7683(78)90052-5) [DOI] [Google Scholar]
- 60.Stamenovic D, Fredberg JJ, Wang N, Butler JP, Ingber DE. 1996. A microstructural approach to cytoskeletal mechanics based on tensegrity. J. Theor. Biol. 181, 125–136. ( 10.1006/jtbi.1996.0120) [DOI] [PubMed] [Google Scholar]
- 61.Ingber DE, Prusty D, Sun ZQ, Betensky H, Wang N. 1995. Cell shape, cytoskeletal mechanics, and cell cycle control in angiogenesis. J. Biomech. 28, 1471–1484. ( 10.1016/0021-9290(95)00095-x) [DOI] [PubMed] [Google Scholar]
- 62.Ingber DE, Bojanowski K, Chen C, Huang S, Maniotis A. 1996. Cellular tensegrity: an architectural basis for control of cell shape and morphogenesis. Mol. Biol. Cell 7, 1975–1975. [Google Scholar]
- 63.Ingber DE, et al. 1994. Cellular tensegrity: Exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int. Rev. Cytol. 150, 173–224. [DOI] [PubMed] [Google Scholar]
- 64.McGarry JG, Prendergast PJ. 2004. A three-dimensional finite element model of an adherent eukaryotic cell. Eur. Cells Mater. 7, 27–33. discussion 33–24. [DOI] [PubMed] [Google Scholar]
- 65.De Santis G, Lennon AB, Boschetti F, Verhegghe B, Verdonck P, Prendergast PJ. 2011. How can cells sense the elasticity of a substrate? an analysis using a cell tensegrity model. Eur. Cells Mater. 22, 202–213. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen BV, Wang QG, Kuiper NJ, El Haj AJ, Thomas CR, Zhang Z. 2001. Biomechanical properties of single chondrocytes and chondrons determined by micromanipulation and finite-element modelling. J. R. Soc. Interface 7, 1723–1733. ( 10.1098/rsif.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rivlin RS, Saunders DW. 1951. Large elastic deformations of isotropic materials VIII. Experiments on the deformation of rubber. Phil. Trans. R. Soc. Lond. A 328, 565–584. ( 10.1098/rsta.1951.0004) [DOI] [Google Scholar]
- 68.Ogden RW. 1998. Nonlinear elastic deformations. New York, NY: Dover. [Google Scholar]
- 69.Ogden RW. 1972. Large deformation isotropic elasticity – on the correlation of theory and experiment for incompressible rubberlike solids. Proc. R. Soc. Lond. A 326, 565–584. ( 10.1098/rspa.1972.0026) [DOI] [Google Scholar]
- 70.Biot M. 1941. General theory of three-dimensional consolidation. J. Appl. Phys. 12, 155–164. ( 10.1063/1.1712886) [DOI] [Google Scholar]
- 71.Strange DGT, Fletcher TL, Tonsomboon K, Brawn H, Zhao X, Oyen ML. 2013. Separating poroviscoelastic deformation mechanisms in hydrogels. Appl. Phys. Lett. 102, 031913 ( 10.1063/1.4789368) [DOI] [Google Scholar]
- 72.Oyen ML. 2013. Nanoindentation of biological and biomimetic materials. Exp. Tech. 37, 73–87. ( 10.1111/j.1747-1567.2011.00716.x) [DOI] [Google Scholar]
- 73.Wu JZ, Herzog W, Epstein M. 1998. Evaluation of the finite element software ABAQUS for biomechanical modelling of biphasic tissues. J. Biomech. 31, 165–169. ( 10.1016/S0021-9290(97)00117-6) [DOI] [PubMed] [Google Scholar]
- 74.Hu Y, Zhao X, Vlassak JJ, Suo Z. 2010. Using indentation to characterize the poroelasticity of gels. Appl. Phys. Lett. 96 121904 ( 10.1063/1.3370354) [DOI] [Google Scholar]
- 75.Mow VC, Kuei SC, Lai WM, Armstrong CG. 1980. Biphasic creep and stress-relaxation of articular-cartilage in compression: theory and experiments. J. Biomech. Eng. Trans. ASME 102, 73–84. ( 10.1115/1.3138202) [DOI] [PubMed] [Google Scholar]
- 76.Bowen RM. 1980. Incompressible porous-media models by use of the theory of mixtures. Int. Eng. Sci. 18, 1129–1148. ( 10.1016/0020-7225(80)90114-7) [DOI] [Google Scholar]
- 77.Setton LA, Zhu WB, Mow VC. 1993. The biphasic poroviscoelastic behavior of articular-cartilage: role of the surface zone in governing the compressive behavior. J. Biomech. 26, 581–592. ( 10.1016/0021-9290(93)90019-b) [DOI] [PubMed] [Google Scholar]
- 78.Gu WY, Lai WM, Mow VC. 1998. A mixture theory for charged-hydrated soft tissues containing multi-electrolytes: passive transport and swelling behaviors. J. Biomech. Eng. Trans. ASME 120, 169–180. ( 10.1115/1.2798299) [DOI] [PubMed] [Google Scholar]
- 79.Manual ASUs. 2012. Hibbitt, Karlsson and Sorensen, Inc. [Google Scholar]
- 80.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. 2001. Scaling the microrheology of living cells. Phys. Rev. Lett. 87, 148102 ( 10.1103/PhysRevLett.87.148102) [DOI] [PubMed] [Google Scholar]
- 81.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Taback NA, Millet EJ, Fredberg JJ. 2003. Time scale and other invariants of integrative mechanical behavior in living cells. Phys. Rev. E 68, 041914 ( 10.1103/PhysRevE.68.041914) [DOI] [PubMed] [Google Scholar]
- 82.Doerner M, Nix W. 1986. A method for interpreting the data from depth-sensing indentation instruments. J. Mater Res. 1, 601–609. ( 10.1557/JMR.1986.0601) [DOI] [Google Scholar]
- 83.Johnson KL. 1985. Contact mechanics. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 84.Sneddon I. 1965. The relationship between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 3, 47–57. ( 10.1016/0020-7225(65)90019-4) [DOI] [Google Scholar]
- 85.Charras GT, Williams BA, Sims SM, Horton MA. 2004. Estimating the sensitivity of mechanosensitive ion channels to membrane strain and tension. Biophys. J. 87, 2870–2884. ( 10.1529/biophysj.104.040436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. 2005. Non-equilibration of hydrostatic pressure in blebbing cells. Nature 435, 365–369. ( 10.1038/nature03550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng L, Xia X, Yu W, Scriven LE, Gerberich WW. 2000. Flat-punch indentation of viscoelastic material. J. Polym. Sci. B Polym. Phys. 38, 10–22. () [DOI] [Google Scholar]
- 88.Cheng L, Xia X, Scriven LE, Gerberich WW. 2005. Spherical-tip indentation of viscoelastic material. Mech. Mater. 37, 213–226. ( 10.1016/j.mechmat.2004.03.002) [DOI] [Google Scholar]
- 89.Cheng YT, Ni WY, Cheng CM. 2005. Determining the instantaneous modulus of viscoelastic solids using instrumented indentation measurements. J. Mater. Res. 20, 3061–3071. ( 10.1557/jmr.2005.0389) [DOI] [Google Scholar]
- 90.Cheng YT, Cheng CM. 2006. Relationship between contact stiffness, contact depth, and mechanical properties for indentation in linear viscoelastic solids using axisymmetric indenters. Struct. Control Health Monit. 13, 561–569. ( 10.1002/stc.127) [DOI] [Google Scholar]
- 91.Oyen ML. 2006. Analytical techniques for indentation of viscoelastic materials. Phil. Mag. 86, 5625–5641. ( 10.1080/14786430600740666) [DOI] [Google Scholar]
- 92.Ward KA, Li WI, Zimmer S, Davis T. 1991. Viscoelastic properties of transformed cells: role in tumor cell progression and metastasis formation. Biorheology 28, 301–313. [DOI] [PubMed] [Google Scholar]
- 93.Rosenbluth MJ, Crow A, Shaevitz JW, Fletcher DA. 2008. Slow stress propagation in adherent cells. Biophys. J. 95, 6052–6059. ( 10.1529/biophysj.108.139139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baaijens FPT, Trickey WR, Laursen TA, Guilak F. 2005. Large deformation finite element analysis of micropipette aspiration to determine the mechanical properties of the chondrocyte. Ann. Biomed. Eng. 33, 494–501. ( 10.1007/s10439-005-2506-3) [DOI] [PubMed] [Google Scholar]
- 95.Moeendarbary E, Valon L, Fritzsche M, Harris AR, Moulding DA, Thrasher AJ, Stride E, Mahadevan L, Charras GT. 2013. The cytoplasm of living cells behaves as a poroelastic material. Nat. Mater. 12, 253–261. ( 10.1038/nmat3517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalyanam S, Yapp RD, Insana MF. 2009. Poro-viscoelastic behavior of gelatin hydrogels under compression-implications for bioelasticity imaging. J. Biomech. Eng. Trans. ASME 131, 081005 ( 10.1115/1.3127250) [DOI] [PubMed] [Google Scholar]
- 97.Cui YH, Wang X, Zhang YX, He FJ. 2010. Poro-viscoelastic properties of anisotropic cylindrical composite materials. Phil. Mag. 90, 1197–1212. ( 10.1080/14786430903317253) [DOI] [Google Scholar]
- 98.Liu K, Ovaert TC. 2011. Poro-viscoelastic constitutive modeling of unconfined creep of hydrogels using finite element analysis with integrated optimization method. J. Mech. Behav. Biomed. Mater. 4, 440–450. ( 10.1016/j.jmbbm.2010.12.005) [DOI] [PubMed] [Google Scholar]
- 99.Guilak F, Haider MA, Setton LA, Laursen TA, Baaijens FPT. 2006. Multiphasic models of cell mechanics. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 100.Alcaraz J, Buscemi L, Grabulosa M, Trepat X, Fabry B, Farre R, Navajas D. 2003. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys. J. 84, 2071–2079. ( 10.1016/S0006-3495(03)75014-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rotsch C, Jacobson K, Radmacher M. 1999. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated by using atomic force microscopy. Proc. Natl Acad. Sci. USA 96, 921–926. ( 10.1073/pnas.96.3.921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Charras GT, Horton MA. 2002. Determination of cellular strains by combined atomic force microscopy and finite element modeling. Biophys. J. 83, 858–879. ( 10.1016/S0006-3495(02)75214-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Charras GT, Horton MA. 2002. Single cell mechanotransduction and its modulation analyzed by atomic force microscope indentation. Biophys. J. 82, 2970–2981. ( 10.1016/S0006-3495(02)75638-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoh JH, Schoenenberger CA. 1994. Surface-morphology and mechanical-properties of mdck monolayers by atomic-force microscopy. J. Cell Sci. 107, 1105–1114. [DOI] [PubMed] [Google Scholar]
- 105.Acerbi I, Luque T, Gimenez A, Puig M, Reguart N, Farre R, Navajas D, Alcaraz J. 2012. Integrin-specific mechanoresponses to compression and extension probed by cylindrical flat-ended AFM tips in lung cells. PLoS ONE 7, e32261 ( 10.1371/journal.pone.0032261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koay EJ, Shieh AC, Athanasiou KA. 2003. Creep indentation of single cells. J. Biomech. Eng. Trans. ASME 125, 334–341. ( 10.1115/1.1572517) [DOI] [PubMed] [Google Scholar]
- 107.Darling EM, Topel M, Zauscher S, Vail TP, Guilak F. 2008. Viscoelastic properties of human mesenchymally-derived stem cells and primary osteoblasts, chondrocytes, and adipocytes. J. Biomech. 41, 454–464. ( 10.1016/j.jbiomech.2007.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Darling EM, Zauscher S, Block JA, Guilak F. 2007. A thin-layer model for viscoelastic, stress-relaxation testing of cells using atomic force microscopy: do cell properties reflect metastatic potential? Biophys. J. 92, 1784–1791. ( 10.1529/biophysj.106.083097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chan EP, Hu Y, Johnson PM, Suo Z, Stafford CM. 2012. Spherical indentation testing of poroelastic relaxations in thin hydrogel layers. Soft Matter 8, 1492–1498. ( 10.1039/c1sm06514a) [DOI] [Google Scholar]
- 110.Tu Y, Gazis D. 1964. The contact problem of a plate pressed between two spheres. J. Appl. Mech. 31, 659–666. ( 10.1115/1.3629728) [DOI] [Google Scholar]
- 111.Chen WT. 1971. Computation of stresses and displacements in a layered elastic medium. Int. J. Eng. Sci. 9, 775–800. ( 10.1016/0020-7225(71)90072-3) [DOI] [Google Scholar]
- 112.Chen W, Engel P. 1972. Impact and contact stress analysis in multilayer media. Int. J. Solids Struct. 8, 1257–1281. ( 10.1016/0020-7683(72)90079-0) [DOI] [Google Scholar]
- 113.Rosenbluth MJ, Lam WA, Fletcher DA. 2006. Force microscopy of nonadherent cells: A comparison of leukemia cell deformability. Biophys. J. 90, 2994–3003. ( 10.1529/biophysj.105.067496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ng L, Hung H-H, Sprunt A, Chubinskaya S, Ortiz C, Grodzinsky A. 2007. Nanomechanical properties of individual chondrocytes and their developing growth factor-stimulated pericellular matrix. J. Biomech. 40, 1011–1023. ( 10.1016/j.jbiomech.2006.04.004) [DOI] [PubMed] [Google Scholar]
- 115.Kailas L, Ratcliffe EC, Hayhurst EJ, Walker MG, Foster SJ, Hobbs JK. 2009. Immobilizing live bacteria for AFM imaging of cellular processes. Ultramicroscopy 109, 775–780. ( 10.1016/j.ultramic.2009.01.012) [DOI] [PubMed] [Google Scholar]
- 116.Chen J. 2013. Understanding the nanoindentation mechanisms of a microsphere for biomedical applications. J. Phys. D, Appl. Phys. 46, 495303 ( 10.1088/0022-3727/46/49/495303) [DOI] [Google Scholar]
- 117.Jaasma MJ, Jackson WM, Keaveny TM. 2006. Measurement and characterization of whole-cell mechanical behavior. Ann. Biomed. Eng. 34, 748–758. ( 10.1007/s10439-006-9081-0) [DOI] [PubMed] [Google Scholar]
- 118.Johnson KL, Kendall K, Roberts AD. 1971. Surface energy and contact of elastic solids. Proc. R. Soc. Lond. A 324, 301–313. ( 10.1098/rspa.1971.0141) [DOI] [Google Scholar]
- 119.Derjaguin BV, Muller VM, Toporov YP. 1975. Effect of contact deformations on adhesion of particles. J. Colloid Interface Sci. 53, 314–326. ( 10.1016/0021-9797(75)90018-1) [DOI] [Google Scholar]
- 120.Maugis D. 1996. On the contact and adhesion of rough surfaces. J. Adhesion Sci. Technol. 10, 161–175. () [DOI] [Google Scholar]
- 121.Sen S, Subramanian S, Discher DE. 2005. Indentation and adhesive probing of a cell membrane with AFM: theoretical model and experiments. Biophys. J. 89, 3203–3213. ( 10.1529/biophysj.105.063826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lin DC, Horkay F. 2008. Nanomechanics of polymer gels and biological tissues: a critical review of analytical approaches in the Hertzian regime and beyond. Soft Matter 4, 669–682. ( 10.1039/b714637j) [DOI] [PubMed] [Google Scholar]
- 123.Martins RP, Finan JD, Guilak F, Lee DA. 2012. Mechanical regulation of nuclear structure and function. Annu. Rev. Biomed. Eng. 14, pp. 431–455. ( 10.1146/annurev-bioeng-071910-124638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vichare S, Inamdar MM, Sen S. 2012. Influence of cell spreading and contractility on stiffness measurements using AFM. Soft Matter 8, 10 464–10 471. ( 10.1039/c2sm26348c) [DOI] [PubMed] [Google Scholar]
- 125.Lai WM, Hou JS, Mow VC. 1991. A triphasic theory for the swelling and deformation behaviors of articular-cartilage. J. Biomech. Eng. Trans. ASME 113, 245–258. ( 10.1115/1.2894880) [DOI] [PubMed] [Google Scholar]
- 126.Harris AK, Wild P, Stopak D. 1980. Silicone-rubber substrata: new wrinkle in the study of cell locomotion. Science 208, 177–179. ( 10.1126/science.6987736) [DOI] [PubMed] [Google Scholar]


