Abstract
Cuticular proteins (CPs) are crucial components of the insect cuticle. Although numerous genes encoding cuticular proteins have been identified in known insect genomes to date, their functions in maintaining insect body shape and adaptability remain largely unknown. In the current study, positional cloning led to the identification of a gene encoding an RR1-type cuticular protein, BmorCPR2, highly expressed in larval chitin-rich tissues and at the mulberry leaf-eating stages, which is responsible for the silkworm stony mutant. In the Dazao-stony strain, the BmorCPR2 allele is a deletion mutation with significantly lower expression, compared to the wild-type Dazao strain. Dysfunctional BmorCPR2 in the stony mutant lost chitin binding ability, leading to reduced chitin content in larval cuticle, limitation of cuticle extension, abatement of cuticle tensile properties, and aberrant ratio between internodes and intersegmental folds. These variations induce a significant decrease in cuticle capacity to hold the growing internal organs in the larval development process, resulting in whole-body stiffness, tightness, and hardness, bulging intersegmental folds, and serious defects in larval adaptability. To our knowledge, this is the first study to report the corresponding phenotype of stony in insects caused by mutation of RR1-type cuticular protein. Our findings collectively shed light on the specific role of cuticular proteins in maintaining normal larval body shape and will aid in the development of pest control strategies for the management of Lepidoptera.
Keywords: Bombyx mori, stony mutant, RR1-type cuticular protein, cuticle physical properties, larval morphological characteristics and adaptability
THE cuticle covering the entire body surface of insects not only participates in defense against pathogens and adverse environmental factors, but is also indispensable for constructing and maintaining external morphological characteristics and locomotion during the entire developmental process (Wigglesworth 1957; Delon and Payre 2004; Moussian et al. 2005). Therefore, the cuticle greatly enhances survival ability and adaptability of insects, ensuring its continued existence as one of the most successful life forms in the animal kingdom.
The cuticle is a complex composite material mainly comprising chitin fibers and proteins (Andersen et al. 1995; Moussian 2010). Chitin is the polymer of β-1,4-linked N-acetyl-d-glucosamine (Gilbert 2011, Chap. 7). In procuticles, chitin fibers are arranged in laminae in an antiparallel manner and superimpose each other, forming sheets of fibrils that are stacked in a helicoidal fashion, maintaining cuticle structure, elasticity, and stability (Bouligand 1965; Neville and Luke 1969; Moussian 2010). In terrestrial insects, the chitin content is positively correlated with body size, suggesting a close relationship with cuticle extension and expansion (Merzendorfer and Zimoch 2003; Lease and Wolf 2010).
Cuticular proteins (CPs), the principal structural constituents of cuticle, are encoded by more than 100 genes in known insect genomes (Andersen et al. 1995; Willis et al. 2005; Weinstock et al. 2006; Karouzou et al. 2007; Cornman et al. 2008; Futahashi et al. 2008; Richards et al. 2008; Cornman and Willis 2009; Gilbert 2011, Chap. 5) and form a proteinaceous matrix in which chitin fibers are embedded (Neville 1975; Papandreou et al. 2010). CP–chitin interactions are necessary for the normal structure and physical properties of cuticle (Andersen et al. 1995; Rebers and Willis 2001; Karouzou et al. 2007; Cornman et al. 2008; Moussian 2010). Over half of the known cuticular proteins contain chitin-binding domains, including the Rebers & Riddiford (R&R) motif (two major groups, RR-1 and RR-2, and a minor form, RR-3), Tweedle motif, and chtDB2 domain (Rebers and Riddiford 1988; Magkrioti et al. 2004; Cornman et al. 2008; Jasrapuria et al. 2010; Tang et al. 2010; Willis 2010; Andersen 2011; Gilbert 2011, Chap. 5). Chitin-binding cuticular proteins are widely distributed at different development stages and play roles in the physical properties of the cuticle ( Rebers and Riddiford 1988; Guan et al. 2006; Soares et al. 2007; Okamoto et al. 2008; Togawa et al. 2008; Charles 2010; Gilbert 2011, Chap. 5; Arakane et al. 2012; Jasrapuria et al. 2012). These proteins often have crucial biological functions, such as body-shape determination, cuticle integrity and mechanical properties maintenance, movement ability, and adaptability of the insect (Guan et al. 2006; Arakane et al. 2012; Jasrapuria et al. 2012).
The soft, flexible cuticle of Lepidoptera larvae not only bears pressure from the internal contents on the integument to stabilize the long cylindrical body, but is also conducive to movement and expanding feeding and survival areas (Carter and Locke 1993; Brackenbury 1997; Lin et al. 2009). However, the mechanisms by which cuticular proteins participate in body-shape stability and adaptability of Lepidoptera is presently unclear. Here, we focus on the silkworm as a Lepidoptera model to investigate the above issues. Positional cloning led to the identification of a gene encoding a RR1-type cuticular protein, BmorCPR2, responsible for the silkworm stony mutant. In the mutant, dysfunctional BmorCPR2 induces significantly decreased cuticle chitin content, abnormal distribution of internode and intersegmental fold, and marked reduction in tensile property of the cuticle. Accordingly, the conflict between growing body-cavity contents and opposing lower cuticle capacity gradually increases, leading to body hardness and tightness. The variant intersegmental fold also bulges out due to enhanced internal pressure, resulting in a malformed body shape. Additionally, the hard body and bulges seriously affect the adaptability of the mutant, such as crooking and shock-buffering abilities. Therefore, characterization of this gene and its underlying effects may contribute to our understanding of the role of cuticular proteins in maintaining of external morphological characteristics of Lepidoptera larvae and control of Lepidoptera pests.
Materials and Methods
Silkworm strains
The wild-type strains—Dazao, 05-111, 06-072, 20-230, Xiafang, 01-070, 16-060, 05-050, 16-100, 16-105, C108, 06-920, 04-702—and stony mutant strains—Dazao-stony (near isogenic line of Dazao) and 12-220 (donor parent of stony locus)—used in this research were obtained from the silkworm gene bank in Southwest University. The near isogenic lines have been backcrossed with Dazao over 24 generations; other wild-type and stony mutant strains inbred at least 20 generations. Silkworms were reared on fresh mulberry leaves under a 12 hr/12 hr light/dark photoperiod at 24°.
Phenotype analysis, physiology test, and behavior assays
The phenotypes of Dazao and Dazao-stony individuals and excrement appearance were recorded using a digital camera (Canon EOS 5D Mark III). The cuticle was dissected, and adhesive tissues were scraped off and cleaned with ddH2O. Internode, anterior, and posterior parts of internode, intersegmental fold of treated cuticle were divided and measured using a stereo microscope (Nikon SMZ1500), digital camera, Adobe Photoshop CS3, and image J software. Based on the principle of buoyancy, we surveyed the exact larval body volume in a volumetric cylinder. Additionally, midgut contents from these individuals were dislodged, lightly cleaned with ddH2O, and torrified at 60°. The ratio of midgut contents mass to individual volumes analyzed. For the physiology tests (dropping experiment; the flexibility and nesting ability of segments) and behavior assays, detailed operation are listed in Supporting Information, File S1.
Positional cloning
Mapping of stony locus and bioinformatic analysis of candidate regions were performed, as described previously (Dai et al. 2010). In Bm_nscaf2827 (chromosome 8), we identified polymorphic PCR markers between the parents, Dazao-stony and C108, and assessed these in BC1F individuals. In BC1M progeny, individuals exhibiting stony phenotype (homozygosis on stony locus) were genotyped. The primers used for mapping are listed in Table S1.
DNA sequence cloning
Total RNAs were extracted from the integuments of wild type (Dazao and other WT strains) and stony mutant (Dazao-stony and stony parent strain, 12-220) on day 4 of the fifth-instar larvae using TRIzol (Invitrogen), according to the manufacturer’s instructions. The 5′-UTR and 3′-UTR of BmorCPR2 and BmorCPR3 were amplified using the GeneRacer Kit (Invitrogen). We additionally amplified the genome sequence ∼2.78 and ∼0.83 kb upstream of BmorCPR2. PCR products were cloned into the pMD19-T vector for sequencing. The primers used for cloning are listed in Table S1.
Expression profile of BmorCPR2
Semiquantitative RT–PCR was performed as described previously (Dai et al. 2010). The development stages include hatching to moth stage. Tissues of Dazao examined on day 4 of the fifth-instar larvae included head, integument, trachea, malpighian tubes, midgut, gonads, fat body, anterior/middle/posterior silk gland, nerves, muscle, and hemocyte. Primers designed for RT–PCR are shown in Table S1. The BmActin3 gene was used as an internal control.
20-Hydroxyecdysone (20E) treatment
Day 4 of the fifth-instar larvae (wild type) were treated with 20E (dissolved in 10% ethanol, injected with 3 μg per individual) or 10% ethanol (control), and BmorCPR2 expression detected at 3 and 24 hr after injection.
Quantitative RT–PCR
Quantitative RT–PCR was performed as described previously (Dai et al. 2010), using the StepOneTM real-time PCR xystem (ABI). Primers used for qRT–PCR are listed in Table S1.
Quantification of chitin content
The larval segment cuticle of Dazao, Dazao-stony, internode, and the intersegmental fold of Dazao and Dazao-stony larvae, anterior and the posterior parts of the internode of Dazao, and posterior parts plus intersegmental fold of Dazao were dissected. After eliminating adherent tissue completely and cleaning with ddH2O, materials were dried under 60° and weighed with an analytical balance (METTLER-MS105DU). Extraction and determination of chitin were performed according to the protocol of Kunyan Zhu (Zhang and Zhu 2006) with slight modifications. All determinations were performed on three or four biological replicates for each sample.
SDS–PAGE and chitin binding
The cuticles of day 5 of the fifth-instar Dazao and Dazao-stony larvae were dissected, and adherent tissue were removed and cleaned with nuclease-free and protease-free water. Total proteins were extracted according to the method of Tang et al. (2010) and detected using 15% SDS–PAGE. For the chitin binding experiment, detailed operations are listed in File S1.
Liquid chromatography tandem mass spectrometry analysis
Selected gel bands were excised and subjected to liquid chromatography tandem mass spectrometry (LC–MS/MS) analysis (LTQ VELOS, Thermo Finnigan, San Jose, CA). Detailed operation are listed in File S1.
RNA interference
dsBmorCPR2 and dsRed were synthetized using the RiboMAX large-scale RNA production system-T7 (Promega) based on the PCR templates according to the manufacturer’s instructions. The injection time selected according to the BmorCPR2 expression pattern was the eating-less period of fourth instar (day 3 of the fourth instar to the period of molting). dsRNA was diluted to 10 μg/μl, and a dose of 110 μg was administered to each individual. Larvae at 16 hr of fourth molting and beginning of fifth instar were used to detect BmorCPR2 and BmLcp22 expression. Individuals from the dsBmorCPR2 group at the beginning of the fifth instar were employed for counting interference efficiency. Meanwhile, chitin contents of the interference and control groups were determined. Primers for PCR are listed in Table S1.
Tensile property of larval cuticles
The cuticles of day 5 of the fifth instar Dazao and Dazao-stony larvae were dissected and adherent tissue was removed, followed by cleaning with ddH2O. The cuticle was spread smooth and trimmed into a rectangle. Tensile properties were determined in the longitudinal direction (head to tail), whereby the length of the rectangular pattern was from semilunar to star marking, and the width was taken as the distance between both sides of the spiracle on the dorsal side (spread smooth). Tensile properties in the transversal direction were determined as follows: the length of the rectangular pattern represents the distance between both sides of the spiracle on the dorsal side (spread smooth), while the width is from the start of the third abdominal segment to the end of the fourth abdominal segment. The sample was incubated for 30 min at room temperature and 80% relative humidity and was rapidly fixed between two grips of the DMA Q800 Dynamic Mechanical analyzer. Samples were stretched according to the strain–stress mode (at ambient temperature, stress varied linearly at a constant rate) to test their mechanical properties. All determinations were performed on three biological replicates for each sample.
Results and Discussion
Stony mutant larva displays altered morphologic characteristics with deficiency in adaptability
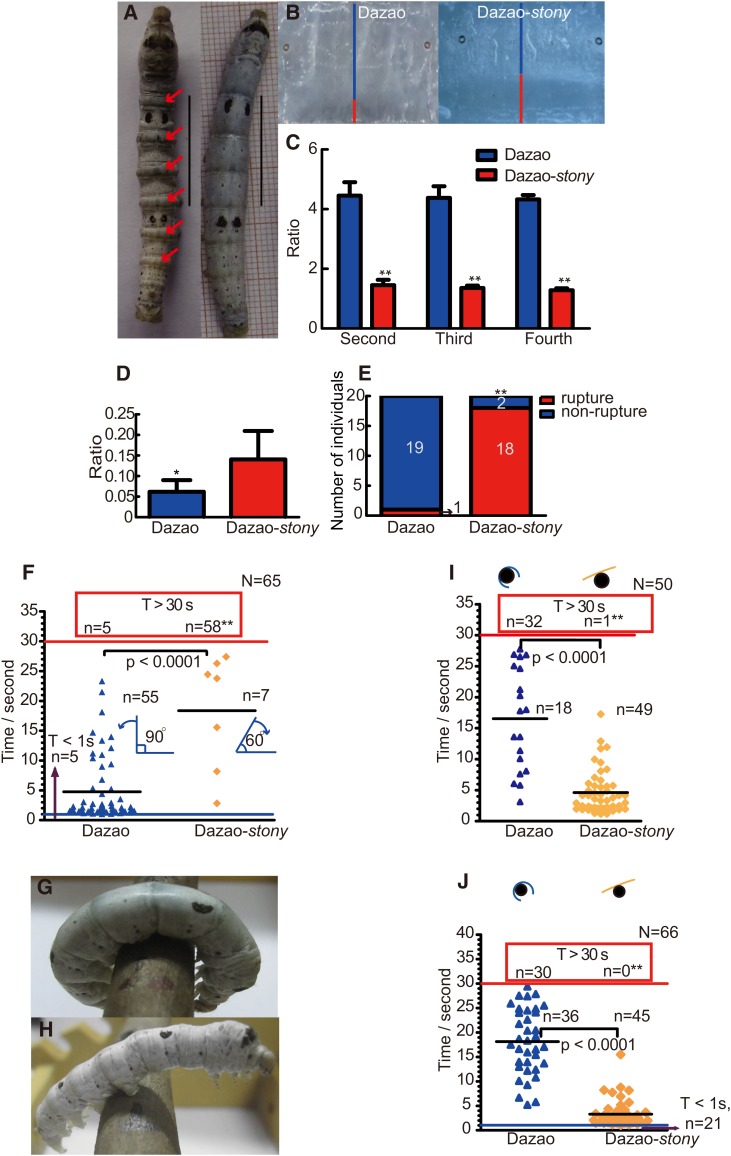
The peculiar body shape of stony is mostly evident in the post margins of every larval segment as distinct bulges, and the mutant is more slender with a smaller body size (Figure 1A). Dissection of stony mutant larvae revealed a higher ratio of midgut content mass to body volume, compared to wild type (Figure 1D). However, the ratio of internodes containing internal contents in every segment was significantly lower than that of wild type (Figure 1, B and C). Furthermore, the tails of the fifth-instar larvae usually cocked up when satiated with mulberry leaf (Figure S1A). The rectocele appeared easily upon defecation, and smaller amounts of excrement were produced (Figure S1, B and C). These phenotypic and physiological characteristics suggest stronger pressure in the body of stony mutant corresponding to hardness and tightness to touch. In the dropping experiment, 90% stony individuals (with smaller gravitational potential energy, GPE) breached the cuticle, accompanied by outflow of internal tissues and hemolymph, while only one wild-type individual displayed similar symptoms (Figure 1E, Figure S1, D and E, File S1, and File S2). The data indicate that the stiff, tight body of stony significantly lowers its ability to buffer shock. Air injection showed nestification (the somite shortening, namely, the anterior part of the latter segment shrinks to the posterior part of the former one) between every two conjoint segments of wild type, leading to shorter larvae (Figure S1, F and G). However, nesting was not observed for the stony mutant owing to the bulges produced by intersegmental fold of its abdominal segment, signifying deficiency in flexibility (Figure S1, H and I).
Figure 1.
Characterization of Dazao-stony mutant. (A) Phenotype of the dorsal side of Dazao (right) and Dazao-stony (left). Red arrows indicate bulges. Scale bar, 2 cm. (B) Dorsal cuticle anatomy characteristics of Dazao and Dazao-stony. The blue and red lines represent the length spanned by internode and intersegmental fold, respectively. (C) Ratios between the length of internode and intersegmental fold in the second, third, and fourth abdomen segments of Dazao and Dazao-stony, respectively (n = 5). (D) Ratio of the midgut content mass and larval volume between Dazao and Dazao-stony (n = 6). Data represent mean values ±SD. Student’s t-test; *, P < 0.05; **, P < 0.01 (C and D). (E) Statistics of breached Dazao and Dazao-stony larvae in dropping experiment. (χ2-test; **, P < 0.01.). (F) Analysis of the larval abdominal crooking test. Both red and blue lines represent threshold time. The numbers in the red box indicate individuals that cannot crook to abdomen in 30 sec. (χ2-test; **, P < 0.01). The counterclockwise and clockwise arrowheads represent the crooking angles in Dazao and Dazao-stony, respectively. Both black lines represent the average time of abdomen bending between Dazao and Dazao-stony within the threshold time. (Mann–Whitney U-test, P < 0.0001). (G and H) Phenotypes of grasping mallet of Dazao and Dazao-stony, respectively. (I and J) Analysis of grasping tests on the large and small diameter mallets of Dazao and Dazao-stony, respectively. Both the red and blue lines signify threshold time. The numbers in the red box represent individuals that grasp for >30 sec. (χ2-test; **, P < 0.01). Both black lines represent the average time of grasping between Dazao and Dazao-stony within the threshold time (Mann–Whitney U-test, P < 0.0001). The black solid circle represents the cross-section of the mallet. Blue and yellow curves represent the ventral bending of Dazao and Dazao-stony, respectively.
Based on these results and known kinematic characteristics of caterpillars (Brackenbury 1997; Lin et al. 2009), we designed a series of behavioral experiments to test the athletic ability of stony mutant. The abdominal crooking test revealed that >90% wild-type individuals easily crook to abdomen within 30 sec, with a crooking angle of >90°; however, ∼89% stony individuals were unable to perform crooking within 30 sec, even over prolonged times, and the remaining stony individuals with crooking ability performed the activity over a longer time and could not crook to >60°, and the stony mutants displayed a severe reverse angle bending in the crooking test (Figure 1F, Figure S1, H and I, and File S3). Since the crooking ability of the abdomen is weak, irrespective of mallet diameter, it is difficult for the stony mutant to grasp, and almost all individuals dropped from the mallet within 30 sec due to loss of balance (Figure 1, G–J, File S4, and File S5). Moreover, within the threshold time (30 sec), the average grasping time of dropped stony individuals was significantly shorter than that of wild type (Figure 1, I and J, File S4, and File S5). Subsequent inversion and food inducement tests showed degeneration of turning over and crawling abilities in the mutant (Figure S1, J, and K, File S6, File S7, and File S8). In view of the abnormal body shape and adaptability deficiency, we speculate that the gene responsible for the mutant phenotype is associated with cuticle abnormality.
The stony locus encodes BmorCPR2
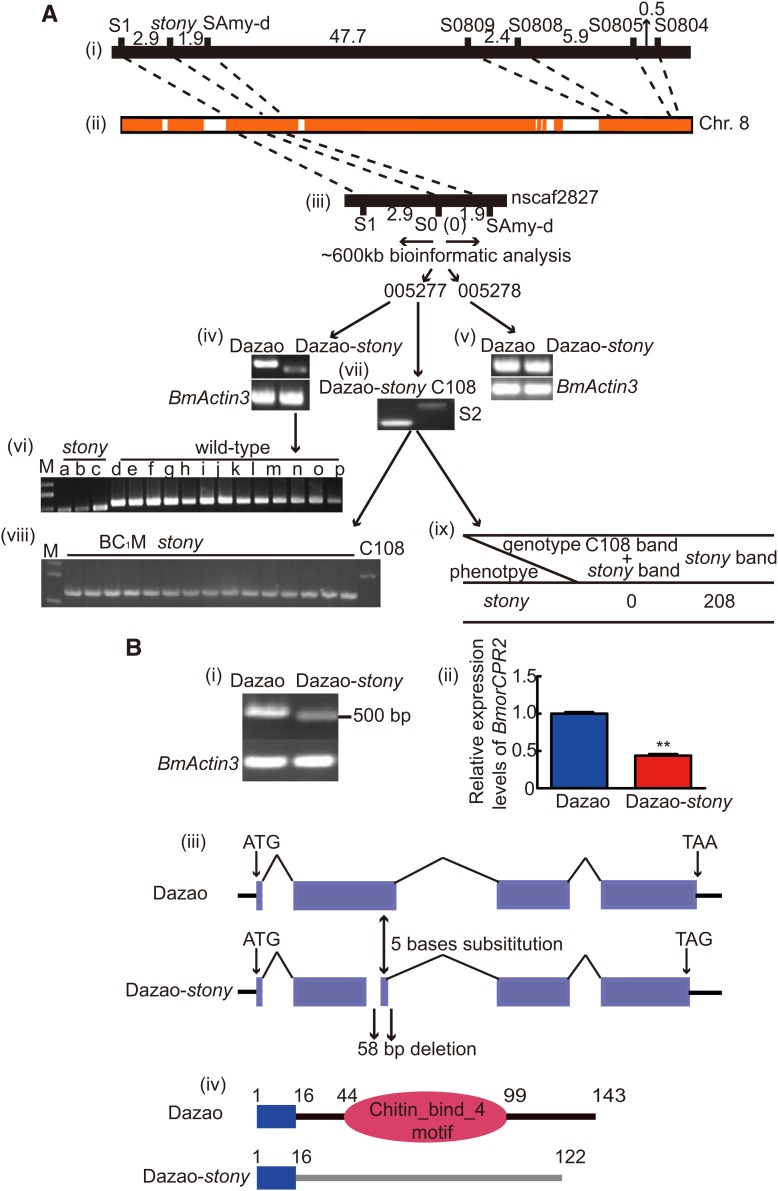
We mapped the stony locus within a ∼1.5-Mb region on Bm_nscaf2827 using 208 BC1 individuals (stony phenotype) (Figure 2A-i and Figure 2A-ii). Subsequently, we designated the polymorphism marker, S0, at the ∼1544-kb position on Bm_nscaf2827 (reference as 1 cM ∼ 300 kb in silkworm (Yamamoto et al. 2008) and the genetic distance between stony and SAmy-d in the linkage map). Further linkage analysis showed that the S0 marker is tightly linked with the stony locus (Figure 2A-iii). We analyzed the upstream and downstream sequences of S0 in the silkworm database. Close to the S0 marker, we observed two predicted gene models encoding RR1-type chitin-binding cuticular proteins that play an important role in construction of the soft insect cuticle (Andersen et al. 1995, 2011; Togawa et al. 2008; Moussian 2010; Willis 2010) (Figure 2A-iii). Full-length cDNA of BGIBMGA005278 (BmorCPR3) was not different in wild type and stony, and the gene expression patterns were relatively similar (Figure 2A-v, and Figure S2). However, cDNA of BGIBMGA005277, previously designated BmorCPR2 or Larval cuticular protein 17 (Nakato et al. 1997; Futahashi et al. 2008), had an obvious deletion, and its expression was significantly reduced in Dazao-stony (Figure 2A-iv). The amplified BGIBMGA005277 cDNA fragment was assessed in two stony (Dazao-stony, 12-220) and 13 wild-type strains (Dazao, 05-111, 06-072, 20-230, Xiafang, 01-070, 16-060,119 05-050, 16-100, 16-105, C108, 06-920, 04-702) with different genetic backgrounds. The results indicate that deletion occurs specifically in the stony mutants (Figure 2A-vi). Simultaneously, we designed the polymorphism marker, S2, according to the genome sequence of BmorCPR2 (Figure 2A-vii). Genotyping of the marker revealed no recombination between S2 and the stony locus (Figure 2, A-viii and -ix). Accordingly, we conclude that BmorCPR2 is a candidate gene responsible for the stony mutant. Full-length BmorCPR2 cDNA (accession no. KF672849.1) is 547 bp encoding 143 amino acids residues and contains a typical chitin-binding domain (Figure 2B-i, d, and Figure S3). Orthologs of this gene are widely distributed in different Lepidopterous insects (identities range from 40 to 75%), indicative of conservation (Figure S3B, and Table S4). However, the BmorCPR2 transcript contains a 58-bp deletion and 5-bp single-nucleotide substitution in the second exon in Dazao-stony, resulting in a frameshift and premature stop codon (Figure 2, B-iii and C, and Figure S3A). In the aberrant transcript (accession no. KF672850.1), the entire chitin-binding domain was lost (Figure 2B-iv and Figure S3A). In addition, deletion of BM2A-like transposon located ∼2.78 kb upstream and insertion of Ins11-like transposon ∼0.83 kb upstream of BmorCPR2, combining the premature termination codon, led to significantly reduced levels of BmorCPR2 in Dazao-stony (Figure 2B-ii and Figure S4) (Chang et al. 2007; Feschotte 2008).
Figure 2.
Genetic basis of the stony mutant. (A) Positional cloning of stony locus. (i) Preliminary mapping of stony locus. (ii) Location of markers on the physical map of chromosome 8. The yellow and white regions represent scaffolds and gaps, respectively. (iii) Fine mapping of stony locus. (iv v) Transcript analysis of BGIBMGA005277 and BGIBMGA005278 in Dazao and Dazao-stony, respectively. (vi) Transcript analysis of BGIBMGA005277 among different stony and wild-type strains (a–c) stony mutant strains; (a and b) Dazao-stony; (c) 12-220; (d–p) wild-type strains (Dazao, 05-111, 06-072, 20-230, Xiafang, 01-070, 16-060, 05-050, 16-100, 16-105, C108, 06-920, 04-702)). (vii) The polymorphism marker S2. (viii–ix) Genotyping analysis between S2 and stony locus. (B) Scheme of BmorCPR2 transcripts of Dazao and Dazao-stony. (i) Full-length BmorCPR2 cDNA in Dazao and Dazao-stony. (ii) Relative expression of BmorCPR2 between Dazao and Dazao-stony in larval cuticles (mean values ±SD; Student’s t-test; **, P < 0.01, n = 3). (iii) Transcripts of BmorCPR2 from Dazao and Dazao-stony. (iv) Domain organization of BmorCPR2 in Dazao and Dazao-stony.
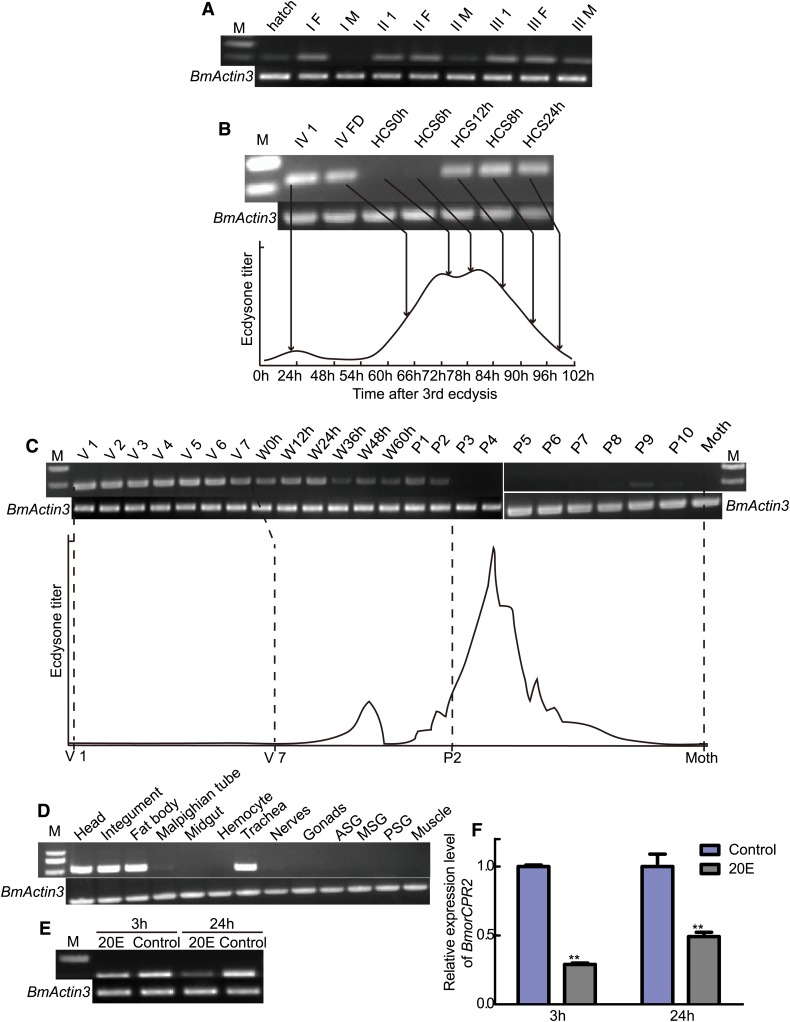
High expression of the BmorCPR2 gene during the interval of larval molting is negatively correlated with the titer of 20E, indicating that the expression pattern of BmorCPR2 is synchronized with the procuticle (the chitinous cuticle) development during larval stages (Mizoguchi et al. 2001; Kiguchi 1981) (Figure 3, A, B, C, E, and F). Additionally, expression of BmorCPR2 was reduced from the wandering stage to the early stage of pupation, and in sequential development stages, BmorCPR2 expression was reduced significantly or to nearly undetectable levels (Figure 3C). These findings coincide with the visible obvious phenotype in the molting interval of stony larvae. In chitin-rich tissues, such as head, integument, and trachea (Gilbert 2011, Chap. 5), BmorCPR2 was also highly expressed, in keeping with the chitin-binding domain (Figure 3D). However, the reason underlying high expression of BmorCPR2 in fat body remains unclear (Figure 3D).
Figure 3.
Expression profiles of BmorCPR2 in Dazao. BmActin3 was used as the internal control for RT–PCR. (A–C) Temporal expression profiles of BmorCPR2 from the hatch to moth stage. I–II, larvae from first to third instar. (F) Feeding stage. M, molting stage. IV 1, day 1 of the fourth-instar larvae (20 hr after third ecdysis). FD, feeding activity decline stage (about 64 hr after third ecdysis), HCS0-24h, hours after head capsule slippage (about 75–99 hr after third ecdysis). V 1–V 7, days 1–7 of the fifth-instar larvae. W0h–W60h, 0–60 hr of the wandering stage; P1–P10, days 1 to 10 of pupation. The developmental changes in hemolymph ecdysteroid titer are based on the data as previously described (Mizoguchi et al. 2001; Kiguchi 1981). (D) Spatial expression of BmorCPR2 on day 4 of the fifth-instar larvae in Dazao. ASG, MSG, and PSG: anterior, middle, and posterior silk gland, respectively. (E and F) Expression patterns of BmorCPR2 after 20E treatment. Data are presented as mean values ±SD. Student’s t-test, **, P < 0.01, n = 3.
BmorCPR2 affects cuticle chitin content and larval molting via effects on chitin binding
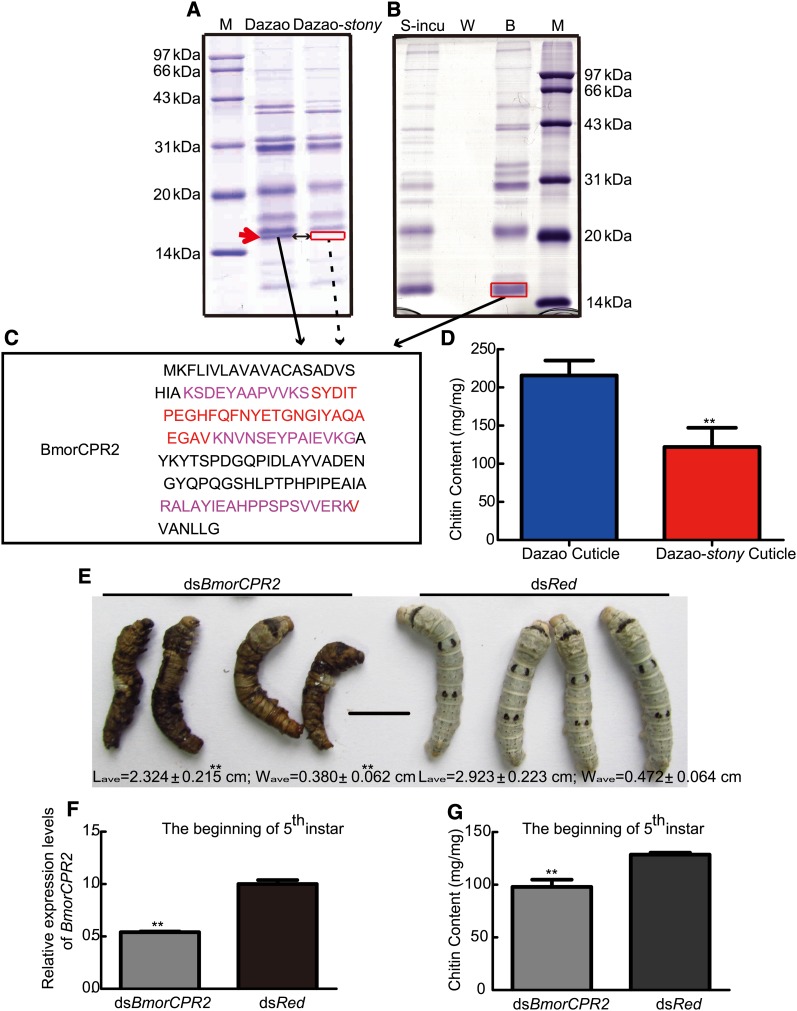
Total proteins extracted from larval cuticle of Dazao and Dazao-stony showed that the mutant protein is devoid of a band at ∼15.3 kDa, compared with wild-type Dazao (Figure 4A and Table S2). Mass spectrometry findings confirmed that this band represents BmorCPR2 protein (a major component), which is absent at the corresponding position in the gel in stony, indicative of defective BmorCPR2 protein in the mutant (Figure 4A, C; Table S2). Chitin binding assay of total proteins extracted from the wild-type cuticle revealed ∼15.3-kDa protein bands in chitin bead deposits, signifying binding of these proteins to chitin (Figure 4B). Meanwhile, mass spectrometry results revealed that the electrophoretic bands contain BmorCPR2, validating the capacity of BmorCPR2 to bind chitin (Figure 4, B and C, and Table S2). These findings are consistent with the structural characteristics of the protein and results of Tang et al. (Rebers and Riddiford 1988; Futahashi et al. 2008; Tang et al. 2010; Willis 2010; Andersen 2011). Subsequently, we assayed the chitin content of Dazao and Dazao-stony larval cuticles (day 5 of the fifth instar). Notably, the cuticle chitin content in Dazao was ∼1.76 times that in Dazao-stony (Figure 4D). Naked chitin fibers need to interact with chitin-binding protein to stabilize the helicoidal layers of cuticle (Rebers and Riddiford 1988; Rebers and Willis 2001; Merzendorfer and Zimoch 2003; Moussian 2010). The largest member of these chitin-binding proteins is the CPR family, which may contribute to coordinating the interactions between chitin and the proteinaceous matrix (Rebers and Riddiford 1988; Rebers and Willis 2001; Togawa et al. 2004; Magkrioti et al. 2004; Tang et al. 2010; Willis 2010; Andersen 2011), and RR-type chitin-binding protein, chitin, and chitinase are colocalized in the procuticle (Moussian et al. 2006; Chaudhari et al. 2011, 2013; Arakane et al. 2012). The recent study on Tribolium castaneum indicated that the TcCPR4 (a RR1-type cuticular protein) distribute the region nearby chitin laminae and can affect the chitin content (Arakane 2013). Based on these results, we hypothesized that BmorCPR2 plays an important role in maintaining chitin content of the cuticle. However, it remains to be established whether BmorCPR2 protects chitin through combining and packaging chitin fibers, similar to the chitin-binding protein, knickkopf (Moussian et al. 2006; Chaudhari et al. 2011). RNAi experiments were performed to specifically reduce the expression levels of BmorCPR2 (Figure 4F and Figure S5, A and B). Our results revealed that 42% of the individuals in the dsBmorCPR2 group display molting difficulties at the beginning of the fifth-instar larvae (dsRed as a control) (Figure 4E; Table S3). Moreover, this phenotype occurred more frequently in the stony mutant, compared with wild type (Figure S1L). Individuals injected with dsBmorCPR2 that displayed molting difficulty were hard to touch, had smaller body size, and became dark owing to effusion of blood (these individuals were still alive) (Figure 4E). After peeling the old cuticle of individuals in the abnormal interference group, we observed that internal organs break new cuticles and overflow (data not shown). The chitin content in the new cuticle of dsBmorCPR2 individuals (with RNAi phenotype) was significantly lower than that of the control group (dsRed as a control) (Figure 4G). The process of cuticle extension and increasing of body size require chitin as crude material (Merzendorfer and Zimoch 2003; Lease and Wolf 2010), and therefore simultaneously, larval molting is a critical period for the formation and growth of new cuticles. Accordingly, we propose that suppression of BmorCPR2 decreases the chitin content and level of BmorCPR2, resulting in limitation of remodeling and extension of the larval cuticle and weakened body cavity capacity. Increasing internal pressure makes the body tight and hard to touch (Carter and Locke 1993; Merzendorfer and Zimoch 2003). In this case, when the larvae molt, abdominal contraction and powerful pressure within the body lead to elongation-limited cuticle rupture, bleeding, and organ overflow. Based on these results, we conclude that the BmorCPR2 gene plays important roles in the maintenance of chitin content of larval cuticle as well as extension of new cuticles and increase of body size during the molting stage.
Figure 4.
Biochemical function of BmorCPR2. (A) SDS–PAGE profiles of larval cuticle total proteins extracted from Dazao and Dazao-stony. The red arrow represents BmorCPR2, which was absent in Dazao-stony. M, molecular mass markers; type, midrange protein molecular weight markers (Shanghai Generay Biotech). (B) Chitin-binding assay with cuticle total proteins of Dazao. S-incu, supernatant removed via centrifuging after incubation. W, second collected eluent after centrifugation. B, liquid obtained from centrifuged chitin resin treated with denaturant. Red boxes indicate the gels used for LC–MS/MS analysis. Solid arrows represent BmorCPR2 detected using LC–MS/MS; dotted arrows indicate BmorCPR2 that is not detectable with LC–MS/MS. M, molecular mass markers; type, midrange protein molecular weight markers (Shanghai Generay Biotech). (C) Corresponding LC–MS/MS peptides of the identified protein (BmorCPR2) in cuticle proteins (red and purple) and chitin binding assay (purple). (D) Comparison of the cuticle chitin contents between Dazao and Dazao-stony. (n = 4). (E) Phenotypes of larvae at the beginning of fifth instar subjected to RNAi. Scale bar, 1 cm. Lave and Wave, the average length and width (the third abdomen segment), respectively, of larvae. (n = 10). (F) Relative expression levels of BmorCPR2 at the beginning of fifth-instar larvae subjected to RNAi (n = 3; dsRed as a control). (G) Comparison of the cuticle chitin content at the beginning of fifth-instar larvae between dsBmorCPR2 and control groups (n = 4; dsRed as a control). Data represent means ±SD. Student’s t-test, ** P < 0.01 (D–G).
BmorCPR2 is involved in maintaining the tensile property of the larval cuticle
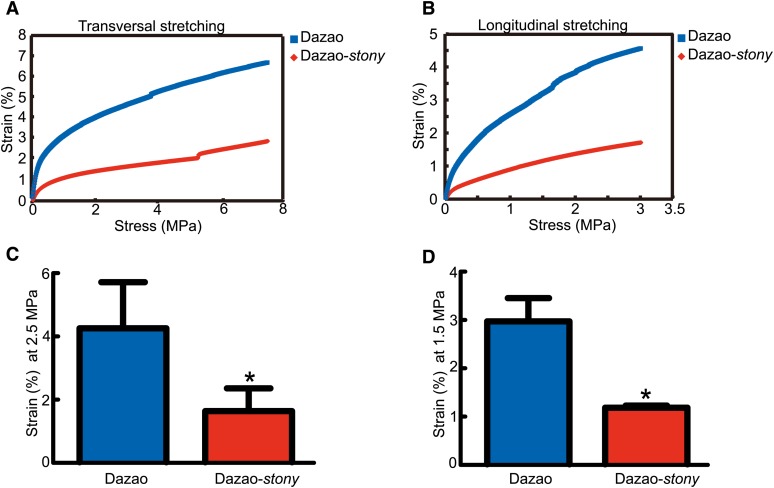
The insect cuticle requires elongation and tensile properties to accommodate the increasing body cavity content during growth and development (Wigglesworth 1957; Locke 1970; Richards and Davies 1977; Moussian 2010). The tensile property of the cuticle in Dazao and Dazao-stony larvae were examined (Figure S6). Compared to Dazao, Dazao-stony needed more stress under the same strain conditions in the longitudinal and transversal (Figure 5, A and B). Within the strictly linear relationship between stress and strain, when longitudinal tensile stress was 1.5 MPa, the strain of Dazao-stony was only one-third that of wild type, and at 2.5-MPa transverse stretch stress, strain of the mutant was also far lower than that of wild type (Figure 5, C and D). In insects, chitin content and chitin–cuticular protein interactions are essential for maintaining the normal mechanical properties of the cuticle. In Dazao-stony, dysfunctional BmorCPR2 affects the chitin content as well as interactions between chitin and BmorCPR2, in turn, significantly attenuating the tensile properties of the cuticle and further decreasing body cavity capacity. This was similar to the phenotype characteristics of Manduca sexta when reducing larval cuticle tensile property (via treatment with cyromazine) (Reynolds and Blakey 1989; Kotze and Reynolds 1990). Under these situations, greater pressure in the body cavity caused by reduced cuticle tensile properties may be another vital factor for the hard body and defective athletic ability of the stony mutant.
Figure 5.
Effects of BmorCPR2 on mechanical properties of larval cuticle. (A) Relationship between stress and strain with transversal stretching. (B) Relationship between stress and strain with longitudinal stretching. (C) Dazao and Dazao-stony cuticles at transversal stress of 2.5 MPa. (D) Dazao and Dazao-stony cuticles at longitudinal stress of 1.5 MPa. Data represent mean values ±SD. Student’s t-test, *, P < 0.05, n = 3 (C–D).
Dysfunction of BmorCPR2 may cause the appearance of posterior internode similar to intersegmental fold in stony mutant
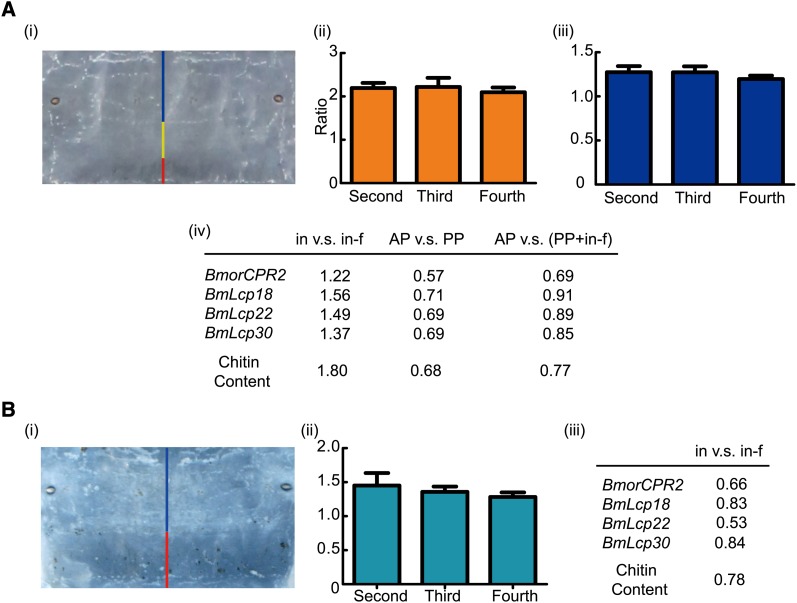
To determine the reasons underlying the abnormal ratio between internode and intersegmental folds in stony, we investigated the expression patterns of the related cuticular proteins and chitin content in different parts of the dorsal segment cuticle in detail. The internode of Dazao is further divided into anterior part (AP) and posterior part (PP) (Figure 6A-i, -ii, -iii). BmorCPR2 displayed similar expression patterns to BmLcp18, BmLcp22, and BmLcp30, three known larval RR1-type chitin-binding cuticular protein genes (Nakato et al. 1997; Togawa et al. 2004), in different regions of Dazao larval cuticle. All genes were expressed more highly in internodes than in intersegmental folds. They also had higher expression in PP, specifically, in the posterior part of the segment (including PP and intersegmental fold) than in AP (Figure 6A-iv and Figure S7A-i, -ii, -iii). Analogous patterns were additionally obtained with regard to chitin content in these regions (Figure 6A-iv and Figure S7A-iv, -v, -vi). Compared to the intersegmental fold, higher gene expression and chitin levels are required in the internode to fit growth (expanded circle area) and satisfy the increasing demand to hold body-cavity contents. In particular, the higher gene expression and chitin content in PP than in AP corresponded well with its enhanced flexibility and tensile property. Among the four cuticle genes examined, only BmorCPR2 displayed sequence differences between Dazao and Dazao-stony (Figure 2, Figure S3, and Figure S4). The Dazao-stony strain was backcrossed with Dazao for >24 generations, suggesting that at least 99.99% (1 − (1/2)24) of the genetic composition is identical between Dazao and Dazao-stony. Theoretically, the expression patterns of BmLcp18, BmLcp22, and BmLcp30 and content distribution of chitin should be similar in different cuticle positions between Dazao and Dazao-stony. However, the expression patterns of the four genes and chitin content in Dazao-stony internode and intersegmental fold are opposite to those in Dazao (Figure 6B), similar to the cases in Figure 5A-iv [AP vs. (PP + in-f), intersegmental fold (in-f)] and Figure S7A-iii and -vi. Therefore, we speculate that the phenotypic appearance of PP is converted to that of the intersegmental fold in stony mutant (Figure 1, B and C, Figure 6A-iii and -iv, Figure 6B, and Figure S7). This finding signifies that the greater part of intersegmental fold selected may be the posterior margin of the corresponding internode, resulting from dysfunctional BmorCPR2 (Figure S7C). In stony mutant, conversion from PP to the intersegmental fold reduces the circle area of the cuticle significantly, leading to lower ability of internodes to contain internal tissues and organs, and consequently, it has a tighter larval body. Simultaneously, the strong internal pressure extrapolates the abnormal intersegmental fold (enlarged) and generates bulges and, in turn, abnormal body shape in the stony mutant (Figure 1, A–C). Moreover, cuticle flexibility and larval athletic ability are impeded (Figure 1, F–J, and Figure S1, F–K). In Manduca sexta larvae, bulgy intersegmental membrane (via treatment with cyromazine) also leads to larval movement and cuticle flexibility impairment (Reynolds and Blakey 1989; Kotze and Reynolds 1990, 1991). Further research is warranted to determine the precise nature of interactions (localization and possible crosslinking) between BmorCPR2 and chitin in different parts of the segment at the ultrastructural level.
Figure 6.
Gene expression profiles and chitin distribution in various parts of segmental cuticle in Dazao and Dazao-stony. (A) Cuticular gene expression profiles and chitin distribution in different parts of Dazao dorsal segment cuticle. (i) Images of various parts of the dorsal segment cuticle in Dazao. Blue, yellow, and red lines represent the AP and PP of the internode (in), and the intersegmental fold (in-f), respectively. (ii) Ratio of the AP and PP lengths in the second, third, and fourth abdomen segments of Dazao (n = 5). (iii)Ratio of AP length and remaining parts in the second, third, and fourth abdomen segments of Dazao (n = 5). (iv)Ratios of genes expression levels and chitin content in different parts of Dazao dorsal segment cuticle. (B) Cuticular gene expression profiles and chitin distribution in different parts of Dazao-stony segment. (i) Images of various parts of the dorsal segment cuticle in Dazao-stony. The blue and red lines represent the internode (in) and intersegmental fold (in-f), respectively. (ii) Ratio of the internode and intersegmental fold lengths in the second, third, and fourth abdomen segments of Dazao-stony (n = 5). (iii) ratios of genes expression levels and chitin content in different parts of Dazao dorsal segment cuticle. Further details can be found in the Figure S7.
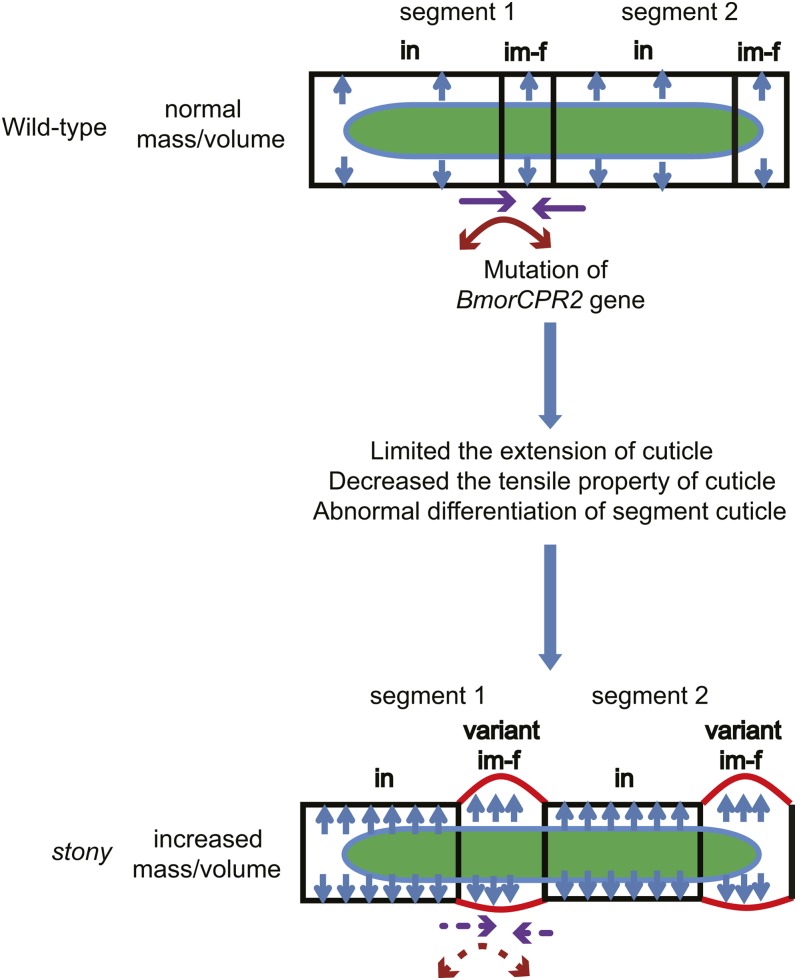
The model to explain the phenotype of the stony mutant
Based on the data obtained, we proposed a model to explain the phenotype of the stony mutant as follows: lack of normal BmorCPR2 protein induces a reduction in the cuticle chitin content, which limits growth and extension of the cuticle. Meanwhile, disruption of the BmorCPR2 gene causes the posterior part of the internode to switch to intersegmental fold in appearance, leading to reduced surrounding internode area and cuticle tensile property. Accumulation of these variations results in intense antagonism between the gradual increase in internal contents and growth-limited cuticle and consequently, an obvious increase in the internal pressure of larval body cavity, causing the larval body to become stiff, tight, and hard and reducing its ability to buffer shock. Simultaneously, due to strong pressure in the body cavity, the posterior of each segment is pushed outward, causing abnormal body-shape and athletic ability defects (Figure 7). In Manduca sexta, the levels and incorporation of glycine of cuticular protein in larvae treated with cyromazine are reduced (Kotze and Reynolds 1991), and the phenotype and physiological characteristics of treated larvae similar to those of the stony mutant (Reynolds and Blakey 1989; Kotze and Reynolds 1990) (Figure 1, Figure 4, Figure 5, and Figure S1, E–K). Accordingly, we speculate that the cyromazine target is associated with BmorCPR2 or candidate genes of other body-shape mutants with similar phenotypic and physiological characteristics to stony in silkworm. We aim to verify this hypothesis in subsequent analyses.
Figure 7.
Schematic overview of the phenotype of stony mutant. Dysfunctional BmorCPR2 leads to cuticle extend blockage, decrease in tensile property, and abnormal differentiation of various parts in the segment cuticle, resulting in significant increase of the internal pressure of the body cavity and pushing out of the increscent and variant intersegmental fold. These factors lead to tightness and hardness, abnormal body shape, and marked decrease in adaptability of larvae. in, internode; im-f, intersegmental fold. The green region shows midgut content. Blue arrows indicate internal pressure of the body cavity, and the solid purple arrow represents telescopic movement between segments. The dotted purple arrow represents the deficiency of telescopic movement between segments. Red solid and dotted circular arrows represent normal crooking and deficiency of crooking capacity of the ventral side, respectively.
Conclusion
The number of chitin-binding cuticular proteins in different insect genomes varies significantly (Magkrioti et al. 2004; Weinstock et al. 2006; Karouzou et al. 2007; Cornman et al. 2008; Futahashi et al. 2008; Richards et al. 2008; Willis 2010; Gilbert 2011, Chap. 5). Even within the same insect, they are selectively switched on or off at different developmental stages as well as in cuticles with different physical properties. Therefore, the chitin-binding cuticular proteins involved in modeling and maintaining body shape differ in identity and function according to the insect and developmental stage (Soares et al. 2007; Okamoto et al. 2008; Togawa et al. 2008; Charles 2010), and our results show that mutation of the BmorCPR2 gene alters the overall morphology of larvae, validating its role in body-shape determination in silkworm. These findings suggest that the number, expression patterns, and functional diversification of chitin-binding cuticular proteins represent key factors in understanding the diversity of insect body shape and provide a reference to analyze the molecular basis for other body-shape mutants in silkworm. In addition, BmorCPR2 homologs are abundant in Lepidoptera insects, including agricultural pests. Unlike domesticated silkworm, wild Lepidoptera larvae lack human protection, and their survival depends on normal differentiation, structure, and function of the cuticle. If wild Lepidoptera larvae appear to have a similar phenotype to stony, their adaptability will be seriously affected, with lethal consequences. Determination of the characteristics of BmorCPR2 may thus provide a reference for potential utilization of these cuticular protein types in pest control strategies for Lepidoptera.
Supplementary Material
Acknowledgments
We thank Professor Kunyan Zhu and professor Jianzhen Zhang for chitin extraction and quantification, Dr. Yuanhao Li and Dr. Songyuan Wu for valuable advice, and M. S. Kunpeng Lu for cuticle dissection. We are extremely grateful to Ms. Yanyan wang for her guidance on Dynamic Mechanical Analyzer analysis. This work was supported by National Basic Research 973 Program of China Grant (No. 2012CB114600), Hi-Tech Research and Development 863 Program of China Grant (2013AA102507), the National Natural Science Foundation of China (Nos. 31302038 and 31072088), Natural Science Foundation Project of ChongQing(CSTC) (No. cstc2013jcyjA0634), Fundamental Research Funds for the Central Universities in China (No. XDJK2013A001), and Par-Eu Scholars Program.
Footnotes
Communicating editor: B. Sullivan
Literature Cited
- Andersen S. O., 2011. Are structural proteins in insect cuticles dominated by intrinsically disordered regions? Insect Biochem. Mol. Biol. 41: 620–627. [DOI] [PubMed] [Google Scholar]
- Andersen S. O., Hojrup P., Roepstorff P., 1995. Insect cuticular proteins. Insect Biochem. Mol. Biol. 25: 153–176. [DOI] [PubMed] [Google Scholar]
- Arakane Y., Lomakin J., Gehrke S. H., Hiromasa Y., Tomich J. M., et al. , 2012. Formation of rigid, non-flight forewings (elytra) of a beetle requires two major cuticular proteins. PLoS Genet. 8: e1002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakane Y., 2013. Functional genomics of TcCPR4 belongs to RR-1 CP family in the red flourbeetle, Tribolium castaneum. Fourth International Conference of Insect Physiology, Biochemistry and Molecular Biology, Nanjing, China. Available at: http://www.meeting.edu.cn/meeting/webmedia/jingpin/ipmb2013/pic/abstract.pdf. [Google Scholar]
- Bouligand Y., 1965. On a twisted fibrillar arrangement common to several biologic structures. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D 261: 4864–4867. [PubMed] [Google Scholar]
- Brackenbury J., 1997. Caterpillar kinematics. Nature 390: 453. [Google Scholar]
- Carter D., Locke M., 1993. Why caterpillars do not grow short and fat. Int. J. Insect Morphol. Embryol. 22: 81–102. [Google Scholar]
- Chang Y. F., Imam J. S., Wilkinson M. F., 2007. The nonsense-mediated decay RNA surveillance pathway. Annu. Rev. Biochem. 76: 51–74. [DOI] [PubMed] [Google Scholar]
- Charles J. P., 2010. The regulation of expression of insect cuticle protein genes. Insect Biochem. Mol. Biol. 40: 205–213. [DOI] [PubMed] [Google Scholar]
- Chaudhari S. S., Arakane Y., Specht C. A., Moussian B., Boyle D. L., et al. , 2011. Knickkopf protein protects and organizes chitin in the newly synthesized insect exoskeleton. Proc. Natl. Acad. Sci. USA 108: 17028–17033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari S. S., Arakane Y., Specht C. A., Moussian B., Kramer K. J., et al. , 2013. Retroactive maintains cuticle integrity by promoting the trafficking of Knickkopf into the procuticle of Tribolium castaneum. PLoS Genet. 9: e1003268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornman R. S., Willis J. H., 2009. Annotation and analysis of low-complexity protein families of Anopheles gambiae that are associated with cuticle. Insect Mol. Biol. 18: 607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornman R. S., Togawa T., Dunn W. A., He N., Emmons A. C., et al. , 2008. Annotation and analysis of a large cuticular protein family with the R&R Consensus in Anopheles gambiae. BMC Genomics 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai F. Y., Qiao L., Tong X. L., Cao C., Chen P., et al. , 2010. Mutations of an arylalkylamine-N-acetyltransferase, Bm-iAANAT, are responsible for silkworm melanism mutant. J. Biol. Chem. 285: 19553–19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delon I., Payre F., 2004. Evolution of larval morphology in flies: get in shape with shavenbaby. Trends Genet. 20: 305–313. [DOI] [PubMed] [Google Scholar]
- Feschotte C., 2008. Transposable elements and the evolution of regulatory networks. Nat. Rev. Genet. 9: 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futahashi R., Okamoto S., Kawasaki H., Zhong Y. S., Iwanaga M., et al. , 2008. Genome-wide identification of cuticular protein genes in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 38: 1138–1146. [DOI] [PubMed] [Google Scholar]
- Gilbert L. I., 2011. Insect Molecular Biology and Biochemistry. Academic Press, New York. [Google Scholar]
- Guan X., Middlebrooks B. W., Alexander S., Wasserman S. A., 2006. Mutation of TweedleD, a member of an unconventional cuticle protein family, alters body shape in Drosophila. Proc. Natl. Acad. Sci. USA 103: 16794–16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasrapuria S., Arakane Y., Osman G., Kramer K. J., Beeman R. W., et al. , 2010. Genes encoding proteins with peritrophin A-type chitin-binding domains in Tribolium castaneum are grouped into three distinct families based on phylogeny, expression and function. Insect Biochem. Mol. Biol. 40: 214–227. [DOI] [PubMed] [Google Scholar]
- Jasrapuria S., Specht C. A., Kramer K. J., Beeman R. W., Muthukrishnan S., 2012. Gene families of cuticular proteins analogous to peritrophins (CPAPs) in Tribolium castaneum have diverse functions. PLoS ONE 7: e49844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karouzou M. V., Spyropoulos Y., Iconomidou V. A., Cornman R. S., Hamodrakas S. J., et al. , 2007. Drosophila cuticular proteins with the R&R Consensus: annotation and classification with a new tool for discriminating RR-1 and RR-2 sequences. Insect Biochem. Mol. Biol. 37: 754–760. [DOI] [PubMed] [Google Scholar]
- Kiguchi K., 1981. Ecdysteroid levels and developmental events during larval moulting in the silkworm, Bombyx mori. J. Insect Physiol. 27: 805–812. [Google Scholar]
- Kotze, A. C., and S. E. Reynolds, 1990 Mechanical properties of the cuticle of Manduca sexta larvae treated with cyromazine. Pestic. Biochem. Physiol. 38: 267–272.
- Kotze, A. C., and S. E. Reynolds, 1991 An examination of cuticle chitin and protein in cyromazine-affected Manduca sexta larvae. Pestic. Biochem. Physiol. 41: 14–20.
- Lease H. M., Wolf B. O., 2010. Exoskeletal chitin scales isometrically with body size in terrestrial insects. J. Morphol. 271: 759–768. [DOI] [PubMed] [Google Scholar]
- Lin H. T., Dorfmann A. L., Trimmer B. A., 2009. Soft-cuticle biomechanics: a constitutive model of anisotropy for caterpillar integument. J. Theor. Biol. 256: 447–457. [DOI] [PubMed] [Google Scholar]
- Locke M., 1970. The molt/intermolt cycle in the epidermis and other tissues of an insect Calpodes ethlius (Lepidoptera, Hesperiidae). Tissue Cell 2: 197–223. [DOI] [PubMed] [Google Scholar]
- Magkrioti C. K., Spyropoulos I. C., Iconomidou V. A., Willis J. H., Hamodrakas S. J., 2004. cuticleDB: a relational database of arthropod cuticular proteins. BMC Bioinformatics 5: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzendorfer H., Zimoch L., 2003. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 206: 4393–4412. [DOI] [PubMed] [Google Scholar]
- Mizoguchi A., Y. Ohashi, K. Hosoda, J. Ishibashi, and H. Kataoka, 2001. Developmental profile of the changes in the prothoracicotropic hormone titer in hemolymph of the silkworm Bombyx mori: correlation with ecdysteroid secretion. Insect Biochem. Mol. Biol. 31: 349–358. [DOI] [PubMed] [Google Scholar]
- Moussian B., 2010. Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem. Mol. Biol. 40: 363–375. [DOI] [PubMed] [Google Scholar]
- Moussian B., Schwarz H., Bartoszewski S., Nusslein-Volhard C., 2005. Involvement of chitin in exoskeleton morphogenesis in Drosophila melanogaster. J. Morphol. 264: 117–130. [DOI] [PubMed] [Google Scholar]
- Moussian B., Tang E., Tonning A., Helms S., Schwarz H., et al. , 2006. Drosophila Knickkopf and Retroactive are needed for epithelial tube growth and cuticle differentiation through their specific requirement for chitin filament organization. Development 133: 163–171. [DOI] [PubMed] [Google Scholar]
- Nakato H., Takekoshi M., Togawa T., Izumi S., Tomino S., 1997. Purification and cDNA cloning of evolutionally conserved larval cuticle proteins of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 27: 701–709. [DOI] [PubMed] [Google Scholar]
- Neville A. C., 1975. Biology of the Arthropod Cuticle. Springer-Verlag, New York. [Google Scholar]
- Neville A. C., Luke B. M., 1969. A two-system model for chitin-protein complexes in insect cuticles. Tissue Cell 1: 689–707. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Futahashi R., Kojima T., Mita K., Fujiwara H., 2008. Catalogue of epidermal genes: genes expressed in the epidermis during larval molt of the silkworm Bombyx mori. BMC Genomics 9: 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou N. C., Iconomidou V. A., Willis J. H., Hamodrakas S. J., 2010. A possible structural model of members of the CPF family of cuticular proteins implicating binding to components other than chitin. J. Insect Physiol. 56: 1420–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebers J. E., Riddiford L. M., 1988. Structure and expression of a Manduca sexta larval cuticle gene homologous to Drosophila cuticle genes. J. Mol. Biol. 203: 411–423. [DOI] [PubMed] [Google Scholar]
- Rebers J. E., Willis J. H., 2001. A conserved domain in arthropod cuticular proteins binds chitin. Insect Biochem. Mol. Biol. 31: 1083–1093. [DOI] [PubMed] [Google Scholar]
- Reynolds S. E., Blakey J. K., 1989. Cyromazine causes decreased cuticle extensibility in larvae of the tobacco hornworm, Manduca sexta. Pestic. Biochem. Physiol. 35: 251–258. [Google Scholar]
- Richards O. W., Davies R. G., 1977. IMMS' General Textbook of Entomology. Halsted Press, New York. [Google Scholar]
- Richards S., Gibbs R. A., Weinstock G. M., Brown S. J., Denell R., et al. , 2008. The genome of the model beetle and pest Tribolium castaneum. Nature 452: 949–955. [DOI] [PubMed] [Google Scholar]
- Soares M. P., Elias-Neto M., Simoes Z. L., Bitondi M. M., 2007. A cuticle protein gene in the honeybee: expression during development and in relation to the ecdysteroid titer. Insect Biochem. Mol. Biol. 37: 1272–1282. [DOI] [PubMed] [Google Scholar]
- Tang L., Liang J., Zhan Z., Xiang Z., He N., 2010. Identification of the chitin-binding proteins from the larval proteins of silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 40: 228–234. [DOI] [PubMed] [Google Scholar]
- Togawa T., Nakato H., Izumi S., 2004. Analysis of the chitin recognition mechanism of cuticle proteins from the soft cuticle of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 34: 1059–1067. [DOI] [PubMed] [Google Scholar]
- Togawa T., Dunn W. A., Emmons A. C., Nagao J., Willis J. H., 2008. Developmental expression patterns of cuticular protein genes with the R&R Consensus from Anopheles gambiae. Insect Biochem. Mol. Biol. 38: 508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock G. M., Robinson G. E., Gibbs R. A., Weinstock G. M., Worley K. C., et al. , 2006. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 443: 931–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigglesworth V. B., 1957. The physiology of insect cuticle. Annu. Rev. Entomol. 2: 37–54. [Google Scholar]
- Willis J. H., 2010. Structural cuticular proteins from arthropods: annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem. Mol. Biol. 40: 189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis J. H., Iconomidou V. A., Smith R. F., Hamodrakas S. J., 2005. Cuticular proteins, pp. 79–110 in Comprehensive Molecular Insect Science, Vol. 4, edited by Gilbert L. I., Iatrou K., Gill S. S. Elsevier, Oxford, UK. [Google Scholar]
- Yamamoto K., Nohata J., Kadono-Okuda K., Narukawa J., Sasanuma M., et al. , 2008. A BAC-based integrated linkage map of the silkworm Bombyx mori. Genome Biol. 9: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhu K. Y., 2006. Characterization of a chitin synthase cDNA and its increased mRNA level associated with decreased chitin synthesis in Anopheles quadrimaculatus exposed to diflubenzuron. Insect Biochem. Mol. Biol. 36: 712–725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.