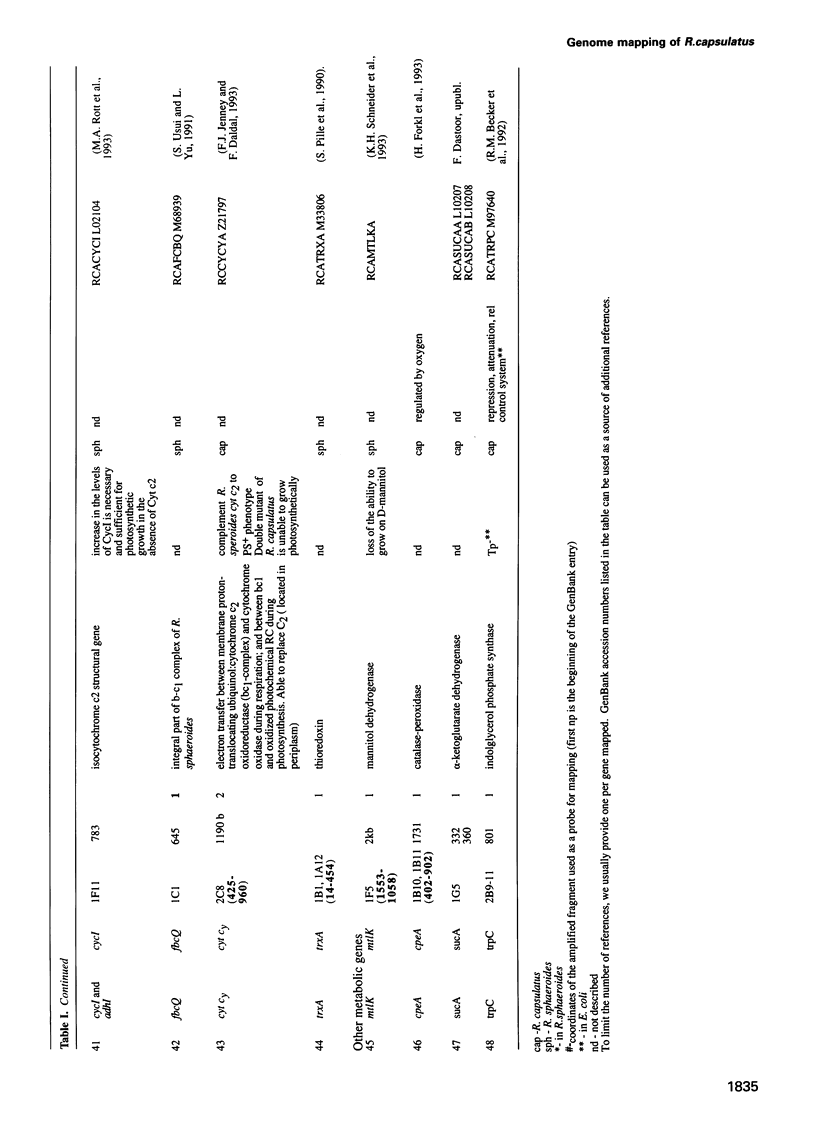
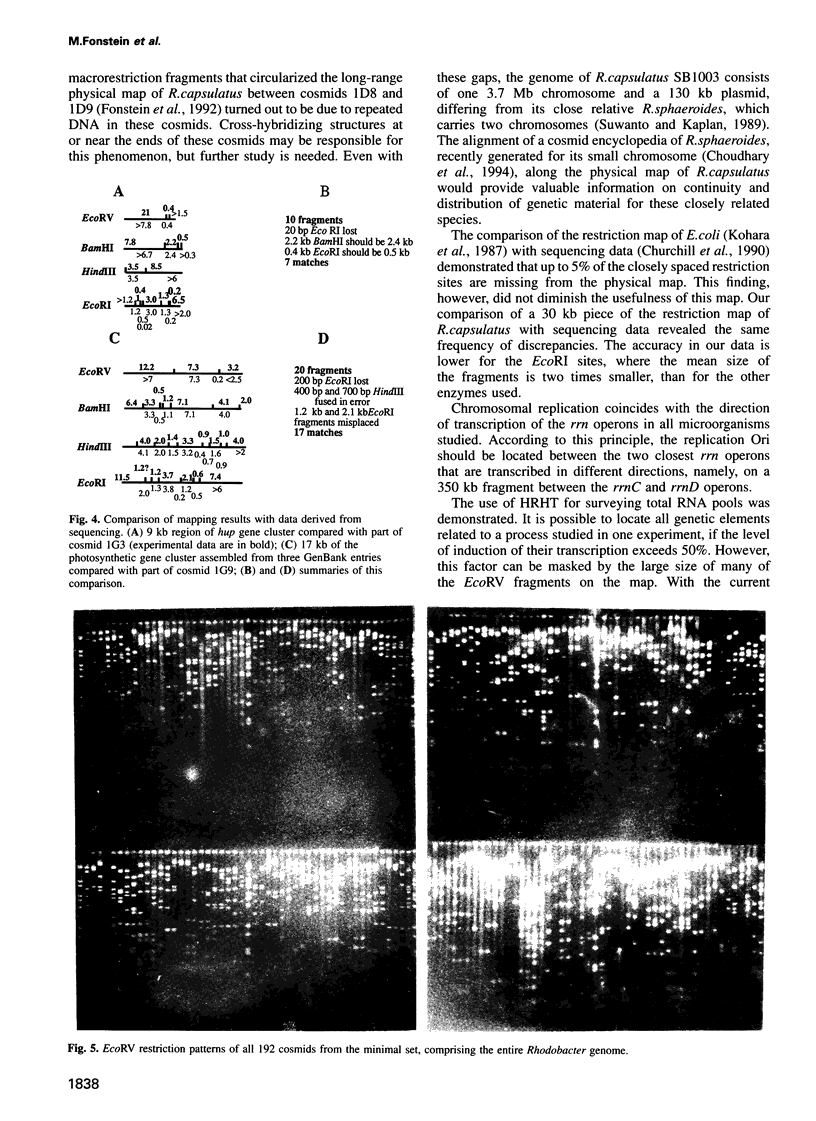
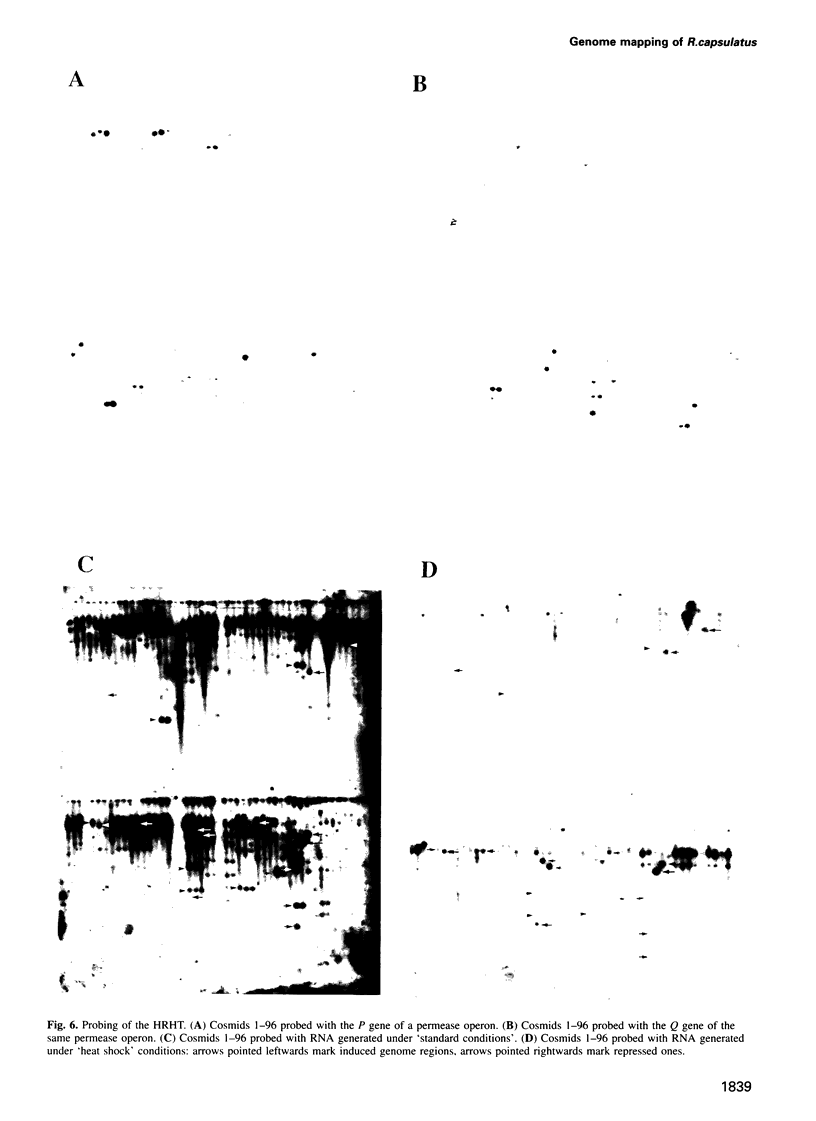
Abstract
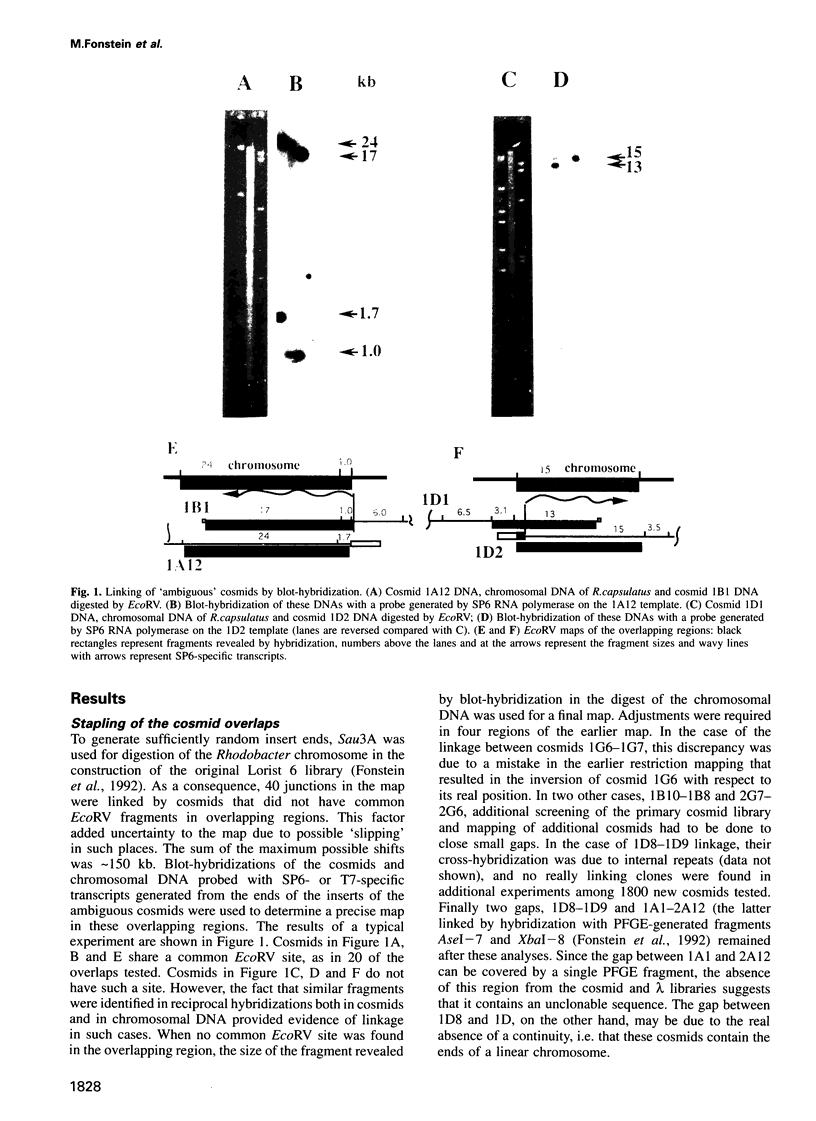
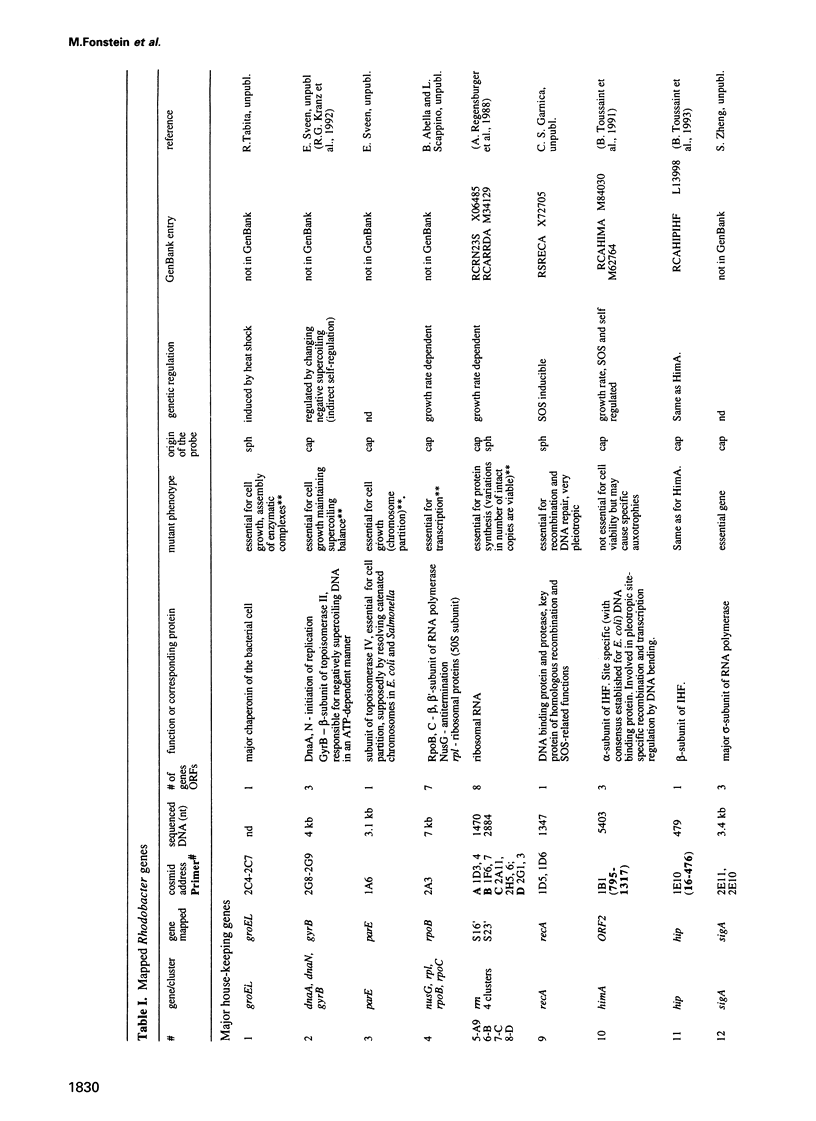
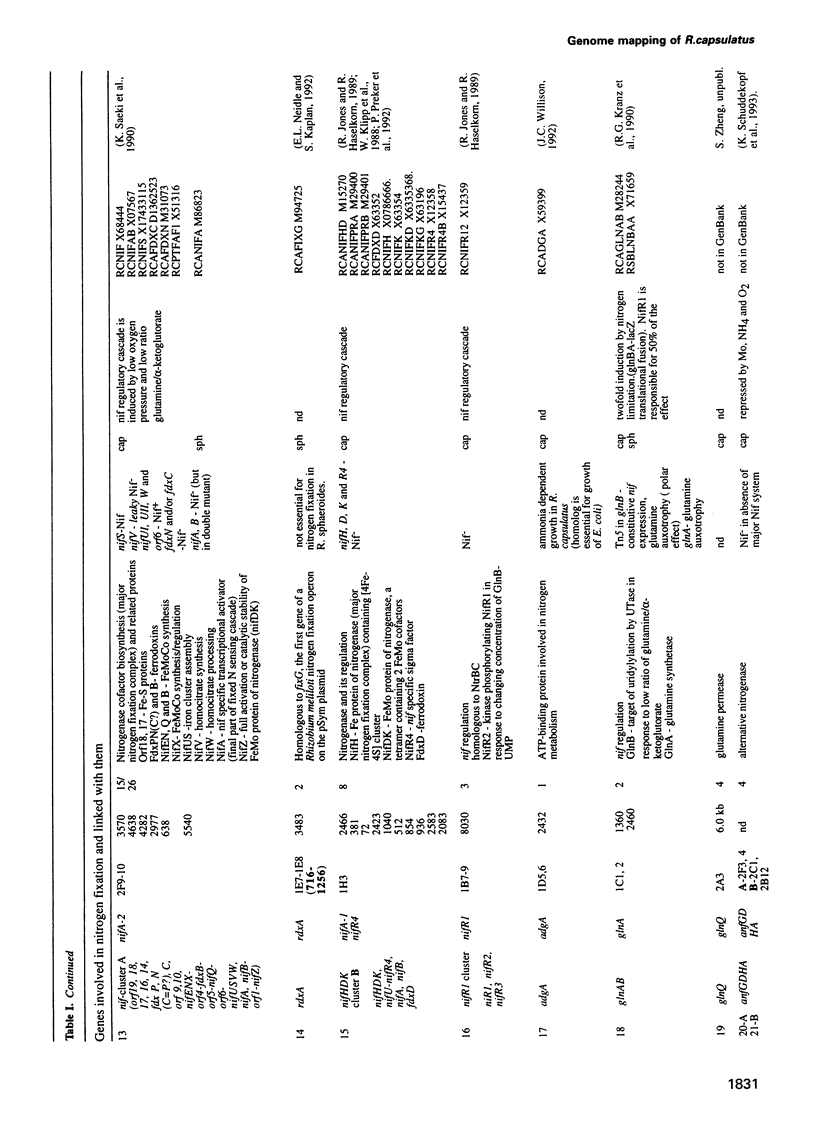
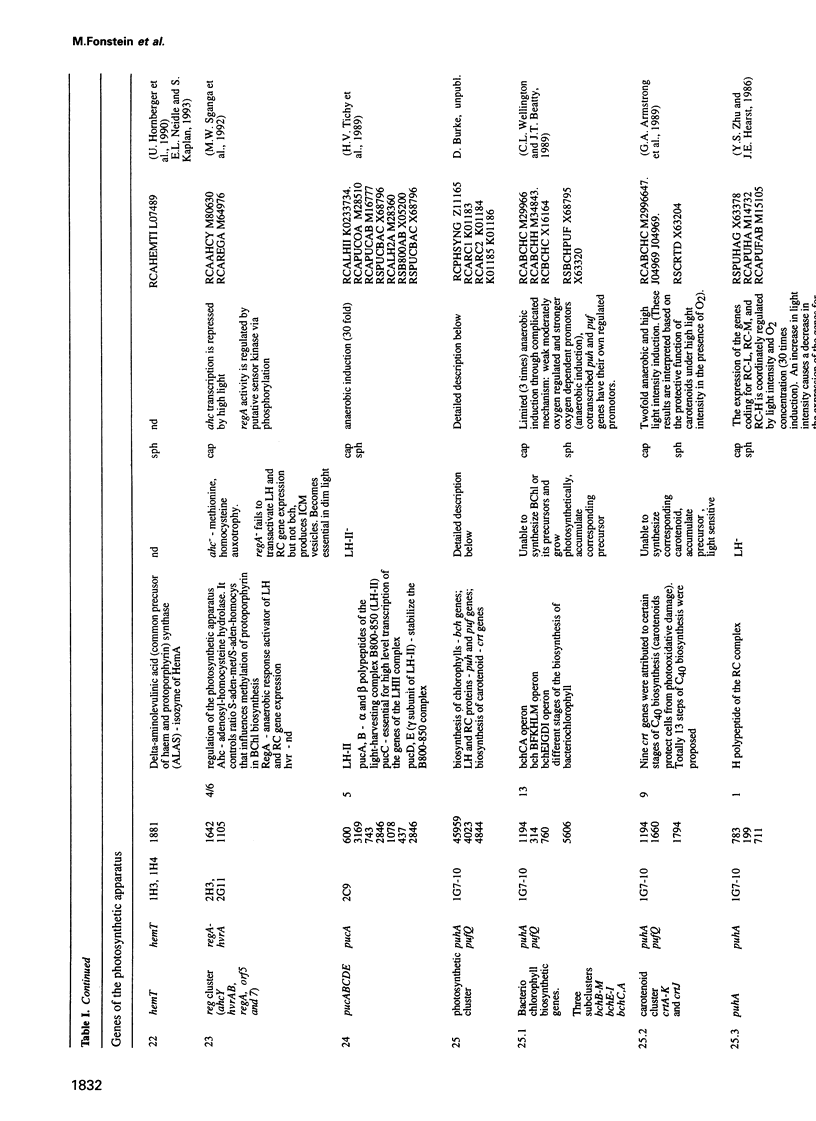
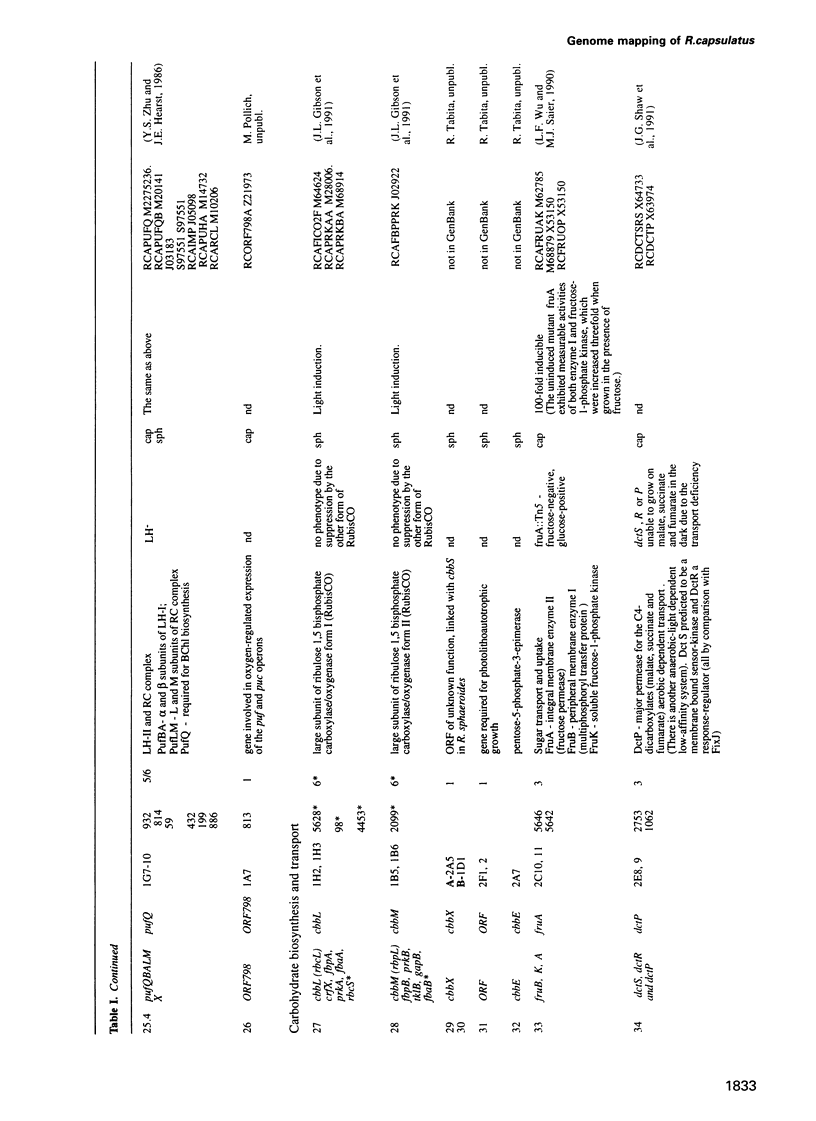
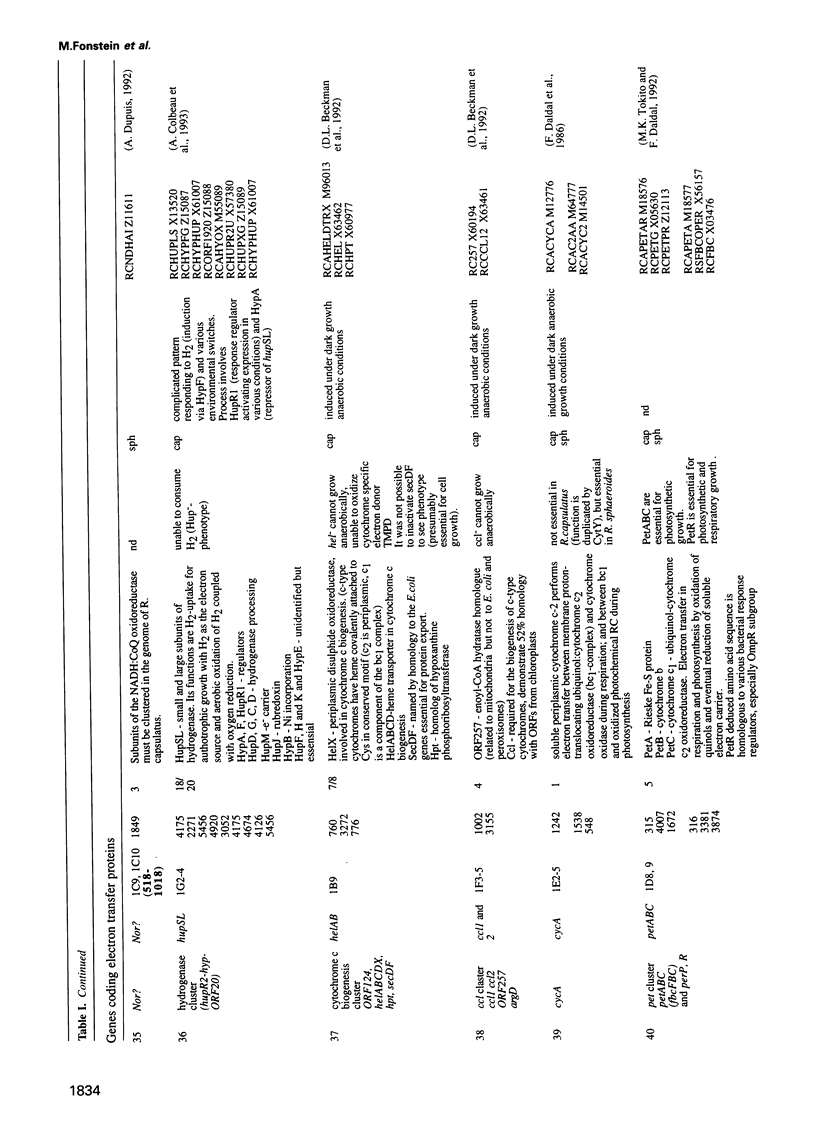
Cosmids from a library containing Rhodobacter capsulatus DNA fragments were previously ordered in two contigs: one corresponding to the chromosome and one to a 134 kb plasmid. This map contained 40 regions connected only by colony hybridization. To confirm the linkage and correct the map, the actual sizes of the overlaps were determined by blot-hybridization with Rhodobacter chromosomal DNA and by mapping of additional cosmids. Several revisions of the earlier map include single cosmid shifts and inversions. One additional gap in a cosmid contig was also found, raising the possibility that the chromosome is not a contiguous circle. About 2500 additional EcoRI,BamHI and HindIII restriction sites were added to the 560 EcoRV sites previously mapped onto the Rhodobacter chromosome, increasing the resolution of the physical map to the size of individual genes. Twenty-five new markers were located on the genetic map. The 48 markers now mapped represent nearly 300 genes and ORFs cloned from different species of Rhodobacter. The orientation of transcription of the four rrn operons was established using 16S rRNA- and 23S rRNA-specific probes and digestion with the rare-cutting enzyme, CeuI. Gel blots of 192 cosmids of the miniset of R.capsulatus digested with EcoRV were prepared. Such a hybridization template represents the whole genome cut into 560 DNA fragments varying in size from 0.4 to 25 kb. This template was used for high-resolution mapping of single genes, analysis of total genomic DNAs from related Rhodobacter strains and differentially expressed RNAs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong G. A., Alberti M., Leach F., Hearst J. E. Nucleotide sequence, organization, and nature of the protein products of the carotenoid biosynthesis gene cluster of Rhodobacter capsulatus. Mol Gen Genet. 1989 Apr;216(2-3):254–268. doi: 10.1007/BF00334364. [DOI] [PubMed] [Google Scholar]
- Azevedo V., Alvarez E., Zumstein E., Damiani G., Sgaramella V., Ehrlich S. D., Serror P. An ordered collection of Bacillus subtilis DNA segments cloned in yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6047–6051. doi: 10.1073/pnas.90.13.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Rudzik M., Young D. A., Marrs B. L. Sequence of the indoleglycerol phosphate synthase (trpC) gene from Rhodobacter capsulatus. J Bacteriol. 1992 Aug;174(16):5482–5484. doi: 10.1128/jb.174.16.5482-5484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman D. L., Trawick D. R., Kranz R. G. Bacterial cytochromes c biogenesis. Genes Dev. 1992 Feb;6(2):268–283. doi: 10.1101/gad.6.2.268. [DOI] [PubMed] [Google Scholar]
- Birkenbihl R. P., Vielmetter W. Cosmid-derived map of E. coli strain BHB2600 in comparison to the map of strain W3110. Nucleic Acids Res. 1989 Jul 11;17(13):5057–5069. doi: 10.1093/nar/17.13.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukanov N. O., Berg D. E. Ordered cosmid library and high-resolution physical-genetic map of Helicobacter pylori strain NCTC11638. Mol Microbiol. 1994 Feb;11(3):509–523. doi: 10.1111/j.1365-2958.1994.tb00332.x. [DOI] [PubMed] [Google Scholar]
- Charlebois R. L., Schalkwyk L. C., Hofman J. D., Doolittle W. F. Detailed physical map and set of overlapping clones covering the genome of the archaebacterium Haloferax volcanii DS2. J Mol Biol. 1991 Dec 5;222(3):509–524. doi: 10.1016/0022-2836(91)90493-p. [DOI] [PubMed] [Google Scholar]
- Chuang S. E., Daniels D. L., Blattner F. R. Global regulation of gene expression in Escherichia coli. J Bacteriol. 1993 Apr;175(7):2026–2036. doi: 10.1128/jb.175.7.2026-2036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill G. A., Daniels D. L., Waterman M. S. The distribution of restriction enzyme sites in Escherichia coli. Nucleic Acids Res. 1990 Feb 11;18(3):589–597. doi: 10.1093/nar/18.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbeau A., Richaud P., Toussaint B., Caballero F. J., Elster C., Delphin C., Smith R. L., Chabert J., Vignais P. M. Organization of the genes necessary for hydrogenase expression in Rhodobacter capsulatus. Sequence analysis and identification of two hyp regulatory mutants. Mol Microbiol. 1993 Apr;8(1):15–29. doi: 10.1111/j.1365-2958.1993.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Daldal F., Cheng S., Applebaum J., Davidson E., Prince R. C. Cytochrome c(2) is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., Kaplan S. Genetic techniques in rhodospirillaceae. Methods Enzymol. 1991;204:459–485. doi: 10.1016/0076-6879(91)04024-i. [DOI] [PubMed] [Google Scholar]
- Dupuis A. Identification of two genes of Rhodobacter capsulatus coding for proteins homologous to the ND1 and 23 kDa subunits of the mitochondrial Complex I. FEBS Lett. 1992 Apr 20;301(2):215–218. doi: 10.1016/0014-5793(92)81250-p. [DOI] [PubMed] [Google Scholar]
- Eiglmeier K., Honoré N., Woods S. A., Caudron B., Cole S. T. Use of an ordered cosmid library to deduce the genomic organization of Mycobacterium leprae. Mol Microbiol. 1993 Jan;7(2):197–206. doi: 10.1111/j.1365-2958.1993.tb01111.x. [DOI] [PubMed] [Google Scholar]
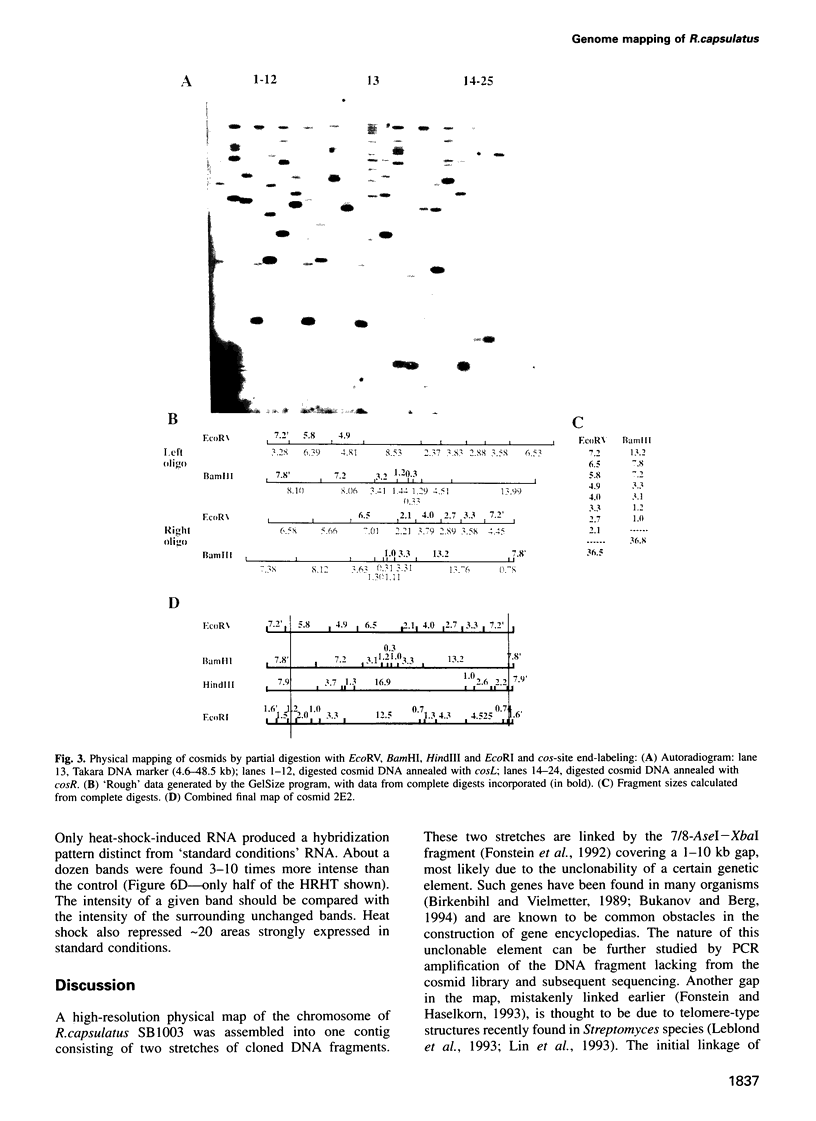
- Fonstein M., Haselkorn R. Chromosomal structure of Rhodobacter capsulatus strain SB1003: cosmid encyclopedia and high-resolution physical and genetic map. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2522–2526. doi: 10.1073/pnas.90.6.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonstein M., Nikolskaya T., Zaporojets D., Nikolsky Y., Kulakauskas S., Mironov A. Tn10-mediated inversions fuse uridine phosphorylase (udp) and rRNA genes of Escherichia coli. J Bacteriol. 1994 Apr;176(8):2265–2271. doi: 10.1128/jb.176.8.2265-2271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonstein M., Zheng S., Haselkorn R. Physical map of the genome of Rhodobacter capsulatus SB 1003. J Bacteriol. 1992 Jun;174(12):4070–4077. doi: 10.1128/jb.174.12.4070-4077.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkl H., Vandekerckhove J., Drews G., Tadros M. H. Molecular cloning, sequence analysis and expression of the gene for catalase-peroxidase (cpeA) from the photosynthetic bacterium Rhodobacter capsulatus B10. Eur J Biochem. 1993 May 15;214(1):251–258. doi: 10.1111/j.1432-1033.1993.tb17918.x. [DOI] [PubMed] [Google Scholar]
- Gibson J. L., Falcone D. L., Tabita F. R. Nucleotide sequence, transcriptional analysis, and expression of genes encoded within the form I CO2 fixation operon of Rhodobacter sphaeroides. J Biol Chem. 1991 Aug 5;266(22):14646–14653. [PubMed] [Google Scholar]
- Hill C. W., Gray J. A. Effects of chromosomal inversion on cell fitness in Escherichia coli K-12. Genetics. 1988 Aug;119(4):771–778. doi: 10.1093/genetics/119.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger U., Liebetanz R., Tichy H. V., Drews G. Cloning and sequencing of the hemA gene of Rhodobacter capsulatus and isolation of a delta-aminolevulinic acid-dependent mutant strain. Mol Gen Genet. 1990 May;221(3):371–378. doi: 10.1007/BF00259402. [DOI] [PubMed] [Google Scholar]
- Höpfl P., Ludwig W., Schleifer K. H. Complete nucleotide sequence of a 23S ribosomal RNA gene from Rhodobacter capsulatus. Nucleic Acids Res. 1988 Mar 25;16(5):2343–2343. doi: 10.1093/nar/16.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenney F. E., Jr, Daldal F. A novel membrane-associated c-type cytochrome, cyt cy, can mediate the photosynthetic growth of Rhodobacter capsulatus and Rhodobacter sphaeroides. EMBO J. 1993 Apr;12(4):1283–1292. doi: 10.1002/j.1460-2075.1993.tb05773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. A., Wong W. K., Beatty J. T. Expression of cellulase genes in Rhodobacter capsulatus by use of plasmid expression vectors. J Bacteriol. 1986 Aug;167(2):604–610. doi: 10.1128/jb.167.2.604-610.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R., Haselkorn R. The DNA sequence of the Rhodobacter capsulatus ntrA, ntrB and ntrC gene analogues required for nitrogen fixation. Mol Gen Genet. 1989 Feb;215(3):507–516. doi: 10.1007/BF00427050. [DOI] [PubMed] [Google Scholar]
- Klipp W., Masepohl B., Pühler A. Identification and mapping of nitrogen fixation genes of Rhodobacter capsulatus: duplication of a nifA-nifB region. J Bacteriol. 1988 Feb;170(2):693–699. doi: 10.1128/jb.170.2.693-699.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kranz R. G., Beckman D. L., Foster-Hartnett D. DNA gyrase activities from Rhodobacter capsulatus: analysis of target(s) of coumarins and cloning of the gyrB locus. FEMS Microbiol Lett. 1992 May 15;72(1):25–32. doi: 10.1016/0378-1097(92)90484-6. [DOI] [PubMed] [Google Scholar]
- Kranz R. G., Pace V. M., Caldicott I. M. Inactivation, sequence, and lacZ fusion analysis of a regulatory locus required for repression of nitrogen fixation genes in Rhodobacter capsulatus. J Bacteriol. 1990 Jan;172(1):53–62. doi: 10.1128/jb.172.1.53-62.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuspa A., Vollrath D., Cheng Y., Kaiser D. Physical mapping of the Myxococcus xanthus genome by random cloning in yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8917–8921. doi: 10.1073/pnas.86.22.8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond P., Redenbach M., Cullum J. Physical map of the Streptomyces lividans 66 genome and comparison with that of the related strain Streptomyces coelicolor A3(2). J Bacteriol. 1993 Jun;175(11):3422–3429. doi: 10.1128/jb.175.11.3422-3429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. S., Kieser H. M., Hopwood D. A., Chen C. W. The chromosomal DNA of Streptomyces lividans 66 is linear. Mol Microbiol. 1993 Dec;10(5):923–933. doi: 10.1111/j.1365-2958.1993.tb00964.x. [DOI] [PubMed] [Google Scholar]
- Liu S. L., Hessel A., Sanderson K. E. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6874–6878. doi: 10.1073/pnas.90.14.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Kaplan S. 5-Aminolevulinic acid availability and control of spectral complex formation in hemA and hemT mutants of Rhodobacter sphaeroides. J Bacteriol. 1993 Apr;175(8):2304–2313. doi: 10.1128/jb.175.8.2304-2313.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Kaplan S. Rhodobacter sphaeroides rdxA, a homolog of Rhizobium meliloti fixG, encodes a membrane protein which may bind cytoplasmic [4Fe-4S] clusters. J Bacteriol. 1992 Oct;174(20):6444–6454. doi: 10.1128/jb.174.20.6444-6454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pille S., Chuat J. C., Breton A. M., Clément-Métral J. D., Galibert F. Cloning, nucleotide sequence, and expression of the Rhodobacter sphaeroides Y thioredoxin gene. J Bacteriol. 1990 Mar;172(3):1556–1561. doi: 10.1128/jb.172.3.1556-1561.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preker P., Hübner P., Schmehl M., Klipp W., Bickle T. A. Mapping and characterization of the promoter elements of the regulatory nif genes rpoN, nifA1 and nifA2 in Rhodobacter capsulatus. Mol Microbiol. 1992 Apr;6(8):1035–1047. doi: 10.1111/j.1365-2958.1992.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Rackwitz H. R., Zehetner G., Murialdo H., Delius H., Chai J. H., Poustka A., Frischauf A., Lehrach H. Analysis of cosmids using linearization by phage lambda terminase. Gene. 1985;40(2-3):259–266. doi: 10.1016/0378-1119(85)90048-4. [DOI] [PubMed] [Google Scholar]
- Riley M. Functions of the gene products of Escherichia coli. Microbiol Rev. 1993 Dec;57(4):862–952. doi: 10.1128/mr.57.4.862-952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott M. A., Witthuhn V. C., Schilke B. A., Soranno M., Ali A., Donohue T. J. Genetic evidence for the role of isocytochrome c2 in photosynthetic growth of Rhodobacter sphaeroides Spd mutants. J Bacteriol. 1993 Jan;175(2):358–366. doi: 10.1128/jb.175.2.358-366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki K., Miyatake Y., Young D. A., Marrs B. L., Matsubara H. A plant-ferredoxin-like gene is located upstream of ferredoxin I gene (fdxN) of Rhodobacter capsulatus. Nucleic Acids Res. 1990 Feb 25;18(4):1060–1060. doi: 10.1093/nar/18.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüddekopf K., Hennecke S., Liese U., Kutsche M., Klipp W. Characterization of anf genes specific for the alternative nitrogenase and identification of nif genes required for both nitrogenases in Rhodobacter capsulatus. Mol Microbiol. 1993 May;8(4):673–684. doi: 10.1111/j.1365-2958.1993.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Sganga M. W., Aksamit R. R., Cantoni G. L., Bauer C. E. Mutational and nucleotide sequence analysis of S-adenosyl-L-homocysteine hydrolase from Rhodobacter capsulatus. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6328–6332. doi: 10.1073/pnas.89.14.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. G., Hamblin M. J., Kelly D. J. Purification, characterization and nucleotide sequence of the periplasmic C4-dicarboxylate-binding protein (DctP) from Rhodobacter capsulatus. Mol Microbiol. 1991 Dec;5(12):3055–3062. doi: 10.1111/j.1365-2958.1991.tb01865.x. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Suwanto A., Kaplan S. Physical and genetic mapping of the Rhodobacter sphaeroides 2.4.1 genome: presence of two unique circular chromosomes. J Bacteriol. 1989 Nov;171(11):5850–5859. doi: 10.1128/jb.171.11.5850-5859.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichy H. V., Oberlé B., Stiehle H., Schiltz E., Drews G. Genes downstream from pucB and pucA are essential for formation of the B800-850 complex of Rhodobacter capsulatus. J Bacteriol. 1989 Sep;171(9):4914–4922. doi: 10.1128/jb.171.9.4914-4922.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokito M. K., Daldal F. petR, located upstream of the fbcFBC operon encoding the cytochrome bc1 complex, is homologous to bacterial response regulators and necessary for photosynthetic and respiratory growth of Rhodobacter capsulatus. Mol Microbiol. 1992 Jun;6(12):1645–1654. doi: 10.1111/j.1365-2958.1992.tb00889.x. [DOI] [PubMed] [Google Scholar]
- Toussaint B., Bosc C., Richaud P., Colbeau A., Vignais P. M. A mutation in a Rhodobacter capsulatus gene encoding an integration host factor-like protein impairs in vivo hydrogenase expression. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10749–10753. doi: 10.1073/pnas.88.23.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint B., Delic-Attree I., De Sury D'Aspremont R., David L., Vinçon M., Vignais P. M. Purification of the integration host factor homolog of Rhodobacter capsulatus: cloning and sequencing of the hip gene, which encodes the beta subunit. J Bacteriol. 1993 Oct;175(20):6499–6504. doi: 10.1128/jb.175.20.6499-6504.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieselmann B. A., Charlebois R. L. Transcriptionally active regions in the genome of the archaebacterium Haloferax volcanii. J Bacteriol. 1992 Jan;174(1):30–34. doi: 10.1128/jb.174.1.30-34.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui S., Yu L. Subunit IV (Mr = 14,384) of the cytochrome b-c1 complex from Rhodobacter sphaeroides. Cloning, DNA sequencing, and ubiquinone binding domain. J Biol Chem. 1991 Aug 25;266(24):15644–15649. [PubMed] [Google Scholar]
- Wellington C. L., Beatty J. T. Promoter mapping and nucleotide sequence of the bchC bacteriochlorophyll biosynthesis gene from Rhodobacter capsulatus. Gene. 1989 Nov 30;83(2):251–261. doi: 10.1016/0378-1119(89)90111-x. [DOI] [PubMed] [Google Scholar]
- Wenzel R., Herrmann R. Cloning of the complete Mycoplasma pneumoniae genome. Nucleic Acids Res. 1989 Sep 12;17(17):7029–7043. doi: 10.1093/nar/17.17.7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison J. C. An essential gene (efg) located at 38.1 minutes on the Escherichia coli chromosome. J Bacteriol. 1992 Sep;174(17):5765–5766. doi: 10.1128/jb.174.17.5765-5766.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison J. C. Biochemical genetics revisited: the use of mutants to study carbon and nitrogen metabolism in the photosynthetic bacteria. FEMS Microbiol Rev. 1993 Jan;10(1-2):1–38. doi: 10.1111/j.1574-6968.1993.tb05862.x. [DOI] [PubMed] [Google Scholar]
- Wu L. F., Saier M. H., Jr Nucleotide sequence of the fruA gene, encoding the fructose permease of the Rhodobacter capsulatus phosphotransferase system, and analyses of the deduced protein sequence. J Bacteriol. 1990 Dec;172(12):7167–7178. doi: 10.1128/jb.172.12.7167-7178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Hu N. T., Marrs B. L. Characterization of the gene transfer agent made by an overproducer mutant of Rhodopseudomonas capsulata. J Mol Biol. 1979 Jun 25;131(2):157–168. doi: 10.1016/0022-2836(79)90071-8. [DOI] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J Bacteriol. 1976 May;126(2):619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Hearst J. E. Regulation of expression of genes for light-harvesting antenna proteins LH-I and LH-II; reaction center polypeptides RC-L, RC-M, and RC-H; and enzymes of bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus by light and oxygen. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7613–7617. doi: 10.1073/pnas.83.20.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]