Abstract
Lipids in the skin are the most diverse in the entire human body. Their bioactivity in health and disease is underexplored. Prostaglandin D2 has recently been identified as a factor which is elevated in the bald scalp of men with androgenetic alopecia and has the capacity to decrease hair lengthening. An enzyme which synthesizes it, prostaglandin D2 synthase (PTGDS or lipocalin-PGDS) is hormone responsive in multiple other organs. PGD2 has two known receptors, GPR44 and PTGDR. GPR44 was found to be necessary for the decrease in hair growth by PGD2. This creates an exciting opportunity to perhaps create novel treatments for androgenetic alopecia which inhibit the activity of PTGDS, PGD2 or GPR44. This review discusses the current knowledge surrounding PGD2 and future steps needed to translate these findings into novel therapies for patients with androgenetic alopecia.
Introduction
Male pattern baldness, or androgenetic alopecia (AGA), is the cause of much anguish. Because hair is so tied to a person’s physical appearance and sexual identity, its loss motivates a search for treatments. Current treatments are based on pathophysiological models which have not significantly gone beyond Aristotle’s observation that “eunuchs do not go bald.” Therefore modern science has extended this observation to demonstrate the importance of testosterone, and treatments against 5-α-reductase type II (finasteride) are currently a mainstay of treatment(1). However, serendipity has also shaped the alopecia armamentarium with the observation that minoxidil, originally used to treat hypertension, also causes hair growth, thus prompting its addition as a treatment for AGA(2).
Besides the above two therapies and, more successfully, hair transplantation, little else is effectively used to augment hair growth in AGA(3). This is partly because our knowledge of the pathogenesis is still meager and offers few rationale targets for therapy. Recently, however, work from our group and others have added to the understanding of AGA and suggested novel therapies(4–6). We have demonstrated that Prostaglandin D2 is elevated in male balding scalp and that it functionally inhibits hair lengthening through its receptor GPR44, which directly suggests treatments for male pattern hair loss. This review will discuss both the advancements and future questions which must be addressed before we really understand whether these novel insights into AGA can be translated into meaningful interventions for patients.
One recent product to stimulate hair growth has been bimatoprost (Latisse). Bimatoprost is a prostaglandin analogue of Prostaglandin F2a and is prescribed to augment the growth of eyelash hair, with extensive efforts to extend this for the treatment of scalp alopecias(7, 8). Latanoprost and Bimatoprost were both used to decrease ocular pressure in glaucoma and were incidentally noted to cause hair lengthening(9–11). There exists a precedence for suspecting the importance of lipids in hair follicle function(12, 13), particularly in the context of the sebaceous gland and scarring alopecias (14–16). However, the discovery of the potency for latanoprost and bimatoprost to induce human hair lengthening remains some of the strongest evidence to implicate prostaglandins in hair follicle function.
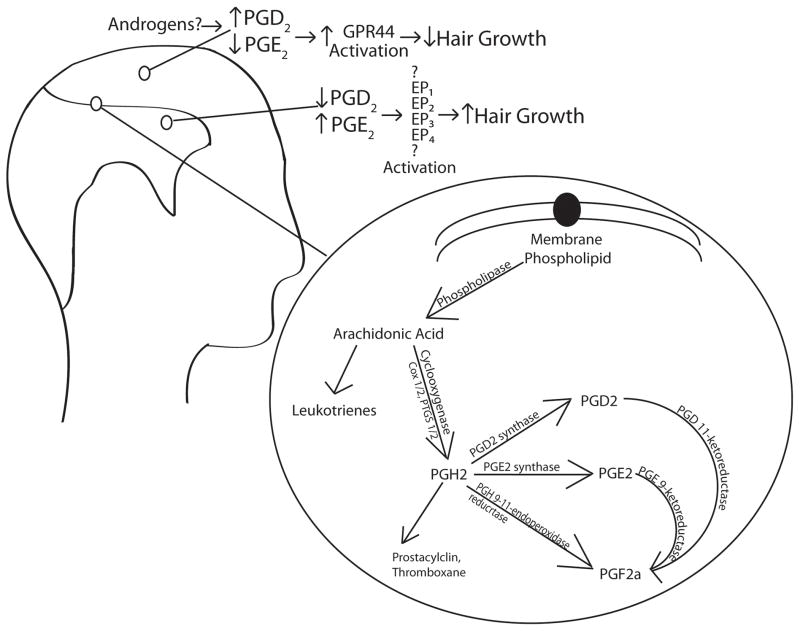
The synthesis and metabolism of prostaglandins or eicosanoids is a very actively studied area, not only given its importance in skin biology such as inflammation(17). The cascade begins with membrane phospholipids which are metabolized by phospholipase into arachidonic acid. This lipid is the substrate for a host of different pathways including the leukotrienes, but also is a substrate for the cyclooxygenase enzymes (COX1/2 or PTGS ½). The cyclooxygenase enzymes produce PGH2 which is converted into selectively different prostaglandin species by specific synthases. Multiple isoforms of synthases can act to create each individual prostaglandin species. For example, PGD2 has at least 2 synthase enzymes using PGH2 as a substrate(18). PGF2a can also be synthesized from PGH2 also through the enzyme PGH 9-11-endoperoxide reductase, but also from PGE2 or PGD2 via the enzymes PGE 9-ketoreductase and PGD 11-ketoreductase(19, 20) respectively. Prostaglandin synthesis enzymes are expressed in the skin diffusely including hair follicles(20, 21) and sebaceous glands(22). Complicating understanding of these pathways is that minutely different lipid compound substrates with very similar chemical structures can often still be metabolized by these same enzymes leading to distinct products with undetermined biologic significance.
The metabolism of prostaglandins is similarly complex. Two important proteins for prostaglandin metabolism include 15-PGDH and SLC02A1 or PGT. 15-PGDH is present in the hair follicle(23), catabolizes prostaglandins(24), and has been shown to be a tumor suppressor(25, 26) given PGE2 pro-carcinogenic effects(27, 28). Similarly, SLC02A1 or PGT functions as a prostaglandin transporter which is important for the uptake and/or clearance of prostaglandins and when mutated in human syndromes includes skin symptoms(29). Given the plethora of potential chemical reactions on prostaglandins and the number of incompletely characterized lipid metabolizing enzymes in the human genome, it is very likely that many important pathways are yet to be defined.
Brain type lipocalin Prostaglandin D2 synthase (PTGDS) in AGA
PTGDS role in hair biology was initially identified in an unbiased screen comparing bald versus haired scalp in male AGA patients, using the patient’s own haired scalp as an internal control for every analysis(4). It was one of the most abundant transcripts in bald scalp compared to haired scalp. PTGDS is a prostaglandin synthase enzyme which acts downstream of the cyclooxgygenase enzymes as mentioned above (30). PTGDS generates PGD2(31–34). Therefore this array finding suggested that PGD2, the product of PTGDS, would be elevated in bald scalp of men.
PTGDS was confirmed to be elevated in bald scalp by mRNA, protein and also its enzymatic product, PGD2. Importantly, its increase was confirmed by mass spectrometry which has been shown to be superior to conventional ELISA based methods which use marginally specific antibodies for the selective identification of individual prostaglandin species(35). We showed that the normal expression of PTGDS matched the temporal and spatial expression of hair follicle areas which were lost in the dying phase of the hair cycle. PGD2 levels peaked 7 fold higher than baseline levels immediately preceding catagen. PTGDS was expressed in the outer root sheath inferior to the bulge.
Having confirmed the correlation between PGD2 and alopecia, the most critical question was its functional relevance. The next goal was functional efforts to identify the role of PGD2. All the above amounts to a correlation; the main difficulty of most array studies is that causality is not clear. Any items found to be elevated in bald scalp might be a product of that state rather than a cause of it. Therefore it was important to demonstrate that PGD2 indeed did decrease hair lengthening in both man and mouse. Therefore, we tested the effect of PGD2 on mouse models with inactivating mutations in each of the receptors for PGD2— PTGDR(DP-1) or GPR44 (DP-2). While PTGDR was not required for PGD2 to inhibit hair growth, GPR44 was required. Recent work from our group has shown that just as PGD2 and GPR44 inhibit hair growth, they also inhibit hair follicle regeneration after wounding(36). Current work is now focused on demonstrating that in human follicles, already identified GPR44 inhibitors also can reverse the hair growth inhibition by PGD2. Recent work has also confirmed that the hematopoietic type of PGD2 synthase (HPGDS) also correlates with areas of hair loss(37).
Reasons why PTGDS involvement in AGA makes sense
All the above is good evidence for the importance of PGD2 in the pathogenesis of AGA. The next question is the exact degree of importance. Is PGD2 the dominant agent which is downstream of testosterone and inhibits hair growth? Or is it one of many agents and itself only a minor contributor to AGA. The following observations support that PGD2 might be a dominant player, though clearly more work is required (see below).
-
Prostaglandins are already known to modulate hair function.
Prostaglandins have already been shown to be important in hair follicle function(7), and even are clinically useful. The best examples are analogues of PGF2a which have been shown in people to increase hair growth. In many ways reminiscent of the discovery of minoxidil, they were not rationally discovered but do give investigators a clue that the pathways which normally control hair lengthening involve prostaglandins. Also, mice overexpressing the COX2 enzyme develop alopecia(38, 39), though mice generally do not show AGA. Therefore the discovery of PTGDS on an unbiased screen is consistent with the above.
-
Prostaglandins often have opposing functions.
Prostaglandins typically control bodily functions in a “yin and yang” manner where they have opposing functions(30). This explains the observation that despite the involvement of prostaglandins in a multitude of organ systems, COX inhibitors like aspirin typically have very restricted clinical effects— because they will inhibit both system activators and system inhibitors with a net negligible effect. For example in the case of bronchial muscle tone, PGD2 increases contraction while PGE2 increases relaxation. In the case of hair, it is known that PGE2 and PGF2a stimulate hair growth, while PGD2 inhibits hair growth(9, 10, 40). This discovery therefore fits current models of prostaglandin function.
-
PTGDS is hormonally responsive in other systems
PTGDS is present in seminal fluid and epididymis. PTGDS expression has been shown to decrease with castration and increase after androgen reintroduction(41–43). But PGDS is also estrogen responsive in some systems(44). Finally, although PGD2 has not been tested, PGE2 has been shown to induce male mating behavior circuits in the developing brain (45). Given low androgen receptor levels in keratinocytes (46–49), an effect of androgens on PTGDS expression might be indirect. Therefore it is clear hormones frequently modulate prostaglandin levels which would be consistent with their role in AGA.
-
PGD2 receptors are in the outer root sheath of the hair follicle.
Early reports are consistent with receptor’s localization within among other areas to the upper and lower outer root sheath region as well as the dermal papilla (21). Therefore the location of receptors is appropriate to imagine a functional importance for hair follicle activity.
-
PGD2 might explain sebaceous hyperplasia in AGA
Although controversial, PGD2 and its non-enzymatic break down product 15-deoxy-delta 12,14-prostagladin J2 are published as ligands for PPARgamma(50, 51). PPARgamma is a known master transcription factor for adipose development and also supports sebaceous gland function(52). Therefore it is tempting to consider that the high levels of PGD2 might explain the dramatic increase in sebaceous gland size in AGA. Whether the sebaceous gland hyperactivity is just a byproduct of AGA pathogenesis, or instead actively promotes alopecia is not fully known and also requires more study.
-
Lipid based pathways are underexplored area of biology
Given that lipid biology is comparatively understudied to genes and proteins, it is possible that it has been overlooked in this very common condition.
-
Minoxidil has effects on prostaglandins
In early literature on the mechanism of minoxidil’s effect on reducing blood pressure, it was noted that it has the capacity to increase PGE2, which has been shown to be reduced in AGA (53–55). Although not proven, minoxidil’s effects on prostaglandins would be consistent with their aberrant regulation in AGA.
-
Recent genetic analysis show associations of AGA with GPR44 which approach significance.
Although not fully statistically significant, a recent GWAS dataset of AGA was analyzed for associations to the PGD2 pathway(56). GPR44 was nominally significant (p=0.03). The authors point out that larger sample sets might improve this association.
-
Classic developmental pathways controlling hair morphogenesis likely will not be directly affected in a disease like AGA.
Many potent developmental pathways have been shown to modulate hair follicle function (wnts, shh for example(57)). However, if these pathways were directly modulated by androgens in AGA for example, the phenotype of AGA would likely be considerably broader. A more plausible model is that AGA will modulate upstream pathways which only indirectly modulate these very powerful developmental pathways. Prostaglandins might be that modulator factor.
Outstanding questions the field must solve
-
Replication by other groups.
Given the novelty of these findings, it will be important that other groups independently assess PGD2 using highly accurate methods such as mass spectrometry. If PGD2 is elevated in bald scalp in those studies, other questions include: Are the elevations of PGD2 heterogenous within areas of the scalp? What are the PGD2 levels in early versus late AGA disease?
-
Do prostaglandins simply modulate hair growth rates or actually hair cycling?
Most hair biologists believe the final length of a hair shaft is determined mostly on the time spent in the anagen growth phase versus the other phases of the hair cycle. Interestingly, published data with prostaglandins are more consistent with modulations on hair follicle growth rate and not cycling. If the case, this is an underappreciated mechanism of regulating hair growth. However, detailed studies to test this have not been performed.
-
More in depth descriptions of PGD2 in hair biology
There are a host of important questions to answer. How does the prostaglandin D2 pathway change during the human hair cycle? Although much of the previous work on the fluctuations in the PGD2 pathway during the hair cycle have been done on mouse, more work investigating human hair cycle fluctuations are important. Similarly, is PGD2 not involved in senile alopecia? Does PGD2 induce recruitment of inflammatory infiltrates seen in AGA; can fibrosis be recapitulated after PGD2 exposure?
-
Create mouse genetic models of increased PGD2 in the skin.
Although a mouse with elevations of PGD2 demonstrates alopecia (COX2 mouse), it also has increases in other prostaglandin species as well. Does a mouse with unique elevations of PGD2 also demonstrate alopecia?
-
Demonstration that in skin PTGDS is hormonally responsive.
-
Do women have similar increases in PGD2?
All the original work on PGD2 in alopecia was done on males, but do females with patterned alopecia show similar changes?
-
Visualization of where PGD2 is located.
Methods exist which combine mass spectrometry with 2-D samples to identify localization of lipid species(58). Using these techniques, in what compartment is PGD2 elevated?
-
Does PGD2 inhibition help AGA? While multiple GPR44 inhibitors are marketed, their individual selectivity for GPR44 is quite variable. If optimum GPR44 inhibition occurs, can hair miniaturization be reversed?
To patients with AGA this is the only question of concern. Many groups are actively searching for GPR44 inhibitors already. However, there are important issues in designing a therapy which might be effective for AGA. One issue is timing: must inhibition of the pathway being in puberty when circulating androgens increase to prevent any increases of PGD2? Is the inhibition of PGD2 on hair growth reversible? Although GPR44 therapies are being designed for allergic diseases, their usefulness in AGA might be compromised if the necessary schedule and delivery are different for inhibiting GPR44 in the hair follicle. The larger the number of candidate compounds to test for GPR44 inhibition in the hair follicle, the greater likelihood of success. If existing candidates for GPR44 inhibition fail to prove useful, the search for new candidates will be more pertinent. Given the likely important locus of expression of GPR44 in keratinocytes, the development of keratinocyte reporter cell lines for GPR44 activity would allow for large scale screening of compounds to inhibit this pathway and provide a pipeline for potential small molecule candidates.
If appropriate GPR44 inhibition can be achieved, the most important of all the above questions will address the reversibility of AGA in human subjects.
Conclusions and Perspective
An outline is emerging for a fundamental role of prostaglandins in modulating hair function. Inhibition of PTGDS might help male pattern hair loss, conceivably with agents as simple as selenium chloride(59). But considering this arena as much larger and given that the skin has one of the most diverse repertoire of lipids in the entire human body(60), the possibilities are vast for identifying bioactive lipids which will inhibit or promote hair growth. The tools are limited, but the task is simple: just find the needle in the haystack.
Figure 1.
Schematic of PGD2 pathway in alopecia
Depicted is our current understanding of prostaglandin synthesis and changes in alopecia.
Acknowledgments
This work was supported by R01AR064297 to LG from NIAMS/NIH. The authors wish to thank Drs. George Cotsarelis and Mayumi Ito for useful discussions on this topic. LG wrote the paper and AN designed the figure and edited the manuscript.
Footnotes
References
- 1.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341(7):491–7. doi: 10.1056/NEJM199908123410706. Epub 1999/08/12. [DOI] [PubMed] [Google Scholar]
- 2.Price VH. Treatment of hair loss. N Engl J Med. 1999;341(13):964–73. doi: 10.1056/NEJM199909233411307. Epub 1999/09/25. [DOI] [PubMed] [Google Scholar]
- 3.Sawaya ME, Hordinsky MK. Advances in alopecia areata and androgenetic alopecia. Adv Dermatol. 1992;7:211–26. discussion 27. Epub 1992/01/01. [PubMed] [Google Scholar]
- 4.Garza LA, Liu Y, Yang Z, Alagesan B, Lawson JA, Norberg SM, et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012;4(126):126ra34. doi: 10.1126/scitranslmed.3003122. Epub 2012/03/24 4/126/126ra34 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garza LA, Yang CC, Zhao T, Blatt HB, Lee M, He H, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121(2):613–22. doi: 10.1172/JCI4447844478. Epub 2011/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22(3):168–71. doi: 10.1111/exd.12024. Epub 2012/09/29. [DOI] [PubMed] [Google Scholar]
- 7.Khidhir KG, Woodward DF, Farjo NP, Farjo BK, Tang ES, Wang JW, et al. The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013;27(2):557–67. doi: 10.1096/fj.12-218156. Epub 2012/10/30. fj.12-218156 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodward DF, Tang ES, Attar M, Wang JW. The biodisposition and hypertrichotic effects of bimatoprost in mouse skin. Exp Dermatol. 2013;22(2):145–8. doi: 10.1111/exd.12071. Epub 2013/01/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnstone MA, Albert DM. Prostaglandin-induced hair growth. Surv Ophthalmol. 2002;47 (Suppl 1):S185–202. doi: 10.1016/s0039-6257(02)00307-7. Epub 2002/09/03 S0039625702003077 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Sasaki S, Hozumi Y, Kondo S. Influence of prostaglandin F2alpha and its analogues on hair regrowth and follicular melanogenesis in a murine model. Exp Dermatol. 2005;14(5):323–8. doi: 10.1111/j.0906-6705.2005.00270.x. Epub 2005/04/28. EXD270 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Wolf R, Matz H, Zalish M, Pollack A, Orion E. Prostaglandin analogs for hair growth: great expectations. Dermatol Online J. 2003;9(3):7. Epub 2003/09/04. [PubMed] [Google Scholar]
- 12.Skolnik P, Eaglstein WH, Ziboh VA. Human essential fatty acid deficiency: treatment by topical application of linoleic acid. Arch Dermatol. 1977;113(7):939–41. Epub 1977/07/01. [PubMed] [Google Scholar]
- 13.Menton DN. The effects of essential fatty acid deficiency on the skin of the mouse. Am J Anat. 1968;122(2):337–55. doi: 10.1002/aja.1001220211. Epub 1968/03/01. [DOI] [PubMed] [Google Scholar]
- 14.Gates AH, Karasek M. Hereditary Absence of Sebaceous Glands in the Mouse. Science. 1965;148(3676):1471–3. doi: 10.1126/science.148.3676.1471. Epub 1965/06/11. 148/3676/1471 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23(3):268–70. doi: 10.1038/15446. Epub 1999/11/05. [DOI] [PubMed] [Google Scholar]
- 16.Sundberg JP, Boggess D, Sundberg BA, Eilertsen K, Parimoo S, Filippi M, et al. Asebia-2J (Scd1(ab2J)): a new allele and a model for scarring alopecia. Am J Pathol. 2000;156(6):2067–75. doi: 10.1016/S0002-9440(10)65078-X. Epub 2000/06/15. S0002-9440(10)65078-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolaou A. Eicosanoids in skin inflammation. Prostaglandins Leukot Essent Fatty Acids. 2013;88(1):131–8. doi: 10.1016/j.plefa.2012.03.009. Epub 2012/04/24. S0952-3278(12)00041-5 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Urade Y, Watanabe K, Hayaishi O. Prostaglandin D, E, and F synthases. J Lipid Mediat Cell Signal. 1995;12(2–3):257–73. doi: 10.1016/0929-7855(95)00032-l. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe K. Prostaglandin F synthase. Prostaglandins Other Lipid Mediat. 2002;68–69:401–7. doi: 10.1016/s0090-6980(02)00044-8. Epub 2002/11/16. [DOI] [PubMed] [Google Scholar]
- 20.Colombe L, Vindrios A, Michelet JF, Bernard BA. Prostaglandin metabolism in human hair follicle. Exp Dermatol. 2007;16(9):762–9. doi: 10.1111/j.1600-0625.2007.00586.x. Epub 2007/08/19. EXD586 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Colombe L, Michelet JF, Bernard BA. Prostanoid receptors in anagen human hair follicles. Exp Dermatol. 2008;17(1):63–72. doi: 10.1111/j.1600-0625.2007.00639.x. Epub 2007/11/17. EXD639 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Alestas T, Ganceviciene R, Fimmel S, Muller-Decker K, Zouboulis CC. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med (Berl) 2006;84(1):75–87. doi: 10.1007/s00109-005-0715-8. Epub 2006/01/03. [DOI] [PubMed] [Google Scholar]
- 23.Michelet JF, Colombe L, Gautier B, Gaillard O, Benech F, Pereira R, et al. Expression of NAD+ dependent 15-hydroxyprostaglandin dehydrogenase and protection of prostaglandins in human hair follicle. Exp Dermatol. 2008;17(10):821–8. doi: 10.1111/j.1600-0625.2008.00706.x. Epub 2008/03/11. EXD706 [pii] [DOI] [PubMed] [Google Scholar]
- 24.Fincham N, Camp R. Novel prostaglandin dehydrogenase in rat skin. Biochem J. 1983;212(1):129–34. doi: 10.1042/bj2120129. Epub 1983/04/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai HH, Chi X, Tong M. Regulation of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) by non-steroidal anti-inflammatory drugs (NSAIDs) Prostaglandins Other Lipid Mediat. 2011;96(1–4):37–40. doi: 10.1016/j.prostaglandins.2011.06.005. Epub 2011/07/19. S1098-8823(11)00045-1 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Yan M, Rerko RM, Platzer P, Dawson D, Willis J, Tong M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci U S A. 2004;101(50):17468–73. doi: 10.1073/pnas.0406142101. Epub 2004/12/03 0406142101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansari KM, Rundhaug JE, Fischer SM. Multiple signaling pathways are responsible for prostaglandin E2-induced murine keratinocyte proliferation. Mol Cancer Res. 2008;6(6):1003–16. doi: 10.1158/1541-7786.MCR-07-2144. Epub 2008/06/24. 6/6/1003 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sung YM, He G, Hwang DH, Fischer SM. Overexpression of the prostaglandin E2 receptor EP2 results in enhanced skin tumor development. Oncogene. 2006;25(40):5507–16. doi: 10.1038/sj.onc.1209538. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki T, Niizeki H, Shimizu A, Shiohama A, Hirakiyama A, Okuyama T, et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68(1):36–44. doi: 10.1016/j.jdermsci.2012.07.008. Epub 2012/08/22. S0923-1811(12)00240-X [pii] [DOI] [PubMed] [Google Scholar]
- 30.Goodman LS, Gilman A, Hardman JG, Gilman AG, Limbird LE. Goodman & Gilman’s the pharmacological basis of therapeutics. 9. xxi. New York: McGraw-Hill, Health Professions Division; 1996. p. 1905. [1] p. folded plate p. [Google Scholar]
- 31.Eguchi N, Minami T, Shirafuji N, Kanaoka Y, Tanaka T, Nagata A, et al. Lack of tactile pain (allodynia) in lipocalin-type prostaglandin D synthase-deficient mice. Proc Natl Acad Sci U S A. 1999;96(2):726–30. doi: 10.1073/pnas.96.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287(5460):2013–7. doi: 10.1126/science.287.5460.2013. [DOI] [PubMed] [Google Scholar]
- 33.Qu WM, Huang ZL, Xu XH, Aritake K, Eguchi N, Nambu F, et al. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci U S A. 2006;103(47):17949–54. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urade Y, Eguchi N. Lipocalin-type and hematopoietic prostaglandin D synthases as a novel example of functional convergence. Prostaglandins & Other Lipid Mediators. 2002;68–69:375–82. doi: 10.1016/s0090-6980(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 35.Bell-Parikh LC, Ide T, Lawson JA, McNamara P, Reilly M, FitzGerald GA. Biosynthesis of 15-deoxy-delta12,14-PGJ2 and the ligation of PPARgamma. J Clin Invest. 2003;112(6):945–55. doi: 10.1172/JCI18012112/6/945. Epub 2003/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson AM, Loy DE, Lawson JA, Katseff AS, Fitzgerald GA, Garza LA. Prostaglandin D2 Inhibits Wound-Induced Hair Follicle Neogenesis through the Receptor, Gpr44. J Invest Dermatol. 2013;133(4):881–9. doi: 10.1038/jid.2012.398. Epub 2012/11/30. jid2012398 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larson AR, Zhan Q, Johnson E, Fragoso AC, Wan M, Murphy GF. A prostaglandin d-synthase-positive mast cell gradient characterizes scalp patterning. J Cutan Pathol. 2013 doi: 10.1111/cup.12286. [DOI] [PubMed] [Google Scholar]
- 38.Muller-Decker K, Leder C, Neumann M, Neufang G, Bayerl C, Schweizer J, et al. Expression of cyclooxygenase isozymes during morphogenesis and cycling of pelage hair follicles in mouse skin: precocious onset of the first catagen phase and alopecia upon cyclooxygenase-2 overexpression. J Invest Dermatol. 2003;121(4):661–8. doi: 10.1046/j.1523-1747.2003.12473.x. [DOI] [PubMed] [Google Scholar]
- 39.Bol DK, Rowley RB, Ho CP, Pilz B, Dell J, Swerdel M, et al. Cyclooxygenase-2 overexpression in the skin of transgenic mice results in suppression of tumor development. Cancer Res. 2002;62(9):2516–21. [PubMed] [Google Scholar]
- 40.Geng L, Hanson WR, Malkinson FD. Topical or systemic 16, 16 dm prostaglandin E2 or WR-2721 (WR-1065) protects mice from alopecia after fractionated irradiation. Int J Radiat Biol. 1992;61(4):533–7. doi: 10.1080/09553009214551291. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H, Ma H, Ni H, Ma XH, Mills N, Yang ZM. Expression and regulation of lipocalin-type prostaglandin d synthase in rat testis and epididymis. Biol Reprod. 2004;70(4):1088–95. doi: 10.1095/biolreprod.103.022079. [DOI] [PubMed] [Google Scholar]
- 42.O’Shaughnessy PJ, Johnston H, Willerton L, Baker PJ. Failure of normal adult Leydig cell development in androgen-receptor-deficient mice. J Cell Sci. 2002;115(Pt 17):3491–6. doi: 10.1242/jcs.115.17.3491. [DOI] [PubMed] [Google Scholar]
- 43.Zhu H, Ma H, Ni H, Ma XH, Mills N, Yang ZM. L-prostaglandin D synthase expression and regulation in mouse testis and epididymis during sexual maturation and testosterone treatment after castration. Endocrine. 2004;24(1):39–45. doi: 10.1385/ENDO:24:1:039. [DOI] [PubMed] [Google Scholar]
- 44.Otsuki M, Gao H, Dahlman-Wright K, Ohlsson C, Eguchi N, Urade Y, et al. Specific regulation of lipocalin-type prostaglandin D synthase in mouse heart by estrogen receptor beta. Mol Endocrinol. 2003;17(9):1844–55. doi: 10.1210/me.2003-0016. [DOI] [PubMed] [Google Scholar]
- 45.Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7(6):643–50. doi: 10.1038/nn1254. Epub 2004/05/25. nn1254 [pii] [DOI] [PubMed] [Google Scholar]
- 46.Choudhry R, Hodgins MB, Van der Kwast TH, Brinkmann AO, Boersma WJ. Localization of androgen receptors in human skin by immunohistochemistry: implications for the hormonal regulation of hair growth, sebaceous glands and sweat glands. J Endocrinol. 1992;133(3):467–75. doi: 10.1677/joe.0.1330467. Epub 1992/06/01. [DOI] [PubMed] [Google Scholar]
- 47.Itami S, Kurata S, Sonoda T, Takayasu S. Interaction between dermal papilla cells and follicular epithelial cells in vitro: effect of androgen. Br J Dermatol. 1995;132(4):527–32. Epub 1995/04/01. [PubMed] [Google Scholar]
- 48.Pelletier G, Ren L. Localization of sex steroid receptors in human skin. Histol Histopathol. 2004;19(2):629–36. doi: 10.14670/HH-19.629. Epub 2004/03/17. [DOI] [PubMed] [Google Scholar]
- 49.Jave-Suarez LF, Langbein L, Winter H, Praetzel S, Rogers MA, Schweizer J. Androgen regulation of the human hair follicle: the type I hair keratin hHa7 is a direct target gene in trichocytes. J Invest Dermatol. 2004;122(3):555–64. doi: 10.1111/j.0022-202X.2004.22336.x. Epub 2004/04/17. JID22336 [pii] [DOI] [PubMed] [Google Scholar]
- 50.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83(5):803–12. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 51.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83(5):813–9. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 52.Trivedi NR, Cong Z, Nelson AM, Albert AJ, Rosamilia LL, Sivarajah S, et al. Peroxisome proliferator-activated receptors increase human sebum production. J Invest Dermatol. 2006;126(9):2002–9. doi: 10.1038/sj.jid.5700336. [DOI] [PubMed] [Google Scholar]
- 53.Michelet JF, Commo S, Billoni N, Mahe YF, Bernard BA. Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. J Invest Dermatol. 1997;108(2):205–9. doi: 10.1111/1523-1747.ep12334249. Epub 1997/02/01. [DOI] [PubMed] [Google Scholar]
- 54.Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37(5):867–74. doi: 10.1016/0006-2952(88)90174-8. [DOI] [PubMed] [Google Scholar]
- 55.Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150(2):186–94. doi: 10.1111/j.1365-2133.2004.05785.x. Epub 2004/03/05. 5785 [pii] [DOI] [PubMed] [Google Scholar]
- 56.Heilmann S, Nyholt DR, Brockschmidt FF, Hillmer AM, Herold C, Maan C, et al. No genetic support for a contribution of prostaglandins to the aetiology of androgenetic alopecia. Br J Dermatol. 2013;169(1):222–4. doi: 10.1111/bjd.12292. Epub 2013/03/02. [DOI] [PubMed] [Google Scholar]
- 57.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118(2):216–25. doi: 10.1046/j.0022-202x.2001.01670.x. Epub 2002/02/14 1670 [pii] [DOI] [PubMed] [Google Scholar]
- 58.Chaurand P, Norris JL, Cornett DS, Mobley JA, Caprioli RM. New developments in profiling and imaging of proteins from tissue sections by MALDI mass spectrometry. J Proteome Res. 2006;5(11):2889–900. doi: 10.1021/pr060346u. Epub 2006/11/04. [DOI] [PubMed] [Google Scholar]
- 59.Lee B, Hirst JJ, Walker DW. Prostaglandin D synthase in the prenatal ovine brain and effects of its inhibition with selenium chloride on fetal sleep/wake activity in utero. J Neurosci. 2002;22(13):5679–86. doi: 10.1523/JNEUROSCI.22-13-05679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicolaides N. Skin lipids: their biochemical uniqueness. Science. 1974;186(4158):19–26. doi: 10.1126/science.186.4158.19. Epub 1974/10/04. [DOI] [PubMed] [Google Scholar]