Abstract
The N-ethylmaleimide-Sensitive Factor (NSF) was one of the initial members of the ATPases Associated with various cellular Activities Plus (AAA+) family. In this review, we discuss what is known about the mechanism of NSF action and how that relates to the mechanisms of other AAA+ proteins. Like other family members, NSF binds to a protein complex (i.e., SNAP-SNARE complex) and utilizes ATP hydrolysis to affect the conformations of that complex. SNAP-SNARE complex disassembly is essential for SNARE recycling and sustained membrane trafficking. NSF is a homo-hexamer; each protomer is composed of an N-terminal domain, NSF-N, and two adjacent AAA-domains, NSF-D1 and NSF-D2. Mutagenesis analysis has established specific roles for many of the structural elements of NSF-D1, the catalytic ATPase domain, and NSF-N, the SNAP-SNARE binding domain. Hydrodynamic analysis of NSF, labeled with (Ni2+-NTA)2-Cy3, detected conformational differences in NSF, in which the ATP-bound conformation appears more compact than the ADP-bound form. This indicates that NSF undergoes significant conformational changes as it progresses through its ATP-hydrolysis cycle. Incorporating these data, we propose a sequential mechanism by which NSF uses NSF-N and NSF-D1 to disassemble SNAP-SNARE complexes. We also illustrate how analytical centrifugation might be used to study other AAA+ proteins.
Keywords: NSF, Membrane Trafficking, ATPase, SNAP, SNARE
1. Introduction
ATPases Associated with various cellular Activities Plus (AAA+) family, so named by Kunau et al. [1], is one of the largest protein superfamilies. Proteins within the AAA+ family contain one or two copies of an homologous ATP-binding domain consisting of 200–250 amino acids (called AAA domain, AAA module or AAA cassette) [2]. The first published sequence of an AAA+ protein was that of the porcine Valosin-Containing Protein (VCP) in 1987 [3]. Since then, AAA+ proteins have been found in all organisms and have been shown to participate in a diverse array of cellular processes, including membrane fusion/transport, organelle biogenesis, proteolysis, protein disaggregation/refolding, DNA replication, DNA transcription, DNA recombination and other processes [4–10].
AAA+ proteins are involved directly or indirectly in a rapidly increasing number of human diseases. Axonemal dynein defects lead to primary ciliary dyskinesia [4]. A dominantly inherited syndrome of inclusion body myopathy associated with Paget's disease of the bone and frontotemporal dementia is caused by mutations in p97/VCP [11]. The loss of a glutamic acid residue in the ER-lumenal AAA+ protein torsinA is responsible for most cases of early-onset, autosomal-dominant, primary dystonia [6]. The human orthologue of Pex1p is mutated in > 70% of Zellweger’s type peroxisome-biogenesis disorders [2]. Decreased production of N-ethylmaleimide-Sensitive Factor (NSF) is associated with epilepsy [12]. ANCCA, an androgen receptor coactivator, is highly expressed in prostate cancer [13]. In many cases (excepting dynein), the AAA+ proteins are involved in the disassembly of some cellular proteins or protein complexes, and their dysfunction or loss of function contributes to these diseases.
2. Structural Features and Function of the AAA+ Proteins
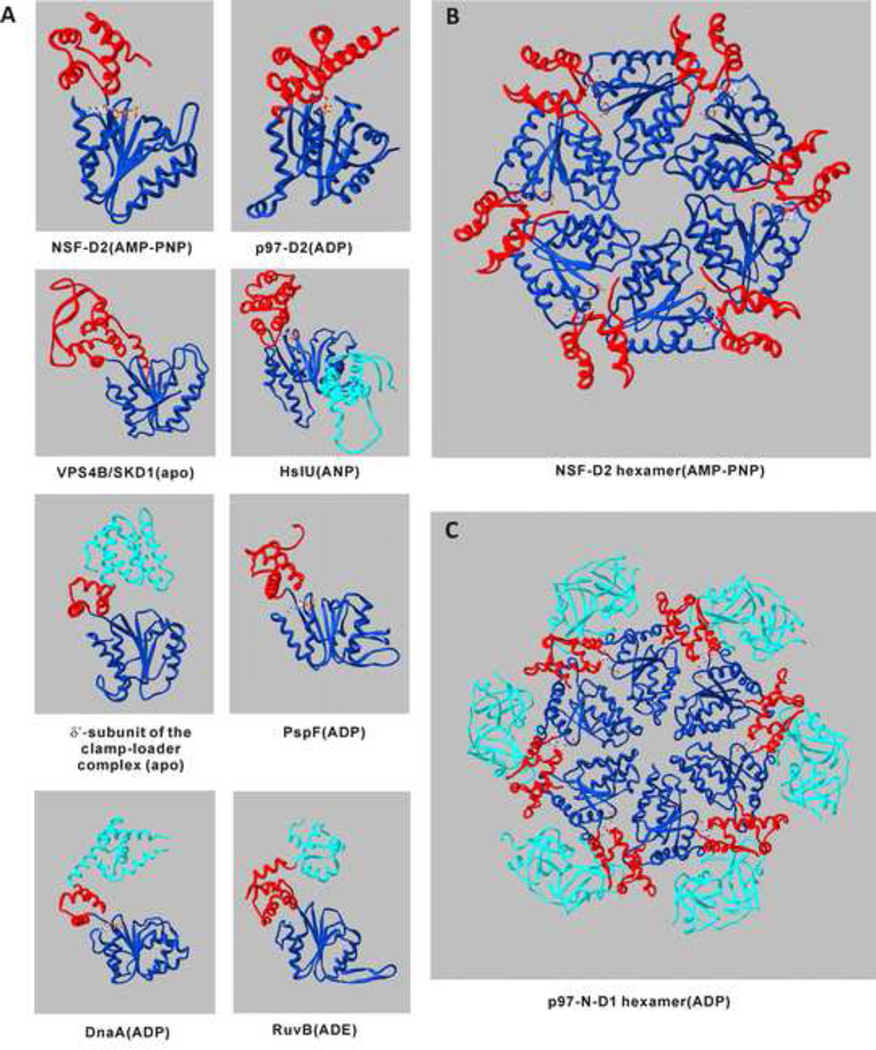
The hallmark of the AAA+ proteins is the AAA domain. Based on the number of AAA domains, the family is divided into two subfamilies: Type I (one AAA domain) and Type II (two AAA domains). Most of the AAA+ proteins contain one domain (such as those involved in proteolysis), while the proteins involved in membrane fusion or organelle biogenesis normally contain two domains [14]. By sequence alignment and crystal structure comparison, the AAA domain is highly conserved [2, 10, 14–18]. It consists of an N-terminal α/β subdomain (dark blue in Figure 1) and a C-terminal α-helical subdomain (red in Figure 1). The numbers of α-helices around the central five-stranded parallel β-sheet and in the C-terminal subdomain vary. Classical AAA+ proteins assemble into closed ring-shaped oligomers (generally hexamers) with a narrow central pore (Figure 1B and Figure 1C). Beside the conserved AAA domain, the AAA+ proteins contain various accessory domains (light blue in Figure 1), which are either attached to the AAA domains or inserted into the AAA domains. These accessory domains are thought to interact with the substrates of the AAA+ proteins [19–24].
Figure 1. Overall Fold of AAA+ Proteins.
Panel A: Ribbon diagram of the AAA domains of NSF-D2 (Chinese Hamster Overy cells) (1D2N), p97-D2 (mice) (3CF0), SKD1/VPS4B (human) (1XWI), HslU (E. coli) (1DO2), δ’-subunit of the clamp-loader complex (E. coli) (1A5T), PspF (E. coli) (2C98), DnaA (Aquifex aeolicus) (1L8Q), RuvB (Thermus thermophilus) (1HQC). In each diagram, α/β subdomain and α-helical subdomain of the AAA module are colored dark blue and red, respectively. The additional I domain of HslU, C domains of δ’-subunit of the clamp-loader complex and RuvB, and additional domain IV of DnaA are shown in light blue. Nucleotides are shown in stick presentation. AMP-PNP: non-hydrolyzable ATP analogue. ADE: adenine. ANP: phosphoaminophosphonic acid-adenylate ester. Panel B: Hexamer of NSF-D2. Panel C: Hexamer of p97-N-D1. The additional N domain is colored in light blue. All the images are based on PDB code of each protein and were generated using Swiss PDB viewer.
Although they have diverse functions, AAA+ proteins all undergo conformational changes as they hydrolyze ATP. Through these conformational changes, they convert the chemical energy from ATP hydrolysis into mechanical work. Three models have been proposed to explain the actions of the closed-ring oligomeric AAA+ proteins: 1) Threading, the substrate polypeptide or polynucleotide is threaded into the central pore of the AAA+ protein’s ring; 2) Prying, the substrate complex is bound at multiple sites and pulled apart; 3) Walking, the substrate is passed from one binding site to the next generating a next movement of the substrate or AAA+ protein [2, 4, 25].
In this review, we will focus on one of the AAA+ proteins, the N-ethylmaleimide-Sensitive Factor (NSF/Sec18p yeast homologue), which is involved in membrane trafficking.
3. N-ethylmaleimide-Sensitive Factor (NSF)
N-ethylmaleimide-Sensitive Factor (NSF) is one of the first members of the AAA+ family and was identified during early molecular analysis of vesicular trafficking between Golgi cisternae [26]. NSF is a homo-hexamer [27], with each protomer of the homo-hexamer having three domains: the N-terminal domain (NSF-N, 1–205), the first ATP-binding domain (NSF-D1, 206–477), and the second ATP-binding domain (NSF-D2, 478–744) [19, 28, 29]. The structure of NSF-D2 was the first AAA domain to be determined [30, 31].
The well-documented role of NSF in vesicular transport is logically based on its interactions with SNAP-binding Receptor, SNAREs [32] and Soluble NSF Attachment Proteins, SNAPs [33], although several studies point to additional roles for NSF [34, 35]. Recent studies also show that NSF may not be constitutively active, but may instead be regulated by several different mechanisms, including inactivation by S-nitrosylation and phosphorylation [36–39].
3.1 SNAREs and SNAPs
SNARE proteins are the minimal machinery for membrane fusion [40]. SNAREs are classified into vesicle (v)- and target-membrane (t)-SNAREs according to their localizations [32] or Arginine (R)- and Glutamine (Q)-SNAREs based on a key residue in the center of their SNARE domains [41]. There are two types of t-SNAREs: Syntaxin-type and SNAP-25-type. All SNARE proteins characteristically contain conserved heptad repeats of approximately 60–70 residues termed the SNARE motif. SNAP-25-like SNAREs contain two such motifs. Structural information from neuronal SNARE complexes shows that the SNARE motifs assemble into parallel, coiled-coil, four-helix bundles by burying the hydrophobic residues inside the core (Figure 2). Three of the helices are contributed by t-SNAREs (1 from Syntaxin and 2 from SNAP-25), with the other helix provided by the v-SNARE. The coiled-coil is composed of 15 hydrophobic layers arranged perpendicular to the axis of the helical bundle, and contains a central hydrophilic zero-layer with one R- and three Q-residues. The surface of the complex is highly grooved with several charged regions [42, 43].
Figure 2. NSF-SNAP-SNARE Particle.
The spark-plug shape of NSF-SNAP-SNARE complex with the two rings of NSF at the wider end and the SNAP-SNARE complex at the thinner end. Each element of the NSF-SNAP-SNARE complex is created from the available crystal structures. The two ATP-binding domains of NSF (D1 and D2) in white are modeled from the NSF-D2 structure (1D2N). A trimer of NSF-N domains, in red, is based on the three-in-three-out model of May et al. 1999 (1QDN). Three yeast α-SNAPs (Sec17p, 1QQE) are depicted in yellow. The coiled-coil SNARE complex is depicted with Syntaxin 1a in light blue, synaptobrevin/VAMP-2, in magenta, and SNAP-25 in dark blue (2BUO). The images were created with Swiss PDB viewer and rendered with Pov-Ray.
Clary et al. [33] determined that NSF required a peripheral membrane protein adaptor to bind Golgi membranes. This protein was called Soluble NSF Attachment Protein or SNAP. In mammals, there are three SNAPs: α, β, and γ. Human α-SNAP and β-SNAP share ~ 85% sequence identity; γ-SNAP only shares 20–25% sequence identity. Both α-SNAP and γ-SNAP are ubiquitous proteins while β-SNAP is a regional-specific brain isoform [44]. In yeast, there is only one, Sec17p, which is 35% identical to human α-SNAP and 22% to γ-SNAP [45]. Since there is large sequence variation among the SNARE family, it seems likely that α-SNAP primarily recognizes the overall shape of the coiled-coil structure but not specific residues. A recent study shows that α-SNAP can inhibit exocytosis at a step downstream of NSF-driven SNARE complex disassembly, revealing a second action of α-SNAP on membrane fusion. It binds to the SNARE motif of free Syntaxin 1, blocking the trans-SNARE complex formation [46].
The crystal structure of Sec17p shows that it contains an N-terminal twisted sheet of α-helical hairpins with a protruding C-terminal, α-helical globular bundle (Figure 2). One edge of the twisted sheet is longer than the other, forming concave and convex faces in the structure. The concave face has a distribution of negative charges, which is most pronounced at the extreme C-terminus. Mutagenesis data show SNAP-SNARE complex interactions involve positively-charged α-SNAP residues distributed over the concave surface of its twisted sheet domain [47]. The crystal structure of γ-SNAP from Brachydanio rerio has been solved. This orthologue has 73% sequence identity to human γ-SNAP, while it is only 21% homologous to human α-SNAP and 22% homologous to Sec17p. From the crystal structure, γ-SNAP adopts a fold very similar to that of Sec17p, although less twisted. The N-terminal and C-terminal portions of γ-SNAP align very well with the corresponding regions of Sec17p and the major difference occurs in the middle of the two proteins. γ-SNAP possesses a group of positively-charged residues on the concave side, which is similar to the residues important for binding of α-SNAP to SNARE complexes. The C-termini of α-SNAP and γ-SNAP are conserved, especially the highly-conserved, penultimate leucine, which is crucial for NSF binding and stimulation of its ATPase [48]. However, α- and γ-SNAP are not equivalent since γ-SNAP has lower specific activity in both Golgi transport and NSF binding assays and it cannot rescue the sec17ts defect (though α-SNAP can) [33].
3.2 Canonical Function of NSF
NSF was one of the first proteins specifically linked to membrane fusion [49] and is critical for most of the heterotypic fusion and some of the homotypic fusion in cells [50–52]. The heightened expression of NSF throughout the central nervous system indicates a central function of NSF in regulating neurotransmitter release [53, 54], although it is required in all cells. Expressing the dominant-negative mutant of NSF (NSF-E329Q) in mammalian cells disrupts the Golgi apparatus, inhibits sulfated glycosaminoglycan (GAG) synthesis and causes cell death [55]. Prominent cell death in many sensory organs and build-up endoplasmic reticulum (ER)-like membrane in hypothalamic neuron are observed in zebrafish expressing a nsf mutant [56].
In yeast, the NSF equivalent is encoded by the Sec18 gene [57]. Sec18p is 48% identical to NSF [49] and is required for multiple steps of membrane trafficking in yeast [58]. At restrictive temperature, the temperature-sensitive mutant, sec18-1ts, exhibits exaggerated ER morphology and the accumulation of small vesicle-like structures [59, 60]. Expression of a dominant-negative mutant of Sec18p (Sec18-109p) interferes with ER-Golgi membrane traffic, causing the accumulation of a fenestrated, membranous structure [61].
Unlike other species, Drosophila expresses two NSF isoforms: dNSF1, which is the dominant isoform in the adult central nervous system and dNSF2, which shows a much broader distribution [62]. dNSF1 null flies die as pharate adults but dNSF2 deletion is lethal at or before the first instar stage [63]. A point mutation in the dNSF1 gene generates a temperature-sensitive mutant comatose, which has a severe defect in synaptic transmission, exhibiting a paralytic phenotype at restrictive temperature [64, 65]. A clear redistribution of Syntaxin and SNAP-25 away from the neurotransmitter release sites (active zone) of dorsal longitudinal flight muscle neuromuscular synapses is observed at the consecutively stimulated comatose synapses, suggesting that NSF maintains a pool of free t-SNAREs at the active zone [66].
Although the importance of NSF is unquestionable, the precise role of this key protein in the membrane fusion process has been intensely debated. Two models have been proposed. One is the pre-fusion model, which posits that NSF disassembles the SNARE complex before fusion, initiating the formation of trans-SNARE complex [67–70]. The other is the post-fusion model, which posits that NSF is required to resolve cis-SNARE complexes, thus allowing individual SNAREs to recycle for addition rounds of fusion. Currently, the post-fusion role of NSF is more accepted since in the comatose mutant, several rounds of vesicle fusion occur before the depletion of free SNAREs causes neurotransmission to cease [66, 71–73]. However, given that the membrane fusion process can be envisioned as a cycle, the role of NSF can be seen as either the final event of one round of a cycle or the initiating phase of the next.
3.3 Structural Studies on NSF
Each protomer of the NSF homo-hexamer contains one N-terminal domain (NSF-N), followed by two conserved, Walker-type, nucleotide-binding domains (termed NSF-D1 and NSF-D2). NSF-N is a kidney-shaped domain composed of two subdomains: NA (a.a. 1–83) and NB (a.a. 87–201), which are joined by a short linker [74, 75] (Figure 3A). NA is made of six β strands arranged in a barrel with two “ψ loops” containing short α helices (α1 and α2) extending over the top. NB is an α/β roll, where four β strands wrap around a single amphipathic α-helix. A cleft between NA and NB is formed and two small hydrophobic clusters are located at either end of the cleft. The surface of NSF-N possesses an overall positive charge and contains three grooves, groove 1, 2 and 3 [74, 75].
Figure 3. Structure of NSF-N and NSF-D2.
Panel A: The crystal structure of the N-domain of NSF. NA in light blue, NB in light purple, and the hinge region in white. The groove 3 is shown in dark blue. Mutated residues are indicated. Red amino acids are positions where mutations are inhibitory (no binding or less than 30% of wild-type NSF binding), those in yellow are partially inhibitory (30–70% of wild-type NSF binding), and those in green have little effect (more than 70% of wild-type NSF binding). The images are based on 1QDN and were generated using Swiss PDB viewer and rendered with Pov-Ray. Panel B: Crystal structure of NSF-D2 hexamer. The subunits are shown in pink, aqua or yellow. AMP-PNP coordinated by Mg2+ is shown in space-fill representation. Six loops in the central pore are indicated in black. The boxed region is enlarged and the β-sheet core subdomain and α-helical cap subdomain from one pink subunit are labeled. The aqua-blue loop above the pink subunit is a section from the adjacent subunit. Conserved motifs are also indicated: green for P loop (Walker A); purple for Walker B box; light blue for Sensor 1; dark green for Sensor 2; dark blue for Arginine Fingers. The asterisk indicates the Arginine Fingers from the adjacent subunit. The images are based on 1D2N and were created with Swiss PDB viewer and rendered with Pov-Ray.
NSF-D2 is an AAA domain based on its structure and sequence [30, 31] (Figure 3B). The structure of NSF-D1 has not been determined, but its sequence homology to NSF-D2 indicates that it is also a typical AAA domain. Overall, each AAA domain consists of two subdomains: an N-terminal nucleotide-binding subdomain (residues 505–676 for NSF-D2), and a C-terminal α-helical subdomain (residues 677–750 for NSF-D2). The N-terminal subdomain (which contains the Walker A and B box motifs) has a wedge shape with a central five-stranded parallel β-sheet. The C-terminal subdomain is composed of four α helices, which lies above the nucleotide-binding domain and contributes several residues to nucleotide binding.
In the hexamer, the nucleotide-binding pocket is located at the interface between adjacent protomers. Several conserved motifs contribute to the nucleotide-binding pocket, including the classical Walker A, Walker B, Sensor 1, Sensor 2 and Arginine Fingers motifs (Figure 3B). Walker A and Walker B motifs are two motifs having the pattern GXXXXGKT/S and (R/K)XXXGXXXL/VhhhhDE, respectively (X: any amino acid; h: hydrophobic amino acid). They are highly conserved in nucleotide-binding proteins [76]. The conserved lysine residues (266, 549) in the two Walker A boxes of NSF are crucial for ATP-binding [29, 77]. The aspartic acid in the DEXX sequence of the Walker B box is thought to coordinate a Mg2+ ion that is needed for ATP hydrolysis, whereas the glutamate is required to activate water for the hydrolysis reaction. The Second Region of Homology (SRH) is another sequence motif that is highly conserved in AAA+ proteins [2]. Sensor 1 is at the N-terminus of the SRH and often contains a threonine/asparagine pair [17]. At its C-terminus are two arginine residues termed Arginine Fingers [78], which are thought to be critical for nucleotide hydrolysis similar to Arginine Fingers of GTPase Activating Proteins (GAP) [78]. The α-helical subdomain of the nucleotide-binding domains contains a motif, called Sensor 2, which is composed of residues that are positioned adjacent to the ATP-binding site. This motif, often contains a conserved arginine (e.g. GAR in ClpA, ClpB [79] and Hsp104 [80]; DLR in Cdc6p [81]) that interacts with the γ-phosphate of ATP. This is absent in NSF-D1 (ELE) and is replaced with a lysine in NSF-D2 (GIK).
The six protomers of NSF form two stacked homo-hexameric ring [82]. Based on the structure of the NSF-D2, there is a central pore which could be contiguous with a predicted pore in NSF-D1 [30, 31, 83]. This pore is thought to be important for substrate binding and processing in other AAA+ proteins [2] and a conserved motif ΦhG (aromatic-hydrophobic-glycine) in one of the central pore loops are involved in substrate engagement in ClpB and p97 [84–86].
3.4 NSF’s ATPase Activity
The intrinsic ATPase activity of NSF is very low [28]. NSF-N has been proposed to exert some control over NSF’s ATPase activity because antibodies against to it cause a two-fold increase in hydrolytic activity [87]. Binding to immobilized α-SNAP stimulates the ATPase activity [88]; however, maximal stimulation of ATPase activity is only achieved when both α-SNAP and SNARE complexes are included [89]. The penultimate leucine of α-SNAP is critical for this activity [90, 91]. Consistently, the L294A mutation of α-SNAP is unable to mediate NSF-SNAP-SNARE complex disassembly. NSF-D1 accounts for the majority of basal and SNAP-SNARE-stimulated ATPase activity [29, 92]. Mutations in the ATP-binding site of domain D1 (K266A and E329Q) cause a 70–80% decrease in ATPase activity relative to wild type NSF [29]. Mutations in the NSF-D2 decrease ATPase activity, but only minimally [29].
3.5 Mechanism of NSF Action
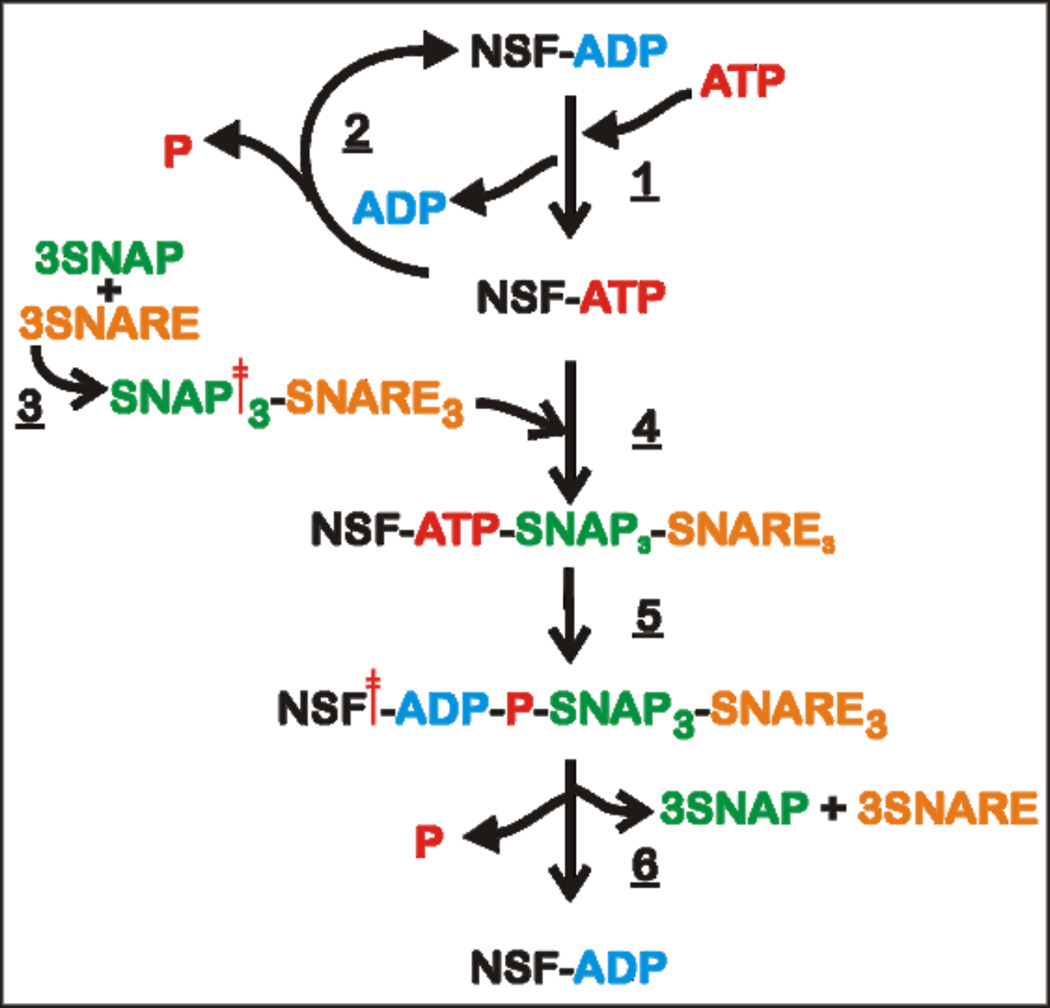
To simplify the model of the NSF-mediated disassembly of SNAP-SNARE complexes, we propose an enzymatic cycle outlined in Figure 4. Cytosolic NSF can exist in either the ADP- or ATP-bound form. Since the binding constants of both NSF-D1 (15 – 20 µM) and NSF-D2 (30 – 40 nM) for ATP [77] are orders of magnitude lower than the concentration of cytosolic ATP ( ~ milimolar range), it is expected that the ATP-bound form predominates (Step 1). The bound ATP can be hydrolyzed to ADP and Pi at an extremely slow rate in solution, based on the low basal ATPase activity of NSF (Step 2) [88]. In the presence of SNARE complexes, α-SNAPs interact with SNARE complex (Step 3) and recruit SNAP-SNARE complex to NSF (Step 4). Only ATP-bound NSF is competent for this binding. Once bound, the SNAP-SNARE complex stimulates the ATPase activity of NSF (Step 5). ATP hydrolysis couples to conformational changes in NSF, which ultimately catalyze the disassembly of SNAP-SNARE complex (Step 6).
Figure 4. Scheme of NSF Action.
Cytosolic NSF binds to ATP (Step 1) and the ATP-bound NSF slowly undergoes unproductive nucleotide hydrolysis (Step 2). Three α-SNAPs bind to SNARE complex (Step 3). In the ATP-bound state, NSF is competent to bind the SNAP-SNARE complex (Step 4). Upon binding, SNAP-SNARE complexes stimulate nucleotide hydrolysis by inducing a structural change in the nucleotide-binding site of NSF-D1 (Step 5). ATP hydrolysis or, more probably, release of the phosphate results in conformational changes of NSF, generating the driving force required to unwind the SNAP-SNARE complex (Step 6).
Identification of the specific elements important for NSF’s ATPase and substrate-binding, and detection of the conformational changes in NSF induced by ATP binding and hydrolysis, are critical for understanding NSF’s mechanism of action. The mutagenesis analyses of NSF-N and NSF-D1 identified possible SNAP-SNARE binding sites, showing that they could be located on a positively-charged surface on NSF-N and in the conserved residues in NSF-D1’s central pore. The important motifs surrounding the nucleotide-binding pocket of NSF-D1: Walker A, Walker B, Sensor 1, Sensor 2 and Arginine Fingers, each plays a discrete role in the overall of function of NSF. The Walker A and Sensor 1 are important for ATP binding; Walker B is essential for ATP hydrolysis; Sensor 2 and Arginine Fingers are important for SNAP-SNARE complex-stimulated ATPase activity and complex disassembly. Mutations in the Walker B motif and in the Arginine Fingers have a dominant-negative effect in cells, suggesting that both elements are important for inter-protomer communications [86]. These studies establish the roles for specific parts of the NSF proteins and elucidate its catalytic mechanism.
Moreover, for the first time, the conformational differences between NSF-ATP and NSF-ADP have been detected in solution [93, 94]. These findings begin to define the specific structural changes that occur during each step of NSF’s ATPase cycle. Such data should also be applicable to other AAA+ proteins which use ATP binding/hydrolysis and adaptor proteins or accessory domains to carry out their cellular functions.
3.5.1 ATP Binding in NSF-D1 (Step 1)
As shown in Figure 4, the first step in the NSF ATPase cycle is one where ATP binds to NSF. Since the NSF-D2 has a very low ATPase activity and a significantly higher affinity for ATP than ADP [77], it is reasonable to assume that the ATPase cycle is only occurring in the NSF-D1 domain. The Walker A and Sensor 1 regions of NSF-D1 are critical for ATP binding [29, 86], suggesting both motifs adopt conformations able to accommodate the γ-phosphate group of ATP. The side chain of the conserved lysine in Walker A (K266) forms hydrogen bonds with β- and γ-phosphate oxygen atoms; the side chain of the conserved asparagine in Sensor 1 (N374) forms a hydrogen bond with the γ-phosphate oxygen atom (Figure 3B). The K266A mutation is defective in ATP binding [29] and N374A/D mutations diminished the intrinsic ATPase activity of NSF [86]. The conserved lysine is also present in the Walker A motif of NSF-D2 (K549). The position of K549 in the crystal structure of NSF-D2 and the phenotype of the K549A mutant are as expected if the lysine residue plays a role in nucleotide binding, as proposed for K266 [29–31]. This lysine residue in Walker A motif is highly conserved in the AAA+ proteins [10, 17] and mutation of this residue inhibits nucleotide binding in several AAA+ proteins [2, 95–104].
Sensor 1 is thought to discriminate between bound ATP and ADP by forming a hydrogen bond with the γ-phosphate of ATP. In the structure of NSF-D2, the conserved asparagine (N374) of Sensor 1 is a serine (S655), which is involved in a hydrogen-bond network that positions a water molecule as a potential nucleophile for ATP-hydrolysis [31]. The conserved asparagine (sometimes threonine) residue in Sensor 1 is present in most AAA+ proteins [10, 17] and the hydrogen bond between its side chain and the γ-phosphate of ATP has been reported in other AAA+ proteins. In the crystal structure of bacterial processivity clamp loader γ-complex, bacterial RuvB or its eukaryotic homologue RuvBL1, threonine or asparagine residue in Sensor 1 forms a hydrogen bond with the γ-phosphate of ATP [105–107]. In mouse SKD1/VPS4B, N279 interacts with the γ-phosphate of ATP but has no connection with any phosphate when ADP is bound [108]. In FtsH, the side chain of N302 moves into the active site only when it senses the γ-phosphate of AMP-PNP [109]. In general, mutation of this residue in Sensor 1 appears to affect nucleotide binding or hydrolysis [61, 110–113].
3.5.2 SNAP-SNARE Binding to ATP-Charged NSF (Step 4)
Walker A and Sensor 1 in NSF-D1 are important not only for ATP binding, but also for inducing a SNAP-SNARE-binding-competent state, since the K266A and N374A/D mutants show almost no binding to SNAP-SNARE complexes [29, 86]. The binding of SNAP-SNARE complex to NSF (Step 4 in Figure 4) involves the NSF-N domain and central pore region of NSF-D1 domain [19, 86]. These two portions of NSF must be affected by the nucleotide bound in the NSF-D1 site, either directly or indirectly, since mutations in the Sensor 1 and the Walker A motif negatively affect SNAP-SNARE binding.
3.5.2.1 NSF-N
The N-domain is essential for protein substrate binding for both NSF/Sec18p [19, 35, 114], and its close relatives, p97/Cdc48p, archaeal homolog VAT, and PEX1 [103, 115–117]. The crystal structures of the N-terminal domains of NSF, Sec18p, p97, VAT and PEX1 bear a striking resemblance, although sequence identity of NSF-N to Sec18p-N is only around 27% and that of NSF-N to other homologs is less than 10% [74, 75, 114, 118–120]. Overall, their N domains contain two subdomains: N-terminal subdomain (NA), which forms a double-ψ β-barrel (DPBB) motif, and C-terminal subdomain (NB), which assumes an α/β roll. Commonly, DPBB motifs contain substrate-binding sites in the first ψ-loop [121]. Our results showed the positively-charged residues in the groove 3 of NSF-N were important for SNAP-SNARE binding (Figure 3A) [86]. Interestingly, groove 3 contains the first NA ψ-loop and is located on the top part of the cleft between the NA and NB subdomains. A similar basic groove is present on the N-domain of Sec18p [114] and a G89D mutation (in the first NA ψ-loop of Sec18p) inhibits the interaction of Sec18p with Sec17p [122]. The positively-charged surface that lines the upper face of the cleft is also found in p97 and its archaeal homologue, VAT, but the binding sites for p97 adaptor proteins, p47 and Ufd1-Npl4, are located on the lower face of the cleft [20, 123]. The upper face of the cleft in PEX1 is acidic [119] and the phospholipid-binding interface is also in the lower part of the PEX1-N [117].
How many N-domains in NSF hexamer are required for SNAP-SNARE binding? Our in vivo assays show that the mixed hexamer mutants, R67A and Y83F, expressed in HeLa cells, have no dominant-negative phenotype, which is in good agreement with the conclusion that NSF hexamers do not need a full complement of functional N domains for activity [19, 86]. This conclusion is also consistent with the “3-in, 3-out” model for NSF-N function, where it is proposed that only three N-domain-containing protomers are needed to interact with three α-SNAPs [75]. Consistently, it has been proposed that three p47 adaptor molecules bind to three of the six p97-N domains based on the EM structure of p97-p47 complex [124].
The full-length Secp18p in the AMP-PNP state binds to a cation exchange column while the protein under hydrolytic conditions (ADP or ATP/Mg2+) does not. This might indicate that conformational changes in NSF upon ATP binding expose a basic surface. One could interpret this to mean that NSF-N is being repositioned into a competent state for binding to the negatively-charged C-terminus of α-SNAP [90, 114]. The masking or exposure of the basic groove by the movement of NSF-N could be controlled by an arginine residue, R67, in this groove. Of all the positively-charged residues identified as important for SNAP-SNARE binding, only NSF R67 is highly conserved in Sec18p, p97, Cdc48p and VAT (but not in PEX1) [86]. In p97, the equivalent R89 (p97-NA) is located in the positively-charged surface that interacts with either Q261 in the p97-D1 α/β subdomain [118] or Y203 in the p97-N-D1 linker [125]. It has been proposed that this interaction controls the position of p97-N with respect to the rest of the protein [126]. More importantly, the most prevalent mutations, R95G or R155H/C/P, in the dominantly inherited syndrome of inclusion body myopathy associated with Paget's disease of the bone and frontotemporal dementia are also located in the same surface [11, 119]. If this interaction is conserved in NSF, the disruption in SNAP-SNARE complex binding caused by the R67A/E mutations does not necessarily mean that R67 is the binding site. Instead it could be a latch (as proposed for p97) that controls the position of the NSF-N. The orientation of NSF-N controlled by R67 is one of the future questions that will require analysis of the conformational changes occurring in the R67A/E mutations of NSF in the ADP- and ATP-bound states.
3.5.2.2 Central Pore in NSF-D1
The other region that appears to be involved in SNAP-SNARE binding is the central pore in NSF-D1 [86]. The conserved ΦhG motif is part of a loop which forms a hydrophobic patch above the central pore (Figure 3B) and is responsible for substrate binding and/or translocation by AAA+ proteins that use the threading-through mechanism [2, 25]. This conserved motif (especially the conserved aromatic residue) is only present in the catalytically active AAA domain of NSF/Sec18p, p97/Cdc48p, PEX1/Pex1p and PEX6/Pex6p (YhG in NSF/Sec18p-D1; WhG in p97/Cdc48p-D2; Y/FhG in PEX1/Pex1p-D2; YhG in PEX6/Pex6p-D2). The WhG motif is also present in the central pore loops of VPS4A/VPS4B/Vps4p. These proteins are all involved in the disassembly of protein complexes. The narrow diameter of the central pores in NSF or p97 makes the translocation of the substrates seem unlikely [2, 125, 127]. Our studies show that the hydrophobic patch (Y296 to G298) in one of the loops that protrudes into the central pore of NSF-D1 might mediate interactions with the SNAP-SNARE complex. The mutants in this motif (Y296A/F, G298A, Y296A/G298A, Y296F/G298A) affected SNAP-SNARE binding, though to different extents [86]. The Y296A (but not Y296F) and the G298A mutants were defective on SNAP-SNARE complex disassembly [86], suggesting the aromatic residue and glycine residue in the NSF-D1 pore may assist in complex dissociation. Mutagenesis studies of p97 and VAT suggest that the hydrophobic patch has a similar function to that in the AAA+ proteases. The aromatic residue is thought to be involved in substrate binding and unfolding [85, 103, 128]. The pore loop of VPS4A/VPS4B is also proposed to be used for contacting substrates or disassembly of the Endosomal Sorting Complexes Required for Transport (ESCRT)-III complex [21, 129].
The reason for the utilization of two structural elements, N-domain and the central pore, in substrate binding is currently unknown. Recently, it is reported the N-domains of ClpA and ClpX are utilized for substrate reorganization. This is thought to serve as a docking site for various adaptor proteins and to be involved in local unfolding of substrates [130, 131]. Once bound, the substrates are delivered into the central pore for unfolding and translocation. For p97, it is proposed that p97-N is the binding sites for the adaptor proteins and the real substrate binding sites are in the p97-D2 pore region [85]. For NSF, it is possible that three α-SNAPs coat the outside of the SNARE complex and first interact with three N-domains. This could position the SNAP-SNARE complex close to the exterior surface of the D1 ring and hence, close to the pore region of the D1 domain. Once in this position, the N-termini of the SNARE complex could be inserted into the pore and where they could make contact with the conserved hydrophobic patch (Figure 5). The movement of this central pore loop during the ATP hydrolysis cycle could then be used to help disassemble the SNARE complex.
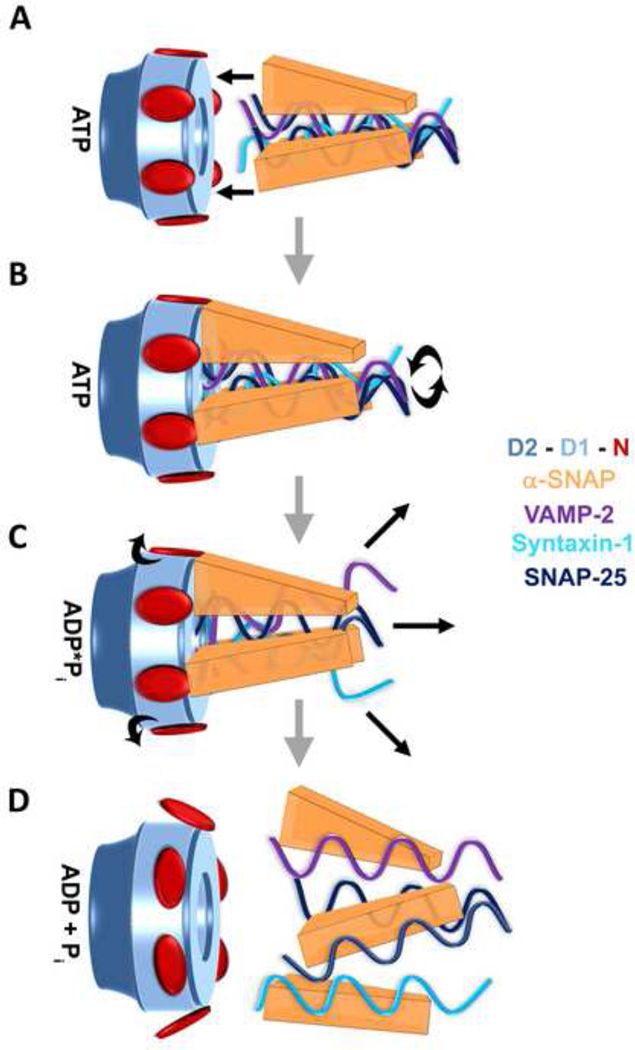
Figure 5. Model of SNAP-SNARE Complex Disassembly by NSF.
Panel A: After membrane fusion, SNAREs form a four-helical bundle (VAMP-2 in purple, Syntaxin 1 in light blue and SNAP-25 in dark blue) and three α-SNAPs (orange) are coated along its length. Panel B: α-SNAPs recruit SNARE to the ATP-bound NSF (NSF-N in red, NSF-D1 in sky-blue and NSF-D2 in blue-gray). The three C-termini of α-SNAPs associate with three NSF-N domains and the N-terminal regions of the SNAREs contact the central pore of NSF-D1. The binding of SNAP-SNARE complex induces conformational changes in NSF-D1, which catalyze ATP hydrolysis. Panel C: After ATP hydrolysis, the chemical energy converts to physical changes in NSF, including NSF-N domains. The movements of NSF-N domains are then coupled to α-SNAPs, which act as a socket to disassemble the SNARE complex. Panel D: NSF returns to ADP-bound state, with free SNAPs and SNAREs.
3.5.3 SNAP-SNARE-Stimulated ATPase (Step 5)
The binding of SNAP-SNARE complexes to NSF stimulates its ATPase activity in Step 5. Arginine Fingers are thought to play a role in this step. Arginine Fingers were first noted in the structure of the Ras/Ras-GTPase-activating protein (Ras-GAP) complex [78]. The positively-charged guanidinium group of R789 in Ras-GAP interacts with the fluoride of the GDP-AlFx in the nucleotide-binding site and the main chain carbonyl oxygen forms a hydrogen bond with a glutamine residue (Q61) of Ras’ switch II region. This arginine is thought to participate in catalysis by stabilizing the transition state and orienting the catalytic machinery during GTP hydrolysis. A similar interaction has been reported in other small G protein, i.e. Arf6-ArfGAP complex [132]. In NSF, Arginine Fingers come from the neighboring subunit (Blue subunit in Figure 3B). It is possible that SNAP-SNARE complex binding to the NSF-N and central pore of NSF-D1 causes a conformational change in the NSF-D1 of the hexamer which positions the Arginine Fingers from neighboring subunits into the nucleotide-binding pocket. This would result in R385 interacting with the γ-phosphate of ATP and R388 forming a salt bridge with E329 in the Walker B motif of an adjacent subunit (Pink one in Figure 3B). The first interaction polarizes the γ-phosphate and stabilizes the transition state (ADP·Pi); the second one positions E329 correctly to polarize an attacking water molecule. Both would accelerate ATP hydrolysis. The R385A and R388A mutations didn’t affect the basal ATPase but did blunt SNAP-SNARE complex-stimulated ATPase activity in vitro [86], suggesting the effects of Arginine Fingers on NSF ATPase activity depend on NSF engagement with SNAP-SNARE complexes. The defect in disassembly of SNAP-SNARE complexes [86] caused by R385A and R388A mutations could be related to a deficit in stimulating NSF’s ATPase activity. The trans-action of Arginine Fingers is critical for NSF function, since the R385A mutant had a dominant-negative effect in vivo (as did Walker B motif mutant E329Q) [86]. The E329Q and R385A mutants were incorporated into mixed-hexamers and caused appreciable cytotoxicity and alterations in Golgi complex morphology. NSF-D2 is required for oligomerization [19], but has neither conserved Arginine Fingers nor does it catalyze significant ATP hydrolysis.
The Arginine Fingers motif is conserved in other AAA+ proteins. In p97-D1, R359 from a neighboring protomer interacts with the γ-phosphate of ATP and R362 forms a salt bridge with E305 in the Walker B motif on an adjacent subunit [118]. In mouse SKD/VPS4B, R290 and R291 promote hexamerization through the interaction with γ-phosphate of ATP [108]. R169 in the processivity clamp loader γ-complex participates in interactions with D36 and Q32 which are located in the adjacent subunit [107]. An interaction between Arginine Fingers and Walker B motif is also suggested in the MCM protein, where ATPase activity of the R473A (Arginine Finger) is rescued by co-incubation with the D404A (Walker B) mutant. The significance of Arginine Fingers in AAA+ family was first noted for FtsH where R312 and R315 mutants abolish ATPase activity [100]. Subsequently, the importance of Arginine Fingers has been shown for many AAA+ proteins [5, 97, 108, 133–137]. Mutations of this conserved motif not only affect ATPase activity but also disrupt hexamerization. Mutations in Arginine Fingers abolish hexamerization in ClpB [97] and decrease cooperative helicase activity in MCM [133]. In p97-D1, the R362E mutation partially disrupts the hexamer and R359E/R362E double mutants are predominantly monomers [104]. R638A mutation in p97-D2 decreases ATPase activity ~ 100-fold [85]. In SKD1/VPS4B, R290A/R291A mutations disrupt the ATP-dependent oligomerization [108].
3.5.4 ATP Hydrolysis (Step 6)
In the last step of NSF’s catalytic cycle, ATP is hydrolyzed by NSF-D1; the chemical energy from ATP hydrolysis causes the large conformational changes in NSF, which are coupled to SNAP-SNARE complex disassembly (Figure 4).
3.5.4.1 ATP Hydrolysis in the Protomer
ATP is hydrolyzed in the individual nucleotide-binding pocket and the Walker B motif is critical for this step [29]. The side chain of E329 in Walker B motif serves to activate a water molecule by making the oxygen more electronegative. The activated water molecule becomes a better nucleophile and attacks the γ-phosphate of ATP ([31] and Figure 3B). The E329Q mutant shows ~ 70% decrease of basal ATPase activity and no SNAP-SNARE-stimulated ATPase activity, but no SNARE-SNARE binding defect [29]. These results demonstrate Walker B motif is not responsible for ATP and SNAP-SNARE complex binding but is essential for ATP hydrolysis. This glutamate residue in the Walker B motif is highly conserved in AAA+ proteins and mutation of this residue in other family members shows the same phenotype as that in NSF [2]. It has been reported that this residue is present in two different configurations in AAA+ proteins: one that mediates the low basal ATPase activity and a second that mediates the stimulated ATPase activity. This inter-conversion between states is thought to be controlled by binding to protein substrates. In this proposal this residue is called a “glutamate switch” [138]. As discussed above, this switch is probably affected by the electrostatic interactions between this glutamate residue and the Arginine Fingers, which could be repositioned by NSF binding to SNAP-SNARE complex.
3.5.4.2 The Cycle of ATP Hydrolysis in the Hexamer
To better understand the coupling between conformational changes of NSF and SNARE complex disassembly, we also need to consider how ATP hydrolysis occurs in the oligomeric ring. In AAA+ proteins, three possibilities have been proposed: rotational, synchronized, and sequential [4]. In the rotational model, ATP hydrolysis occurs in three subunits and the other three subunits are always inactive; in the synchronized model, all the subunits are occupied and all hydrolyze ATP simultaneously; in the sequential model, all the subunits are active but the nucleotide-bound state of each subunit alternates as one goes around the hexamer. Several studies support the sequential model in other AAA+ oligomers. First of all, not all of the six subunits need to bind ATP simultaneously. Each proteasome-activating nucleotidase (PAN) hexamer only binds four ATPγSs [99] and only half of the sites are available for nucleotide binding in p97 [125]; Secondly, the communication between neighboring subunits during ATP hydrolysis has been reported in ClpX, RuvB, MCM [98, 139–142]. This has been elegantly shown in the hetero-oligomeric m-AAA preoteases. By co-expressing Yta10 and Yta12 subunits in yeast, the authors made a hetero-oligomeric m-AAA protease with alternating Yta10 and Yta12 subunits. Mutation (Yta10-WT/Yta12-K394A) in the Walker A motif of Yta12 subunits (no nucleotide binding) inhibits ATPase activity up to ~ 70%; however, mutation (Yta10-WT/Yta12-E448Q) in the Walker B motif of Yta12 subunits (trapped-ATP) completely abolishes the ATPase activity of the hetero-hexamer. Using a gain-of-function, genetic screen, the authors identified the Arginine Fingers and the conserved ΦhG of the pore as being involved in inter-subunit communications. In the proposed model, Arginine Fingers in Yta10 subunit sense the nucleotide state in the adjacent Yta12 subunit and ATP-hydrolysis in Yta12 subunit changes the conformation of its pore loop, releasing the substrate. The alternating ATP hydrolysis in different subunits generates a rotational power stroke in the substrate, unfolding and translocating the substrate through the pore.
Although the translocation mechanism is unlikely for NSF, the functions of the Walker B motif and Arginine Fingers in inter-protomer communication suggest that NSF could hydrolyze ATP in a sequential model. Only the E329Q and R385A mutants were toxic when expressed in HeLa cells. Given that both mutants did form mixed-hexamers [86], it seems plausible that inclusion of a mutant protomer “poisons” the hexamer, thus leading to cytotoxicity. This would imply some concerted mechanism to interconnect protomers. NSF’s Arginine Fingers could connect two adjacent protomers by interacting with ATP’s γ-phosphate and/or participating in electrostatic interactions with E329 (Figure 3B), as proposed above. Other interactions with Arginine Fingers are also possible, since the mixture of Arginine Fingers and Walker A mutants partially restores the ATPase activity of RuvB, p97, and MCM [104, 133, 139]. Nucleotide binding studies show that NSF contains two different classes of nucleotide binding sites: high affinity ATP-binding sites in NSF-D2 and low affinity sites in NSF-D1. Each NSF protomer binds to one ATP in both its high or in its low affinity sites but no cooperative ATP binding has been detected [77]. To further delve into the cooperative hydrolysis of ATP, future studies will need to focus on: 1) using Isothermal Titration Calorimetry (ITC) to precisely determine the number of available nucleotide binding sites in the NSF hexamer; 2) making complementary mutations in the Walker A motif, Arginine Fingers and the central pore to screen for mutations that can restore NSF-E329Q mutant’s activity (ATP hydrolysis, cell viability).
3.5.5 Conformational Dynamics during NSF’s ATPase Cycle (Step 6)
The NSF-mediated disassembly of SNAP-SNARE complexes occurs in a series of steps (Figure 4). These steps are reliant on specific conformational changes in NSF upon ATP binding, hydrolysis, and/or exchange of ADP for ATP. A full understanding of ATP-dependent SNARE complex disassembly will require the resolution of the structural changes associated with each step.
3.5.5.1 Conformational Changes in NSF-N and NSF-D1 Domains
The different conformation of NSF in ATP- or ADP-bound states was first reported using deep-freeze etch electron microscopy (EM) [82]. When placed on mica sheets, NSF appears as a hexamer with a six-fold radial symmetry. NSF-D1 and NSF-D2 form a double-stacked ring structure, which is ~10 nm high. Since the NSF-D2 has minor ATPase activity and is responsible for oligomeriztion of NSF, it is believed NSF-D2 constitutively exists in the ATP-bound state. The dramatic changes seen are identified as involving the NSF-D1 and NSF-N. In the presence of nonhydrolyzable ATP, the ring diameter of NSF-D1 is ~13 nm with six domains flared outward away from the central pore (these were interpreted to be NSF-N domains). In the ADP-bound state, the putative N domains are packed back onto the double-ring structure and the NSF-D1 ring is dilated to ~16 nm. However, the quick freeze step and the effects of the mica on NSF could affect the NSF structure. The nucleotide-dependent conformational changes in NSF-N and NSF-D1 domains are not detected in NSF-E329Q by the higher resolution electron cryomicroscopy [83]. Another way to detect the conformational changes in NSF is via crystallization in different nucleotide states; but this has so far been plagued by technical problems. Only crystal structures of NSF fragments (NSF-N and NSF-D2) are available [30, 31, 74, 75].
A third method is analytical ultracentrifugation. However, the traditional analytical methods, relying on protein absorbance (~280 nm), are severely affected by the presence of high concentrations of nucleotide in the NSF buffer. Typically, the OD260 of 0.5 mM ATP is ~7. Because of this interference and low signal to noise, NSF was first thought to be a trimer [29] and had similar sedimentation coefficients in either ADP- or ATP-bound states [27]. To solve this problem, NSF was labeled with an optical probe, (Ni2+-NTA)2-Cy3 (λabs = 550 nm), which binds to its N-terminal His6-tag. The conformational changes of NSF have been primarily studied by sedimentation velocity with (Ni2+-NTA)2-Cy3 labeled NSF in the presence of different nucleotides [93, 94]. By analyzing the NSF-AMP-PNP- and NSF-ADP-bound states in parallel, the subtle differences of NSF in two different nucleotide states are easily detected. NSF-AMPPNP sediments faster with a calculated s20,W larger than NSF-ADP. The smaller f/f0 in NSF-AMP-PNP also indicates that NSF exists in a more compact state before it hydrolyzes ATP. Since the f/f0 value for a spherical protein is ~ 1.2, the shapes of NSF either in AMP-PNP-bound state (f/f0 = 1.4) or ADP-bound state (f/f0 = 1.47) are more ellipsoidal. The data from these ultracentrifugation experiments offer different conclusion from what has been reported by electron microscopy, where the ADP-bound state was more compact [82]. This difference is perhaps due to the effects of the mica on NSF in the ATP-bound state. If the NSF-N-D1 portion is flexible in the ATP-bound state, it may be able to pack more tightly in solution but may be splayed out by interactions with the mica sheet. These two datasets agree that NSF, in its two nucleotide states, is conformationally different.
Six-fold symmetry is a common feature of many AAA+ proteins, including p97 [118, 125, 143–146], ClpB [147] and Hsp104 [135, 148]. These proteins share a common domain arrangement, with an N domain followed by two AAA domains in each protomer. The cryo-EM structures of these proteins show that they all form double-stacked ring structures. In ClpB and Hsp104, the diameter of the top ring and the size of its central pore vary in response to different nucleotides. The high-resolution crystal structures of p97 in different nucleotide-bound states give some insights into the conformational changes of AAA+ proteins during an ATPase cycle. p97 is the only ATPase with a tandem AAA+ domain for which the crystal structures in its native hexameric state are currently available [125, 127]. ATP hydrolysis by p97 is required for protein dislocation during endoplasmic reticulum-associated degradation (ERAD) [149]. The p97-N is responsible for binding to adaptor proteins, such as p47, which mediates p97 binding to other substrates [20]. Unlike NSF-D2, the p97-D1 domain is important for oligomerization while the p97-D2 domain is the major ATPase domain. Comparing the crystal structures of p97 in AMP-PNP-, ADP-AlFx- (transition state), ADP-bound and apo states, shows that the p97-N domains are mobile and become rigid only in the transition state (ADP-AlFx); the p97-D1 domain is invariant. The linker between p97-D1-D2 and the p97-D2 domain undergo large conformational changes, specifically in the C-terminal α-helical subdomain (D2α) and in the Arginine Finger motifs. During ATP hydrolysis, the conformational changes of p97-D2 are transmitted to the p97-D1 domain by p97-D1-D2 linker, which finally affects the motion of the p97-N domains. Because NSF-N and the major ATPase domain (NSF-D1) are connected, we propose that the conformational changes of NSF are in NSF-N and NSF-D1 domains. Further experiments to study the hydrodynamics of NSF truncation mutants (NSF-D2, NSF-D1D2) by sedimention velocity will be required to confirm our hypothesis.
3.5.5.2 Role of Sensor 2 in NSF-D1
Another region, which is likely to undergo a large conformational change, is the C-terminal α-helical subdomain in NSF-D1, where the Sensor 2 is located. In AAA+ proteins, Sensor 2 lies just above the nucleotide binding site (Figure 3B). A conserved arginine in Sensor 2 of some AAA+ proteins forms a salt bridge with the β-phosphate of ATP and ADP [105–107], allowing discrimination between the nucleotide-bound and apo states. This arginine is absent in NSF-D1 and is replaced with a lysine in NSF-D2 (also in Sec18p). Mutations of three residues (E440, L441, E442) in Sensor 2 of NSF-D1 had only limited effects on basal ATPase activity, suggesting that Sensor 2 plays only a limited role in nucleotide binding. The E440R (but not E440A), L441A and the E442R (but not E442A) mutations did affect SNAP-dependent SNARE complex binding [86] and the presence of a positive charge at that position is detrimental. Since it is unlikely that Sensor 2 is the SNAP-SNARE complex binding site, this phenotype may imply that the role of Sensor 2 is to induce a SNAP-SNARE-binding-competent state. We showed E440A, L441A and E442R (but not E442A) affected the SNAP-SNARE-stimulated ATPase activity and complex disassembly [86]. Similarly, a positively-charged residue in these positions affected both processes. Taken together, these results suggest Sensor 2 plays multiple roles including nucleotide sensing, ATP hydrolysis and complex disassembly (Figure 4). An alternative explanation is that the Sensor 2 region is very flexible and undergoes conformational changes in the different steps of NSF ATPase cycle, which give Sensor 2 different roles. A possible model for the movements of Sensor 2 in different steps of NSF action is: Step 1, the Walker A motif coordinates ATP binding by forming the hydrogen bonds between the side chain of the conserved lysine and the β- and γ-phosphate oxygen atoms. The presence of ATP is sensed by the conserved asparagine in Sensor 1 through a hydrogen bond between its side chain and γ-phosphate oxygen atom. This information would be transferred outside the nucleotide-binding pocket in NSF-D1 to NSF-N, which could be mediated by conformational changes in Sensor 2. The orientation changes of NSF-N relative to NSF-D1 expose the basic surface in groove 3 for α-SNAP binding. Step 5, after NSF binding to α-SNAP through NSF-N, this information would be transferred back into the nucleotide-binding pocket again through the Sensor 2 region, causing the movement of the Arginine Fingers (the binding of SNAP-SNARE complex to central pore loop in NSF-D1 could also have such effects) to the appropriate position; thus promoting ATP hydrolysis. Step 6, after ATP hydrolysis, the chemical energy from hydrolysis provides the energy needed for a drastic conformational change of the NSF-D1 α-subdomain where Sensor 2 is located. The movements of NSF-D1 α-subdomain cause the conformational changes of NSF-N, which participates in SNARE complex disassembly through α-SNAPs (discussed below). The fact that mixed-hexamers of Sensor 2 mutants did not have dominant-negative effects in vivo [86], indicates that Sensor 2 may act in cis and is not involved in inter-protomer communication during ATP hydrolysis.
The lack of the conserved arginine in Sensor 2 is also seen in p97/Cdc48p, PEX1/Pex1p, PEX6/Pex6p and VPS4A/VPS4B/Vps4p. Sensor 2 has been reported to mediate conformational changes associated with nucleotide binding and/or hydrolysis in other AAA+ proteins [134]. R296 in the Sensor 2 of the Rubisco activase is critical for conformational changes, as shown by proteolytic protection analysis and intrinsic fluorescence changes [5]. In p97, the D2α region undergoes an order-disorder transition during the hydrolysis cycle [127]. These data suggest that analytical centrifugation analysis of the mutants in Sensor 2 region (E440A, L441A or E442R) in different nucleotide-bound states may prove useful in determining if Sensor 2 plays any role in NSF’s nucleotide-related conformational changes.
3.6 SNARE Complex Disassembly Mechanism
A structural and mechanistic understanding of how the SNAP-SNARE complex is disassembled by NSF remains an open problem. Two models have been proposed: the helicase model and the socket-wrench model [50]. In the helicase model, NSF destabilizes the SNARE complex by pulling one of the SNAREs into its central pore. In the socket wrench model, NSF is a ratchet and the three α-SNAPs are the socket. The mechanical force generated from NSF ATP hydrolysis repositions the NSF-N domains to produce a torque, thus altering the overall conformation of the α-SNAPs. The conformational changes of α-SNAPs are coupled to untwist the SNARE complex [50]. Initial proposals suggested that the central zero-layer of the SNARE complex would be a critical element of complex disassembly. It was thought that hydrating the charged residues would destabilize the hydrophobic core of the SNARE complex, though direct experimental data has not supported this model [150]. Thus it is formally possible that either “pulling” or “twisting” by NSF and α-SNAP is sufficient to disassemble SNARE complexes. Understanding what conformational changes NSF can undergo during nucleotide hydrolysis, may help in distinguishing between these models.
Electron microscopy studies of the NSF-SNAP-SNARE complex show that α-SNAP binds laterally along the SNARE bundle and NSF binds to one end of the SNAP-SNARE complex. In this arrangement, the NSF-N domains would face the C-terminus of α-SNAP and N-terminus of the SNARE complex [83, 151] (Figure 2). NSF looks like a double-layered barrel (NSF-D1 and NSF-D2) with six protrusions extended from the side of NSF-D1 and a cap-like structure (SNAP-SNARE) sits on the top. A structural similarity has been noted in the p97-p47 complex, where three p47 adaptor molecules bind to the N-terminal domains of the p97 hexamer [124]. Superposition of the EM reconstructions of p97-p47 and the NSF-SNAP-SNARE complex shows that p97-p47 is similar to the NSF-SNAP-SNARE complex both in size and overall shape. The p97-p47 complex adopts a barrel-shaped arrangement (p97-D1 and p97-D2) with small densities protruding outwards from the outer tip of the p97-D1 ring (p97-N) and the adaptor proteins (p47) are located on top of the hexamer. Therefore, the possible dislocation mechanism reported for p97 could be used to refine a model for NSF-mediated SNAP-SNARE complex disassembly.
Nucleotide-dependent conformational changes of the p97-p47 complex have been detected in EM analyses. In the presence of AMP-PNP, there is no central hole in the p97-D2 ring. P97-D1 ring has the same diameter as that of p97-D2 ring and stacks on the top of p97-D2. Three p47 molecules sit on the top of the central pore region of p97-D1 ring. In the presence of ADP, a pore of ~ 20Å is seen at the bottom of the p97-D2 ring and the particle is wider at the p97-D1-D2 interface. Three arms corresponding to the N-terminal domains of p47 emerge from the central pore [124]. From the structural studies of p97, it is proposed that changes occurring during ATP hydrolysis in the p97-D2 are transmitted and applied to p97-N. This leads to the conformational changes of the p47 adaptor domains, exerting a pulling force required for protein dislocation.
3.6.1 Possible Model of SNAP-SNARE Complex Disassembly
As shown previously, NSF-N and the conserved loop (Y296/G298) in the pore region of NSF-D1 are involved in SNAP-SNARE binding. Mutations of Y296 and G298 affected the SNAP-SNARE complex disassembly [86]. Based on the structure NSF-D2 [30, 31], the proposed “3-in, 3-out” organization of NSF-N domains [75] and the similarity between NSF and p97, we suggest a model for SNAP-SNARE complex disassembly (Figure 5). In this model, three α-SNAPs interact primarily with three N-domains extended from the NSF hexamer. This positions the SNARE complex in close proximity to the pore region of NSF-D1 domain. In the pore region, the SNARE complex interacts with the hydrophobic patch (Y296/G298) in one of the loops. The interaction sites between NSF-D1 pore and SNARE complex work as a racket, holding the SNARE complex inside the NSF-D1 pore. The SNAP-SNARE binding also induces the conformational changes of Arginine Fingers in NSF-D1, which are moved into the ATP binding sites and interact with the γ-phosphate of the bound ATP. This interaction puts the γ-phosphate into the right position for the glutamate in the Walker B motif to catalyze ATP hydrolysis [138]. After ATP hydrolysis, the chemical energy is converted into physical changes in NSF, including the conformational changes in the Sensor 2 and the central pore loop. The former motif transfers this information to NSF-N domains and the movements of the NSF-N domains are then coupled to α-SNAPs, which act as a socket to disassemble the SNARE complex. The relative movements of the central pore loop in NSF-D1 during ATP hydrolysis may assist in complex disassembly. This model of NSF is speculative but can be useful in interpreting future studies.
3.6.2 Application of this Model to Other AAA+ Protein
Other AAA+ proteins involved in protein complex disassembly are p97, VPS4, and the PEX1-PEX6 complex [115, 152, 153]. Although there are more structural studies of p97 or p97-adaptor complexes, a full understanding of the mechanism(s) of p97 in membrane fusion or ERAD is still currently unavailable. The structural similarity between p97 and NSF helps in making models to describe SNARE complex disassembly. The information from such a model will be useful to understand the mechanisms of both proteins. Compared to p97 and NSF, the VPS4 and PEX1-PEX6 complexes are less studied. In the proposed model of VPS4 function, the Microtubule Interacting and Transport (MIT) motif in VPS4-N binds to the MIT interacting Motif (MIM) motif in the ESCRT-III proteins. This interaction then promotes the hexamerization of VPS4, stimulates VPS4 ATPase activity and brings ESCRT-III proteins into proximity to the central pore of VPS4. At this stage, ESCRT-III proteins enter the central pore and interact with the conserved hydrophobic patch in the central pore loop. ATP hydrolysis causes the movement of this loop, which provides a physical force to dissociate the ESCRT-III complex from endosome membranes thus recycling the individual subunit back [21]. The model of VPS4 disassembly of ESCRT-III complex is similar to what we propose for NSF; however, more evidence is needed to support this model. The functional roles of PEX1 and PEX6 in peroxisome biogenesis have still not been defined. One possible model is that PEX1 and PEX6 form a complex, which is recruited to the peroxisomal membrane through their interaction with PEX26. ATP-dependent conformational changes in the PEX1 and PEX6 complex are used to disengage PEX5 from peroxisomal membrane. PEX5 is then recycled to the cytosol where it binds to more proteins and transports them into peroxisomes [153]. Our work will help in designing experiments to further study VPS4 and PEX1-PEX6 complexes and to understand their mechanisms.
In summary, the research described in this review uncovers more questions than it provides answers. However, with the new Cy-(Ni2+NTA)2-His6 labeling technique [93], it will be now possible to address some of the questions about the conformational changes that NSF undergoes during ATP hydrolysis. This same approach could be applied to other AAA proteins. In addition, it will be possible to examine the assembly of the NSF-SNAP-SNARE complexes temporally, which will greatly aid in understanding the mechanisms of SNARE complex disassembly. Since the AAA domains are extremely well-conserved within the family, elucidation of the basic structure and function of NSF will provide clues to understand the common mechanisms of other AAA+ proteins.
Research highlights.
Compilation of mutagenesis data, new conformational change data, new concept for understanding NSF function
ACKNOWLEDGMENTS
The authors thank Dr. Elena A. Matveeva for her careful reading of this manuscript and her insightful comments.
Abbreviations
- NSF
N-ethylmaleimide sensitive factor
- AAA+
ATPases associated with various cellular activities plus
- SNAP
Soluble NSF attachment protein
- SNARE
SNAP-binding receptor
- ESCRT
Endosomal Sorting Complexes Required for Transport
- AMP-PNP
non-hydrolyzable ATP analogue
- Ni2+-NTA
Nickel (II) -Nitriloacetic Acid
- (Ni2+-NTA)2-Cy3
Ni2+-loaded, bis-NTA-Cy3
- VCP
Valosin-Containing Protein
- ERAD
Endoplasmic Reticulum-Associated Degradation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kunau WH, Beyer A, Franken T, Gotte K, Marzioch M, Saidowsky J, Skaletz-Rorowski A, Wiebel FF. Two complementary approaches to study peroxisome biogenesis in Saccharomyces cerevisiae: forward and reversed genetics. Biochimie. 1993;75:209–224. doi: 10.1016/0300-9084(93)90079-8. [DOI] [PubMed] [Google Scholar]
- 2.Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 3.Koller KJ, Brownstein MJ. Use of a cDNA clone to identify a supposed precursor protein containing valosin. Nature. 1987;325:542–545. doi: 10.1038/325542a0. [DOI] [PubMed] [Google Scholar]
- 4.Ogura T, Wilkinson AJ. AAA+ superfamily ATPases: common structure--diverse function. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Wang D, Portis AR., Jr Identification of critical arginine residues in the functioning of Rubisco activase. Arch Biochem Biophys. 2006;450:176–182. doi: 10.1016/j.abb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L, Wrabl JO, Hayashi AP, Rose LS, Thomas PJ. The torsin-family AAA+ protein OOC-5 contains a critical disulfide adjacent to Sensor-II that couples redox state to nucleotide binding. Mol Biol Cell. 2008;19:3599–3612. doi: 10.1091/mbc.E08-01-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Q, Rife CL, Carlton D, Miller MD, Krishna SS, Elsliger MA, Abdubek P, Astakhova T, Chiu HJ, Clayton T, Duan L, Feuerhelm J, Grzechnik SK, Hale J, Han GW, Jaroszewski L, Jin KK, Klock HE, Knuth MW, Kumar A, McMullan D, Morse AT, Nigoghossian E, Okach L, Oommachen S, Paulsen J, Reyes R, van den Bedem H, Hodgson KO, Wooley J, Deacon AM, Godzik A, Lesley SA, Wilson IA. Crystal structure of a novel archaeal AAA+ ATPase SSO1545 from Sulfolobus solfataricus. Proteins. 2009;74:1041–1049. doi: 10.1002/prot.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulbrich C, Diepholz M, Bassler J, Kressler D, Pertschy B, Galani K, Bottcher B, Hurt E. Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell. 2009;138:911–922. doi: 10.1016/j.cell.2009.06.045. [DOI] [PubMed] [Google Scholar]
- 9.Gradolatto A, Smart SK, Byrum S, Blair LP, Rogers RS, Kolar EA, Lavender H, Larson SK, Aitchison JD, Taverna SD, Tackett AJ. A noncanonical bromodomain in the AAA ATPase protein Yta7 directs chromosomal positioning and barrier chromatin activity. Mol Cell Biol. 2009;29:4604–4611. doi: 10.1128/MCB.00160-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer LM, Leipe DD, Koonin EV, Aravind L. Evolutionary history and higher order classification of AAA+ ATPases. J Struct Biol. 2004;146:11–31. doi: 10.1016/j.jsb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 12.Matveeva EA, Vanaman TC, Whiteheart SW, Slevin JT. Asymmetric accumulation of hippocampal 7S SNARE complexes occurs regardless of kindling paradigm. Epilepsy Res. 2007;73:266–274. doi: 10.1016/j.eplepsyres.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou JX, Guo L, Revenko AS, Tepper CG, Gemo AT, Kung HJ, Chen HW. Androgen-induced coactivator ANCCA mediates specific androgen receptor signaling in prostate cancer. Cancer Res. 2009;69:3339–3346. doi: 10.1158/0008-5472.CAN-08-3440. [DOI] [PubMed] [Google Scholar]
- 14.Beyer A. Sequence analysis of the AAA protein family. Protein Sci. 1997;6:2043–2058. doi: 10.1002/pro.5560061001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogura T, Matsushita-Ishiodori Y, Johjima A, Nishizono M, Nishikori S, Esaki M, Yamanaka K. From the common molecular basis of the AAA protein to various energy-dependent and -independent activities of AAA proteins. Biochem Soc Trans. 2008;36:68–71. doi: 10.1042/BST0360068. [DOI] [PubMed] [Google Scholar]
- 16.Erzberger JP, Berger JM. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu Rev Biophys Biomol Struct. 2006;35:93–114. doi: 10.1146/annurev.biophys.35.040405.101933. [DOI] [PubMed] [Google Scholar]
- 17.Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- 18.Bose D, Joly N, Pape T, Rappas M, Schumacher J, Buck M, Zhang X. Dissecting the ATP hydrolysis pathway of bacterial enhancer-binding proteins. Biochem Soc Trans. 2008;36:83–88. doi: 10.1042/BST0360083. [DOI] [PubMed] [Google Scholar]
- 19.Nagiec EE, Bernstein A, Whiteheart SW. Each domain of the N-ethylmaleimide-sensitive fusion protein contributes to its transport activity. J Biol Chem. 1995;270:29182–29188. doi: 10.1074/jbc.270.49.29182. [DOI] [PubMed] [Google Scholar]
- 20.Dreveny I, Kondo H, Uchiyama K, Shaw A, Zhang X, Freemont PS. Structural basis of the interaction between the AAA ATPase p97/VCP and its adaptor protein p47. EMBO J. 2004;23:1030–1039. doi: 10.1038/sj.emboj.7600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrill SA, Hanson PI. Activation of human VPS4A by ESCRT-III proteins reveals ability of substrates to relieve enzyme autoinhibition. J Biol Chem. 2010;285:35428–35438. doi: 10.1074/jbc.M110.126318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bochtler M, Hartmann C, Song HK, Bourenkov GP, Bartunik HD, Huber R. The structures of HsIU and the ATP-dependent protease HsIU-HsIV. Nature. 2000;403:800–805. doi: 10.1038/35001629. [DOI] [PubMed] [Google Scholar]
- 23.Erzberger JP, Pirruccello MM, Berger JM. The structure of bacterial DnaA: implications for general mechanisms underlying DNA replication initiation. EMBO J. 2002;21:4763–4773. doi: 10.1093/emboj/cdf496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada K, Kunishima N, Mayanagi K, Ohnishi T, Nishino T, Iwasaki H, Shinagawa H, Morikawa K. Crystal structure of the Holliday junction migration motor protein RuvB from Thermus thermophilus, HB8. Proc Natl Acad Sci U S A. 2001;98:1442–1447. doi: 10.1073/pnas.031470598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mogk A, Haslberger T, Tessarz P, Bukau B. Common and specific mechanisms of AAA+ proteins involved in protein quality control. Biochem Soc Trans. 2008;36:120–125. doi: 10.1042/BST0360120. [DOI] [PubMed] [Google Scholar]
- 26.Block MR, Glick BS, Wilcox CA, Wieland FT, Rothman JE. Purification of an Nethylmaleimide-sensitive protein catalyzing vesicular transport. Proc Natl Acad Sci U S A. 1988;85:7852–7856. doi: 10.1073/pnas.85.21.7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleming KG, Hohl TM, Yu RC, Muller SA, Wolpensinger B, Engel A, Engelhardt H, Brunger AT, Sollner TH, Hanson PI. A revised model for the oligomeric state of the Nethylmaleimide-sensitive fusion protein, NSF. J Biol Chem. 1998;273:15675–15681. doi: 10.1074/jbc.273.25.15675. [DOI] [PubMed] [Google Scholar]
- 28.Tagaya M, Wilson DW, Brunner M, Arango N, Rothman JE. Domain structure of an Nethylmaleimide-sensitive fusion protein involved in vesicular transport. J Biol Chem. 1993;268:2662–2666. [PubMed] [Google Scholar]
- 29.Whiteheart SW, Rossnagel K, Buhrow SA, Brunner M, Jaenicke R, Rothman JE. Nethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J Cell Biol. 1994;126:945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu RC, Hanson PI, Jahn R, Brunger AT. Structure of the ATP-dependent oligomerization domain of N-ethylmaleimide sensitive factor complexed with ATP. Nat Struct Biol. 1998;5:803–811. doi: 10.1038/1843. [DOI] [PubMed] [Google Scholar]
- 31.Lenzen CU, Steinmann D, Whiteheart SW, Weis WI. Crystal structure of the hexamerization domain of N-ethylmaleimide-sensitive fusion protein. Cell. 1998;94:525–536. doi: 10.1016/s0092-8674(00)81593-7. [DOI] [PubMed] [Google Scholar]
- 32.Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 33.Clary DO, Griff IC, Rothman JE. SNAPs, a family of NSF attachment proteins involved in intracellular membrane fusion in animals and yeast. Cell. 1990;61:709–721. doi: 10.1016/0092-8674(90)90482-t. [DOI] [PubMed] [Google Scholar]
- 34.Whiteheart SW, Matveeva EA. Multiple binding proteins suggest diverse functions for the N-ethylmaleimide sensitive factor. J Struct Biol. 2004;146:32–43. doi: 10.1016/j.jsb.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Zhao C, Slevin JT, Whiteheart SW. Cellular functions of NSF: not just SNAPs and SNAREs. FEBS Lett. 2007;581:2140–2149. doi: 10.1016/j.febslet.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, Hara MR, Quick RA, Cao W, O'Rourke B, Lowenstein JM, Pevsner J, Wagner DD, Lowenstein CJ. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell. 2003;115:139–150. doi: 10.1016/s0092-8674(03)00803-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huynh H, Bottini N, Williams S, Cherepanov V, Musumeci L, Saito K, Bruckner S, Vachon E, Wang X, Kruger J, Chow CW, Pellecchia M, Monosov E, Greer PA, Trimble W, Downey GP, Mustelin T. Control of vesicle fusion by a tyrosine phosphatase. Nat Cell Biol. 2004;6:831–839. doi: 10.1038/ncb1164. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Cheng K, Gong K, Fu AK, Ip NY. Pctaire1 phosphorylates N-ethylmaleimidesensitive fusion protein: implications in the regulation of its hexamerization and exocytosis. J Biol Chem. 2006;281:9852–9858. doi: 10.1074/jbc.M513496200. [DOI] [PubMed] [Google Scholar]
- 39.Matveeva EA, Whiteheart SW, Vanaman TC, Slevin JT. Phosphorylation of the Nethylmaleimide-sensitive factor is associated with depolarization-dependent neurotransmitter release from synaptosomes. J Biol Chem. 2001;276:12174–12181. doi: 10.1074/jbc.M007394200. [DOI] [PubMed] [Google Scholar]
- 40.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 41.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci U S A. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 43.Antonin W, Fasshauer D, Becker S, Jahn R, Schneider TR. Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat Struct Biol. 2002;9:107–111. doi: 10.1038/nsb746. [DOI] [PubMed] [Google Scholar]
- 44.Whiteheart SW, Griff IC, Brunner M, Clary DO, Mayer T, Buhrow SA, Rothman JE. SNAP family of NSF attachment proteins includes a brain-specific isoform. Nature. 1993;362:353–355. doi: 10.1038/362353a0. [DOI] [PubMed] [Google Scholar]
- 45.Griff IC, Schekman R, Rothman JE, Kaiser CA. The yeast SEC17 gene product is functionally equivalent to mammalian alpha-SNAP protein. J Biol Chem. 1992;267:12106–12115. [PubMed] [Google Scholar]
- 46.Barszczewski M, Chua JJ, Stein A, Winter U, Heintzmann R, Zilly FE, Fasshauer D, Lang T, Jahn R. A novel site of action for alpha-SNAP in the SNARE conformational cycle controlling membrane fusion. Mol Biol Cell. 2008;19:776–784. doi: 10.1091/mbc.E07-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marz KE, Lauer JM, Hanson PI. Defining the SNARE complex binding surface of alpha-SNAP: implications for SNARE complex disassembly. J Biol Chem. 2003;278:27000–27008. doi: 10.1074/jbc.M302003200. [DOI] [PubMed] [Google Scholar]
- 48.Bitto E, Bingman CA, Kondrashov DA, McCoy JG, Bannen RM, Wesenberg GE, Phillips GN., Jr Structure and dynamics of gamma-SNAP: insight into flexibility of proteins from the SNAP family. Proteins. 2008;70:93–104. doi: 10.1002/prot.21468. [DOI] [PubMed] [Google Scholar]
- 49.Wilson DW, Wilcox CA, Flynn GC, Chen E, Kuang WJ, Henzel WJ, Block MR, Ullrich A, Rothman JE. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989;339:355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- 50.Whiteheart SW, Schraw T, Matveeva EA. N-ethylmaleimide sensitive factor (NSF) structure and function. Int Rev Cytol. 2001;207:71–112. doi: 10.1016/s0074-7696(01)07003-6. [DOI] [PubMed] [Google Scholar]
- 51.Wilson KL. NSF-independent fusion mechanisms. Cell. 1995;81:475–477. doi: 10.1016/0092-8674(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 52.Baur T, Ramadan K, Schlundt A, Kartenbeck J, Meyer HH. NSF- and SNARE-mediated membrane fusion is required for nuclear envelope formation and completion of nuclear pore complex assembly in Xenopus laevis egg extracts. J Cell Sci. 2007;120:2895–2903. doi: 10.1242/jcs.010181. [DOI] [PubMed] [Google Scholar]
- 53.Puschel AW, O'Connor V, Betz H. The N-ethylmaleimide-sensitive fusion protein (NSF) is preferentially expressed in the nervous system. FEBS Lett. 1994;347:55–58. doi: 10.1016/0014-5793(94)00505-2. [DOI] [PubMed] [Google Scholar]
- 54.Xu WH, Zhang QR, Denlinger DL. A novel member of the NSF family in the corn earworm, Helicoverpa zea: molecular cloning, developmental expression, and tissue distribution. Biochim Biophys Acta. 2006;1759:186–190. doi: 10.1016/j.bbaexp.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Dalal S, Rosser MF, Cyr DM, Hanson PI. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol Biol Cell. 2004;15:637–648. doi: 10.1091/mbc.E03-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurrasch DM, Nevin LM, Wong JS, Baier H, Ingraham HA. Neuroendocrine transcriptional programs adapt dynamically to the supply and demand for neuropeptides as revealed in NSF mutant zebrafish. Neural Dev. 2009;4:22. doi: 10.1186/1749-8104-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 58.Graham TR, Emr SD. Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J Cell Biol. 1991;114:207–218. doi: 10.1083/jcb.114.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaiser CA, Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990;61:723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- 60.Novick P, Ferro S, Schekman R. Order of events in the yeast secretory pathway. Cell. 1981;25:461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- 61.Steel GJ, Harley C, Boyd A, Morgan A. A screen for dominant negative mutants of SEC18 reveals a role for the AAA protein consensus sequence in ATP hydrolysis. Mol Biol Cell. 2000;11:1345–1356. doi: 10.1091/mbc.11.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boulianne GL, Trimble WS. Identification of a second homolog of N-ethylmaleimide-sensitive fusion protein that is expressed in the nervous system and secretory tissues of Drosophila. Proc Natl Acad Sci U S A. 1995;92:7095–7099. doi: 10.1073/pnas.92.15.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Golby JA, Tolar LA, Pallanck L. Partitioning of N-ethylmaleimide-sensitive fusion (NSF) protein function in Drosophila melanogaster: dNSF1 is required in the nervous system, and dNSF2 is required in mesoderm. Genetics. 2001;158:265–278. doi: 10.1093/genetics/158.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siddiqi O, Benzer S. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1976;73:3253–3257. doi: 10.1073/pnas.73.9.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pallanck L, Ordway RW, Ganetzky B. A Drosophila NSF mutant. Nature. 1995;376:25. doi: 10.1038/376025a0. [DOI] [PubMed] [Google Scholar]
- 66.Kawasaki F, Ordway RW. Molecular mechanisms determining conserved properties of short-term synaptic depression revealed in NSF and SNAP-25 conditional mutants. Proc Natl Acad Sci U S A. 2009;106:14658–14663. doi: 10.1073/pnas.0907144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 68.Morgan A. Classic clues to NSF function. Nature. 1996;382:680. doi: 10.1038/382680a0. [DOI] [PubMed] [Google Scholar]
- 69.Kuner T, Li Y, Gee KR, Bonewald LF, Augustine GJ. Photolysis of a caged peptide reveals rapid action of N-ethylmaleimide sensitive factor before neurotransmitter release. Proc Natl Acad Sci U S A. 2008;105:347–352. doi: 10.1073/pnas.0707197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woodman PG. The roles of NSF, SNAPs and SNAREs during membrane fusion. Biochim Biophys Acta. 1997;1357:155–172. doi: 10.1016/s0167-4889(97)00039-6. [DOI] [PubMed] [Google Scholar]
- 71.Littleton JT, Barnard RJ, Titus SA, Slind J, Chapman ER, Ganetzky B. SNAREcomplex disassembly by NSF follows synaptic-vesicle fusion. Proc Natl Acad Sci U S A. 2001;98:12233–12238. doi: 10.1073/pnas.221450198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.May AP, Whiteheart SW, Weis WI. Unraveling the mechanism of the vesicle transport ATPase NSF, the N-ethylmaleimide-sensitive factor. J Biol Chem. 2001;276:21991–21994. doi: 10.1074/jbc.R100013200. [DOI] [PubMed] [Google Scholar]
- 73.Sanyal S, Tolar LA, Pallanck L, Krishnan KS. Genetic interaction between shibire and comatose mutations in Drosophila suggest a role for snap-receptor complex assembly and disassembly for maintenance of synaptic vesicle cycling. Neurosci Lett. 2001;311:21–24. doi: 10.1016/s0304-3940(01)02125-5. [DOI] [PubMed] [Google Scholar]
- 74.Yu RC, Jahn R, Brunger AT. NSF N-terminal domain crystal structure: models of NSF function. Mol Cell. 1999;4:97–107. doi: 10.1016/s1097-2765(00)80191-4. [DOI] [PubMed] [Google Scholar]
- 75.May AP, Misura KM, Whiteheart SW, Weis WI. Crystal structure of the amino-terminal domain of N-ethylmaleimide-sensitive fusion protein. Nat Cell Biol. 1999;1:175–182. doi: 10.1038/11097. [DOI] [PubMed] [Google Scholar]
- 76.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alphaand beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matveeva EA, He P, Whiteheart SW. N-Ethylmaleimide-sensitive fusion protein contains high and low affinity ATP-binding sites that are functionally distinct. J Biol Chem. 1997;272:26413–26418. doi: 10.1074/jbc.272.42.26413. [DOI] [PubMed] [Google Scholar]
- 78.Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F, Wittinghofer A. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- 79.Lee S, Sowa ME, Watanabe YH, Sigler PB, Chiu W, Yoshida M, Tsai FT. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell. 2003;115:229–240. doi: 10.1016/s0092-8674(03)00807-9. [DOI] [PubMed] [Google Scholar]
- 80.Hattendorf DA, Lindquist SL. Analysis of the AAA sensor-2 motif in the C-terminal ATPase domain of Hsp104 with a site-specific fluorescent probe of nucleotide binding. Proc Natl Acad Sci U S A. 2002;99:2732–2737. doi: 10.1073/pnas.261693199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi N, Tsutsumi S, Tsuchiya T, Stillman B, Mizushima T. Functions of sensor 1 and sensor 2 regions of Saccharomyces cerevisiae Cdc6p in vivo and in vitro. J Biol Chem. 2002;277:16033–16040. doi: 10.1074/jbc.M108615200. [DOI] [PubMed] [Google Scholar]
- 82.Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 83.Furst J, Sutton RB, Chen J, Brunger AT, Grigorieff N. Electron cryomicroscopy structure of N-ethyl maleimide sensitive factor at 11 A resolution. Embo J. 2003;22:4365–4374. doi: 10.1093/emboj/cdg420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weibezahn J, Tessarz P, Schlieker C, Zahn R, Maglica Z, Lee S, Zentgraf H, Weber-Ban EU, Dougan DA, Tsai FT, Mogk A, Bukau B. Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell. 2004;119:653–665. doi: 10.1016/j.cell.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 85.DeLaBarre B, Christianson JC, Kopito RR, Brunger AT. Central pore residues mediate the p97/VCP activity required for ERAD. Mol Cell. 2006;22:451–462. doi: 10.1016/j.molcel.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 86.Zhao C, Matveeva EA, Ren Q, Whiteheart SW. Dissecting the N-ethylmaleimidesensitive factor: required elements of the N and D1 domains. J Biol Chem. 2010;285:761–772. doi: 10.1074/jbc.M109.056739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sumida M, Hong RM, Tagaya M. Role of two nucleotide-binding regions in an Nethylmaleimide-sensitive factor involved in vesicle-mediated protein transport. J Biol Chem. 1994;269:20636–20641. [PubMed] [Google Scholar]
- 88.Morgan A, Dimaline R, Burgoyne RD. The ATPase activity of N-ethylmaleimidesensitive fusion protein (NSF) is regulated by soluble NSF attachment proteins. J Biol Chem. 1994;269:29347–29350. [PubMed] [Google Scholar]
- 89.Matveeva E, Whiteheart SW. The effects of SNAP/SNARE complexes on the ATPase of NSF. FEBS Lett. 1998;435:211–214. doi: 10.1016/s0014-5793(98)01071-0. [DOI] [PubMed] [Google Scholar]
- 90.Barnard RJ, Morgan A, Burgoyne RD. Domains of alpha-SNAP required for the stimulation of exocytosis and for N-ethylmalemide-sensitive fusion protein (NSF) binding and activation. Molecular biology of the cell. 1996;7:693–701. doi: 10.1091/mbc.7.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Barnard RJ, Morgan A, Burgoyne RD. Stimulation of NSF ATPase activity by alpha-SNAP is required for SNARE complex disassembly and exocytosis. J Cell Biol. 1997;139:875–883. doi: 10.1083/jcb.139.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steel GJ, Morgan A. Selective stimulation of the D1 ATPase domain of N-ethylmaleimidesensitive fusion protein (NSF) by soluble NSF attachment proteins. FEBS Lett. 1998;423:113–116. doi: 10.1016/s0014-5793(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 93.Zhao C, Hellman LM, Zhan X, Bowman WS, Whiteheart SW, Fried MG. Hexahistidine-tag-specific optical probes for analyses of proteins and their interactions. Anal Biochem. 2010;399:237–245. doi: 10.1016/j.ab.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hellman LM, Zhao C, Melikishvili M, Tao X, Hopper JE, Whiteheart SW, Fried MG. Histidine-tag-directed chromophores for tracer analyses in the analytical ultracentrifuge. Methods. 2011;54:31–38. doi: 10.1016/j.ymeth.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iwasaki H, Han YW, Okamoto T, Ohnishi T, Yoshikawa M, Yamada K, Toh H, Daiyasu H, Ogura T, Shinagawa H. Mutational analysis of the functional motifs of RuvB, an AAA+ class helicase and motor protein for holliday junction branch migration. Mol Microbiol. 2000;36:528–538. doi: 10.1046/j.1365-2958.2000.01842.x. [DOI] [PubMed] [Google Scholar]
- 96.Schaupp A, Marcinowski M, Grimminger V, Bosl B, Walter S. Processing of proteins by the molecular chaperone Hsp104. J Mol Biol. 2007;370:674–686. doi: 10.1016/j.jmb.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 97.Mogk A, Schlieker C, Strub C, Rist W, Weibezahn J, Bukau B. Roles of individual domains and conserved motifs of the AAA+ chaperone ClpB in oligomerization, ATP hydrolysis, and chaperone activity. J Biol Chem. 2003;278:17615–17624. doi: 10.1074/jbc.M209686200. [DOI] [PubMed] [Google Scholar]
- 98.Augustin S, Gerdes F, Lee S, Tsai FT, Langer T, Tatsuta T. An intersubunit signaling network coordinates ATP hydrolysis by m-AAA proteases. Mol Cell. 2009;35:574–585. doi: 10.1016/j.molcel.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Horwitz AA, Navon A, Groll M, Smith DM, Reis C, Goldberg AL. ATP-induced structural transitions in PAN, the proteasome-regulatory ATPase complex in Archaea. J Biol Chem. 2007;282:22921–22929. doi: 10.1074/jbc.M702846200. [DOI] [PubMed] [Google Scholar]
- 100.Karata K, Inagawa T, Wilkinson AJ, Tatsuta T, Ogura T. Dissecting the role of a conserved motif (the second region of homology) in the AAA family of ATPases. Site-directed mutagenesis of the ATP-dependent protease FtsH. J Biol Chem. 1999;274:26225–26232. doi: 10.1074/jbc.274.37.26225. [DOI] [PubMed] [Google Scholar]
- 101.Babst M, Wendland B, Estepa EJ, Emr SD. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 1998;17:2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krause T, Kunau WH, Erdmann R. Effect of site-directed mutagenesis of conserved lysine residues upon Pas1 protein function in peroxisome biogenesis. Yeast. 1994;10:1613–1620. doi: 10.1002/yea.320101210. [DOI] [PubMed] [Google Scholar]
- 103.Gerega A, Rockel B, Peters J, Tamura T, Baumeister W, Zwickl P. VAT, the thermoplasma homolog of mammalian p97/VCP, is an N domain-regulated protein unfoldase. J Biol Chem. 2005;280:42856–42862. doi: 10.1074/jbc.M510592200. [DOI] [PubMed] [Google Scholar]
- 104.Wang Q, Song C, Irizarry L, Dai R, Zhang X, Li CC. Multifunctional roles of the conserved Arg residues in the second region of homology of p97/valosin-containing protein. J Biol Chem. 2005;280:40515–40523. doi: 10.1074/jbc.M509636200. [DOI] [PubMed] [Google Scholar]
- 105.Putnam CD, Clancy SB, Tsuruta H, Gonzalez S, Wetmur JG, Tainer JA. Structure and mechanism of the RuvB Holliday junction branch migration motor. J Mol Biol. 2001;311:297–310. doi: 10.1006/jmbi.2001.4852. [DOI] [PubMed] [Google Scholar]
- 106.Matias PM, Gorynia S, Donner P, Carrondo MA. Crystal structure of the human AAA+ protein RuvBL1. J Biol Chem. 2006;281:38918–38929. doi: 10.1074/jbc.M605625200. [DOI] [PubMed] [Google Scholar]
- 107.Jeruzalmi D, O'Donnell M, Kuriyan J. Crystal structure of the processivity clamp loader gamma (gamma) complex of E. coli DNA polymerase III. Cell. 2001;106:429–441. doi: 10.1016/s0092-8674(01)00463-9. [DOI] [PubMed] [Google Scholar]
- 108.Inoue M, Kamikubo H, Kataoka M, Kato R, Yoshimori T, Wakatsuki S, Kawasaki M. Nucleotide-dependent conformational changes and assembly of the AAA ATPase SKD1/VPS4B. Traffic. 2008;9:2180–2189. doi: 10.1111/j.1600-0854.2008.00831.x. [DOI] [PubMed] [Google Scholar]
- 109.Suno R, Niwa H, Tsuchiya D, Zhang X, Yoshida M, Morikawa K. Structure of the whole cytosolic region of ATP-dependent protease FtsH. Mol Cell. 2006;22:575–585. doi: 10.1016/j.molcel.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 110.Kawakami H, Ozaki S, Suzuki S, Nakamura K, Senriuchi T, Su'etsugu M, Fujimitsu K, Katayama T. The exceptionally tight affinity of DnaA for ATP/ADP requires a unique aspartic acid residue in the AAA+ sensor 1 motif. Mol Microbiol. 2006;62:1310–1324. doi: 10.1111/j.1365-2958.2006.05450.x. [DOI] [PubMed] [Google Scholar]
- 111.Hattendorf DA, Lindquist SL. Cooperative kinetics of both Hsp104 ATPase domains and interdomain communication revealed by AAA sensor-1 mutants. EMBO J. 2002;21:12–21. doi: 10.1093/emboj/21.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takehara M, Makise M, Takenaka H, Asano T, Mizushima T. Analysis of mutant origin recognition complex with reduced ATPase activity in vivo and in vitro. Biochem J. 2008;413:535–543. doi: 10.1042/BJ20070484. [DOI] [PubMed] [Google Scholar]
- 113.Schumacher J, Joly N, Rappas M, Bradley D, Wigneshweraraj SR, Zhang X, Buck M. Sensor I threonine of the AAA+ ATPase transcriptional activator PspF is involved in coupling nucleotide triphosphate hydrolysis to the restructuring of sigma 54-RNA polymerase. J Biol Chem. 2007;282:9825–9833. doi: 10.1074/jbc.M611532200. [DOI] [PubMed] [Google Scholar]
- 114.Babor SM, Fass D. Crystal structure of the Sec18p N-terminal domain. Proc Natl Acad Sci U S A. 1999;96:14759–14764. doi: 10.1073/pnas.96.26.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yeung HO, Kloppsteck P, Niwa H, Isaacson RL, Matthews S, Zhang X, Freemont PS. Insights into adaptor binding to the AAA protein p97. Biochem Soc Trans. 2008;36:62–67. doi: 10.1042/BST0360062. [DOI] [PubMed] [Google Scholar]
- 116.Aker J, Hesselink R, Engel R, Karlova R, Borst JW, Visser AJ, de Vries SC. In vivo hexamerization and characterization of the Arabidopsis AAA ATPase CDC48A complex using forster resonance energy transfer-fluorescence lifetime imaging microscopy and fluorescence correlation spectroscopy. Plant Physiol. 2007;145:339–350. doi: 10.1104/pp.107.103986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shiozawa K, Goda N, Shimizu T, Mizuguchi K, Kondo N, Shimozawa N, Shirakawa M, Hiroaki H. The common phospholipid-binding activity of the N-terminal domains of PEX1 and VCP/p97. FEBS J. 2006;273:4959–4971. doi: 10.1111/j.1742-4658.2006.05494.x. [DOI] [PubMed] [Google Scholar]
- 118.Zhang X, Shaw A, Bates PA, Newman RH, Gowen B, Orlova E, Gorman MA, Kondo H, Dokurno P, Lally J, Leonard G, Meyer H, van Heel M, Freemont PS. Structure of the AAA ATPase p97. Mol Cell. 2000;6:1473–1484. doi: 10.1016/s1097-2765(00)00143-x. [DOI] [PubMed] [Google Scholar]
- 119.Shiozawa K, Maita N, Tomii K, Seto A, Goda N, Akiyama Y, Shimizu T, Shirakawa M, Hiroaki H. Structure of the N-terminal domain of PEX1 AAA-ATPase. Characterization of a putative adaptor-binding domain. J Biol Chem. 2004;279:50060–50068. doi: 10.1074/jbc.M407837200. [DOI] [PubMed] [Google Scholar]
- 120.Coles M, Diercks T, Liermann J, Groger A, Rockel B, Baumeister W, Koretke KK, Lupas A, Peters J, Kessler H. The solution structure of VAT-N reveals a 'missing link' in the evolution of complex enzymes from a simple betaalphabetabeta element. Curr Biol. 1999;9:1158–1168. doi: 10.1016/S0960-9822(00)80017-2. [DOI] [PubMed] [Google Scholar]
- 121.Castillo RM, Mizuguchi K, Dhanaraj V, Albert A, Blundell TL, Murzin AG. A sixstranded double-psi beta barrel is shared by several protein superfamilies. Structure. 1999;7:227–236. doi: 10.1016/s0969-2126(99)80028-8. [DOI] [PubMed] [Google Scholar]
- 122.Horsnell WG, Steel GJ, Morgan A. Analysis of NSF mutants reveals residues involved in SNAP binding and ATPase stimulation. Biochemistry. 2002;41:5230–5235. doi: 10.1021/bi0160359. [DOI] [PubMed] [Google Scholar]
- 123.Isaacson RL, Pye VE, Simpson P, Meyer HH, Zhang X, Freemont PS, Matthews S. Detailed structural insights into the p97-Npl4-Ufd1 interface. J Biol Chem. 2007;282:21361–21369. doi: 10.1074/jbc.M610069200. [DOI] [PubMed] [Google Scholar]
- 124.Beuron F, Dreveny I, Yuan X, Pye VE, McKeown C, Briggs LC, Cliff MJ, Kaneko Y, Wallis R, Isaacson RL, Ladbury JE, Matthews SJ, Kondo H, Zhang X, Freemont PS. Conformational changes in the AAA ATPase p97-p47 adaptor complex. EMBO J. 2006;25:1967–1976. doi: 10.1038/sj.emboj.7601055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.DeLaBarre B, Brunger AT. Complete structure of p97/valosin-containing protein reveals communication between nucleotide domains. Nat Struct Biol. 2003;10:856–863. doi: 10.1038/nsb972. [DOI] [PubMed] [Google Scholar]
- 126.Brunger AT, DeLaBarre B. NSF and p97/VCP: similar at first, different at last. FEBS Lett. 2003;555:126–133. doi: 10.1016/s0014-5793(03)01107-4. [DOI] [PubMed] [Google Scholar]
- 127.DeLaBarre B, Brunger AT. Nucleotide dependent motion and mechanism of action of p97/VCP. J Mol Biol. 2005;347:437–452. doi: 10.1016/j.jmb.2005.01.060. [DOI] [PubMed] [Google Scholar]
- 128.Rothballer A, Tzvetkov N, Zwickl P. Mutations in p97/VCP induce unfolding activity. FEBS Lett. 2007;581:1197–1201. doi: 10.1016/j.febslet.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 129.Scott A, Chung HY, Gonciarz-Swiatek M, Hill GC, Whitby FG, Gaspar J, Holton JM, Viswanathan R, Ghaffarian S, Hill CP, Sundquist WI. Structural and mechanistic studies of VPS4 proteins. EMBO J. 2005;24:3658–3669. doi: 10.1038/sj.emboj.7600818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Erbse AH, Wagner JN, Truscott KN, Spall SK, Kirstein J, Zeth K, Turgay K, Mogk A, Bukau B, Dougan DA. Conserved residues in the N-domain of the AAA+ chaperone ClpA regulate substrate recognition and unfolding. FEBS J. 2008;275:1400–1410. doi: 10.1111/j.1742-4658.2008.06304.x. [DOI] [PubMed] [Google Scholar]
- 131.Thibault G, Tsitrin Y, Davidson T, Gribun A, Houry WA. Large nucleotide-dependent movement of the N-terminal domain of the ClpX chaperone. EMBO J. 2006;25:3367–3376. doi: 10.1038/sj.emboj.7601223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ismail SA, Vetter IR, Sot B, Wittinghofer A. The structure of an Arf-ArfGAP complex reveals a Ca2+ regulatory mechanism. Cell. 2010;141:812–821. doi: 10.1016/j.cell.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 133.Moreau MJ, McGeoch AT, Lowe AR, Itzhaki LS, Bell SD. ATPase site architecture and helicase mechanism of an archaeal MCM. Mol Cell. 2007;28:304–314. doi: 10.1016/j.molcel.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 134.Ogura T, Whiteheart SW, Wilkinson AJ. Conserved arginine residues implicated in ATP hydrolysis, nucleotide-sensing, and inter-subunit interactions in AAA and AAA+ ATPases. J Struct Biol. 2004;146:106–112. doi: 10.1016/j.jsb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 135.Wendler P, Shorter J, Plisson C, Cashikar AG, Lindquist S, Saibil HR. Atypical AAA+ subunit packing creates an expanded cavity for disaggregation by the protein-remodeling factor Hsp104. Cell. 2007;131:1366–1377. doi: 10.1016/j.cell.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gonciarz MD, Whitby FG, Eckert DM, Kieffer C, Heroux A, Sundquist WI, Hill CP. Biochemical and structural studies of yeast Vps4 oligomerization. J Mol Biol. 2008;384:878–895. doi: 10.1016/j.jmb.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kawakami H, Keyamura K, Katayama T. Formation of an ATP-DnaA-specific initiation complex requires DnaA Arginine 285, a conserved motif in the AAA+ protein family. J Biol Chem. 2005;280:27420–27430. doi: 10.1074/jbc.M502764200. [DOI] [PubMed] [Google Scholar]
- 138.Zhang X, Wigley DB. The 'glutamate switch' provides a link between ATPase activity and ligand binding in AAA+ proteins. Nat Struct Mol Biol. 2008;15:1223–1227. doi: 10.1038/nsmb.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hishida T, Han YW, Fujimoto S, Iwasaki H, Shinagawa H. Direct evidence that a conserved arginine in RuvB AAA+ ATPase acts as an allosteric effector for the ATPase activity of the adjacent subunit in a hexamer. Proc Natl Acad Sci U S A. 2004;101:9573–9577. doi: 10.1073/pnas.0403584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martin A, Baker TA, Sauer RT. Pore loops of the AAA+ ClpX machine grip substrates to drive translocation and unfolding. Nat Struct Mol Biol. 2008;15:1147–1151. doi: 10.1038/nsmb.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Martin A, Baker TA, Sauer RT. Diverse pore loops of the AAA+ ClpX machine mediate unassisted and adaptor-dependent recognition of ssrA-tagged substrates. Mol Cell. 2008;29:441–450. doi: 10.1016/j.molcel.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kress W, Weber-Ban E. The alternating power stroke of a 6-cylinder AAA protease chaperone engine. Mol Cell. 2009;35:545–547. doi: 10.1016/j.molcel.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 143.Rouiller I, DeLaBarre B, May AP, Weis WI, Brunger AT, Milligan RA, Wilson-Kubalek EM. Conformational changes of the multifunction p97 AAA ATPase during its ATPase cycle. Nat Struct Biol. 2002;9:950–957. doi: 10.1038/nsb872. [DOI] [PubMed] [Google Scholar]
- 144.Davies JM, Brunger AT, Weis WI. Improved structures of full-length p97, an AAA ATPase: implications for mechanisms of nucleotide-dependent conformational change. Structure. 2008;16:715–726. doi: 10.1016/j.str.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 145.Davies JM, Tsuruta H, May AP, Weis WI. Conformational changes of p97 during nucleotide hydrolysis determined by small-angle X-Ray scattering. Structure. 2005;13:183–195. doi: 10.1016/j.str.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 146.Huyton T, Pye VE, Briggs LC, Flynn TC, Beuron F, Kondo H, Ma J, Zhang X, Freemont PS. The crystal structure of murine p97/VCP at 3.6A. J Struct Biol. 2003;144:337–348. doi: 10.1016/j.jsb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 147.Lee S, Choi JM, Tsai FT. Visualizing the ATPase cycle in a protein disaggregating machine: structural basis for substrate binding by ClpB. Mol Cell. 2007;25:261–271. doi: 10.1016/j.molcel.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wendler P, Shorter J, Snead D, Plisson C, Clare DK, Lindquist S, Saibil HR. Motor mechanism for protein threading through Hsp104. Mol Cell. 2009;34:81–92. doi: 10.1016/j.molcel.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vij N. AAA ATPase p97/VCP: cellular functions, disease and therapeutic potential. J Cell Mol Med. 2008;12:2511–2518. doi: 10.1111/j.1582-4934.2008.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lauer JM, Dalal S, Marz KE, Nonet ML, Hanson PI. SNARE complex zero layer residues are not critical for N-ethylmaleimide-sensitive factor-mediated disassembly. J Biol Chem. 2006;281:14823–14832. doi: 10.1074/jbc.M512706200. [DOI] [PubMed] [Google Scholar]
- 151.Hohl TM, Parlati F, Wimmer C, Rothman JE, Sollner TH, Engelhardt H. Arrangement of subunits in 20 S particles consisting of NSF, SNAPs, and SNARE complexes. Mol Cell. 1998;2:539–548. doi: 10.1016/s1097-2765(00)80153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shestakova A, Hanono A, Drosner S, Curtiss M, Davies BA, Katzmann DJ, Babst M. Assembly of the AAA ATPase Vps4 on ESCRT-III. Mol Biol Cell. 2010;21:1059–1071. doi: 10.1091/mbc.E09-07-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fujiki Y, Miyata N, Matsumoto N, Tamura S. Dynamic and functional assembly of the AAA peroxins, Pex1p and Pex6p and their membrane receptor Pex26p involved in shuttling of the PTS1 receptor Pex5p in peroxisome biogenesis. Biochem Soc Trans. 2008;36:109–113. doi: 10.1042/BST0360109. [DOI] [PubMed] [Google Scholar]