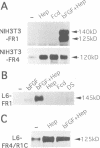
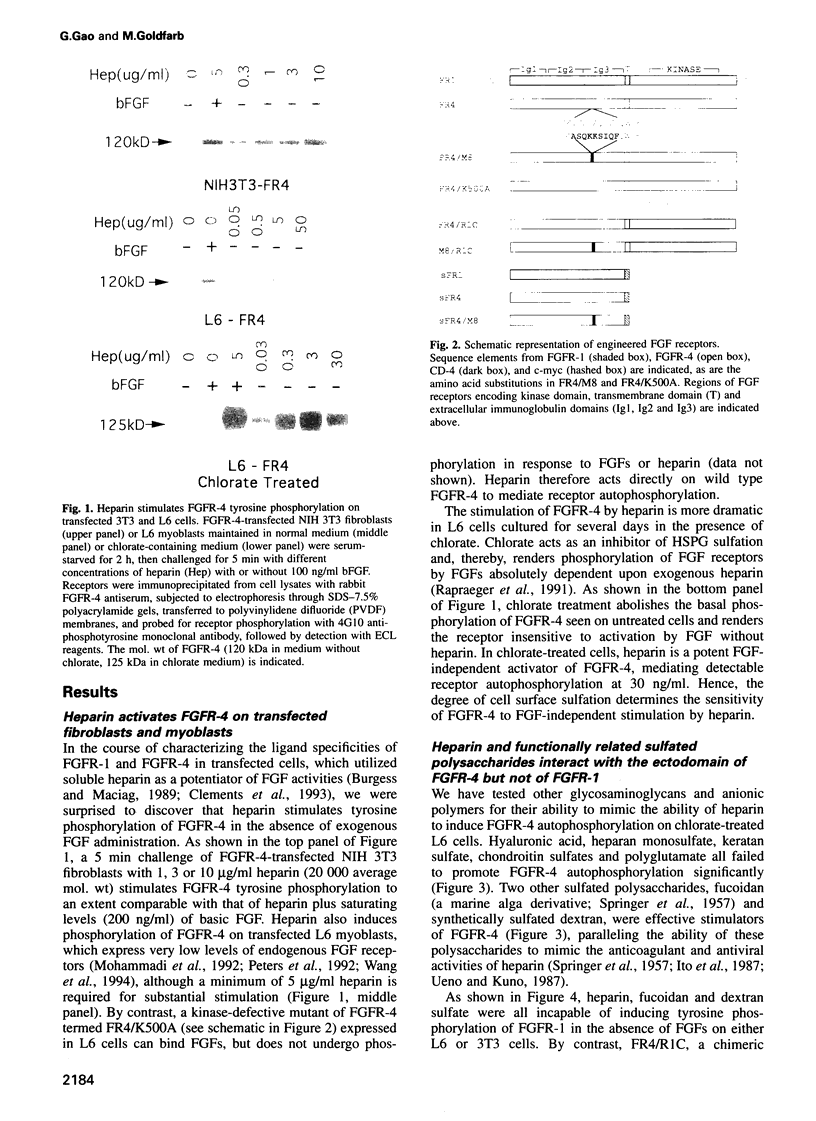
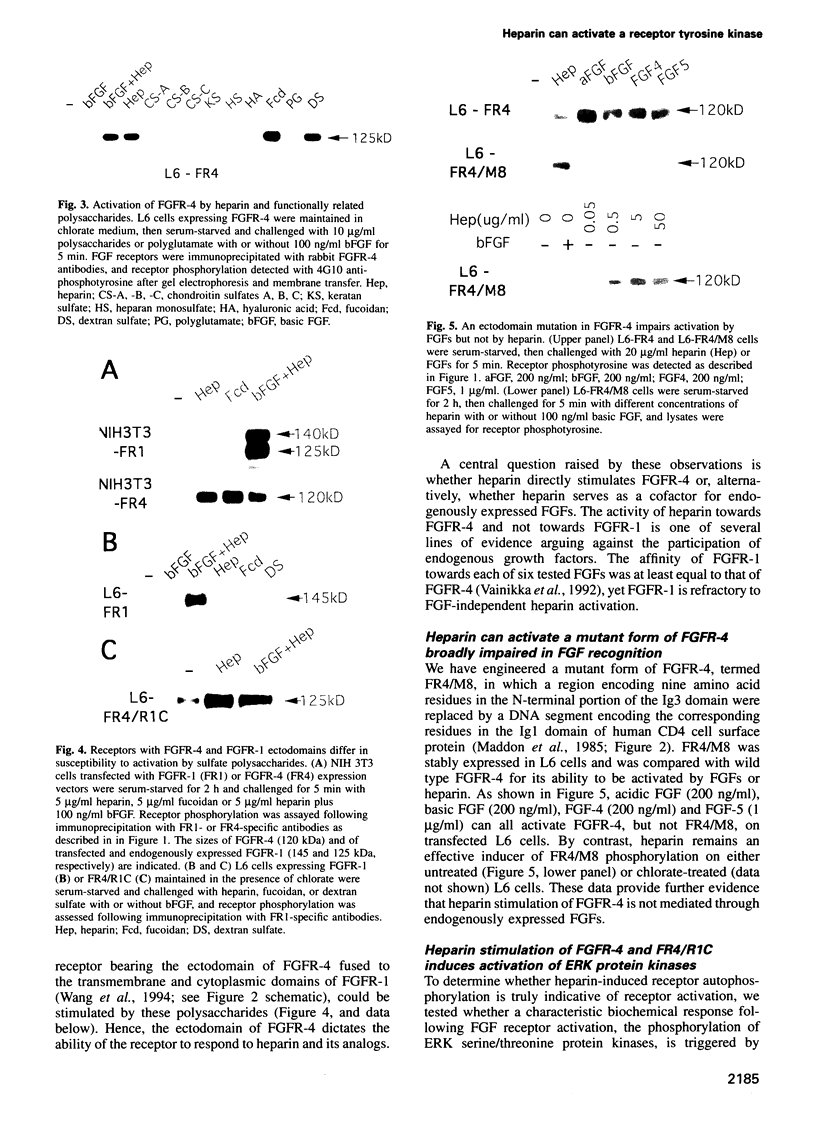
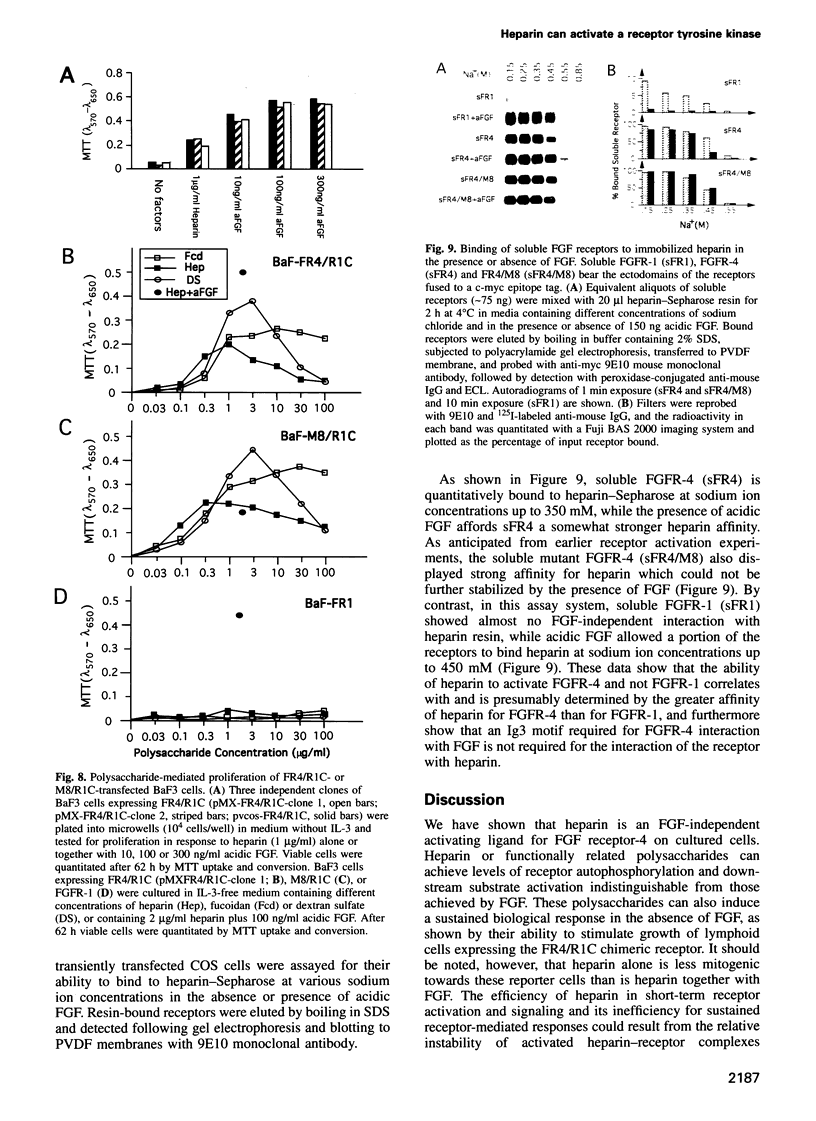
Abstract
Heparin, a densely sulfated glycosaminoglycan produced by mast cells, is best known for its inhibitory effects on the blood coagulation system. Heparin or heparan sulfate proteoglycans are also essential cofactors for the interaction of fibroblast growth factors (FGFs) with their receptor tyrosine kinases (FGFRs). Here we show that heparin is a growth factor-independent activating ligand for FGFR-4. Heparin stimulates FGFR-4 autophosphorylation on transfected myoblasts, fibroblasts and lymphoid cells, and is most potent on cells lacking surface heparan proteoglycan. Two functional analogs of heparin, fucoidan and dextran sulfate, are also activators of FGFR-4, while neither heparin nor its analogs can stimulate FGFR-1 in the absence of FGF. A mutation in the FGFR-4 ectodomain which impairs receptor activation by FGFs does not interfere with activation by heparin, demonstrating that receptor domains required for heparin or FGF activation are not identical. Heparin activation of FGFR-4 or of a chimeric receptor bearing FGFR-4 ectodomain and FGFR-1 cytodomain triggers downstream tyrosine phosphorylation of several signaling proteins, and induces proliferation of cells bearing the chimeric receptor. Consistent with these findings, a soluble FGFR-4 ectodomain has strong FGF-independent affinity for immobilized heparin resin, while soluble FGFR-1 requires FGF for stable heparin interaction. Heparin activation of FGFR-4 is the first example of a mammalian polysaccharide serving as a signaling ligand.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Cardin A. D., Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Cheon H. G., LaRochelle W. J., Bottaro D. P., Burgess W. H., Aaronson S. A. High-affinity binding sites for related fibroblast growth factor ligands reside within different receptor immunoglobulin-like domains. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):989–993. doi: 10.1073/pnas.91.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements D. A., Wang J. K., Dionne C. A., Goldfarb M. Activation of fibroblast growth factor (FGF) receptors by recombinant human FGF-5. Oncogene. 1993 May;8(5):1311–1316. [PubMed] [Google Scholar]
- Crumley G., Bellot F., Kaplow J. M., Schlessinger J., Jaye M., Dionne C. A. High-affinity binding and activation of a truncated FGF receptor by both aFGF and bFGF. Oncogene. 1991 Dec;6(12):2255–2262. [PubMed] [Google Scholar]
- Davis S., Aldrich T. H., Valenzuela D. M., Wong V. V., Furth M. E., Squinto S. P., Yancopoulos G. D. The receptor for ciliary neurotrophic factor. Science. 1991 Jul 5;253(5015):59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- Dell K. R., Williams L. T. A novel form of fibroblast growth factor receptor 2. Alternative splicing of the third immunoglobulin-like domain confers ligand binding specificity. J Biol Chem. 1992 Oct 15;267(29):21225–21229. [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. The fibroblast growth factor family. Cell Growth Differ. 1990 Sep;1(9):439–445. [PubMed] [Google Scholar]
- Guimond S., Maccarana M., Olwin B. B., Lindahl U., Rapraeger A. C. Activating and inhibitory heparin sequences for FGF-2 (basic FGF). Distinct requirements for FGF-1, FGF-2, and FGF-4. J Biol Chem. 1993 Nov 15;268(32):23906–23914. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara M., Tyrrell D. J., Stauber G. B., Brown S., Cousens L. S., Stack R. J. Preparation of affinity-fractionated, heparin-derived oligosaccharides and their effects on selected biological activities mediated by basic fibroblast growth factor. J Biol Chem. 1993 Mar 5;268(7):4675–4683. [PubMed] [Google Scholar]
- Ito M., Baba M., Sato A., Pauwels R., De Clercq E., Shigeta S. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antiviral Res. 1987 Jul;7(6):361–367. doi: 10.1016/0166-3542(87)90018-0. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Busch S. J., Cardin A. D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991 Apr;71(2):481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- Jeske W., Fareed J. Antithrombin III- and heparin cofactor II-mediated anticoagulant and antiprotease actions of heparin and its synthetic analogues. Semin Thromb Hemost. 1993;19 (Suppl 1):241–247. [PubMed] [Google Scholar]
- Kan M., Wang F., Xu J., Crabb J. W., Hou J., McKeehan W. L. An essential heparin-binding domain in the fibroblast growth factor receptor kinase. Science. 1993 Mar 26;259(5103):1918–1921. doi: 10.1126/science.8456318. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Miki T., Fleming T. P., Bottaro D. P., Rubin J. S., Ron D., Aaronson S. A. Expression cDNA cloning of the KGF receptor by creation of a transforming autocrine loop. Science. 1991 Jan 4;251(4989):72–75. doi: 10.1126/science.1846048. [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Dionne C. A., Li W., Li N., Spivak T., Honegger A. M., Jaye M., Schlessinger J. Point mutation in FGF receptor eliminates phosphatidylinositol hydrolysis without affecting mitogenesis. Nature. 1992 Aug 20;358(6388):681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- Montgomery R. I., Lidholt K., Flay N. W., Liang J., Vertel B., Lindahl U., Esko J. D. Stable heparin-producing cell lines derived from the Furth murine mastocytoma. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11327–11331. doi: 10.1073/pnas.89.23.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe V., Ford M. D., Wildschut J. A., Bartlett P. F. Developmental regulation of neural response to FGF-1 and FGF-2 by heparan sulfate proteoglycan. Science. 1993 Apr 2;260(5104):103–106. doi: 10.1126/science.7682010. [DOI] [PubMed] [Google Scholar]
- Ornitz D. M., Leder P. Ligand specificity and heparin dependence of fibroblast growth factor receptors 1 and 3. J Biol Chem. 1992 Aug 15;267(23):16305–16311. [PubMed] [Google Scholar]
- Partanen J., Mäkelä T. P., Eerola E., Korhonen J., Hirvonen H., Claesson-Welsh L., Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991 Jun;10(6):1347–1354. doi: 10.1002/j.1460-2075.1991.tb07654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- SPRINGER G. F., WURZEL H. A., MCNEAL G. M., Jr, ANSELL N. J., DOUGHTY M. F. Isolation of anticoagulant fractions from crude fucoidin. Proc Soc Exp Biol Med. 1957 Feb;94(2):404–409. doi: 10.3181/00379727-94-22960. [DOI] [PubMed] [Google Scholar]
- Stahl N., Yancopoulos G. D. The alphas, betas, and kinases of cytokine receptor complexes. Cell. 1993 Aug 27;74(4):587–590. doi: 10.1016/0092-8674(93)90506-l. [DOI] [PubMed] [Google Scholar]
- Stark K. L., McMahon J. A., McMahon A. P. FGFR-4, a new member of the fibroblast growth factor receptor family, expressed in the definitive endoderm and skeletal muscle lineages of the mouse. Development. 1991 Oct;113(2):641–651. doi: 10.1242/dev.113.2.641. [DOI] [PubMed] [Google Scholar]
- Ueno R., Kuno S. Dextran sulphate, a potent anti-HIV agent in vitro having synergism with zidovudine. Lancet. 1987 Jun 13;1(8546):1379–1379. doi: 10.1016/s0140-6736(87)90681-7. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Vainikka S., Partanen J., Bellosta P., Coulier F., Birnbaum D., Basilico C., Jaye M., Alitalo K. Fibroblast growth factor receptor-4 shows novel features in genomic structure, ligand binding and signal transduction. EMBO J. 1992 Dec;11(12):4273–4280. doi: 10.1002/j.1460-2075.1992.tb05526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. K., Gao G., Goldfarb M. Fibroblast growth factor receptors have different signaling and mitogenic potentials. Mol Cell Biol. 1994 Jan;14(1):181–188. doi: 10.1128/mcb.14.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Duan D. S., de Vries C., Peters K. G., Johnson D. E., Williams L. T. Differential splicing in the extracellular region of fibroblast growth factor receptor 1 generates receptor variants with different ligand-binding specificities. Mol Cell Biol. 1992 Jan;12(1):82–88. doi: 10.1128/mcb.12.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Yayon A., Zimmer Y., Shen G. H., Avivi A., Yarden Y., Givol D. A confined variable region confers ligand specificity on fibroblast growth factor receptors: implications for the origin of the immunoglobulin fold. EMBO J. 1992 May;11(5):1885–1890. doi: 10.1002/j.1460-2075.1992.tb05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Culpepper A., Reddy M., Loveless J., Goldfarb M. Human oncogenes detected by a defined medium culture assay. Oncogene. 1987;1(4):369–376. [PubMed] [Google Scholar]