Abstract
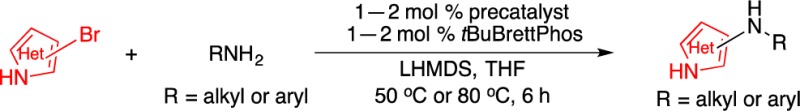
An efficient method for the palladium-catalyzed amination of unprotected bromoimidazoles and bromopyrazoles is presented. The transformation is facilitated by the use of our newly developed Pd precatalyst based on the bulky biarylphosphine ligand tBuBrettPhos (L4). The mild reaction conditions employed allow for the preparation of a broad scope of aminoimidazoles and aminopyrazoles in moderate to excellent yields.
The past decade has seen growing efforts in developing methods toward the functionalization of five-membered nitrogen-containing heterocycles.1 Their unique biological properties and their ability to engage in hydrogen bond interactions has rendered these subunits useful components of medicinally relevant molecules.2 Among these compounds, five-membered aminoheterocycles such as aminoimidazoles3a,3b and aminopyrazoles3c,3d have attracted considerable interest (Figure 1).
Figure 1.
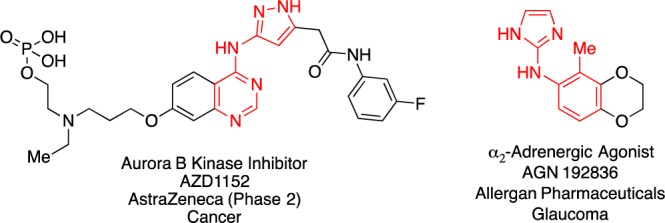
Biologically active compounds containing aminopyrazole and aminoimidazole subunits.
An obvious means to prepare aminoheterocycles is by using the palladium-catalyzed C–N cross-coupling between five-membered heterocyclic halides and amines.4,5 Despite the significant advances in palladium-catalyzed C–N cross-coupling methods, five-membered heterocyclic halides represent difficult coupling partners,6 presumably due to their ability to inhibit and/or deactivate the palladium catalyst.7 While success has been made with halothiophenes and halofurans,8 and very recently haloimidazoles and halopyrazoles,4,8d the use of five-membered heterocycles bearing unprotected NH groups as electrophiles in palladium-catalyzed C–N cross-coupling reactions is comparatively rare9 and limited to specific substrate combinations.4,10 Herein, we present a general method for the palladium-catalyzed amination of a number of unprotected bromoimidazoles and bromopyrazoles.
We initiated our investigation by examining the palladium-catalyzed coupling between test substrates 4-bromo-1H-imidazole and aniline. As depicted in Table 1, we observed dramatic ligand effects in these initial experiments. For example, L1 and L2 were previously reported to be effective for the coupling between 4-bromo-1H-pyrazole and aniline;4,11a however, these ligands proved to be ineffective for the coupling of the 4-bromo-1H-imidazole as the electrophile (entries 1 and 2). Conversely, the use of L3, previously developed for the amidation of five-membered heterocyclic bromides,8d resulted in the full conversion of the bromoimidazole and 77% yield of the corresponding 4-amino adduct (entry 3). The use of a catalyst based on L4(11b) performed comparably, providing the desired amination product in slightly higher yield (85%) under otherwise identical conditions (entry 4). Notably, this process could be carried out at room temperature, affording the product in 87% yield in 12 h. Interestingly, these results contrast with our previous observation that L4 was inferior to the bulkier L3 in the amidation reactions of five-membered heterocyclic bromides.8d However, amines are significantly more nucleophilic than amides, and the use of L3, which bears the large 1-adamantyl substituents on phosphorus, is presumably unnecessary to facilitate reductive elimination. Finally, reactions employing catalysts derived from L5(11c) or L6(11d) yielded no desired product (entries 5 and 6), thus providing further information on the importance of the BrettPhos biaryl backbone framework (such as in L3 and L4) to effect amination of these types of heterocycles.11e
Table 1. Ligand Effects in the Palladium-Catalyzed Amination of 4-Bromo-1H-imidazolea.
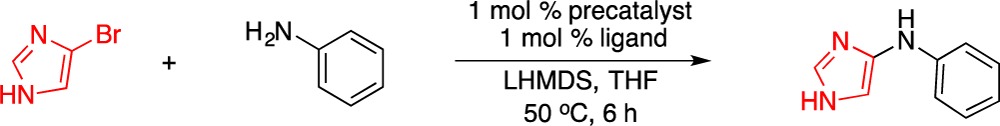
| entry | precatalyst | ligand | conversionb (%) | yieldb (%) |
|---|---|---|---|---|
| 1 | P1 | L1 | 27 | 13 |
| 2 | P2 | L2 | 7 | 0 |
| 3 | P3 | L3 | 100 | 77 |
| 4 | P4 | L4 | 100 | 85 (87)c |
| 5 | P5 | L5 | 4 | 0 |
| 6 | P6 | L6 | 8 | 0 |
Reaction conditions: HetArBr (0.3 mmol), aniline (0.36 mmol), LHMDS (0.66 mmol).
Determined based on the 1H NMR of the crude reaction mixture using 1,3,5-trimethoxybenzene as internal standard, average of two runs.
rt, 12 h.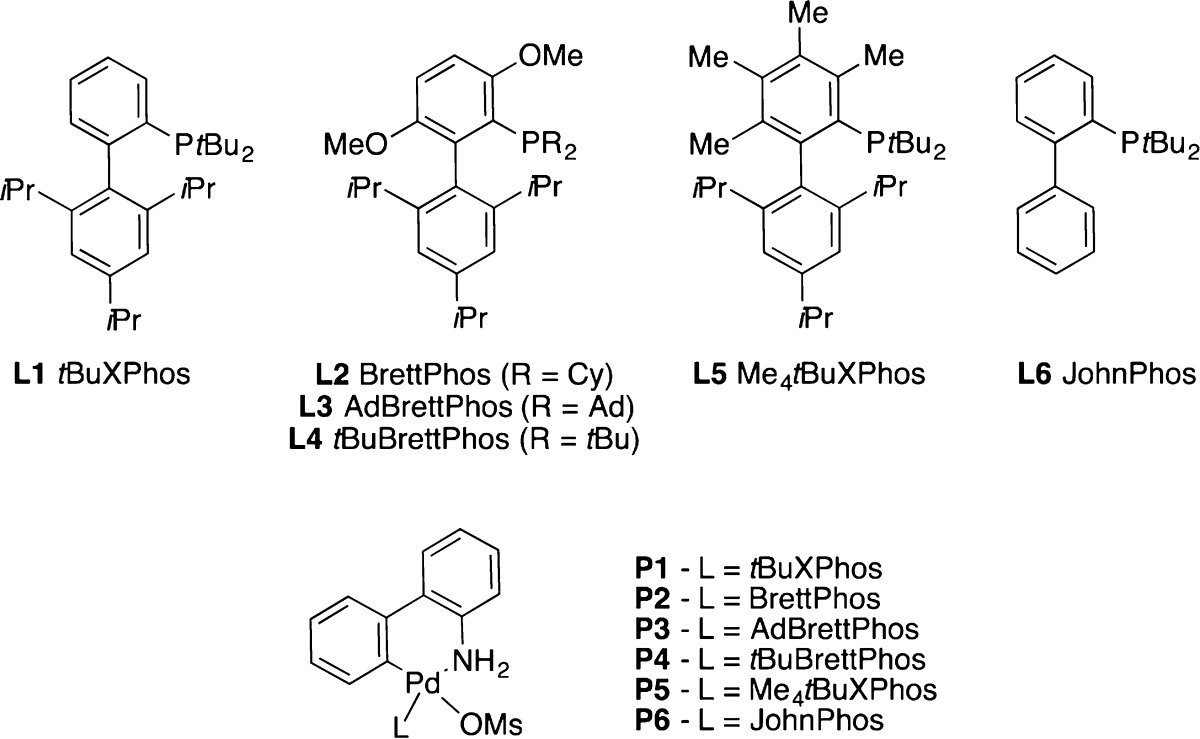
Using this optimized protocol, with P4 (1–2 mol %), L4 (1–2 mol %), and LHMDS (2.2 equiv) in THF,12 we assessed the scope of the amine coupling partners for the amination of 4-bromo-1H-imidazole (Scheme 1). This system was found to be effective for electron-rich (1a and 1b), electron-deficient (1c and 1d), and heteroarylamines (1e, 1f, and 1g).13 Notably, there exist very few examples of preparing 4-aminoimidazoles that have appeared to date.14 In addition, this investigation was extended to the amination of 2-bromo-1H-imidazoles. As shown in Scheme 1, a variety of amine nucleophiles including anilines (1h, 1j, and 1k), alkylamines (1i), and heteroarylamines (1l) underwent efficient arylation to afford 2-aminoimidazoles in good yields.
Scheme 1. Scope of 4- and 2-Bromo-1H-imidazole Coupling.
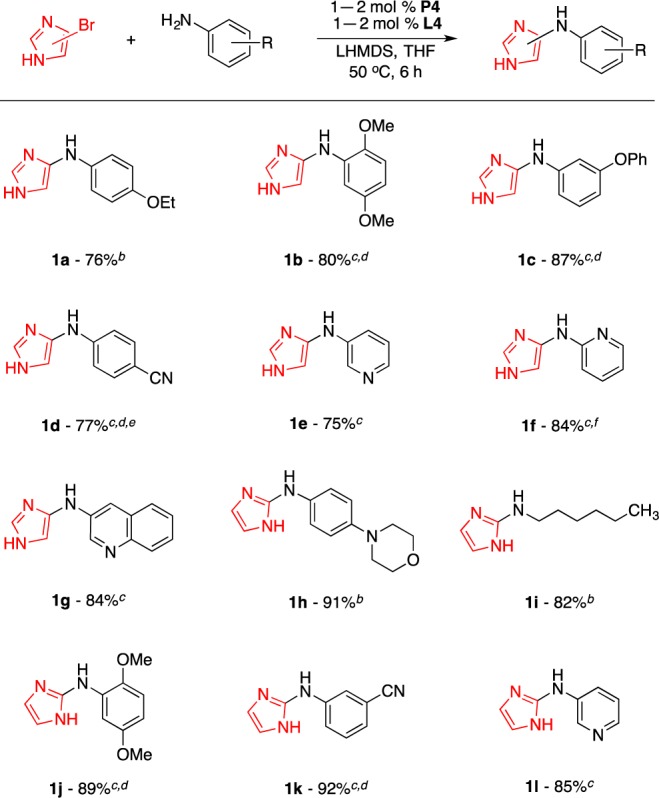
Reaction conditions: HetArBr (1.0 mmol), amine (1.2 mmol), LHMDS (2.2 mmol). Isolated yields are an average of two runs.
P4 (1 mol %), L4 (1 mol %).
P4 (2 mol %), L4 (2 mol %).
80 °C.
12 h.
Amine (1.4 mmol).
Next, we turned our attention to amination reactions using 4- and 3-bromo-1H-pyrazoles as electrophiles. Under the optimized reaction conditions, various aliphatic and aromatic amines of different electronic and steric properties represent useful coupling partners for the transformation (Scheme 2). Substituents such as phenoxy (2c), trifluoromethyl (2d), cyano (2f), morpholinyl (2h), and furanyl (2j) were also well tolerated, although coupling products with aminophenols and aminoacetanilide functional groups could not be prepared in useful yields.
Scheme 2. Scope of 4- and 3-Bromo-1H-pyrazoles Coupling with Aliphatic, Aromatic, and Heteroaromatic Amines.
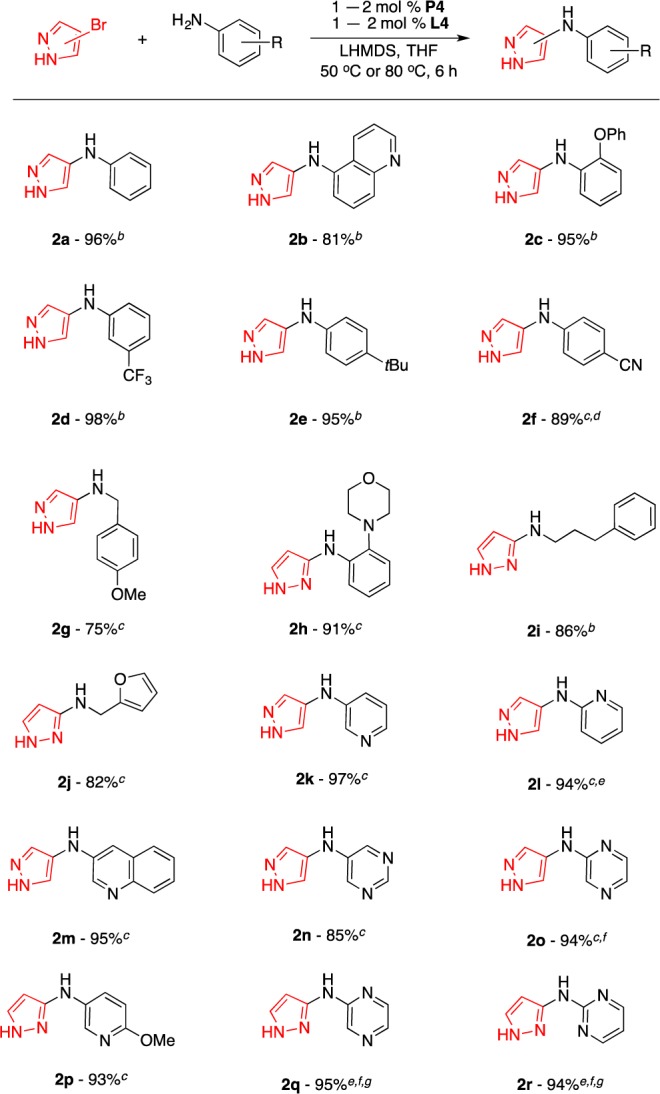
Reaction conditions: HetArBr (1.0 mmol), amine (1.2 mmol), LHMDS (2.2 mmol). Isolated yields are an average of two runs.
P4 (1 mol %), L4 (1 mol %), 50 °C.
P4 (2 mol %), L4 (2 mol %), 80 °C.
12 h.
Amine (1.4 mmol).
16 h.
P4 (4 mol %), L4 (4 mol %).
There has also been an increasing interest in the synthesis of diheteroarylamines,15 as these compounds have displayed promising activity in assays targeting cancer,15a,15b myeloproliferative disorders15c,15d and platelet aggregation.15e Therefore, we evaluated the utility of this protocol toward the synthesis of a variety of diheteroarylamines from 4- and 3-bromo-1H-pyrazoles and a range of interesting heteroaromatic amines (Scheme 2). Various aminoheterocycles such as aminopyridines (2k, 2i, and 2p), aminoquinolines (2m), aminopyrimidines (2n and 2r), and aminopyrazines (2o and 2q) were found to be suitable coupling partners, although in the case of 2q and 2r, higher catalyst loadings and longer reaction times were necessary. Unfortunately, 4-aminopyridine and 8-aminoquinoline were not successfully transformed under our reaction conditions.
In conclusion, we have developed a general method to cross-couple 4- and 2-bromo-1H-imidazoles and 4- and 3-bromo-1H-pyrazoles effectively with aliphatic, aromatic, and heteroaromatic amines. In particular, this method provides facile access to 4-aminoimidazoles and diheteroarylamines. We anticipate that this protocol will find widespread application in a variety of settings to access these types of substituted five-membered heterocycles that are traditionally difficult to prepare.
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health under award no. GM58160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. N.H. thanks the Astellas Foundation for Research on Metabolic Disorders for a Fellowship and is a Japan Society for the Promotion of Science (JSPS) for Young Scientist Research Fellow for which he is grateful. We thank Drs. J. Robb DeBergh (MIT) and Nathan T. Jui (MIT) for help with preparation of this manuscript.
Supporting Information Available
Experimental procedures along with experimental and spectroscopic data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare the following competing financial interest(s): MIT has patents on the ligands and has also filed patents on the methansulfonate precatalysts used in this paper from which S.L.B. and current or former co-workers receive royalty payments.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Hein J. E.; Fokin V. V. Chem. Soc. Rev. 2010, 39, 1302. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Schultz D. M.; Wolfe J. P. Synthesis 2012, 44, 351. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Seiple I. B.; Su S.; Young I. S.; Nakamura A.; Yamaguchi J.; Jørgensen L.; Rodriguez R. A.; O’Malley D. P.l; Gaich T.; Köck M.; Baran P. S. J. Am. Chem. Soc. 2011, 133, 14710. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Li Y.; Geng J.; Liu Y.; Yu S.; Zhao G. ChemMedChem 2013, 8, 27. [DOI] [PubMed] [Google Scholar]; e Baumann M.; Baxendale I. R.; Ley S. V.; Nikbin N. Beilstein J. Org. Chem. 2011, 7, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan P.; Li D.; Chen X.; Liu X.; Clercq E. D. Curr. Med. Chem. 2011, 18, 29. [DOI] [PubMed] [Google Scholar]
- a Sullivan J. D.; Giles R. L.; Looper R. E. Curr. Bioact. Compd. 2009, 5, 39. [Google Scholar]; b Munk S. A.; Harcourt D. A.; Arasasingham P. N.; Burke J. A.; Kharlamb A. B.; Manlapaz C. A.; Padillo E. U.; Roberts D.; Runde E.; Williams L.; et al. J. Med. Chem. 1997, 40, 18. [DOI] [PubMed] [Google Scholar]; c Aggarwal R.; Kumar V.; Kumar R.; Singh S. P. Beilstein J. Org. Chem. 2011, 7, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Mortlock A. A.; Foote K. M.; Heron N. M.; Jung F. H.; Pasquet G.; Lohmann J. M.; Warin N.; Renaud F.; De Savi C.; Roberts N. J.; Johnson T.; Dousson C. B.; Hill G. B.; Perkins D.; Hatter G.; Wilkinson R. W.; Wedge S. R.; Heaton S. P.; Odedra R.; Keen N. J.; Crafter C.; Brown E.; Thompson K.; Brightwell S.; Khatri L.; Brady M. C.; Kearney S.; McKillop D.; Rhead S.; Parry T.; Green S. J. Med. Chem. 2007, 50, 2213. [DOI] [PubMed] [Google Scholar]
- Maiti D.; Fors B. F.; Henderson J. L.; Nakamura Y.; Buchwald S. L. Chem. Sci. 2011, 2, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of arylation of five-membered heterocyclic amines, see:; a Moss T. A.; Addie M. S.; Nowak T.; Waring M. J. Synlett 2012, 23, 285. [Google Scholar]; b Ueda S.; Buchwald S. L. Angew. Chem., Int. Ed. 2012, 51, 10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Surry D. S.; Buchwald S. L. Angew. Chem., Int. Ed. 2008, 47, 6338. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Hartwig J. F. Acc. Chem. Res. 2008, 41, 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shen Q.; Shekhar S.; Stambuli J. P.; Hartwig J. F. Angew. Chem., Int. Ed. 2005, 44, 1371. [DOI] [PubMed] [Google Scholar]; b Shen Q.; Hartwig J. F. J. Am. Chem. Soc. 2007, 129, 7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hooper M. W.; Utsunomiya M.; Hartwig J. F. J. Org. Chem. 2003, 68, 2861. [DOI] [PubMed] [Google Scholar]; b Hooper M. W.; Hartwig J. F. Organometallics 2003, 22, 3394. [Google Scholar]; c Charles M. D.; Schultz P.; Buchwald S. L. Org. Lett. 2005, 7, 3965. [DOI] [PubMed] [Google Scholar]; d Su M.; Buchwald S. L. Angew. Chem., Int. Ed. 2012, 51, 4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Gore V. K.; Ma V. V.; Tamir R.; Gavva N. R.; Treanor J. J. S.; Norman M. H. Bioorg. Med. Chem. Lett. 2007, 17, 5825. [DOI] [PubMed] [Google Scholar]; b McLaughlin M.; Palucki M.; Davies I. W. Org. Lett. 2006, 8, 3311. [DOI] [PubMed] [Google Scholar]
- For examples of C–N cross-coupling reactions using unprotected 4-, 5-, 6-, or 7-halosubstituted indoles, azaindoles, indazoles, benzimidazoles, and benzotriazoles as electrophiles, see:; a Henderson J. L.; McDermott S. M.; Buchwald S. L. Org. Lett. 2010, 12, 4438. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Henderson J. L.; Buchwald S. L. Org. Lett. 2010, 12, 4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Anderson K. W.; Tundel R. E.; Ikawa T.; Altman R. A.; Buchwald S. L. Angew. Chem., Int. Ed. 2006, 45, 6523. [DOI] [PubMed] [Google Scholar]; b Fors B. F.; Dooleweerdt K.; Zeng Q.; Buchwald S. L. Tetrahedron 2009, 65, 6576. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ikawa T.; Barder T. E.; Biscoe M. R.; Buchwald S. L. J. Am. Chem. Soc. 2007, 129, 13001. [DOI] [PubMed] [Google Scholar]; d Wolfe J. P.; Buchwald S. L. Angew. Chem., Int. Ed. 1999, 38, 2413. [DOI] [PubMed] [Google Scholar]; e Fors B. P.; Watson D. A.; Biscoe M. R.; Buchwald S. L. J. Am. Chem. Soc. 2008, 130, 13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The use of 1.2 equiv of LHMDS resulted in 2% yield. See the Supporting Information for details on optimization of reaction conditions.
- The coupling reaction between 4-bromo-1H-imidazole and 3-propylphenylamine proceeded cleanly to product based on 1H NMR and LCMS analysis on the crude reaction mixture, giving a yield between 50 and 69%. However, the coupled product was unstable under various isolation attempts. Decomposition of the desired aminoimidazole product was observed upon reaction workup and column purification.
- a Kang X.; Long W.; Ma C.; Wang Y.; Shen X.; Hu Y.; Tan F.; Wang Y.. Preparation of Pyridinyloxypyridinylaminobenzene Derivatives for Use as Glucokinase Active Modulators. Patent WO 2012062210, 2012.; b Kahraman M.; Borchardt A. J.; Cook T. G.; Davis R. L.; Gardiner E. M. M.; Malecha J. W.; Noble S. A.; Prins T. J.. Benzothiophene Derivatives, Processes for Preparing Them, Pharmaceutical Compositions Containing Them, and Their Use as Inhibitors of Rho Kinase. Patent WO 2008011560, 2008.
- a Tasler S.; Müller O.; Wieber T.; Herz T.; Krauss R.; Totzke F.; Kubbutat M. H. G.; Schächtele C. Bioorg. Med. Chem. Lett. 2009, 19, 1349. [DOI] [PubMed] [Google Scholar]; b Teng M.; Zhu J.; Johnson M. D.; Chen P.; Kornmann J.; Chen E.; Blasina A.; Register J.; Anderes K.; Rogers C.; Deng Y.; Ninkovic S.; Grant S.; Hu Q.; Lundgren K.; Peng Z.; Kania R. S. J. Med. Chem. 2007, 50, 5253. [DOI] [PubMed] [Google Scholar]; c Guan H.; Lamb M. L.; Peng B.; Huang S.; DeGrace N.; Read J.; Hussain S.; Wu J.; Rivard C.; Alimzhanov M.; Bebernitz G.; Bell K.; Ye M.; Zinda M.; Ioannidis S. Bioorg. Med. Chem. Lett. 2013, 23, 3105. [DOI] [PubMed] [Google Scholar]; d Ioannidis S.; Lamb M. L.; Davies A. M.; Almeida L.; Su M.; Bebernitz G.; Ye M.; Bell K.; Alimzhanov M.; Zinda M. Bioorg. Med. Chem. Lett. 2009, 19, 6524. [DOI] [PubMed] [Google Scholar]; e Ruel R.; L’Heureux A.; Thibeault C.; Daris J.; Martel A.; Price L. A.; Wu Q.; Hua J.; Wexler R. R.; Rehfuss R.; Lam P. Y. S. Bioorg. Med. Chem. Lett. 2013, 23, 3519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


