Abstract
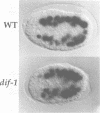
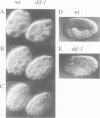
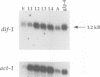
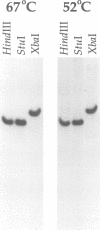
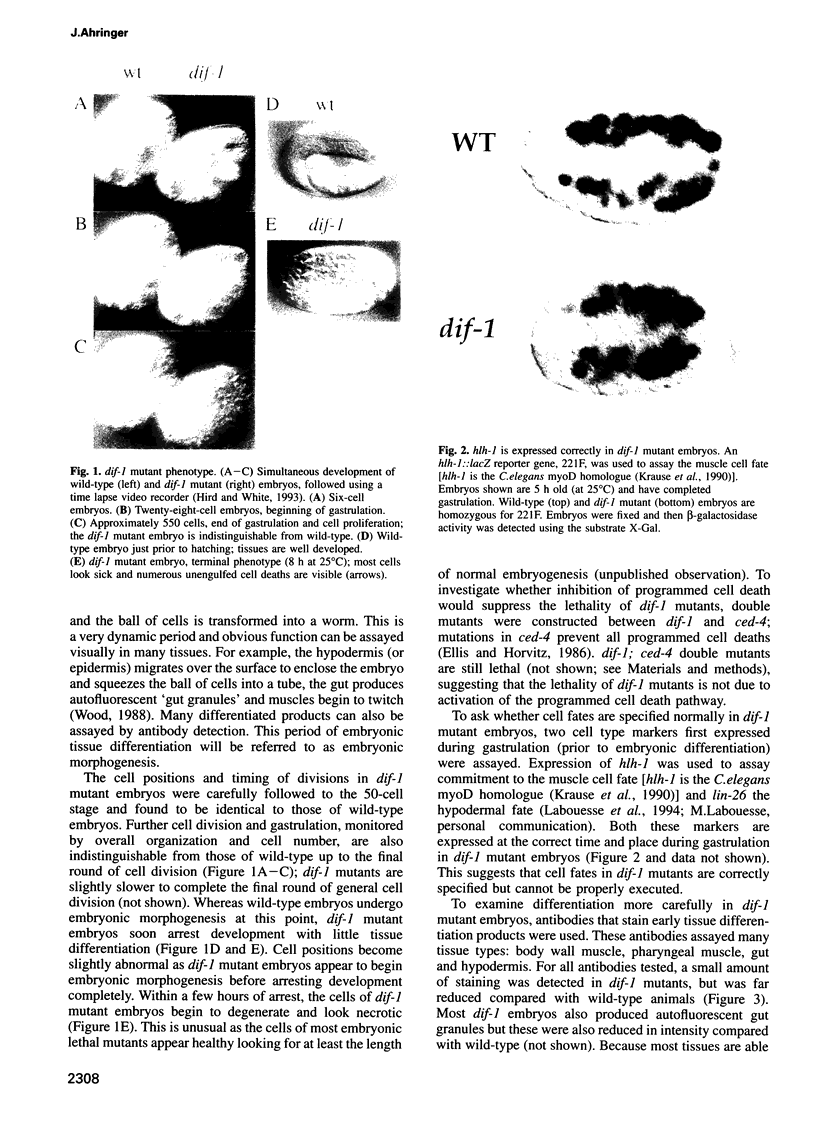
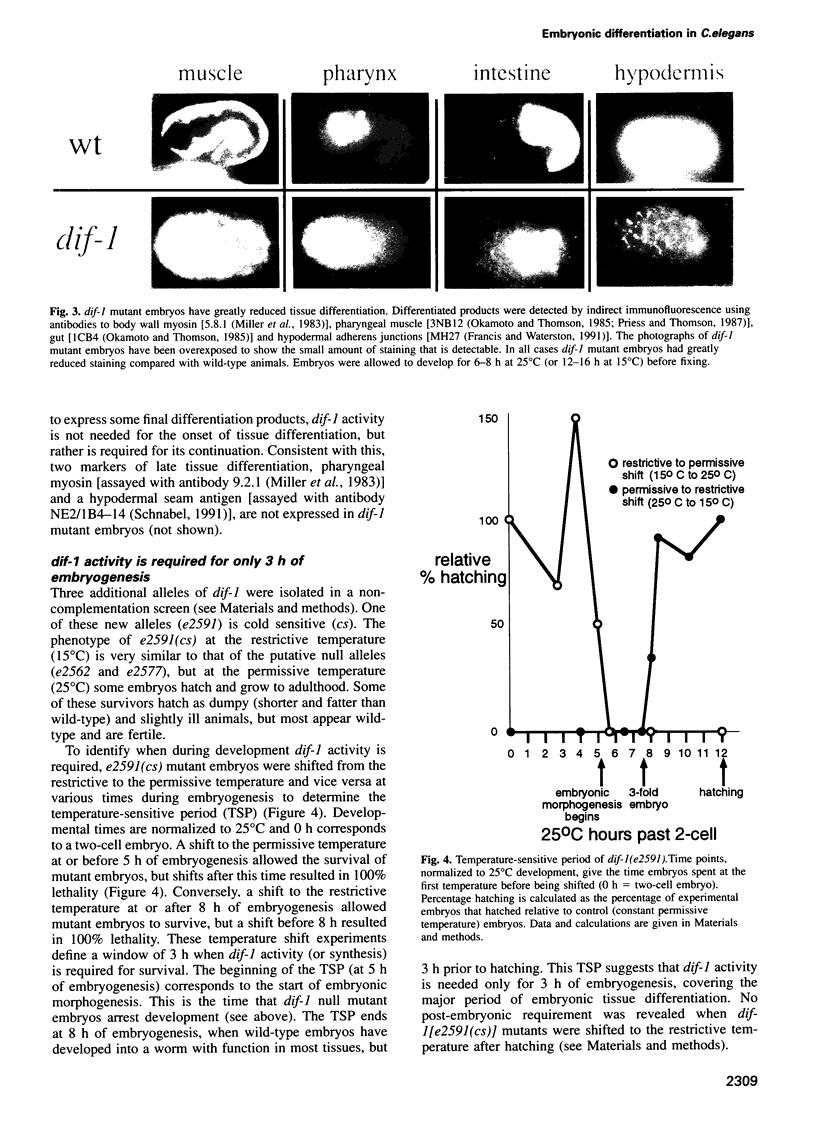
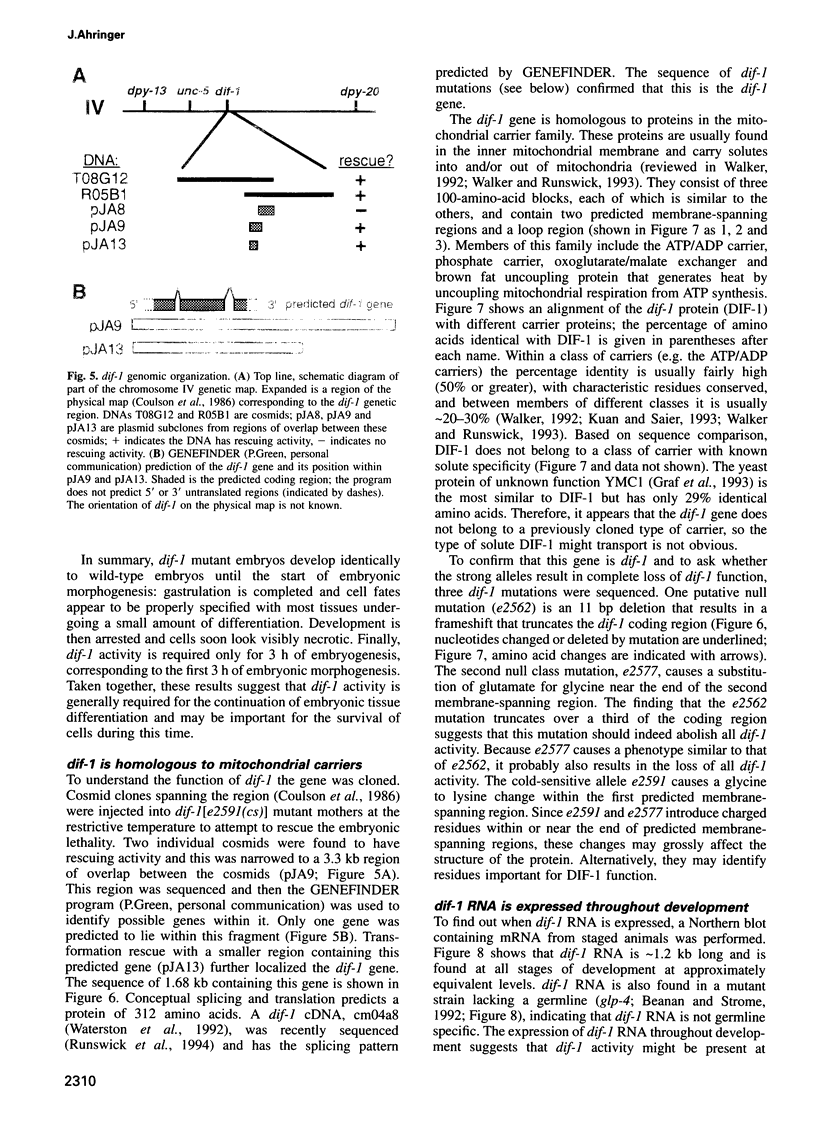
The dif-1 gene was identified in a general screen for maternal-effect embryonic lethal (Mel) mutants. dif-1 mutant embryos complete gastrulation and embryonic cell division normally, but then arrest development with only a small amount of tissue differentiation. Either maternal or zygotic dif-1 activity is sufficient for wild-type development. The temperature-sensitive period of a cold-sensitive dif-1 mutant shows that dif-1 activity is essential only for 3 h, corresponding to the major period of embryonic tissue differentiation, and is not required post-embryonically. The results point to a role for dif-1 in the maintenance of tissue differentiation in the developing embryo, but not for its initiation. Cloning and sequencing of the dif-1 gene revealed that its product is homologous to proteins in the mitochondrial carrier family. Although dif-1 activity is required only during embryogenesis, dif-1 RNA is expressed at all stages of development. In situ hybridization to embryos showed that dif-1 RNA is initially present in all cells of the embryo; this most likely corresponds to maternal dif-1 RNA. Later, the presumable zygotic dif-1 RNA is found only in the gut and hypodermis of the embryo. This tissue-specific expression raises the possibility that the dif-1 protein acts non-cell autonomously and that some communication or molecular transport dependent on DIF-1 takes place during embryonic tissue differentiation. dif-1 is the first mitochondrial carrier homologue known to be needed specifically for a developmental process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler P. N., Charlton J., Jones K. H., Liu J. The cold-sensitive period for frizzled in the development of wing hair polarity ends prior to the start of hair morphogenesis. Mech Dev. 1994 May;46(2):101–107. doi: 10.1016/0925-4773(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Adrian G. S., McCammon M. T., Montgomery D. L., Douglas M. G. Sequences required for delivery and localization of the ADP/ATP translocator to the mitochondrial inner membrane. Mol Cell Biol. 1986 Feb;6(2):626–634. doi: 10.1128/mcb.6.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertson D. G. Formation of the first cleavage spindle in nematode embryos. Dev Biol. 1984 Jan;101(1):61–72. doi: 10.1016/0012-1606(84)90117-9. [DOI] [PubMed] [Google Scholar]
- Beanan M. J., Strome S. Characterization of a germ-line proliferation mutation in C. elegans. Development. 1992 Nov;116(3):755–766. doi: 10.1242/dev.116.3.755. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassard A. M., Bouillaud F., Mattei M. G., Hentz E., Raimbault S., Thomas M., Ricquier D. Human uncoupling protein gene: structure, comparison with rat gene, and assignment to the long arm of chromosome 4. J Cell Biochem. 1990 Jul;43(3):255–264. doi: 10.1002/jcb.240430306. [DOI] [PubMed] [Google Scholar]
- Coulson A., Sulston J., Brenner S., Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis H. M., Horvitz H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986 Mar 28;44(6):817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Fernández M., Fernández E., Rodicio R. ACR1, a gene encoding a protein related to mitochondrial carriers, is essential for acetyl-CoA synthetase activity in Saccharomyces cerevisiae. Mol Gen Genet. 1994 Mar;242(6):727–735. doi: 10.1007/BF00283428. [DOI] [PubMed] [Google Scholar]
- Fiermonte G., Walker J. E., Palmieri F. Abundant bacterial expression and reconstitution of an intrinsic membrane-transport protein from bovine mitochondria. Biochem J. 1993 Aug 15;294(Pt 1):293–299. doi: 10.1042/bj2940293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Files J. G., Carr S., Hirsh D. Actin gene family of Caenorhabditis elegans. J Mol Biol. 1983 Mar 5;164(3):355–375. doi: 10.1016/0022-2836(83)90056-6. [DOI] [PubMed] [Google Scholar]
- Fire A., Harrison S. W., Dixon D. A modular set of lacZ fusion vectors for studying gene expression in Caenorhabditis elegans. Gene. 1990 Sep 14;93(2):189–198. doi: 10.1016/0378-1119(90)90224-f. [DOI] [PubMed] [Google Scholar]
- Francis R., Waterston R. H. Muscle cell attachment in Caenorhabditis elegans. J Cell Biol. 1991 Aug;114(3):465–479. doi: 10.1083/jcb.114.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf R., Baum B., Braus G. H. YMC1, a yeast gene encoding a new putative mitochondrial carrier protein. Yeast. 1993 Mar;9(3):301–305. doi: 10.1002/yea.320090310. [DOI] [PubMed] [Google Scholar]
- Higgins D. G. CLUSTAL V: multiple alignment of DNA and protein sequences. Methods Mol Biol. 1994;25:307–318. doi: 10.1385/0-89603-276-0:307. [DOI] [PubMed] [Google Scholar]
- Hird S. N., White J. G. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J Cell Biol. 1993 Jun;121(6):1343–1355. doi: 10.1083/jcb.121.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jank B., Habermann B., Schweyen R. J., Link T. A. PMP47, a peroxisomal homologue of mitochondrial solute carrier proteins. Trends Biochem Sci. 1993 Nov;18(11):427–428. [PubMed] [Google Scholar]
- Kaplan R. S., Mayor J. A., Wood D. O. The mitochondrial tricarboxylate transport protein. cDNA cloning, primary structure, and comparison with other mitochondrial transport proteins. J Biol Chem. 1993 Jun 25;268(18):13682–13690. [PubMed] [Google Scholar]
- Kemphues K. J., Priess J. R., Morton D. G., Cheng N. S. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988 Feb 12;52(3):311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Wright B., Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982 Jun;93(3):576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Fire A., Harrison S. W., Priess J., Weintraub H. CeMyoD accumulation defines the body wall muscle cell fate during C. elegans embryogenesis. Cell. 1990 Nov 30;63(5):907–919. doi: 10.1016/0092-8674(90)90494-y. [DOI] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan J., Saier M. H., Jr The mitochondrial carrier family of transport proteins: structural, functional, and evolutionary relationships. Crit Rev Biochem Mol Biol. 1993;28(3):209–233. doi: 10.3109/10409239309086795. [DOI] [PubMed] [Google Scholar]
- Labouesse M., Sookhareea S., Horvitz H. R. The Caenorhabditis elegans gene lin-26 is required to specify the fates of hypodermal cells and encodes a presumptive zinc-finger transcription factor. Development. 1994 Sep;120(9):2359–2368. doi: 10.1242/dev.120.9.2359. [DOI] [PubMed] [Google Scholar]
- Mains P. E. Embryonic development in Caenorhabditis elegans. Results Probl Cell Differ. 1992;18:49–90. doi: 10.1007/978-3-540-47191-2_2. [DOI] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991 Dec;10(12):3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., 3rd, Ortiz I., Berliner G. C., Epstein H. F. Differential localization of two myosins within nematode thick filaments. Cell. 1983 Sep;34(2):477–490. doi: 10.1016/0092-8674(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Douglas M. G. Function-based mapping of the yeast mitochondrial ADP/ATP translocator by selection for second site revertants. J Mol Biol. 1993 Apr 20;230(4):1171–1182. doi: 10.1006/jmbi.1993.1234. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Lawson J. E., Klingenberg M., Douglas M. G. Site-directed mutagenesis of the yeast mitochondrial ADP/ATP translocator. Six arginines and one lysine are essential. J Mol Biol. 1993 Apr 20;230(4):1159–1170. doi: 10.1006/jmbi.1993.1233. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Thomson J. N. Monoclonal antibodies which distinguish certain classes of neuronal and supporting cells in the nervous tissue of the nematode Caenorhabditis elegans. J Neurosci. 1985 Mar;5(3):643–653. doi: 10.1523/JNEUROSCI.05-03-00643.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priess J. R. Establishment of initial asymmetry in early Caenorhabditis elegans embryos. Curr Opin Genet Dev. 1994 Aug;4(4):563–568. doi: 10.1016/0959-437x(94)90073-c. [DOI] [PubMed] [Google Scholar]
- Priess J. R., Thomson J. N. Cellular interactions in early C. elegans embryos. Cell. 1987 Jan 30;48(2):241–250. doi: 10.1016/0092-8674(87)90427-2. [DOI] [PubMed] [Google Scholar]
- Rocca W. A., Dorsey F. C., Grigoletto F., Gent M., Roberts R. S., Walker M. D., Easton J. D., Bruno R., Carolei A., Sancesario G. Design and baseline results of the monosialoganglioside early stroke trial. The EST Study Group. Stroke. 1992 Apr;23(4):519–526. doi: 10.1161/01.str.23.4.519. [DOI] [PubMed] [Google Scholar]
- Rosenquist T. A., Kimble J. Molecular cloning and transcript analysis of fem-3, a sex-determination gene in Caenorhabditis elegans. Genes Dev. 1988 May;2(5):606–616. doi: 10.1101/gad.2.5.606. [DOI] [PubMed] [Google Scholar]
- Runswick M. J., Philippides A., Lauria G., Walker J. E. Extension of the mitochondrial transporter super-family: sequences of five members from the nematode worm, Caenorhabditis elegans. DNA Seq. 1994;4(5):281–291. doi: 10.3109/10425179409020854. [DOI] [PubMed] [Google Scholar]
- Schnabel R. Cellular interactions involved in the determination of the early C. elegans embryo. Mech Dev. 1991 Jun;34(2-3):85–99. doi: 10.1016/0925-4773(91)90046-9. [DOI] [PubMed] [Google Scholar]
- Seydoux G., Fire A. Soma-germline asymmetry in the distributions of embryonic RNAs in Caenorhabditis elegans. Development. 1994 Oct;120(10):2823–2834. doi: 10.1242/dev.120.10.2823. [DOI] [PubMed] [Google Scholar]
- Staden R. Staden: comparing sequences. Methods Mol Biol. 1994;25:155–170. doi: 10.1385/0-89603-276-0:155. [DOI] [PubMed] [Google Scholar]
- Strome S. Generation of cell diversity during early embryogenesis in the nematode Caenorhabditis elegans. Int Rev Cytol. 1989;114:81–123. doi: 10.1016/s0074-7696(08)60859-1. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983 Nov;100(1):64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Runswick M. J. The mitochondrial transport protein superfamily. J Bioenerg Biomembr. 1993 Oct;25(5):435–446. doi: 10.1007/BF01108401. [DOI] [PubMed] [Google Scholar]
- Waterston R., Martin C., Craxton M., Huynh C., Coulson A., Hillier L., Durbin R., Green P., Shownkeen R., Halloran N. A survey of expressed genes in Caenorhabditis elegans. Nat Genet. 1992 May;1(2):114–123. doi: 10.1038/ng0592-114. [DOI] [PubMed] [Google Scholar]
- Wilson R., Ainscough R., Anderson K., Baynes C., Berks M., Bonfield J., Burton J., Connell M., Copsey T., Cooper J. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature. 1994 Mar 3;368(6466):32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Edgar L. G. Patterning in the C. elegans embryo. Trends Genet. 1994 Feb;10(2):49–54. doi: 10.1016/0168-9525(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]