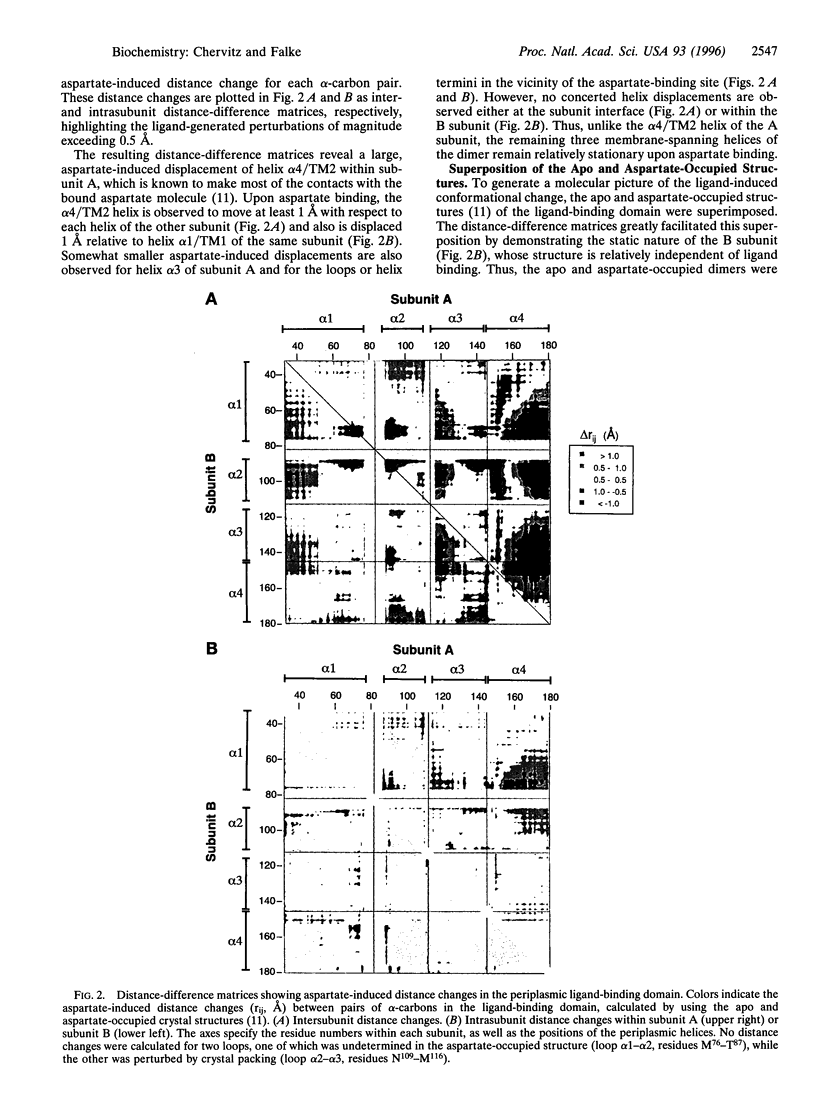
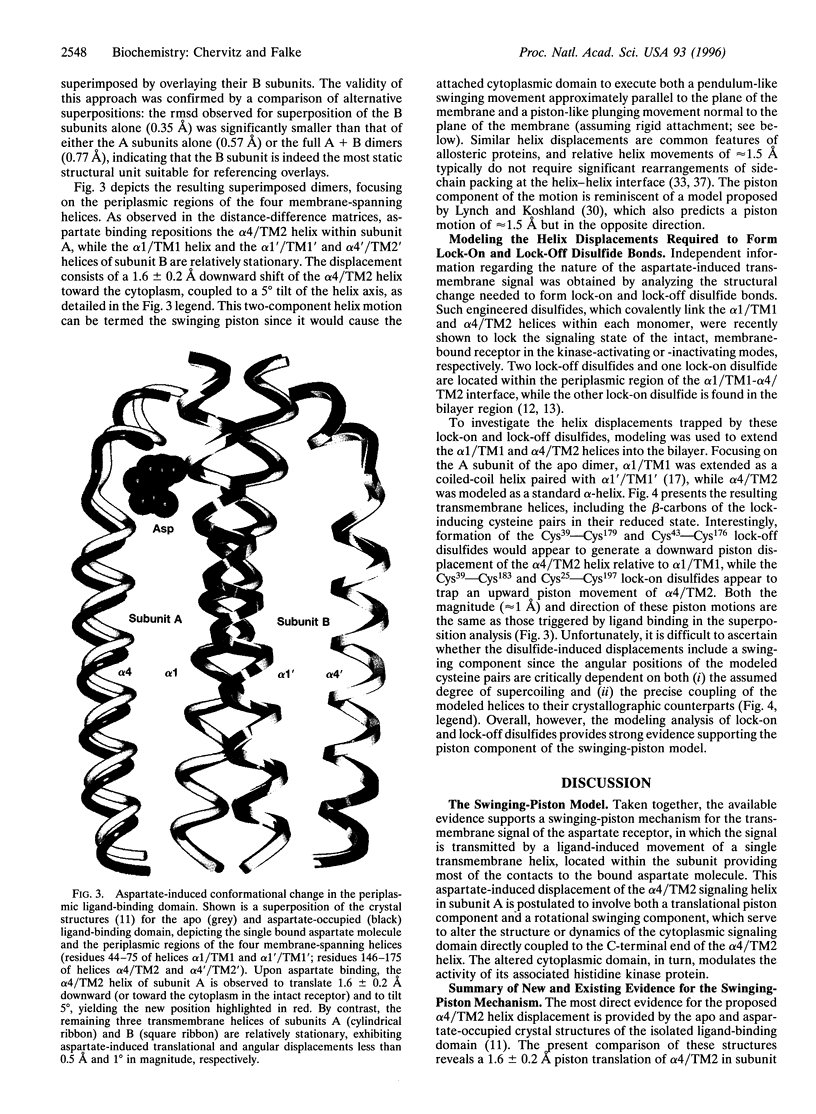
Abstract
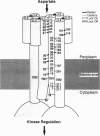
The aspartate receptor of bacterial chemotaxis is representative of a large class of membrane-spanning receptors found in prokaryotic and eukaryotic organisms. These receptors, which regulate histidine kinase pathways and possess two putative transmembrane helices per subunit, appear to control a wide variety of cellular processes. The best characterized subgroup of the two-helix receptor class is the homologous family of chemosensory receptors from Escherichia coli and Salmonella typhimurium, including the aspartate receptor. This receptor binds aspartate, an attractant, in the periplasmic compartment and undergoes an intramolecular, transmembrane conformational change, thereby modulating the autophosphorylation rate of a bound histidine kinase in the cytoplasm. Here, we analyze recent results from x-ray crystallographic, solution 19F NMR, and engineered disulfide studies probing the aspartate-induced structural change within the periplasmic and transmembrane regions of the receptor. Together, these approaches provide evidence that aspartate binding triggers a "swinging-piston" displacement of the second membrane-spanning helix, which is proposed to communicate the signal across the bilayer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alex L. A., Simon M. I. Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet. 1994 Apr;10(4):133–138. doi: 10.1016/0168-9525(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Baumgartner J. W., Kim C., Brissette R. E., Inouye M., Park C., Hazelbauer G. L. Transmembrane signalling by a hybrid protein: communication from the domain of chemoreceptor Trg that recognizes sugar-binding proteins to the kinase/phosphatase domain of osmosensor EnvZ. J Bacteriol. 1994 Feb;176(4):1157–1163. doi: 10.1128/jb.176.4.1157-1163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich K. A., Kaplan N., Hess J. F., Simon M. I. Transmembrane signal transduction in bacterial chemotaxis involves ligand-dependent activation of phosphate group transfer. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1208–1212. doi: 10.1073/pnas.86.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervitz S. A., Falke J. J. Lock on/off disulfides identify the transmembrane signaling helix of the aspartate receptor. J Biol Chem. 1995 Oct 13;270(41):24043–24053. doi: 10.1074/jbc.270.41.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervitz S. A., Lin C. M., Falke J. J. Transmembrane signaling by the aspartate receptor: engineered disulfides reveal static regions of the subunit interface. Biochemistry. 1995 Aug 1;34(30):9722–9733. doi: 10.1021/bi00030a010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson M. A., Biemann H. P., Koshland D. E., Jr, Falke J. J. Attractant- and disulfide-induced conformational changes in the ligand binding domain of the chemotaxis aspartate receptor: a 19F NMR study. Biochemistry. 1994 May 24;33(20):6100–6109. doi: 10.1021/bi00186a009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke J. J., Dernburg A. F., Sternberg D. A., Zalkin N., Milligan D. L., Koshland D. E., Jr Structure of a bacterial sensory receptor. A site-directed sulfhydryl study. J Biol Chem. 1988 Oct 15;263(29):14850–14858. [PubMed] [Google Scholar]
- Falke J. J., Koshland D. E., Jr Global flexibility in a sensory receptor: a site-directed cross-linking approach. Science. 1987 Sep 25;237(4822):1596–1600. doi: 10.1126/science.2820061. [DOI] [PubMed] [Google Scholar]
- Gegner J. A., Graham D. R., Roth A. F., Dahlquist F. W. Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell. 1992 Sep 18;70(6):975–982. doi: 10.1016/0092-8674(92)90247-a. [DOI] [PubMed] [Google Scholar]
- Gerstein M., Lesk A. M., Chothia C. Structural mechanisms for domain movements in proteins. Biochemistry. 1994 Jun 7;33(22):6739–6749. doi: 10.1021/bi00188a001. [DOI] [PubMed] [Google Scholar]
- Kim S. H. "Frozen" dynamic dimer model for transmembrane signaling in bacterial chemotaxis receptors. Protein Sci. 1994 Feb;3(2):159–165. doi: 10.1002/pro.5560030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikos A., Conley M. P., Boyd A., Berg H. C., Simon M. I. Chimeric chemosensory transducers of Escherichia coli. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1326–1330. doi: 10.1073/pnas.82.5.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. F., Burrows G. G., Lebert M. R., Dutton D. P., Hazelbauer G. L. Deducing the organization of a transmembrane domain by disulfide cross-linking. The bacterial chemoreceptor Trg. J Biol Chem. 1994 Nov 25;269(47):29920–29927. [PubMed] [Google Scholar]
- Lee G. F., Dutton D. P., Hazelbauer G. L. Identification of functionally important helical faces in transmembrane segments by scanning mutagenesis. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5416–5420. doi: 10.1073/pnas.92.12.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. F., Lebert M. R., Lilly A. A., Hazelbauer G. L. Transmembrane signaling characterized in bacterial chemoreceptors by using sulfhydryl cross-linking in vivo. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3391–3395. doi: 10.1073/pnas.92.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch B. A., Koshland D. E., Jr Disulfide cross-linking studies of the transmembrane regions of the aspartate sensory receptor of Escherichia coli. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10402–10406. doi: 10.1073/pnas.88.23.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch B. A., Koshland D. E., Jr The fifth Datta Lecture. Structural similarities between the aspartate receptor of bacterial chemotaxis and the trp repressor of E. coli. Implications for transmembrane signaling. FEBS Lett. 1992 Jul 27;307(1):3–9. doi: 10.1016/0014-5793(92)80891-j. [DOI] [PubMed] [Google Scholar]
- Milburn M. V., Privé G. G., Milligan D. L., Scott W. G., Yeh J., Jancarik J., Koshland D. E., Jr, Kim S. H. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science. 1991 Nov 29;254(5036):1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- Milligan D. L., Koshland D. E., Jr Intrasubunit signal transduction by the aspartate chemoreceptor. Science. 1991 Dec 13;254(5038):1651–1654. doi: 10.1126/science.1661030. [DOI] [PubMed] [Google Scholar]
- Milligan D. L., Koshland D. E., Jr Purification and characterization of the periplasmic domain of the aspartate chemoreceptor. J Biol Chem. 1993 Sep 25;268(27):19991–19997. [PubMed] [Google Scholar]
- Milligan D. L., Koshland D. E., Jr Site-directed cross-linking. Establishing the dimeric structure of the aspartate receptor of bacterial chemotaxis. J Biol Chem. 1988 May 5;263(13):6268–6275. [PubMed] [Google Scholar]
- Ninfa E. G., Stock A., Mowbray S., Stock J. Reconstitution of the bacterial chemotaxis signal transduction system from purified components. J Biol Chem. 1991 May 25;266(15):9764–9770. [PubMed] [Google Scholar]
- Pakula A. A., Simon M. I. Determination of transmembrane protein structure by disulfide cross-linking: the Escherichia coli Tar receptor. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Signal transduction schemes of bacteria. Cell. 1993 Jun 4;73(5):857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- Schuster S. C., Swanson R. V., Alex L. A., Bourret R. B., Simon M. I. Assembly and function of a quaternary signal transduction complex monitored by surface plasmon resonance. Nature. 1993 Sep 23;365(6444):343–347. doi: 10.1038/365343a0. [DOI] [PubMed] [Google Scholar]
- Scott W. G., Milligan D. L., Milburn M. V., Privé G. G., Yeh J., Koshland D. E., Jr, Kim S. H. Refined structures of the ligand-binding domain of the aspartate receptor from Salmonella typhimurium. J Mol Biol. 1993 Jul 20;232(2):555–573. doi: 10.1006/jmbi.1993.1411. [DOI] [PubMed] [Google Scholar]
- Scott W. G., Stoddard B. L. Transmembrane signalling and the aspartate receptor. Structure. 1994 Sep 15;2(9):877–887. doi: 10.1016/s0969-2126(94)00088-3. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Lukat G. S., Stock A. M. Bacterial chemotaxis and the molecular logic of intracellular signal transduction networks. Annu Rev Biophys Biophys Chem. 1991;20:109–136. doi: 10.1146/annurev.bb.20.060191.000545. [DOI] [PubMed] [Google Scholar]
- Stoddard B. L., Bui J. D., Koshland D. E., Jr Structure and dynamics of transmembrane signaling by the Escherichia coli aspartate receptor. Biochemistry. 1992 Dec 8;31(48):11978–11983. doi: 10.1021/bi00163a004. [DOI] [PubMed] [Google Scholar]
- Swanson R. V., Alex L. A., Simon M. I. Histidine and aspartate phosphorylation: two-component systems and the limits of homology. Trends Biochem Sci. 1994 Nov;19(11):485–490. doi: 10.1016/0968-0004(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Utsumi R., Brissette R. E., Rampersaud A., Forst S. A., Oosawa K., Inouye M. Activation of bacterial porin gene expression by a chimeric signal transducer in response to aspartate. Science. 1989 Sep 15;245(4923):1246–1249. doi: 10.1126/science.2476847. [DOI] [PubMed] [Google Scholar]
- Yeh J. I., Biemann H. P., Pandit J., Koshland D. E., Kim S. H. The three-dimensional structure of the ligand-binding domain of a wild-type bacterial chemotaxis receptor. Structural comparison to the cross-linked mutant forms and conformational changes upon ligand binding. J Biol Chem. 1993 May 5;268(13):9787–9792. [PubMed] [Google Scholar]