Abstract
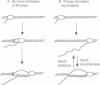
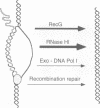
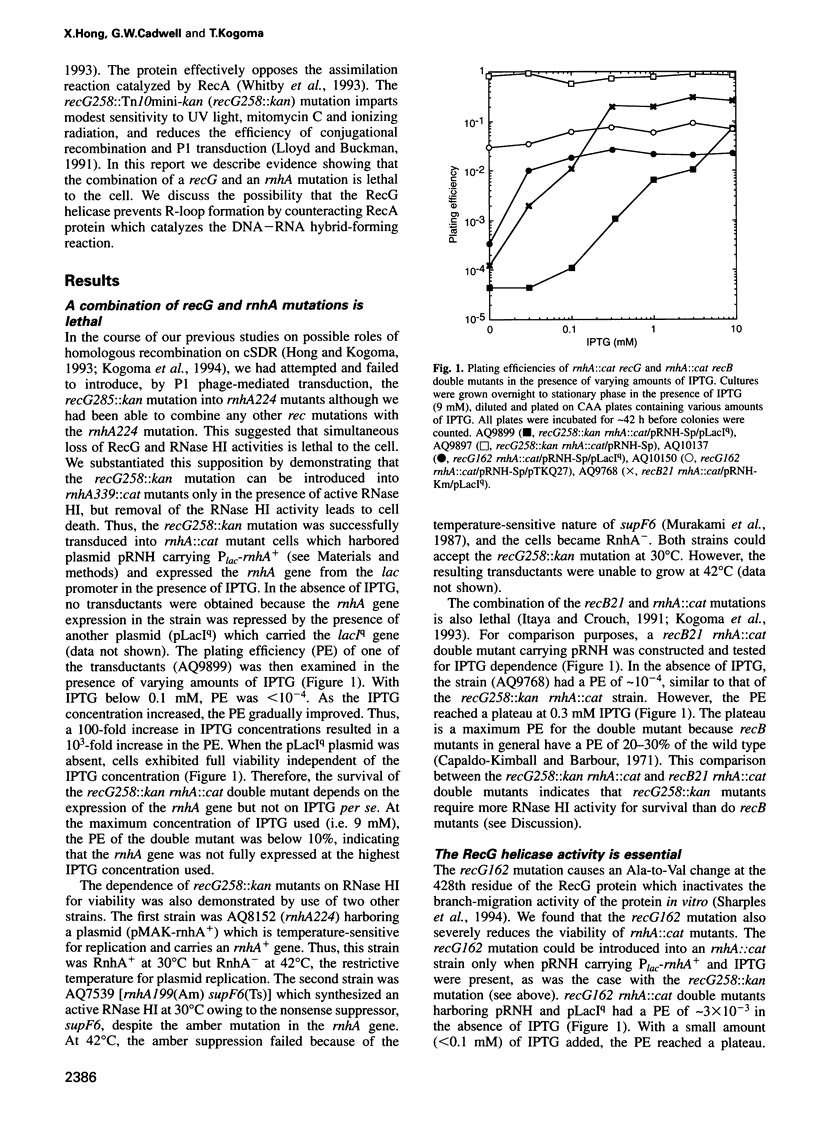
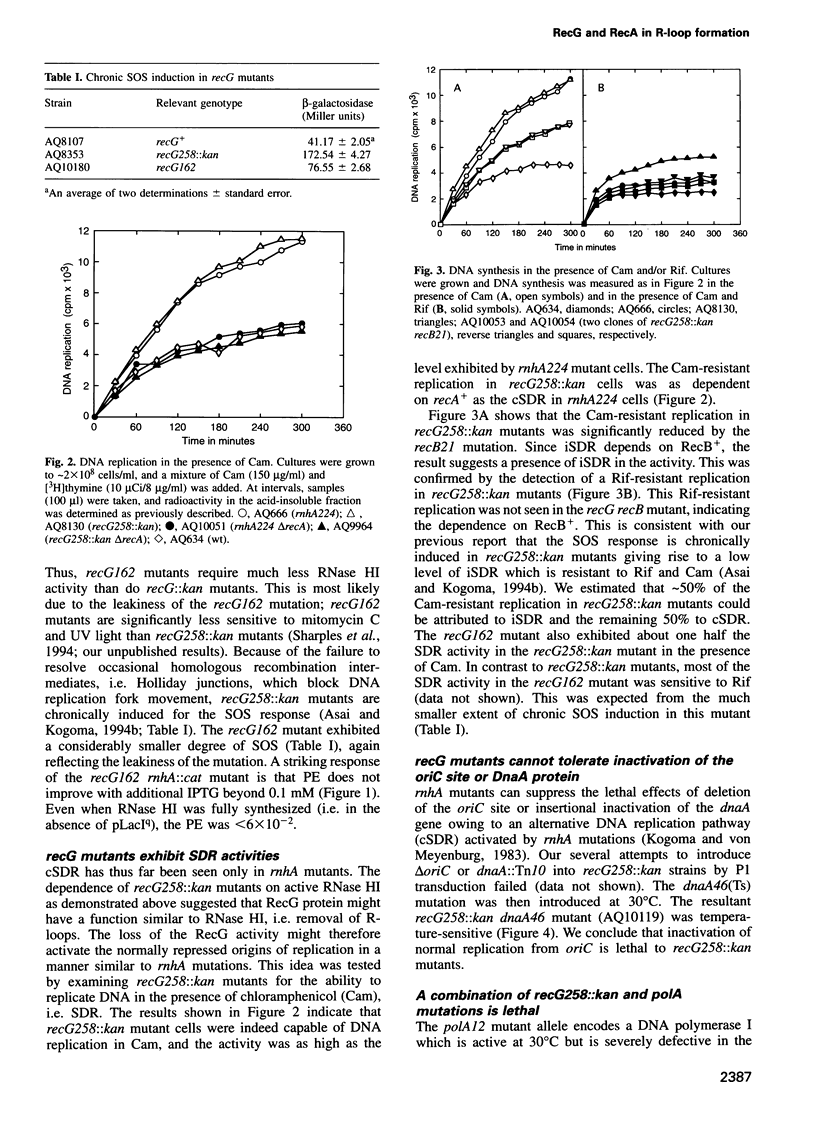
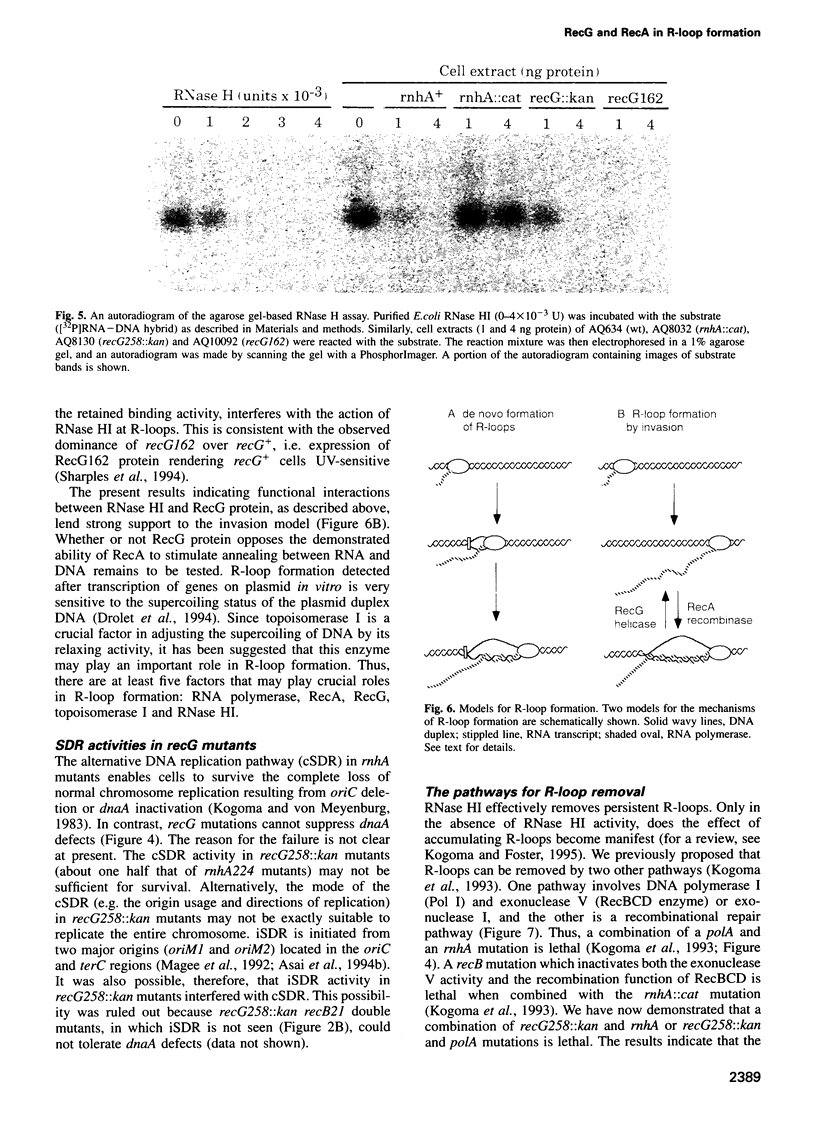
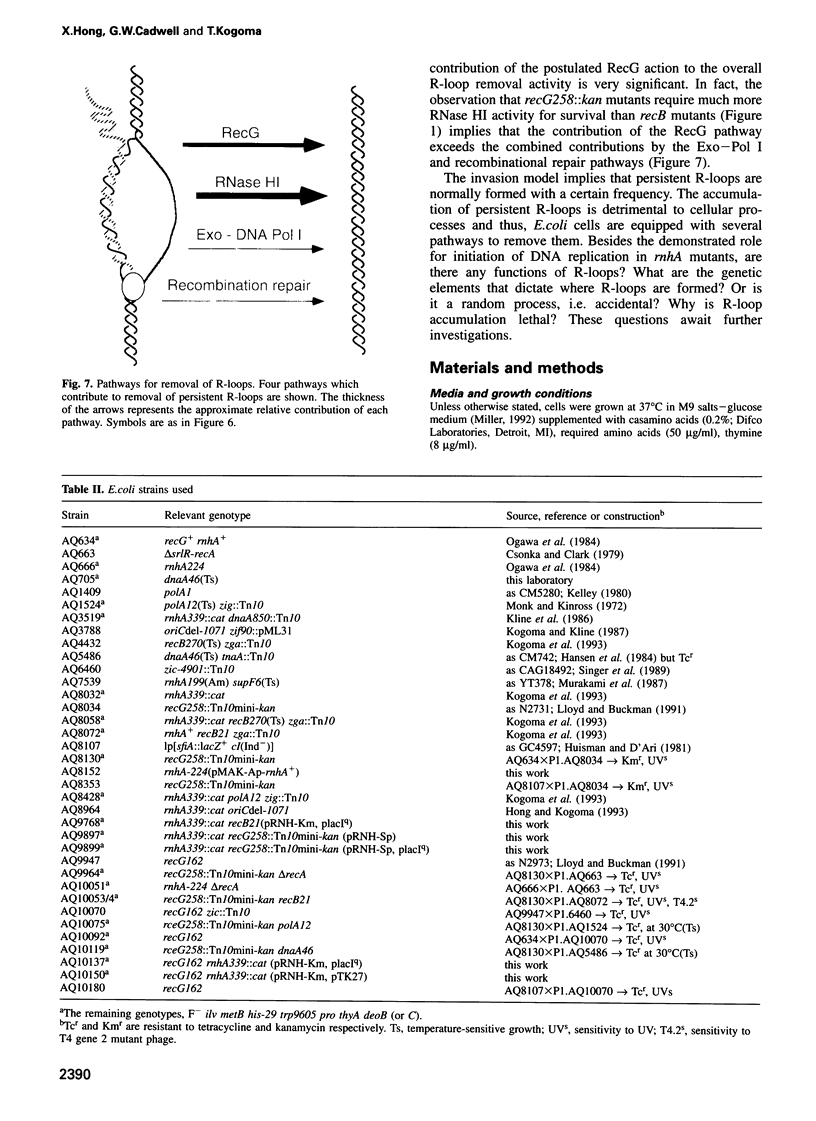
Escherichia coli rnhA mutants devoid of RNase HI exhibit constitutive stable DNA replication, cSDR, which is thought to be initiated from R-loops stabilized in the absence of RNase HI. We found that a combination of an rnhA and a recG mutation is lethal to the cell. recG mutations that inactivate the helicase activity of RecG protein and inhibit reverse branch migration of Holliday junctions impart phenotypes resembling those of rnhA mutants. Thus, recG mutants display cSDR activity, and recG polA double mutants are inviable as are rnhA polA double mutants. These results suggest that the RecG helicase has a role in preventing R-loop formation. A model that R-loops are formed by assimilation of RNA transcripts into the duplex DNA is discussed. The model further postulates that RecA protein catalyzes this assimilation reaction and that RecG protein counteracts RecA in this reaction, resolves R-loops by its helicase activity, or does both.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asai T., Bates D. B., Kogoma T. DNA replication triggered by double-stranded breaks in E. coli: dependence on homologous recombination functions. Cell. 1994 Sep 23;78(6):1051–1061. doi: 10.1016/0092-8674(94)90279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T., Imai M., Kogoma T. DNA damage-inducible replication of the Escherichia coli chromosome is initiated at separable sites within the minimal oriC. J Mol Biol. 1994 Feb 4;235(5):1459–1469. doi: 10.1006/jmbi.1994.1101. [DOI] [PubMed] [Google Scholar]
- Asai T., Kogoma T. D-loops and R-loops: alternative mechanisms for the initiation of chromosome replication in Escherichia coli. J Bacteriol. 1994 Apr;176(7):1807–1812. doi: 10.1128/jb.176.7.1807-1812.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T., Kogoma T. Roles of ruvA, ruvC and recG gene functions in normal and DNA damage-inducible replication of the Escherichia coli chromosome. Genetics. 1994 Aug;137(4):895–902. doi: 10.1093/genetics/137.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T., Sommer S., Bailone A., Kogoma T. Homologous recombination-dependent initiation of DNA replication from DNA damage-inducible origins in Escherichia coli. EMBO J. 1993 Aug;12(8):3287–3295. doi: 10.1002/j.1460-2075.1993.tb05998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Kornberg A. Transcriptional activation of initiation of replication from the E. coli chromosomal origin: an RNA-DNA hybrid near oriC. Cell. 1988 Oct 7;55(1):113–123. doi: 10.1016/0092-8674(88)90014-1. [DOI] [PubMed] [Google Scholar]
- Cao Y., Kogoma T. Requirement for the polymerization and 5'-->3' exonuclease activities of DNA polymerase I in initiation of DNA replication at oriK sites in the absence of RecA in Escherichia coli rnhA mutants. J Bacteriol. 1993 Nov;175(22):7254–7259. doi: 10.1128/jb.175.22.7254-7259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo-Kimball F., Barbour S. D. Involvement of recombination genes in growth and viability of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):204–212. doi: 10.1128/jb.106.1.204-212.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl P. L., Bloom L., Crouch R. J. Isolation and mapping of a mutation in Escherichia coli with altered levels of ribonuclease H. J Bacteriol. 1980 Oct;144(1):28–35. doi: 10.1128/jb.144.1.28-35.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka L. N., Clark A. J. Deletions generated by the transposon Tn10 in the srl recA region of the Escherichia coli K-12 chromosome. Genetics. 1979 Oct;93(2):321–343. doi: 10.1093/genetics/93.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet M., Bi X., Liu L. F. Hypernegative supercoiling of the DNA template during transcription elongation in vitro. J Biol Chem. 1994 Jan 21;269(3):2068–2074. [PubMed] [Google Scholar]
- Hamilton C. M., Aldea M., Washburn B. K., Babitzke P., Kushner S. R. New method for generating deletions and gene replacements in Escherichia coli. J Bacteriol. 1989 Sep;171(9):4617–4622. doi: 10.1128/jb.171.9.4617-4622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. B., Atlung T., Hansen F. G., Skovgaard O., von Meyenburg K. Fine structure genetic map and complementation analysis of mutations in the dnaA gene of Escherichia coli. Mol Gen Genet. 1984;196(3):387–396. doi: 10.1007/BF00436184. [DOI] [PubMed] [Google Scholar]
- Hong X., Kogoma T. Absence of a direct role for RNase HI in initiation of DNA replication at the oriC site on the Escherichia coli chromosome. J Bacteriol. 1993 Oct;175(20):6731–6734. doi: 10.1128/jb.175.20.6731-6734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman O., D'Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981 Apr 30;290(5809):797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- Itaya M., Crouch R. J. A combination of RNase H (rnh) and recBCD or sbcB mutations in Escherichia coli K12 adversely affects growth. Mol Gen Genet. 1991 Jul;227(3):424–432. doi: 10.1007/BF00273933. [DOI] [PubMed] [Google Scholar]
- Itaya M. Isolation and characterization of a second RNase H (RNase HII) of Escherichia coli K-12 encoded by the rnhB gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8587–8591. doi: 10.1073/pnas.87.21.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Formation of an RNA primer for initiation of replication of ColE1 DNA by ribonuclease H. Proc Natl Acad Sci U S A. 1980 May;77(5):2450–2454. doi: 10.1073/pnas.77.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya S., Crouch R. J. DNA sequence of the gene coding for Escherichia coli ribonuclease H. J Biol Chem. 1983 Jan 25;258(2):1276–1281. [PubMed] [Google Scholar]
- Kelley W. S. Mapping of the polA locus of Escherichia coli K12: genetic fine structure of the cistron. Genetics. 1980 May;95(1):15–38. doi: 10.1093/genetics/95.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick D. P., Radding C. M. RecA protein promotes rapid RNA-DNA hybridization in heterogeneous RNA mixtures. Nucleic Acids Res. 1992 Aug 25;20(16):4347–4353. doi: 10.1093/nar/20.16.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick D. P., Rao B. J., Radding C. M. RNA-DNA hybridization promoted by E. coli RecA protein. Nucleic Acids Res. 1992 Aug 25;20(16):4339–4346. doi: 10.1093/nar/20.16.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C., Kogoma T., Tam J. E., Shields M. S. Requirement of the Escherichia coli dnaA gene product for plasmid F maintenance. J Bacteriol. 1986 Oct;168(1):440–443. doi: 10.1128/jb.168.1.440-443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T. Absence of RNase H allows replication of pBR322 in Escherichia coli mutants lacking DNA polymerase I. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7845–7849. doi: 10.1073/pnas.81.24.7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T., Barnard K. G., Hong X. RecA, Tus protein and constitutive stable DNA replication in Escherichia coli rnhA mutants. Mol Gen Genet. 1994 Sep 1;244(5):557–562. doi: 10.1007/BF00583907. [DOI] [PubMed] [Google Scholar]
- Kogoma T. Escherichia coli RNA polymerase mutants that enhance or diminish the SOS response constitutively expressed in the absence of RNase HI activity. J Bacteriol. 1994 Mar;176(5):1521–1523. doi: 10.1128/jb.176.5.1521-1523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T., Hong X., Cadwell G. W., Barnard K. G., Asai T. Requirement of homologous recombination functions for viability of the Escherichia coli cell that lacks RNase HI and exonuclease V activities. Biochimie. 1993;75(1-2):89–99. doi: 10.1016/0300-9084(93)90029-r. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Kline B. C. Integrative suppression of dnaA(Ts) mutations mediated by plasmid F in Escherichia coli is a DnaA-dependent process. Mol Gen Genet. 1987 Dec;210(2):262–269. doi: 10.1007/BF00325692. [DOI] [PubMed] [Google Scholar]
- Kogoma T., Skarstad K., Boye E., von Meyenburg K., Steen H. B. RecA protein acts at the initiation of stable DNA replication in rnh mutants of Escherichia coli K-12. J Bacteriol. 1985 Aug;163(2):439–444. doi: 10.1128/jb.163.2.439-444.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoma T., von Meyenburg K. The origin of replication, oriC, and the dnaA protein are dispensable in stable DNA replication (sdrA) mutants of Escherichia coli K-12. EMBO J. 1983;2(3):463–468. doi: 10.1002/j.1460-2075.1983.tb01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. G., Sharples G. J. Dissociation of synthetic Holliday junctions by E. coli RecG protein. EMBO J. 1993 Jan;12(1):17–22. doi: 10.1002/j.1460-2075.1993.tb05627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. G., Sharples G. J. Processing of recombination intermediates by the RecG and RuvAB proteins of Escherichia coli. Nucleic Acids Res. 1993 Apr 25;21(8):1719–1725. doi: 10.1093/nar/21.8.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee T. R., Asai T., Malka D., Kogoma T. DNA damage-inducible origins of DNA replication in Escherichia coli. EMBO J. 1992 Nov;11(11):4219–4225. doi: 10.1002/j.1460-2075.1992.tb05516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee T. R., Kogoma T. Requirement of RecBC enzyme and an elevated level of activated RecA for induced stable DNA replication in Escherichia coli. J Bacteriol. 1990 Apr;172(4):1834–1839. doi: 10.1128/jb.172.4.1834-1839.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H., Asai T., Kubota Y., Arai K., Kogoma T. Escherichia coli PriA protein is essential for inducible and constitutive stable DNA replication. EMBO J. 1994 Nov 15;13(22):5338–5345. doi: 10.1002/j.1460-2075.1994.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukata H., Tomizawa J. A mechanism of formation of a persistent hybrid between elongating RNA and template DNA. Cell. 1990 Jul 27;62(2):331–338. doi: 10.1016/0092-8674(90)90370-t. [DOI] [PubMed] [Google Scholar]
- Masukata H., Tomizawa J. Control of primer formation for ColE1 plasmid replication: conformational change of the primer transcript. Cell. 1986 Jan 17;44(1):125–136. doi: 10.1016/0092-8674(86)90491-5. [DOI] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Ohmori H., Yura T., Nagata T. Requirement of the Escherichia coli dnaA gene function for ori-2-dependent mini-F plasmid replication. J Bacteriol. 1987 Apr;169(4):1724–1730. doi: 10.1128/jb.169.4.1724-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. FORMATION AND PROPERTIES OF RNA-DNA COMPLEXES. J Mol Biol. 1964 Jul;9:125–142. doi: 10.1016/s0022-2836(64)80095-4. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Pickett G. G., Kogoma T., Kornberg A. RNase H confers specificity in the dnaA-dependent initiation of replication at the unique origin of the Escherichia coli chromosome in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1040–1044. doi: 10.1073/pnas.81.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Rice G. A., Kane C. M., Chamberlin M. J. Footprinting analysis of mammalian RNA polymerase II along its transcript: an alternative view of transcription elongation. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4245–4249. doi: 10.1073/pnas.88.10.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P. Attachment of nascent RNA molecules to superhelical DNA. J Mol Biol. 1975 Nov 5;98(3):565–579. doi: 10.1016/s0022-2836(75)80087-8. [DOI] [PubMed] [Google Scholar]
- Sharples G. J., Whitby M. C., Ryder L., Lloyd R. G. A mutation in helicase motif III of E. coli RecG protein abolishes branch migration of Holliday junctions. Nucleic Acids Res. 1994 Feb 11;22(3):308–313. doi: 10.1093/nar/22.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstad K., Baker T. A., Kornberg A. Strand separation required for initiation of replication at the chromosomal origin of E.coli is facilitated by a distant RNA--DNA hybrid. EMBO J. 1990 Jul;9(7):2341–2348. doi: 10.1002/j.1460-2075.1990.tb07406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Torrey T. A., Kogoma T. Genetic analysis of constitutive stable DNA replication in rnh mutants of Escherichia coli K12. Mol Gen Genet. 1987 Jul;208(3):420–427. doi: 10.1007/BF00328133. [DOI] [PubMed] [Google Scholar]
- Torrey T. A., Kogoma T. Suppressor mutations (rin) that specifically suppress the recA+ dependence of stable DNA replication in Escherichia coliK-12. Mol Gen Genet. 1982;187(2):225–230. doi: 10.1007/BF00331121. [DOI] [PubMed] [Google Scholar]
- Touati D. Transcriptional and posttranscriptional regulation of manganese superoxide dismutase biosynthesis in Escherichia coli, studied with operon and protein fusions. J Bacteriol. 1988 Jun;170(6):2511–2520. doi: 10.1128/jb.170.6.2511-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby M. C., Ryder L., Lloyd R. G. Reverse branch migration of Holliday junctions by RecG protein: a new mechanism for resolution of intermediates in recombination and DNA repair. Cell. 1993 Oct 22;75(2):341–350. doi: 10.1016/0092-8674(93)80075-p. [DOI] [PubMed] [Google Scholar]
- Whitby M. C., Vincent S. D., Lloyd R. G. Branch migration of Holliday junctions: identification of RecG protein as a junction specific DNA helicase. EMBO J. 1994 Nov 1;13(21):5220–5228. doi: 10.1002/j.1460-2075.1994.tb06853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]