Abstract
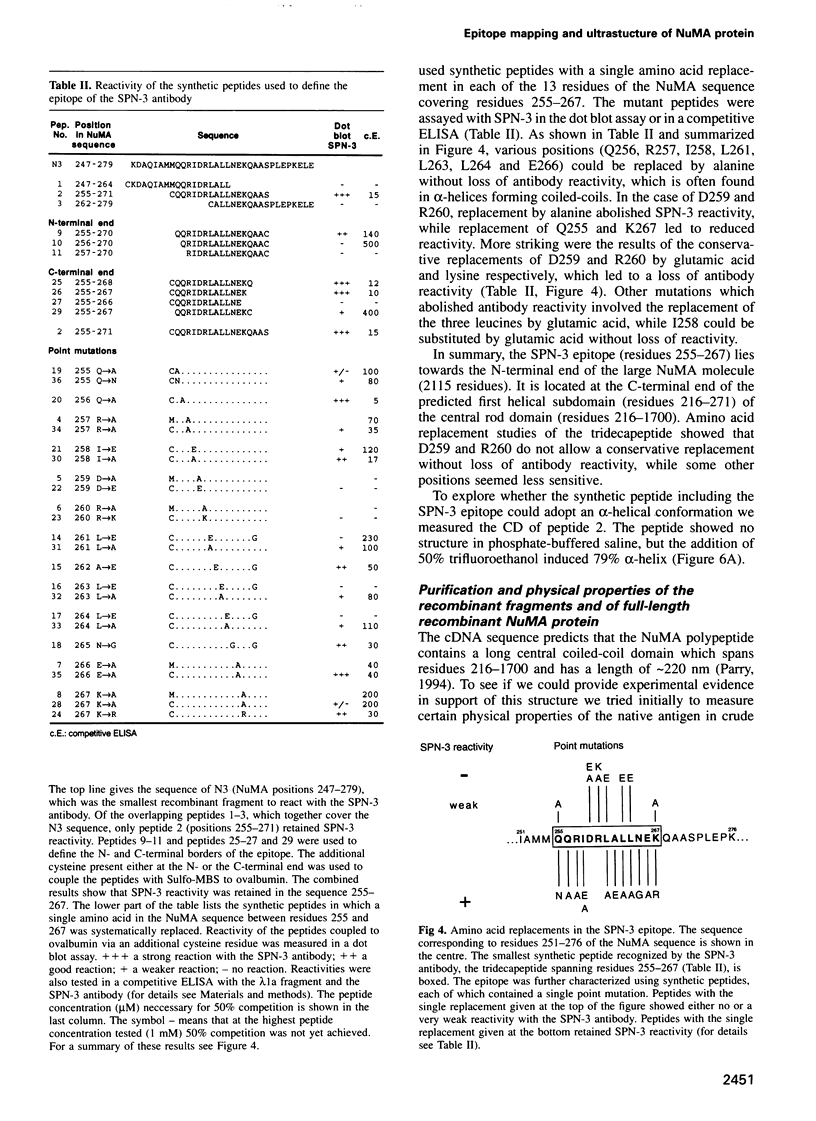
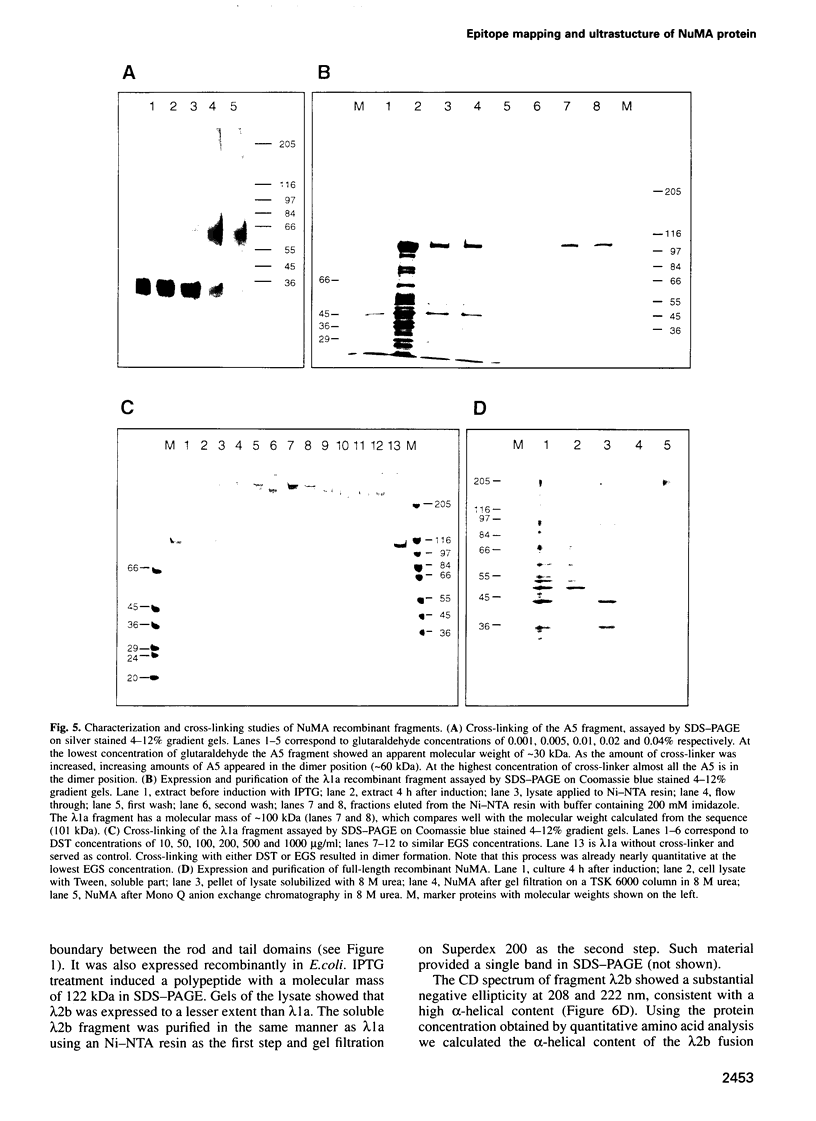
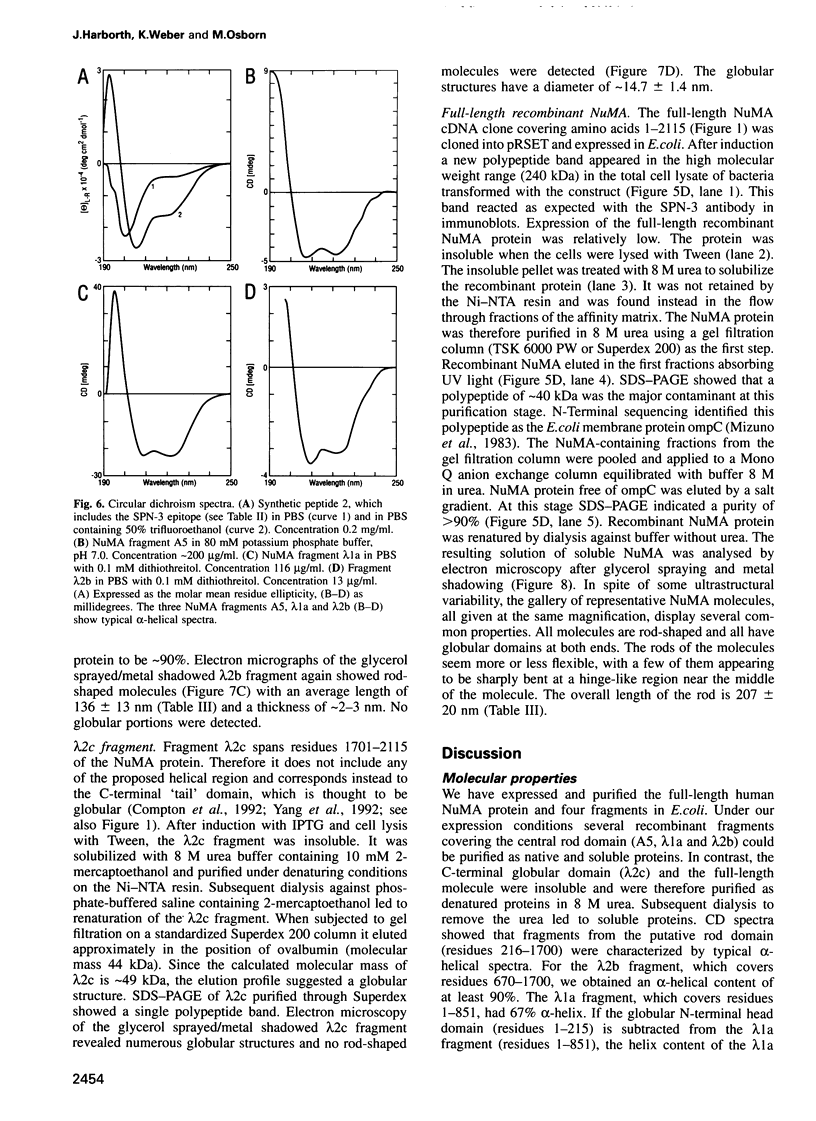
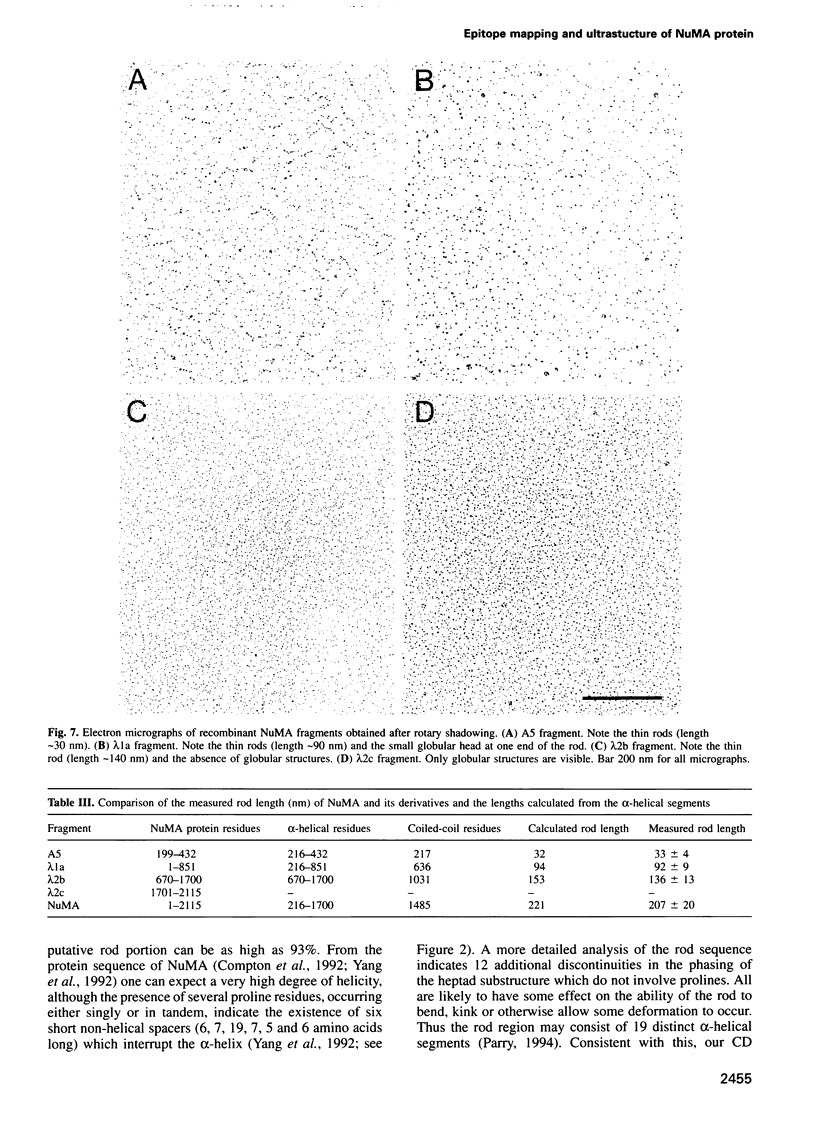
NuMA, a 238 kDa protein present in the nucleus during interphase, translocates to the spindle poles in mitosis. NuMA plays an essential role in mitosis, since microinjection of the NuMA SPN-3 monoclonal antibody causes mitotic arrest and micronuclei formation. We have mapped the approximate position of the epitopes of six monoclonal NuMA antibodies using recombinant NuMA fragments. The SPN-3 epitope has been located to residues 255-267 at the C-terminus of the first helical subdomain of the central rod domain and several residues crucial for antibody binding have been identified. To gain insight into the ultrastructure of NuMA, several defined fragments, as well as the full-length recombinant protein, were expressed in Escherichia coli and purified to homogeneity. They were then characterized by chemical cross-linking, circular dichroism spectra and electron microscopy. The results directly reveal the tripartate structure of NuMA. A long central rod domain is flanked by globular end domains. The rod is 207 nm long and is at least 90% alpha-helical. It reflects a double-stranded coiled-coil with the alpha-helices arranged parallel and in register. The NuMA protein thus forms the longest coiled-coil currently known. Our analyses reveal no indication that recombinant NuMA assembles into filaments or other higher order structures.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Chien C. T., Bartel P. L., Sternglanz R., Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C., Lanar D. E., Parry D. A. Amino acid sequence and structural repeats in schistosome paramyosin match those of myosin. Biosci Rep. 1987 Jan;7(1):11–16. doi: 10.1007/BF01122722. [DOI] [PubMed] [Google Scholar]
- Cohen C., Parry D. A. Alpha-helical coiled coils and bundles: how to design an alpha-helical protein. Proteins. 1990;7(1):1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Laver W. G., Varghese J. N., Baker A. T., Tulloch P. A., Air G. M., Webster R. G. Three-dimensional structure of a complex of antibody with influenza virus neuraminidase. 1987 Mar 26-Apr 1Nature. 326(6111):358–363. doi: 10.1038/326358a0. [DOI] [PubMed] [Google Scholar]
- Compton D. A., Cleveland D. W. NuMA is required for the proper completion of mitosis. J Cell Biol. 1993 Feb;120(4):947–957. doi: 10.1083/jcb.120.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
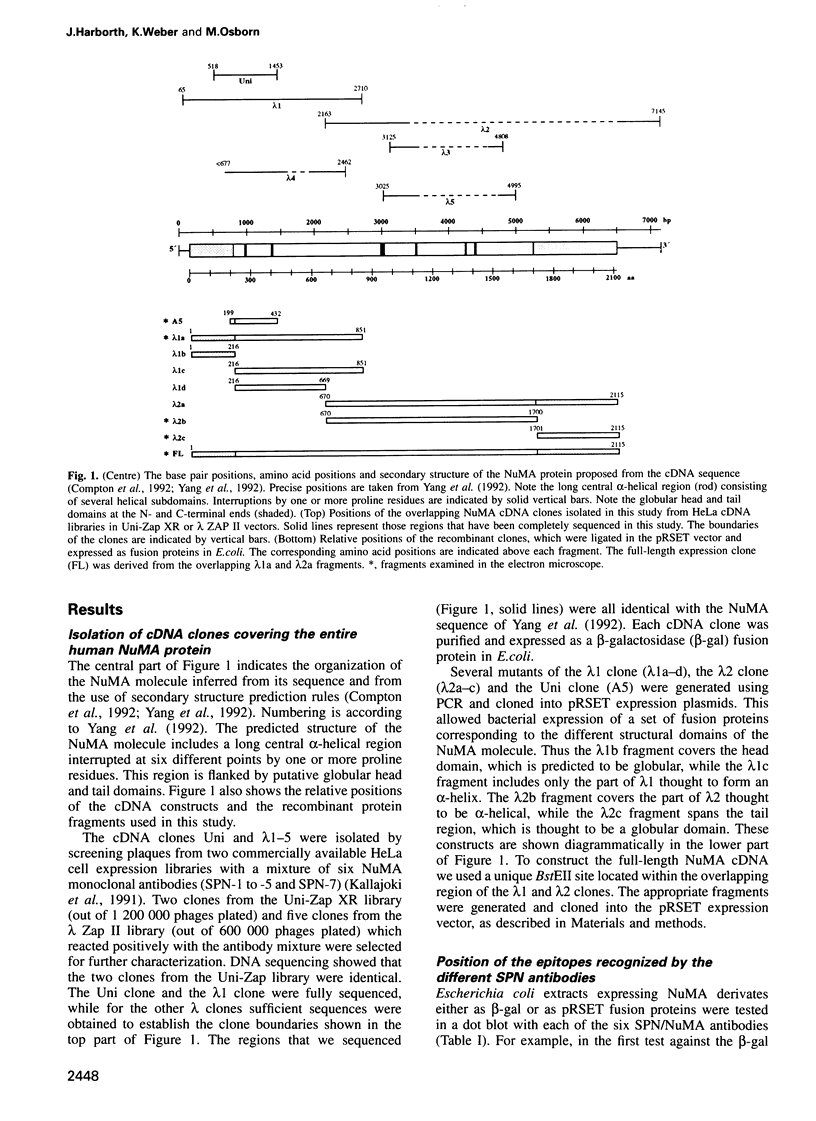
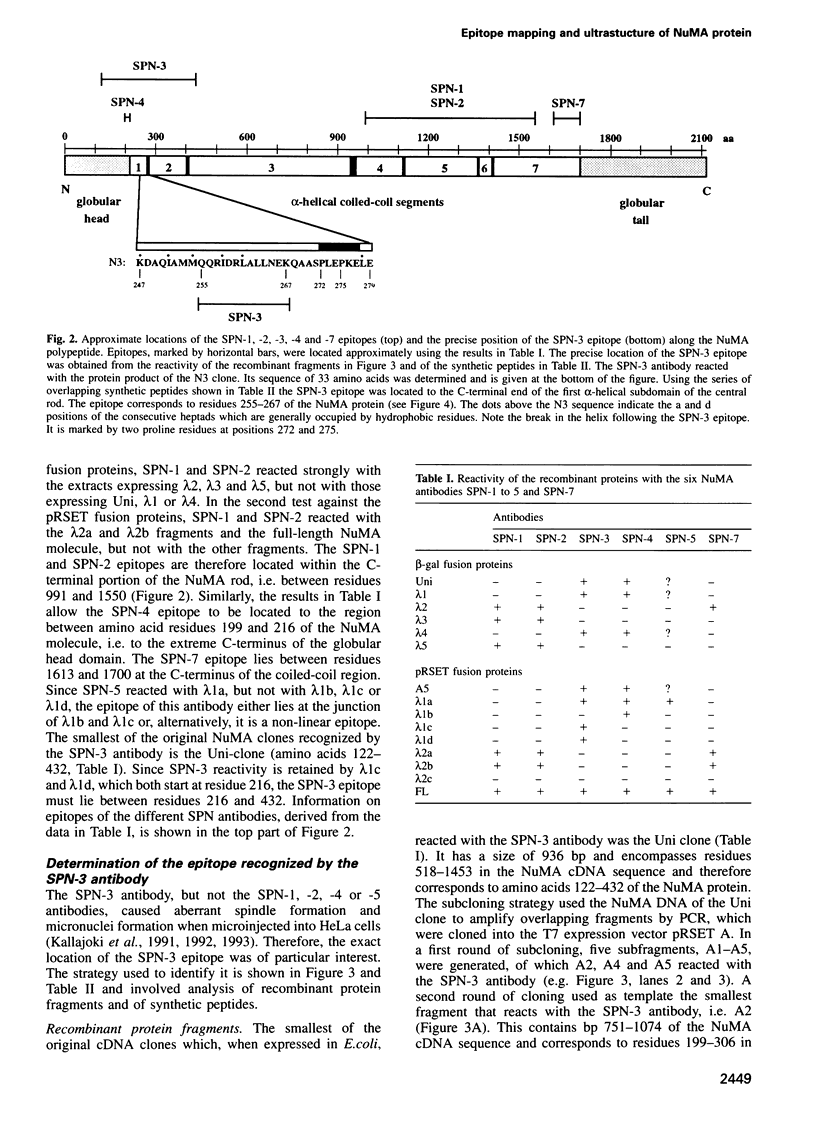
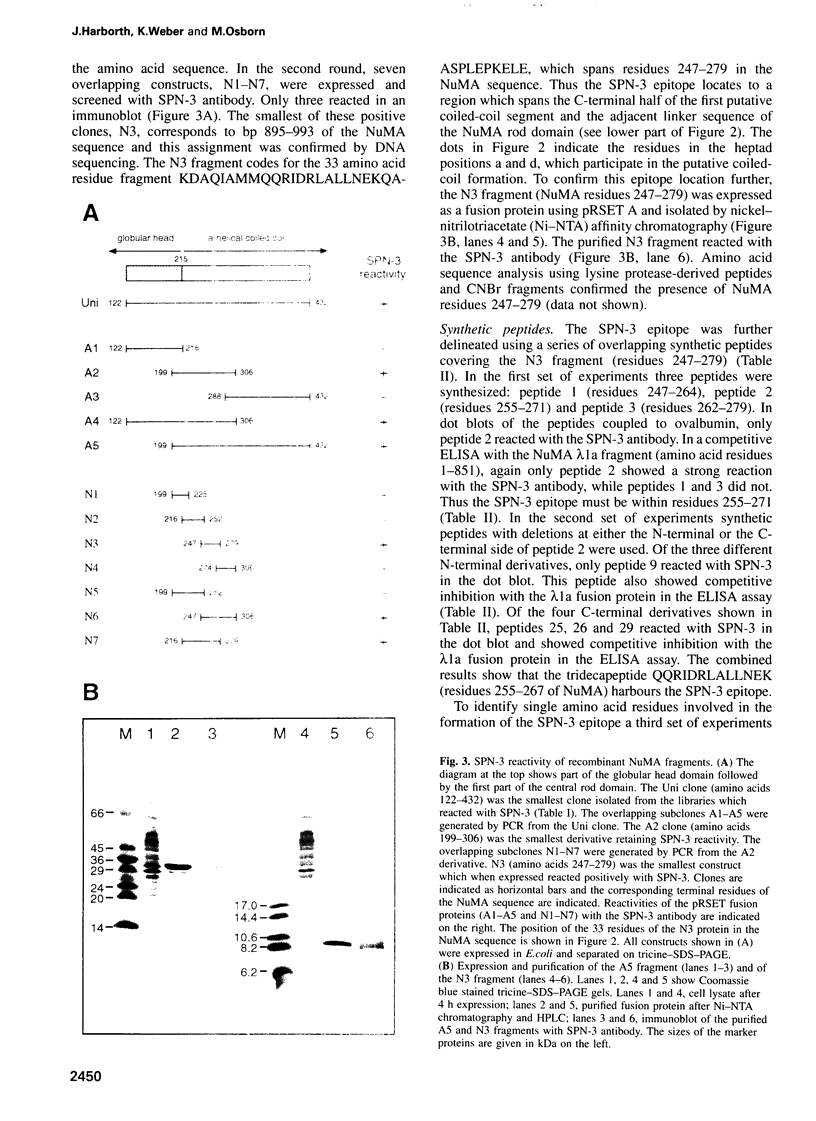
- Compton D. A., Szilak I., Cleveland D. W. Primary structure of NuMA, an intranuclear protein that defines a novel pathway for segregation of proteins at mitosis. J Cell Biol. 1992 Mar;116(6):1395–1408. doi: 10.1083/jcb.116.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton D. A., Yen T. J., Cleveland D. W. Identification of novel centromere/kinetochore-associated proteins using monoclonal antibodies generated against human mitotic chromosome scaffolds. J Cell Biol. 1991 Mar;112(6):1083–1097. doi: 10.1083/jcb.112.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. R. The nucleoskeleton: artefact, passive framework or active site? J Cell Sci. 1988 May;90(Pt 1):1–6. doi: 10.1242/jcs.90.1.1. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- He D. C., Nickerson J. A., Penman S. Core filaments of the nuclear matrix. J Cell Biol. 1990 Mar;110(3):569–580. doi: 10.1083/jcb.110.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins S., Aebi U. Making heads and tails of intermediate filament assembly, dynamics and networks. Curr Opin Cell Biol. 1994 Feb;6(1):25–33. doi: 10.1016/0955-0674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Cook P. R. Visualization of a filamentous nucleoskeleton with a 23 nm axial repeat. EMBO J. 1988 Dec 1;7(12):3667–3677. doi: 10.1002/j.1460-2075.1988.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallajoki M., Harborth J., Weber K., Osborn M. Microinjection of a monoclonal antibody against SPN antigen, now identified by peptide sequences as the NuMA protein, induces micronuclei in PtK2 cells. J Cell Sci. 1993 Jan;104(Pt 1):139–150. doi: 10.1242/jcs.104.1.139. [DOI] [PubMed] [Google Scholar]
- Kallajoki M., Weber K., Osborn M. A 210 kDa nuclear matrix protein is a functional part of the mitotic spindle; a microinjection study using SPN monoclonal antibodies. EMBO J. 1991 Nov;10(11):3351–3362. doi: 10.1002/j.1460-2075.1991.tb04899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallajoki M., Weber K., Osborn M. Ability to organize microtubules in taxol-treated mitotic PtK2 cells goes with the SPN antigen and not with the centrosome. J Cell Sci. 1992 May;102(Pt 1):91–102. doi: 10.1242/jcs.102.1.91. [DOI] [PubMed] [Google Scholar]
- Kroll D. J., Abdel-Malek Abdel-Hafiz H., Marcell T., Simpson S., Chen C. Y., Gutierrez-Hartmann A., Lustbader J. W., Hoeffler J. P. A multifunctional prokaryotic protein expression system: overproduction, affinity purification, and selective detection. DNA Cell Biol. 1993 Jun;12(5):441–453. doi: 10.1089/dna.1993.12.441. [DOI] [PubMed] [Google Scholar]
- Lydersen B. K., Pettijohn D. E. Human-specific nuclear protein that associates with the polar region of the mitotic apparatus: distribution in a human/hamster hybrid cell. Cell. 1980 Nov;22(2 Pt 2):489–499. doi: 10.1016/0092-8674(80)90359-1. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Kuriyama R. Primary structure and microtubule-interacting domain of the SP-H antigen: a mitotic MAP located at the spindle pole and characterized as a homologous protein to NuMA. J Cell Sci. 1993 Jun;105(Pt 2):589–600. doi: 10.1242/jcs.105.2.589. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Leslie R., Kuriyama R. Identification of a minus end-specific microtubule-associated protein located at the mitotic poles in cultured mammalian cells. Eur J Cell Biol. 1991 Apr;54(2):255–267. [PubMed] [Google Scholar]
- McIntosh J. R., Koonce M. P. Mitosis. Science. 1989 Nov 3;246(4930):622–628. doi: 10.1126/science.2683078. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Parry D. A. NuMA/centrophilin: sequence analysis of the coiled-coil rod domain. Biophys J. 1994 Sep;67(3):1203–1206. doi: 10.1016/S0006-3495(94)80589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potschka M., Nave R., Weber K., Geisler N. The two coiled coils in the isolated rod domain of the intermediate filament protein desmin are staggered. A hydrodynamic analysis of tetramers and dimers. Eur J Biochem. 1990 Jul 5;190(3):503–508. doi: 10.1111/j.1432-1033.1990.tb15602.x. [DOI] [PubMed] [Google Scholar]
- Price C. M., Pettijohn D. E. Redistribution of the nuclear mitotic apparatus protein (NuMA) during mitosis and nuclear assembly. Properties of purified NuMA protein. Exp Cell Res. 1986 Oct;166(2):295–311. doi: 10.1016/0014-4827(86)90478-7. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stewart M., Edwards P. Length of myosin rod and its proteolytic fragments determined by electron microscopy. FEBS Lett. 1984 Mar 12;168(1):75–78. doi: 10.1016/0014-5793(84)80209-4. [DOI] [PubMed] [Google Scholar]
- Stick R. cDNA cloning of the developmentally regulated lamin LIII of Xenopus laevis. EMBO J. 1988 Oct;7(10):3189–3197. doi: 10.1002/j.1460-2075.1988.tb03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. K., Tang C. J., Chen Y. L., Wu C. W. Nuclear proteins of the bovine esophageal epithelium. II. The NuMA gene gives rise to multiple mRNAs and gene products reactive with monoclonal antibody W1. J Cell Sci. 1993 Feb;104(Pt 2):249–260. doi: 10.1242/jcs.104.2.249. [DOI] [PubMed] [Google Scholar]
- Tousson A., Zeng C., Brinkley B. R., Valdivia M. M. Centrophilin: a novel mitotic spindle protein involved in microtubule nucleation. J Cell Biol. 1991 Feb;112(3):427–440. doi: 10.1083/jcb.112.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. H., Lambie E. J., Snyder M. NuMA: an unusually long coiled-coil related protein in the mammalian nucleus. J Cell Biol. 1992 Mar;116(6):1303–1317. doi: 10.1083/jcb.116.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. H., Snyder M. The nuclear-mitotic apparatus protein is important in the establishment and maintenance of the bipolar mitotic spindle apparatus. Mol Biol Cell. 1992 Nov;3(11):1259–1267. doi: 10.1091/mbc.3.11.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng C., He D., Brinkley B. R. Localization of NuMA protein isoforms in the nuclear matrix of mammalian cells. Cell Motil Cytoskeleton. 1994;29(2):167–176. doi: 10.1002/cm.970290208. [DOI] [PubMed] [Google Scholar]