Abstract
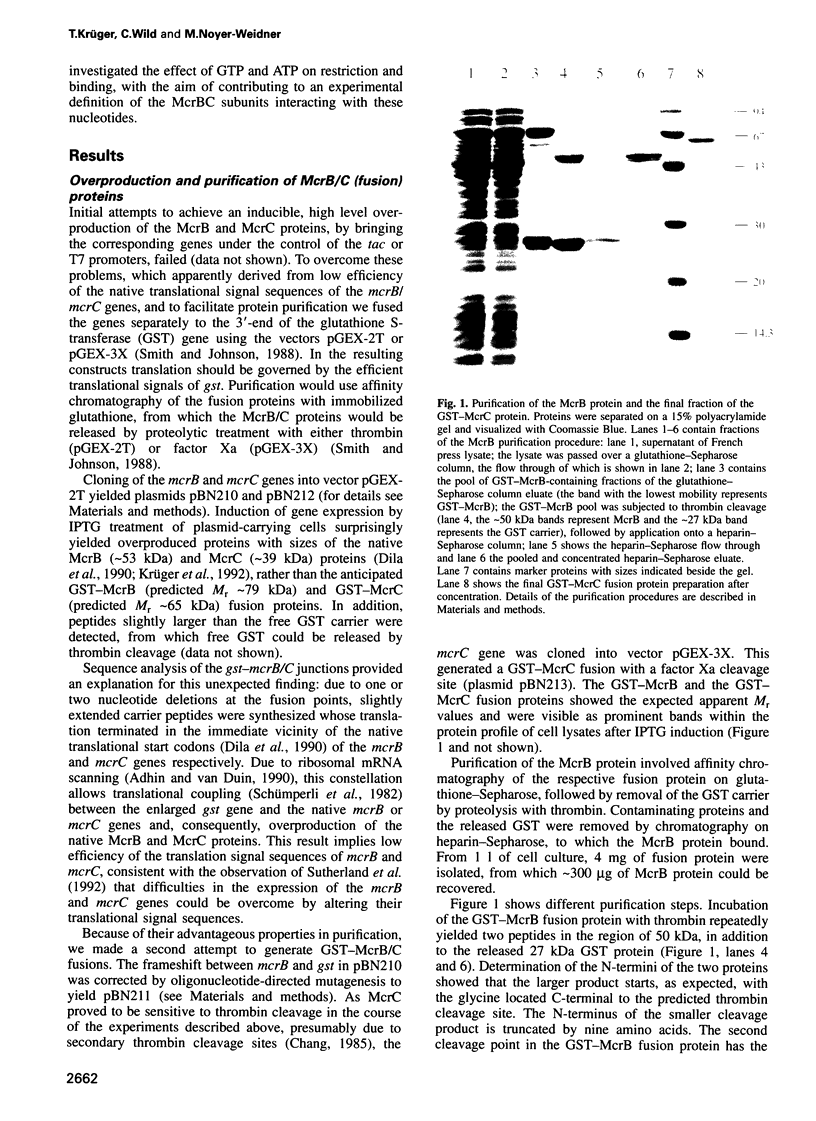
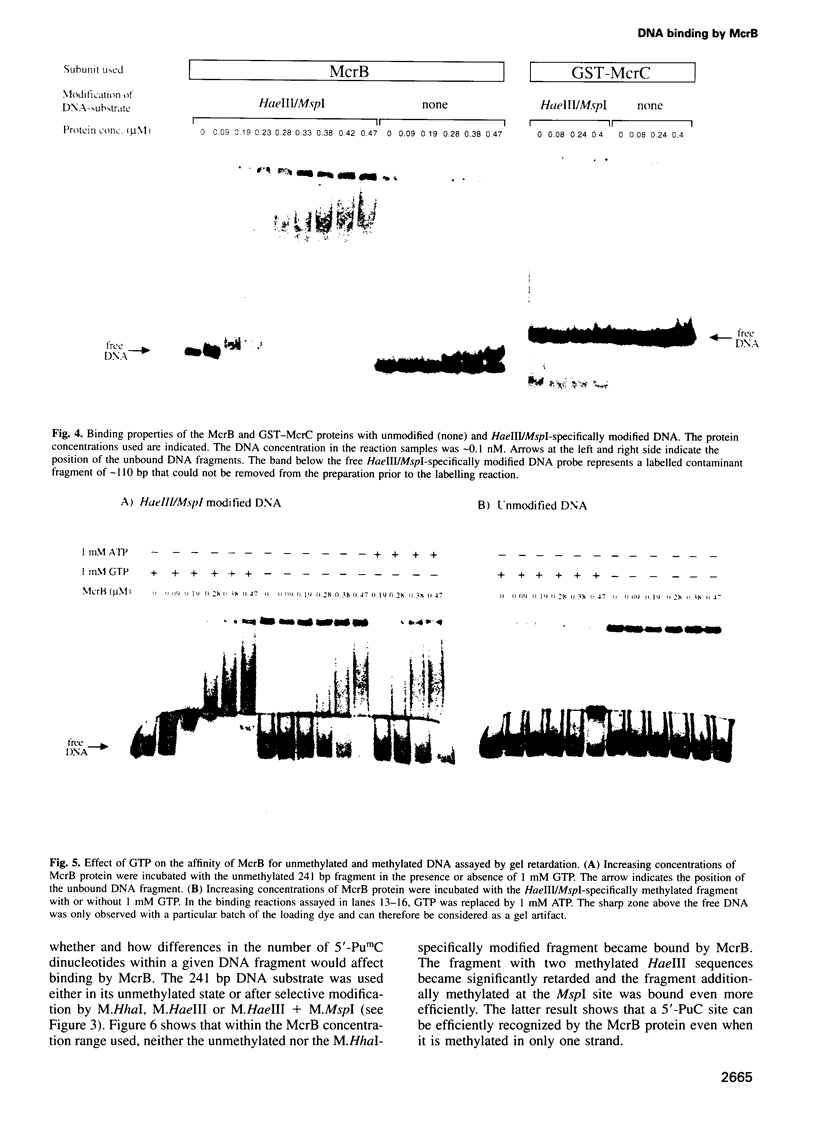
Restriction of DNA by the Escherichia coli K-12 McrBC restriction endonuclease, which consists of the two subunits McrB and McrC, depends on the presence of modified cytosine residues in a special constellation. From previous work by others it was known that restriction of 5-methylcytosine-containing DNA requires two methylated 5'-PuC sites separated by approximately 40-80 non-defined base pairs. Here we show that binding of the McrBC nuclease is mediated exclusively by the McrB subunit. McrB has a low affinity for non-methylated DNA, with which it forms low molecular weight complexes. The affinity for DNA is significantly increased, with variations depending on the sequence context, by hemi- or fully methylated 5'-PuC sites. Binding to such substrates yields high molecular weight complexes, presumably involving several McrB molecules. Methylation at unique 5'-PuC sites can be sufficient to stimulate DNA binding by McrB. As such substrates are not cleaved by the nuclease, restriction apparently requires the coordinated interaction of molecules bound to neighbouring 5'-PumC sites. The binding properties of McrB exhibit some similarities to recently identified eukaryotic proteins interacting in a non-sequence-specific manner with DNA containing methylated 5'-CpG sequences and might point to a common molecular origin of these proteins. In addition to DNA, McrB also binds GTP, an essential cofactor in DNA restriction by McrBC. McrC neither binds to DNA nor modulates the DNA binding potential of McrB. As McrC is essential for restriction it appears to predominantly function in catalysis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhin M. R., van Duin J. Scanning model for translational reinitiation in eubacteria. J Mol Biol. 1990 Jun 20;213(4):811–818. doi: 10.1016/S0022-2836(05)80265-7. [DOI] [PubMed] [Google Scholar]
- Bestor T. H. DNA methylation: evolution of a bacterial immune function into a regulator of gene expression and genome structure in higher eukaryotes. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1235):179–187. doi: 10.1098/rstb.1990.0002. [DOI] [PubMed] [Google Scholar]
- Bestor T., Laudano A., Mattaliano R., Ingram V. Cloning and sequencing of a cDNA encoding DNA methyltransferase of mouse cells. The carboxyl-terminal domain of the mammalian enzymes is related to bacterial restriction methyltransferases. J Mol Biol. 1988 Oct 20;203(4):971–983. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- Bhagwat A. S., Johnson B., Weule K., Roberts R. J. Primary sequence of the EcoRII endonuclease and properties of its fusions with beta-galactosidase. J Biol Chem. 1990 Jan 15;265(2):767–773. [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Chang J. Y. Thrombin specificity. Requirement for apolar amino acids adjacent to the thrombin cleavage site of polypeptide substrate. Eur J Biochem. 1985 Sep 2;151(2):217–224. doi: 10.1111/j.1432-1033.1985.tb09091.x. [DOI] [PubMed] [Google Scholar]
- Dever T. E., Glynias M. J., Merrick W. C. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1814–1818. doi: 10.1073/pnas.84.7.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dila D., Sutherland E., Moran L., Slatko B., Raleigh E. A. Genetic and sequence organization of the mcrBC locus of Escherichia coli K-12. J Bacteriol. 1990 Sep;172(9):4888–4900. doi: 10.1128/jb.172.9.4888-4900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürste J. P., Pansegrau W., Frank R., Blöcker H., Scholz P., Bagdasarian M., Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48(1):119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Hennecke F., Kolmar H., Bründl K., Fritz H. J. The vsr gene product of E. coli K-12 is a strand- and sequence-specific DNA mismatch endonuclease. Nature. 1991 Oct 24;353(6346):776–778. doi: 10.1038/353776a0. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Hofsteenge J. The repressor MDBP-2 is a member of the histone H1 family that binds preferentially in vitro and in vivo to methylated nonspecific DNA sequences. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9499–9503. doi: 10.1073/pnas.89.20.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger T., Grund C., Wild C., Noyer-Weidner M. Characterization of the mcrBC region of Escherichia coli K-12 wild-type and mutant strains. Gene. 1992 May 1;114(1):1–12. doi: 10.1016/0378-1119(92)90700-y. [DOI] [PubMed] [Google Scholar]
- Lacks S. A. Purification and properties of the complementary endonucleases DpnI and DpnII. Methods Enzymol. 1980;65(1):138–146. doi: 10.1016/s0076-6879(80)65019-8. [DOI] [PubMed] [Google Scholar]
- Lacks S., Greenberg B. A deoxyribonuclease of Diplococcus pneumoniae specific for methylated DNA. J Biol Chem. 1975 Jun 10;250(11):4060–4066. [PubMed] [Google Scholar]
- Lewis J. D., Meehan R. R., Henzel W. J., Maurer-Fogy I., Jeppesen P., Klein F., Bird A. Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell. 1992 Jun 12;69(6):905–914. doi: 10.1016/0092-8674(92)90610-o. [DOI] [PubMed] [Google Scholar]
- Meehan R. R., Lewis J. D., Bird A. P. Characterization of MeCP2, a vertebrate DNA binding protein with affinity for methylated DNA. Nucleic Acids Res. 1992 Oct 11;20(19):5085–5092. doi: 10.1093/nar/20.19.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan R. R., Lewis J. D., McKay S., Kleiner E. L., Bird A. P. Identification of a mammalian protein that binds specifically to DNA containing methylated CpGs. Cell. 1989 Aug 11;58(3):499–507. doi: 10.1016/0092-8674(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. 1984 Jun 28-Jul 4Nature. 309(5971):810–812. doi: 10.1038/309810a0. [DOI] [PubMed] [Google Scholar]
- Nan X., Meehan R. R., Bird A. Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2. Nucleic Acids Res. 1993 Oct 25;21(21):4886–4892. doi: 10.1093/nar/21.21.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M., Strzelecka T., Dorner L. F., Schildkraut I., Aggarwal A. K. Structure of restriction endonuclease BamHI and its relationship to EcoRI. Nature. 1994 Apr 14;368(6472):660–664. doi: 10.1038/368660a0. [DOI] [PubMed] [Google Scholar]
- Noyer-Weidner M., Diaz R., Reiners L. Cytosine-specific DNA modification interferes with plasmid establishment in Escherichia coli K12: involvement of rglB. Mol Gen Genet. 1986 Dec;205(3):469–475. doi: 10.1007/BF00338084. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A. Organization and function of the mcrBC genes of Escherichia coli K-12. Mol Microbiol. 1992 May;6(9):1079–1086. doi: 10.1111/j.1365-2958.1992.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Raleigh E. A., Trimarchi R., Revel H. Genetic and physical mapping of the mcrA (rglA) and mcrB (rglB) loci of Escherichia coli K-12. Genetics. 1989 Jun;122(2):279–296. doi: 10.1093/genetics/122.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross T. K., Achberger E. C., Braymer H. D. Nucleotide sequence of the McrB region of Escherichia coli K-12 and evidence for two independent translational initiation sites at the mcrB locus. J Bacteriol. 1989 Apr;171(4):1974–1981. doi: 10.1128/jb.171.4.1974-1981.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D., McKenney K., Sobieski D. A., Rosenberg M. Translational coupling at an intercistronic boundary of the Escherichia coli galactose operon. Cell. 1982 Oct;30(3):865–871. doi: 10.1016/0092-8674(82)90291-4. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Bandyopadhyay P. K. Model for how type I restriction enzymes select cleavage sites in DNA. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4677–4681. doi: 10.1073/pnas.85.13.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supakar P. C., Weist D., Zhang D. L., Inamdar N., Zhang X. Y., Khan R., Ehrlich K. C., Ehrlich M. Methylated DNA-binding protein is present in various mammalian cell types. Nucleic Acids Res. 1988 Aug 25;16(16):8029–8044. doi: 10.1093/nar/16.16.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland E., Coe L., Raleigh E. A. McrBC: a multisubunit GTP-dependent restriction endonuclease. J Mol Biol. 1992 May 20;225(2):327–348. doi: 10.1016/0022-2836(92)90925-a. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yen R. W., Vertino P. M., Nelkin B. D., Yu J. J., el-Deiry W., Cumaraswamy A., Lennon G. G., Trask B. J., Celano P., Baylin S. B. Isolation and characterization of the cDNA encoding human DNA methyltransferase. Nucleic Acids Res. 1992 May 11;20(9):2287–2291. doi: 10.1093/nar/20.9.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. L., Ehrlich K. C., Supakar P. C., Ehrlich M. A plant DNA-binding protein that recognizes 5-methylcytosine residues. Mol Cell Biol. 1989 Mar;9(3):1351–1356. doi: 10.1128/mcb.9.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]








