In 2013, a conditional marketing authorization valid throughout the European Union was issued for bosutinib for the treatment of adult patients with chronic-phase, accelerated-phase, and blast-phase Philadelphia chromosome positive chronic myelogenous leukemia previously treated with one tyrosine kinase inhibitor or more and for whom imatinib, nilotinib, and dasatinib are not considered appropriate treatment options. This publication is a summary of information available on the European Medicines Agency website.
Keywords: Bosutinib, Bosulif, Chronic myelogenous leukemia, EMA, European Medicines Agency
Abstract
On March 27, 2013, a conditional marketing authorization valid throughout the European Union was issued for bosutinib (Bosulif) for the treatment of adult patients with chronic-phase, accelerated-phase, and blast-phase Philadelphia chromosome positive (Ph+) chronic myelogenous leukemia (CML) previously treated with one tyrosine kinase inhibitor or more and for whom imatinib, nilotinib, and dasatinib are not considered appropriate treatment options. Bosutinib is a kinase inhibitor that targets the BCR-ABL kinase. The recommended dose is 500 mg of bosutinib once daily. The main evidence of efficacy for bosutinib was based on a CML subgroup analysis of study 3160A4-200, a phase I/II study of bosutinib in Ph+ leukemia in imatinib-resistant or intolerant CML. The subgroup was defined based on the presence of a BCR-ABL kinase domain mutation that would be expected to confer resistance to dasatinib (F317, E255) or nilotinib (E255, Y253, F359) and expected to have sensitivity to bosutinib or based on the presence of medical conditions or prior toxicities that may predispose the patient to unacceptable risk in the setting of nilotinib or dasatinib therapy. A conditional marketing authorization was granted because of the limited evidence of efficacy and safety currently supporting this last-line indication.
Implications for Practice:
Bosutinib has been approved in the European Union to treat adults with chronic myeloid leukemia (CML). The approval is based on efficacy data from 52 patients from a larger study. Patients in this subgroup were identified as having an unmet medical need, because other tyrosine-kinase inhibitors were not considered appropriate treatment options for them due to disease resistance or the risk of severe side effects. In this subgroup, 18 out of 36 patients with chronic-phase CML had a “major cytogenetic response” to bosutinib, while 7 out of the 16 patients with advanced (accelerated- or blast-phase) CML also had a sufficient response based on other measurements.
Introduction
Chronic myelogenous leukemia (CML) is a hematopoietic stem cell disease characterized by a proliferation of granulocytes and their immature myeloid precursors including blast cells. The disease is caused by a translocation that results in the expression of the breakpoint cluster region-Abelson kinase (BCR-ABL) oncoprotein, which exhibits tyrosine kinase activity that results in the dysregulation of intracellular signal transduction pathways that promote proliferation and genetic instability, suppress apoptosis, and interfere with cellular adhesion.
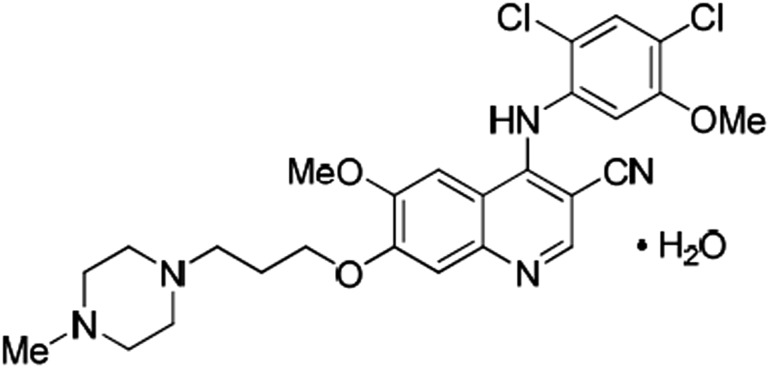
Pfizer Ltd., the applicant company, submitted a new drug application for bosutinib (Bosulif; oral immediate release film-coated tablets) for the treatment of CML to the European Medicines Agency (EMA). Bosutinib (SKI-606; WAY-173606 monohydrate) is a substituted 4-anilinoquinoline-3-carbonitrile tyrosine kinase inhibitor (TKI; Fig. 1) [1].
Figure 1.
Structural formula of bosutinib monohydrate. Chemical name: 3-Quinolinecarbonitrile, 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl) propoxy]-, hydrate (1:1). Molecular formula: C26H29Cl2N5O3⋅H2O.
Three TKIs were available in the European Union for the treatment of CML (imatinib, dasatinib, and nilotinib) at the time of submission of the new drug application for bosutinib. This paper summarizes the scientific review of the application leading to approval of bosutinib in the European Union. The detailed scientific assessment report and product information are available on the EMA website (http://www.ema.europa.eu).
Mechanism of Action and Toxicology
Bosutinib is a member of the class of BCR-ABL kinase inhibitors [2, 3]. Bosutinib binds the kinase domain of BCR-ABL in an active or intermediate conformation [3]. Bosutinib, similar to dasatinib, is also an inhibitor of Src family kinases, which have been associated with disease progression and BCR-ABL-independent forms of imatinib resistance (imatinib and nilotinib do not have broad Src family kinase inhibitory activity) [4, 5].
Bosutinib has shown activity against several mutants from a panel of mutated forms of BCR-ABL associated with imatinib resistance in CML and Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia patients (except for the L248R, G250E, E255K/V, T315I/V, and L248+F3591 mutations) [6].
In in vitro studies, bosutinib inhibited proliferation and survival of established CML cell lines, Ph+ acute lymphoblastic leukemia cell lines, and patient-derived primary primitive CML cells [2, 3]. Bosutinib treatment reduced the size of CML tumors growing in nude mice and inhibited growth of murine myeloid tumors expressing imatinib-resistant forms of Bcr-Abl [2].
In in vitro studies, bosutinib inhibited proliferation and survival of established CML cell lines, Ph+ acute lymphoblastic leukemia cell lines, and patient-derived primary primitive CML cells. Bosutinib treatment reduced the size of CML tumors growing in nude mice and inhibited growth of murine myeloid tumors expressing imatinib-resistant forms of Bcr-Abl.
Repeated-dose toxicity studies of up to 6 months in rats and up to 9 months in dogs revealed the gastrointestinal system to be the primary target organ of toxicity of bosutinib.
Clinical Pharmacology
After administration of bosutinib tablets with a single dose of 500 mg of bosutinib with food in healthy subjects, bosutinib absorption was relatively slow, with a median time-to-peak concentration of 6 hours. Bosutinib exhibited dose-proportional increases in area under the plasma curve and maximum concentration over the dose range of 200–600 mg. Food increased bosutinib maximum concentration 1.8-fold and area under the plasma curve 1.7-fold compared with the fasting state. In general, the volume of distribution was large, suggesting extensive tissue distribution and/or low bioavailability.
Elimination occurred via feces (>91%) with an elimination half-life of 19 hours in cancer patients (34 hours in healthy subjects) after extensive metabolism by liver or intestinal CYP3A4 and occurred only ∼3% via urine. Non-CML patients with hepatic impairment showed about twofold increases of bosutinib exposure and elimination half-life. This was expected because CYP3A4 concentrations are decreased in subjects with severe chronic liver disease (without chronic cholestasis). Bosutinib is contraindicated in patients with hepatic impairment.
Clinical Efficacy
The rationale for the 500-mg dose selection for the efficacy studies was primarily based on one phase I dose escalation study in patients with advanced malignant solid tumors and one phase I/II study of bosutinib in Ph+ leukemia [7, 8]. These studies explored the activity of bosutinib in second- and third-line chronic-phase (CP) CML patients and at more advanced CML stages.
Initially, the applicant company submitted results from the pivotal phase III study 3000-WW to support the authorization of bosutinib in patients with newly diagnosed Ph+ CML in CP. Study 3000-WW was a multinational, multicenter, randomized, open-label, parallel-arm phase III study to compare the efficacy and safety of bosutinib alone to that of imatinib alone in subjects with newly diagnosed chronic-phase CML [9]. The study failed to achieve its primary objective, with complete cytogenetic response (CCyR) at 12 months being 70.0% (95% confidence interval [CI]: 64.3%–75.7%) in the bosutinib group versus 67.9% (95% CI: 62.1%–73.6%), in the imatinib group (p = .601). In an updated analysis, the CCyR rate at 24 months was 57.6% (95% CI: 51.5%–63.7%) in the bosutinib group and 65.1% (95% CI: 59.2%–71.0%) in the imatinib group. Following assessment, bosutinib could not be considered approvable for a first-line line indication in CML, and this indication was withdrawn.
Subsequently, the applicant company restricted the claim to CML subpopulations with unmet medical need, comprising CP, accelerated-phase (AP), and blast-phase (BP) Ph+ CML patients following imatinib and with either nilotinib or dasatinib failure and for whom treatment with the remaining TKI was considered unsuitable. Unsuitability of available treatments was based on the presence of comorbidities such as pleural effusion, medical conditions associated with cardiovascular disease or diabetes, prior history of drug intolerance for similar reasons, or resistant BCR-ABL mutations. This claim was supported by the results of study 3160A4-200, part 2.
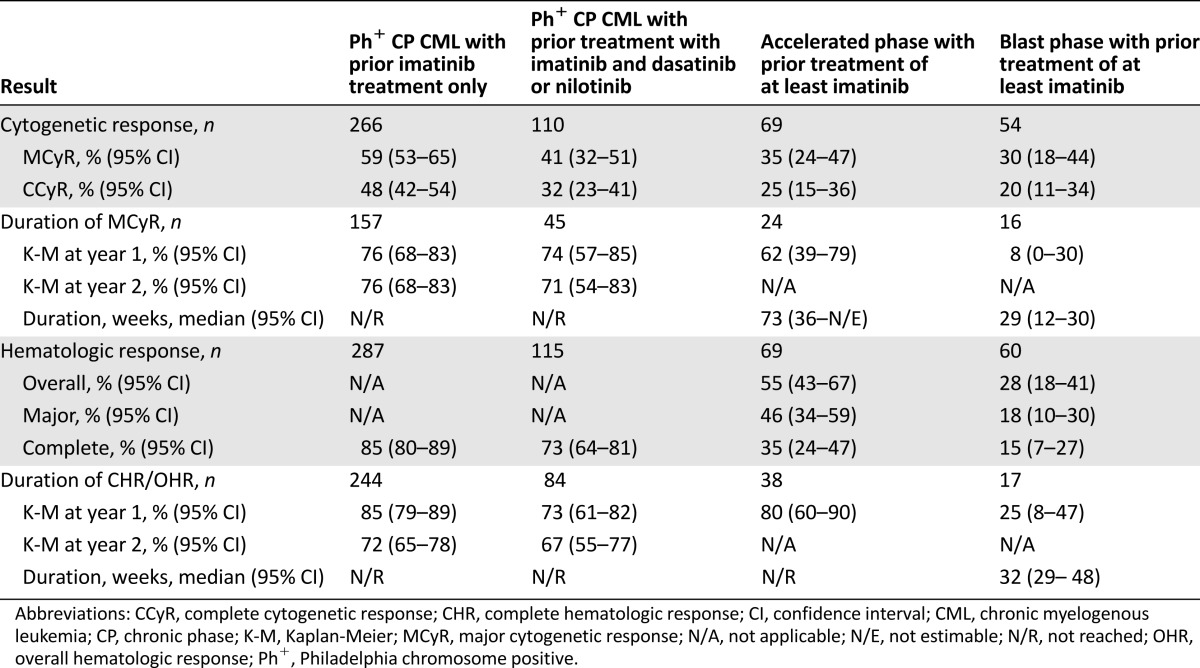
In this study, efficacy was seen in the subpopulation of CP, AP, and BP CML patients who, based on clearly defined criteria, may not be candidates for treatment with at least one of the currently approved TKIs because of intolerance, mutations, or comorbidities (Table 1). In this subgroup (n = 52), major cytogenetic response (MCyR) was observed in 9 of 21 patients with CP CML (43%) following failure of imatinib and one additional TKI (the duration of MCyR ranged from 8 weeks to ≥204 weeks, and treatment duration ranged from 35 weeks to ≥215 weeks as of the March 28, 2011, database snapshot), 9 of 15 with CP CML following failure of imatinib alone (60%; the duration of MCyR ranged from 12 weeks to 155 weeks, and treatment duration ranged from 24 weeks to ≥197 weeks), and response was observed in 4 of 5 patients with AP CML and 3 of 11 patients with BP CML. The efficacy was further supported by the results seen in the larger reference population within study 3160A4-200 (Table 2). There was one patient, with intolerance to all approved TKIs (imatinib, nilotinib, and dasatinib), who achieved CCyR and major molecular response by week 24 during bosutinib therapy and maintained these responses as of the data snapshot at least 1 year after the first dose. Cytogenetic responses were noted in patients who had mutations that would be expected to impart clinical resistance to dasatinib or nilotinib (with the exception of T315I).
Table 1.
Demographic and baseline characteristics of the “unmet medical need” subpopulation in study 3160A4-200
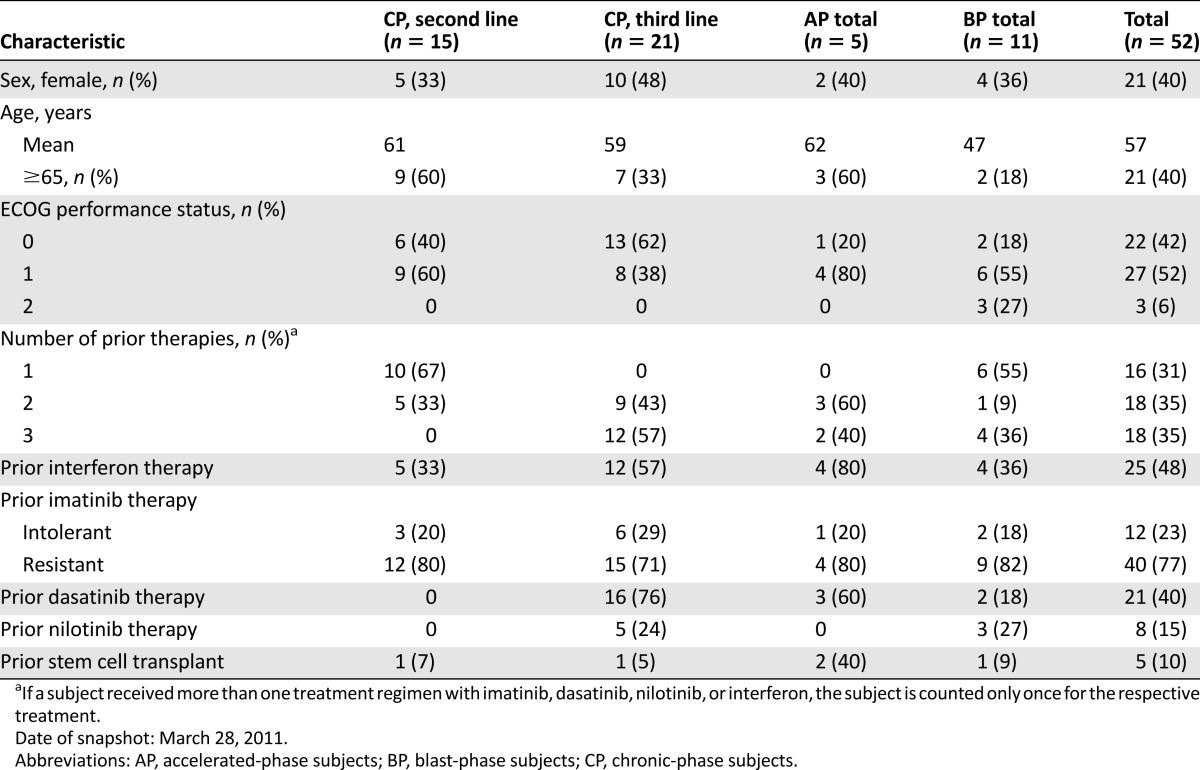
Table 2.
Efficacy results in previously treated patients with chronic and advanced phase CML (Study 3160A4-200)
Clinical Safety
In accordance with signals derived from nonclinical studies, hepatotoxicity, gastrointestinal effects, and cardiac arrhythmias were the most important toxicities associated with bosutinib in the safety database (Table 3).
Table 3.
Summary of most frequent adverse reactions associated with bosutinib (n = 870; 10% cut-off)
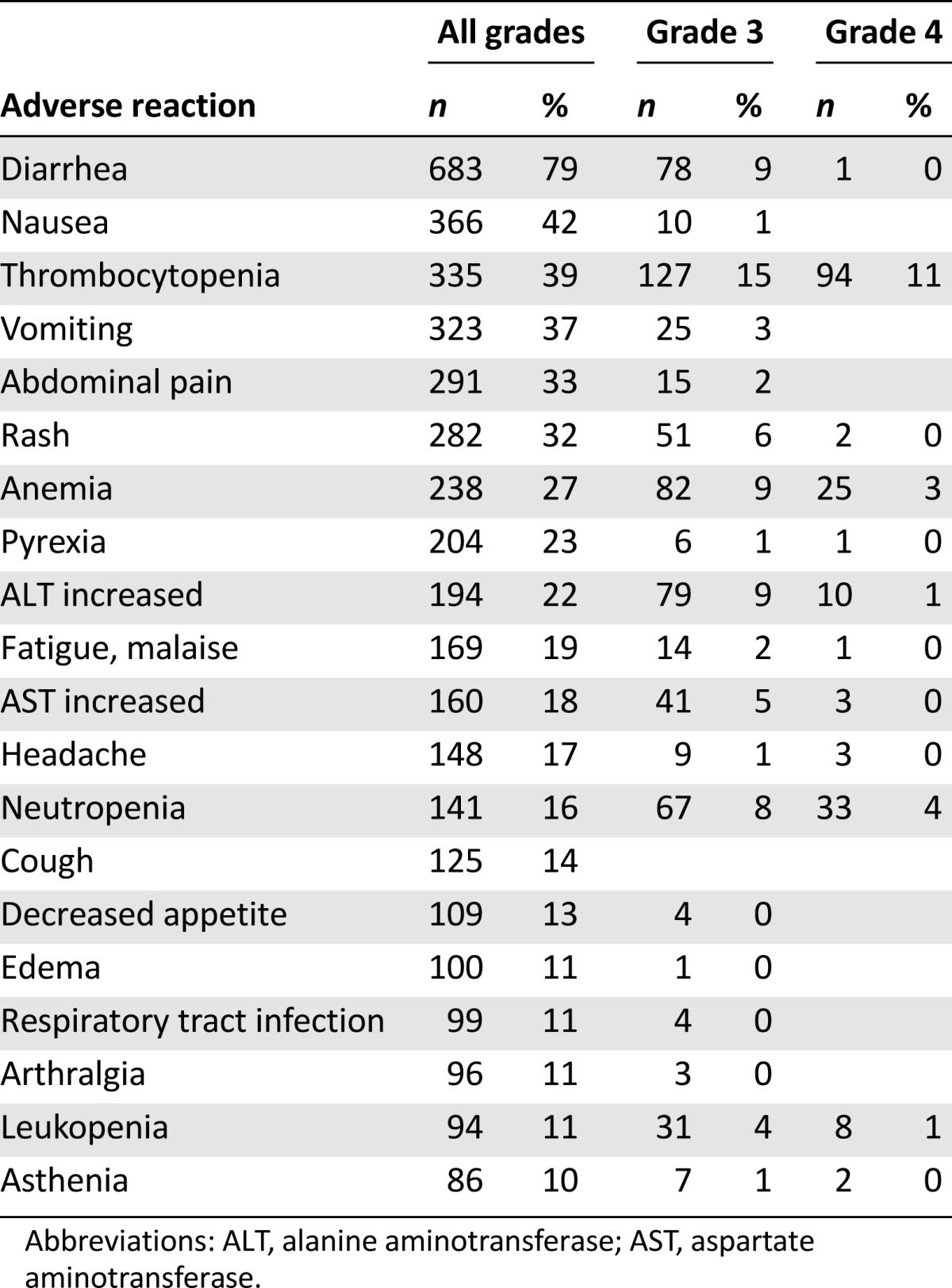
Diarrhea was identified consistently as dose dependent and dose limiting for bosutinib. In study 3000-WW, drug-related diarrhea affected 65.7% versus 17.9% of patients for bosutinib versus imatinib, respectively. In study 3000-WW, drug-related vomiting was observed in 31.5% versus 13.5% of patients for bosutinib versus imatinib, respectively. Cardiac treatment-emergent adverse events were experienced by 21 subjects (8.5%) in the bosutinib arm and 17 subjects (6.8%) in the imatinib arm. Prolonged QT interval was the most frequently occurring cardiac treatment-emergent adverse event and was experienced by six subjects (2.4%) in the bosutinib arm compared with eight subjects (3.2%) in the imatinib arm.
Other important identified risks were hypersensitivity reactions (including anaphylaxis), fluid retention, myelosuppression, respiratory tract infections, bleeding, rash, and pancreatitis or increased lipase.
Benefit-Risk Assessment
For the first-line indication, a positive benefit-risk balance could not be established for bosutinib in view of the pronounced toxicity (particularly hepatotoxicity and gastrointestinal toxicity) compared with imatinib and failure to show superior efficacy.
For the first-line indication, a positive benefit-risk balance could not be established for bosutinib in view of the pronounced toxicity (particularly hepatotoxicity and gastrointestinal toxicity) compared with imatinib and failure to show superior efficacy.
Based on the subgroup analysis presented from study 3160A4-200, bosutinib was associated with clinical benefits in patients who had exhausted all available TKI therapies or for whom treatment with other available TKIs was deemed unsuitable by their physicians. For these patients, there are currently limited or no treatment options, and bosutinib may offer a valuable alternative. Although bosutinib was associated with pronounced toxicity, the safety profile was considered acceptable. Results from a pediatric development in an agreed pediatric investigational plan are expected by 2016.
At the time of assessment, the number of patients treated with bosutinib was small, and the observation time was short, both for the evaluation of efficacy and the evaluation of safety. Consequently, a conditional approval valid throughout the European Union was granted for bosutinib with the restricted indication of adult patients with CP, AP, or BP Ph+ CML previously treated with one TKI or more and for whom imatinib, nilotinib, and dasatinib are not considered appropriate treatment options. A conditional approval obliges the applicant company to submit additional data, with a view to confirming that the benefit-risk balance is positive. The EMA will review new information about bosutinib on an annual basis until all the conditions are fulfilled.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
The scientific assessment as summarized in this report is based on the marketing authorization application submitted by Pfizer Ltd. and on important contributions from, among others, the rapporteur and corapporteur assessment teams, members of the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use, and additional experts. This publication is a summary of the European Public Assessment Report, the summary of product characteristics, and other product information available on the EMA website. Christian Gisselbrecht and Edward Laane are members of the EMA Scientific Advisory Group on Oncology and did not participate in the EMA review of bosutinib. Health care professionals and interested readers are referred to the EMA website for up-to-date information on this marketing authorization (http://www.ema.europa.eu). The authors remain solely responsible for the opinions expressed in this publication.
Footnotes
Editor’s Note: Also in this issue are the results of two trials studying the effectiveness of bosutinib in combination with different aromatase inhibitors in locally advanced or metastatic HR-positive/HER2-negative breast cancer. See Beverly Moy et al., “Bosutinib in Combination With the Aromatase Inhibitor Exemestane” on pages 346–347 and “Bosutinib in Combination With the Aromatase Inhibitor Letrozole” on pages 348–349.
For Further Reading: Firoozeh Alvandi, Virginia E. Kwitkowski, Chia-Wen Ko et al. U.S. Food and Drug Administration Approval Summary: Omacetaxine Mepesuccinate as Treatment for Chronic Myeloid Leukemia. The Oncologist 2014;19:94–99.
Implications for Practice: We report relevant clinical information regarding the approval of a new drug, omacetaxine mepesuccinate (Synribo), for the treatment of adult patients with chronic or accelerated phase chronic myeloid leukemia with resistance and/or intolerance to two or more tyrosine kinase inhibitors. We discuss the disease, available treatment options, proposed mechanisms of action of omacetaxine mepesuccinate, clinical trial data supporting the accelerated approval of omacetaxine mepesuccinate, the approved indication as derived from trials conducted, drug storage and handling limitations, recommended dosing, method of administration, and expected toxicities.
Author Contributions
Data analysis and interpretation: Christoph Unkrig, Harald Enzmann, Jorge Camarero, Arantxa Sancho-Lopez
Manuscript writing: Zahra Hanaizi, Francesco Pignatti
Final approval of manuscript: Francesco Pignatti, Christoph Unkrig, Harald Enzmann, Jorge Camarero, Arantxa Sancho-Lopez, Tomas Salmonson, Christian Gisselbrecht, Edward Laane
Disclosures
The authors indicated no financial relationships.
References
- 1.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Golas JM, Arndt K, Etienne C, et al. SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res. 2003;63:375–381. [PubMed] [Google Scholar]
- 3.Puttini M, Coluccia AM, Boschelli F, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66:11314–11322. doi: 10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]
- 4.Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk Lymphoma. 2008;49:615–619. doi: 10.1080/10428190801896103. [DOI] [PubMed] [Google Scholar]
- 5.Remsing Rix LL, Rix U, Colinge J, et al. Global target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cells. Leukemia. 2009;23:477–485. doi: 10.1038/leu.2008.334. [DOI] [PubMed] [Google Scholar]
- 6.Redaelli S, Mologni L, Rostagno R, et al. Three novel patient-derived BCR/ABL mutants show different sensitivity to second and third generation tyrosine kinase inhibitors. Am J Hematol. 2012;87:E125–E128. doi: 10.1002/ajh.23338. [DOI] [PubMed] [Google Scholar]
- 7.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567–4576. doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury HJ, Cortes JE, Kantarjian HM, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119:3403–3412. doi: 10.1182/blood-2011-11-390120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: Results from the BELA trial. J Clin Oncol. 2012;30:3486–3492. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]