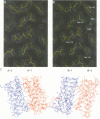
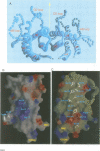
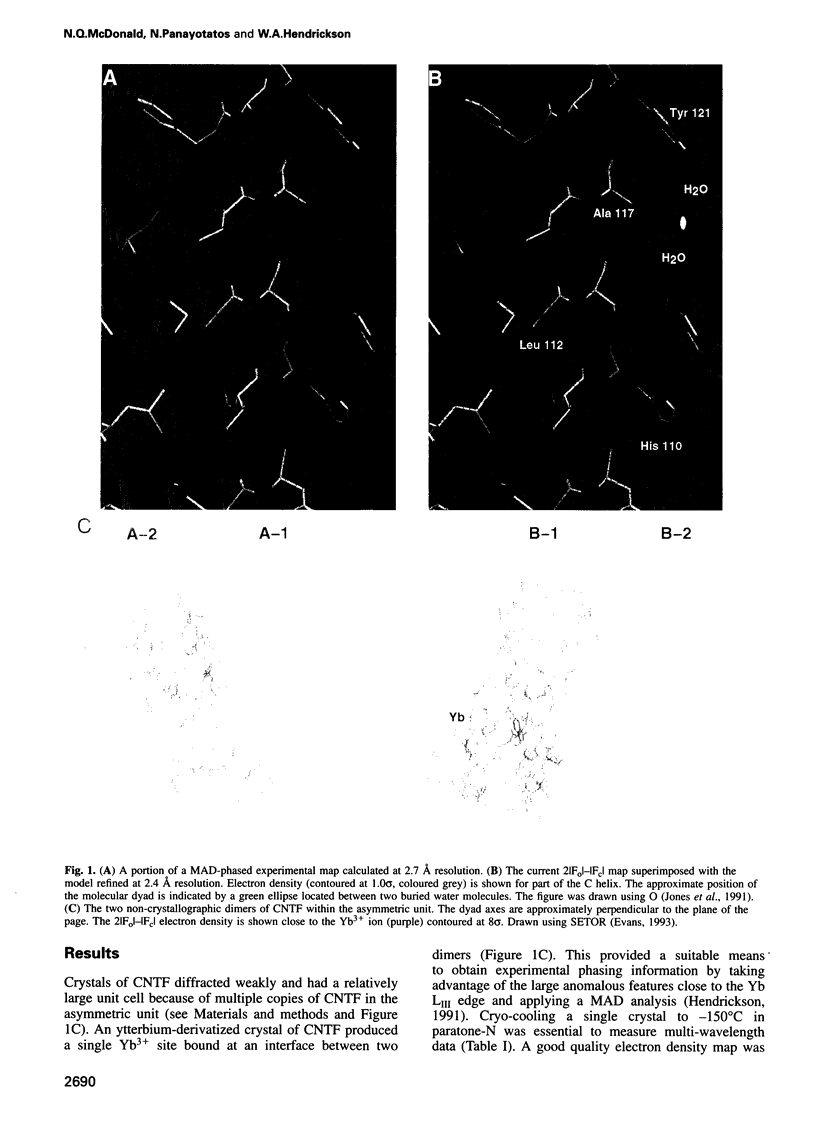
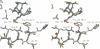Abstract
Ciliary neurotrophic factor (CNTF) promotes the survival and differentiation of developing motor neurons and is a potential therapeutic for treating neurodegeneration and nerve injury. The crystal structure of human CNTF has been determined at 2.4 A resolution using multi-wavelength anomalous diffraction (MAD) phasing from a single Yb3+ ions. The structure reveals that CNTF is dimeric, with a novel anti-parallel arrangement of the subunits, not previously observed for other cytokines. Each subunit adopts a double crossover four-helix bundle fold, in which two helices contribute to the dimer interface, whilst two different helices show pronounced kinks. Analysis of the electrostatic surface of CNTF identified residues within these kinked helices that may contact the CNTF receptor-alpha. Solution experiments show that CNTF dimerizes at concentrations > 40 microM. Such dimers are likely to be relevant to the storage of CNTF in the peripheral nerve given the high concentrations present in this tissue. However, it is unlikely that they play a role in engaging the three distinct receptor subunits that comprise the CNTF receptor, given the low concentration of extracellular CNTF and its high potency.
Full text
PDF
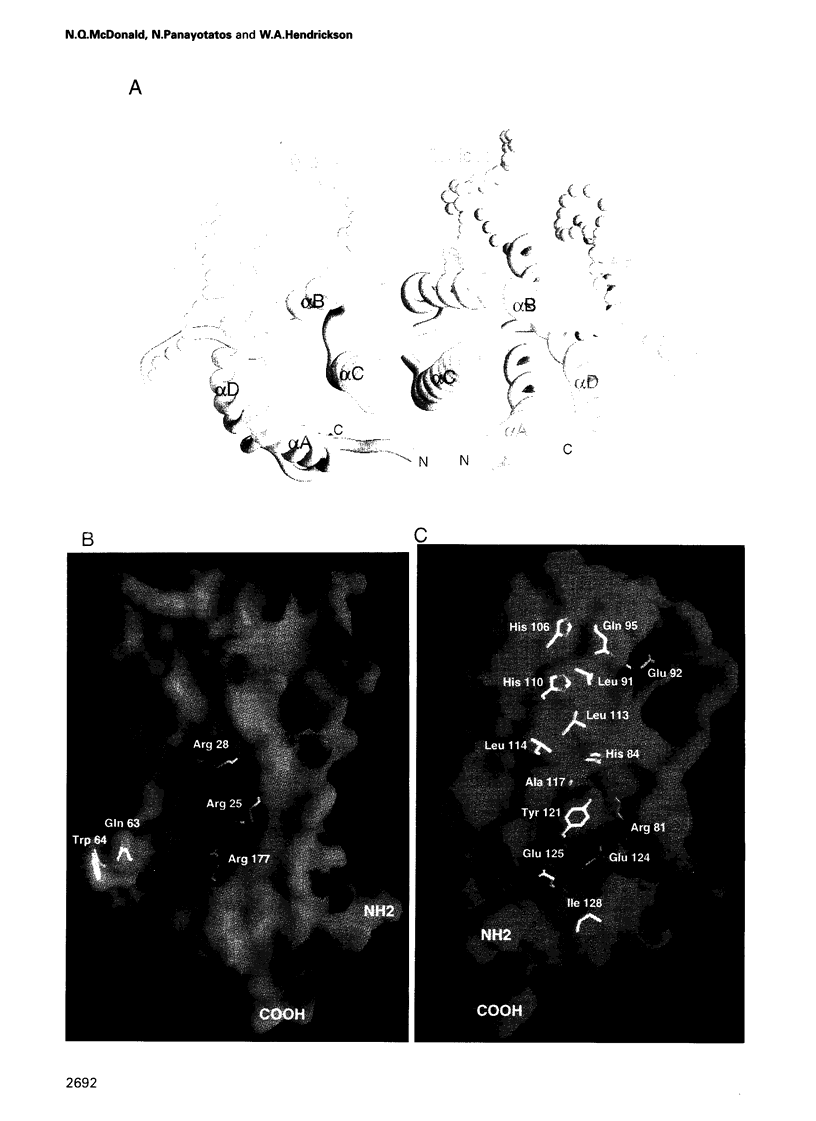
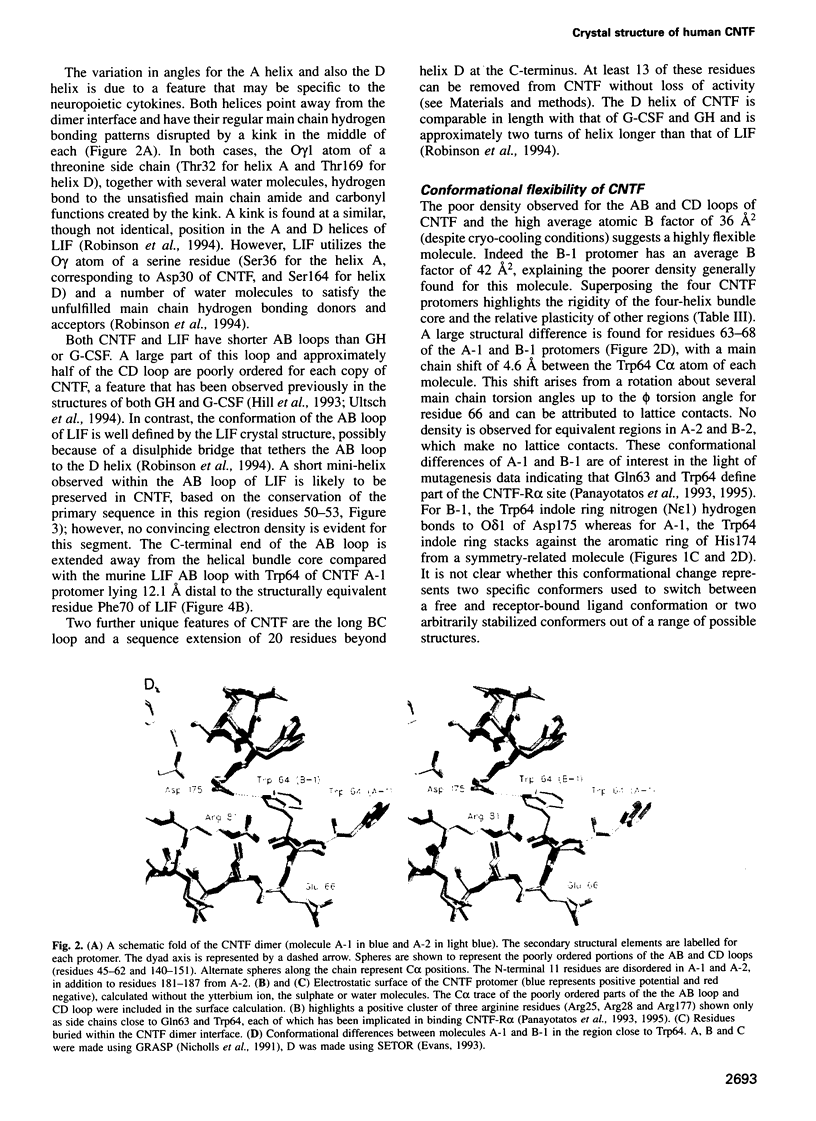
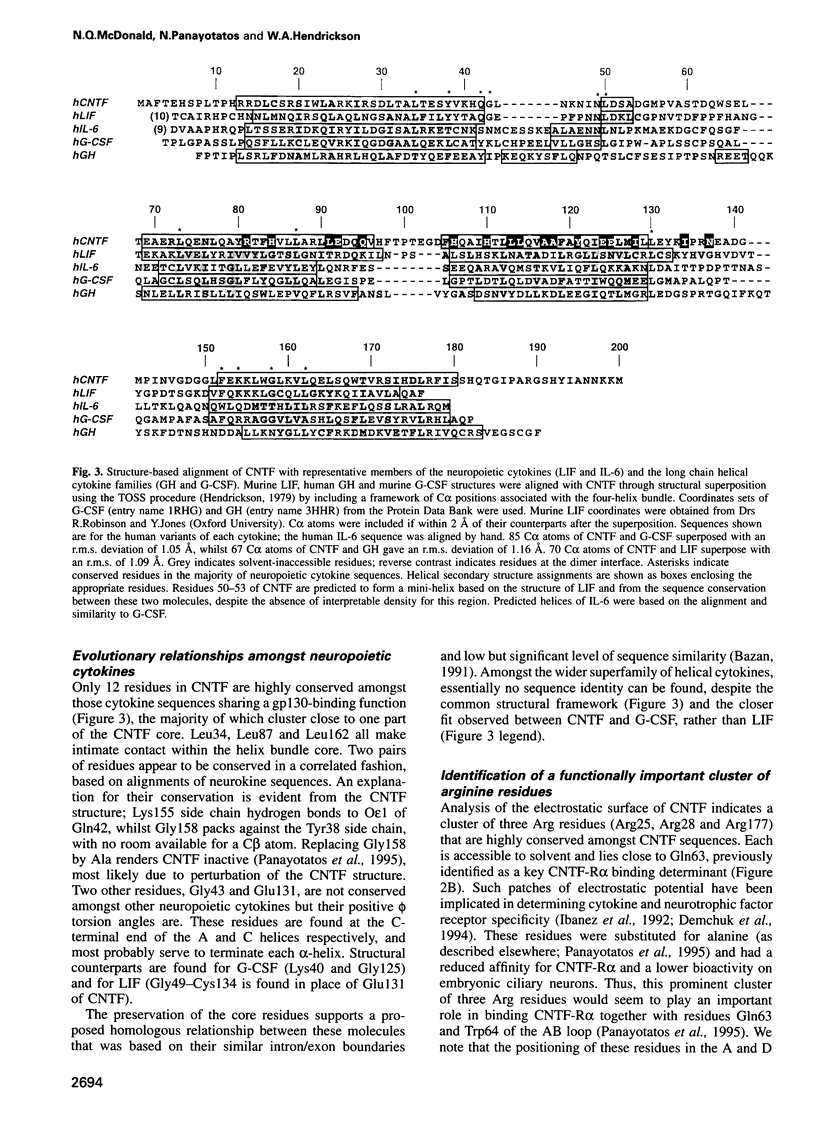


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R. Ciliary neurotrophic factor as an injury factor. Curr Opin Neurobiol. 1993 Oct;3(5):785–789. doi: 10.1016/0959-4388(93)90154-q. [DOI] [PubMed] [Google Scholar]
- Baldwin E. T., Weber I. T., St Charles R., Xuan J. C., Appella E., Yamada M., Matsushima K., Edwards B. F., Clore G. M., Gronenborn A. M. Crystal structure of interleukin 8: symbiosis of NMR and crystallography. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):502–506. doi: 10.1073/pnas.88.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Neuropoietic cytokines in the hematopoietic fold. Neuron. 1991 Aug;7(2):197–208. doi: 10.1016/0896-6273(91)90258-2. [DOI] [PubMed] [Google Scholar]
- Briscoe J., Guschin D., Müller M. Signal transduction. Just another signalling pathway. Curr Biol. 1994 Nov 1;4(11):1033–1035. doi: 10.1016/s0960-9822(00)00236-0. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Mulkerrin M. G., Wells J. A. Dimerization of human growth hormone by zinc. Science. 1991 Aug 2;253(5019):545–548. doi: 10.1126/science.1907025. [DOI] [PubMed] [Google Scholar]
- Davis S., Aldrich T. H., Ip N. Y., Stahl N., Scherer S., Farruggella T., DiStefano P. S., Curtis R., Panayotatos N., Gascan H. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science. 1993 Mar 19;259(5102):1736–1739. doi: 10.1126/science.7681218. [DOI] [PubMed] [Google Scholar]
- Davis S., Aldrich T. H., Stahl N., Pan L., Taga T., Kishimoto T., Ip N. Y., Yancopoulos G. D. LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993 Jun 18;260(5115):1805–1808. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- Davis S., Aldrich T. H., Valenzuela D. M., Wong V. V., Furth M. E., Squinto S. P., Yancopoulos G. D. The receptor for ciliary neurotrophic factor. Science. 1991 Jul 5;253(5015):59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- Demchuk E., Mueller T., Oschkinat H., Sebald W., Wade R. C. Receptor binding properties of four-helix-bundle growth factors deduced from electrostatic analysis. Protein Sci. 1994 Jun;3(6):920–935. doi: 10.1002/pro.5560030607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ealick S. E., Cook W. J., Vijay-Kumar S., Carson M., Nagabhushan T. L., Trotta P. P., Bugg C. E. Three-dimensional structure of recombinant human interferon-gamma. Science. 1991 May 3;252(5006):698–702. doi: 10.1126/science.1902591. [DOI] [PubMed] [Google Scholar]
- Ehlers M., Grötzinger J., deHon F. D., Müllberg J., Brakenhoff J. P., Liu J., Wollmer A., Rose-John S. Identification of two novel regions of human IL-6 responsible for receptor binding and signal transduction. J Immunol. 1994 Aug 15;153(4):1744–1753. [PubMed] [Google Scholar]
- Evans S. V. SETOR: hardware-lighted three-dimensional solid model representations of macromolecules. J Mol Graph. 1993 Jun;11(2):134-8, 127-8. doi: 10.1016/0263-7855(93)87009-t. [DOI] [PubMed] [Google Scholar]
- Fontaine V., Savino R., Arcone R., de Wit L., Brakenhoff J. P., Content J., Ciliberto G. Involvement of the Arg179 in the active site of human IL-6. Eur J Biochem. 1993 Feb 1;211(3):749–755. doi: 10.1111/j.1432-1033.1993.tb17605.x. [DOI] [PubMed] [Google Scholar]
- Friedman B., Scherer S. S., Rudge J. S., Helgren M., Morrisey D., McClain J., Wang D. Y., Wiegand S. J., Furth M. E., Lindsay R. M. Regulation of ciliary neurotrophic factor expression in myelin-related Schwann cells in vivo. Neuron. 1992 Aug;9(2):295–305. doi: 10.1016/0896-6273(92)90168-d. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A. Determination of macromolecular structures from anomalous diffraction of synchrotron radiation. Science. 1991 Oct 4;254(5028):51–58. doi: 10.1126/science.1925561. [DOI] [PubMed] [Google Scholar]
- Hill C. P., Osslund T. D., Eisenberg D. The structure of granulocyte-colony-stimulating factor and its relationship to other growth factors. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5167–5171. doi: 10.1073/pnas.90.11.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáez C. F., Ebendal T., Barbany G., Murray-Rust J., Blundell T. L., Persson H. Disruption of the low affinity receptor-binding site in NGF allows neuronal survival and differentiation by binding to the trk gene product. Cell. 1992 Apr 17;69(2):329–341. doi: 10.1016/0092-8674(92)90413-7. [DOI] [PubMed] [Google Scholar]
- Janin J., Miller S., Chothia C. Surface, subunit interfaces and interior of oligomeric proteins. J Mol Biol. 1988 Nov 5;204(1):155–164. doi: 10.1016/0022-2836(88)90606-7. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kossiakoff A. A., Somers W., Ultsch M., Andow K., Muller Y. A., De Vos A. M. Comparison of the intermediate complexes of human growth hormone bound to the human growth hormone and prolactin receptors. Protein Sci. 1994 Oct;3(10):1697–1705. doi: 10.1002/pro.5560031008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leebeek F. W., Kariya K., Schwabe M., Fowlkes D. M. Identification of a receptor binding site in the carboxyl terminus of human interleukin-6. J Biol Chem. 1992 Jul 25;267(21):14832–14838. [PubMed] [Google Scholar]
- Lindsay R. M., Wiegand S. J., Altar C. A., DiStefano P. S. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994 May;17(5):182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Liu H. X., Radziejewski C., Lottspeich F., Oberthuer W., Wong V., Lindsay R. M., Furth M. E., Panayotatos N. Recombinant human and rat ciliary neurotrophic factors. J Neurochem. 1991 Sep;57(3):1003–1012. doi: 10.1111/j.1471-4159.1991.tb08250.x. [DOI] [PubMed] [Google Scholar]
- May L. T., Santhanam U., Sehgal P. B. On the multimeric nature of natural human interleukin-6. J Biol Chem. 1991 May 25;266(15):9950–9955. [PubMed] [Google Scholar]
- Milburn M. V., Hassell A. M., Lambert M. H., Jordan S. R., Proudfoot A. E., Graber P., Wells T. N. A novel dimer configuration revealed by the crystal structure at 2.4 A resolution of human interleukin-5. Nature. 1993 May 13;363(6425):172–176. doi: 10.1038/363172a0. [DOI] [PubMed] [Google Scholar]
- Mott H. R., Campbell I. D. Four-helix bundle growth factors and their receptors: protein-protein interactions. Curr Opin Struct Biol. 1995 Feb;5(1):114–121. doi: 10.1016/0959-440x(95)80016-t. [DOI] [PubMed] [Google Scholar]
- Nicholls A., Sharp K. A., Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11(4):281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- Nishi R. Neurotrophic factors: two are better than one. Science. 1994 Aug 19;265(5175):1052–1053. doi: 10.1126/science.8066443. [DOI] [PubMed] [Google Scholar]
- Owczarek C. M., Layton M. J., Metcalf D., Lock P., Willson T. A., Gough N. M., Nicola N. A. Inter-species chimeras of leukaemia inhibitory factor define a major human receptor-binding determinant. EMBO J. 1993 Sep;12(9):3487–3495. doi: 10.1002/j.1460-2075.1993.tb06023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotatos N., Everdeen D., Liten A., Somogyi R., Acheson A. Recombinant human CNTF receptor alpha: production, binding stoichiometry, and characterization of its activity as a diffusible factor. Biochemistry. 1994 May 17;33(19):5813–5818. doi: 10.1021/bi00185a020. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Radziejewska E., Acheson A., Pearsall D., Thadani A., Wong V. Exchange of a single amino acid interconverts the specific activity and gel mobility of human and rat ciliary neurotrophic factors. J Biol Chem. 1993 Sep 5;268(25):19000–19003. [PubMed] [Google Scholar]
- Pandit J., Bohm A., Jancarik J., Halenbeck R., Koths K., Kim S. H. Three-dimensional structure of dimeric human recombinant macrophage colony-stimulating factor. Science. 1992 Nov 20;258(5086):1358–1362. doi: 10.1126/science.1455231. [DOI] [PubMed] [Google Scholar]
- Rajarathnam K., Sykes B. D., Kay C. M., Dewald B., Geiser T., Baggiolini M., Clark-Lewis I. Neutrophil activation by monomeric interleukin-8. Science. 1994 Apr 1;264(5155):90–92. doi: 10.1126/science.8140420. [DOI] [PubMed] [Google Scholar]
- Robinson R. C., Grey L. M., Staunton D., Vankelecom H., Vernallis A. B., Moreau J. F., Stuart D. I., Heath J. K., Jones E. Y. The crystal structure and biological function of leukemia inhibitory factor: implications for receptor binding. Cell. 1994 Jul 1;77(7):1101–1116. doi: 10.1016/0092-8674(94)90449-9. [DOI] [PubMed] [Google Scholar]
- Rozwarski D. A., Gronenborn A. M., Clore G. M., Bazan J. F., Bohm A., Wlodawer A., Hatada M., Karplus P. A. Structural comparisons among the short-chain helical cytokines. Structure. 1994 Mar 15;2(3):159–173. doi: 10.1016/s0969-2126(00)00018-6. [DOI] [PubMed] [Google Scholar]
- Savino R., Lahm A., Giorgio M., Cabibbo A., Tramontano A., Ciliberto G. Saturation mutagenesis of the human interleukin 6 receptor-binding site: implications for its three-dimensional structure. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4067–4071. doi: 10.1073/pnas.90.9.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino R., Lahm A., Salvati A. L., Ciapponi L., Sporeno E., Altamura S., Paonessa G., Toniatti C., Ciliberto G. Generation of interleukin-6 receptor antagonists by molecular-modeling guided mutagenesis of residues important for gp130 activation. EMBO J. 1994 Mar 15;13(6):1357–1367. doi: 10.1002/j.1460-2075.1994.tb06389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendtner M., Carroll P., Holtmann B., Hughes R. A., Thoenen H. Ciliary neurotrophic factor. J Neurobiol. 1994 Nov;25(11):1436–1453. doi: 10.1002/neu.480251110. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Kreutzberg G. W., Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990 May 31;345(6274):440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Stöckli K. A., Thoenen H. Synthesis and localization of ciliary neurotrophic factor in the sciatic nerve of the adult rat after lesion and during regeneration. J Cell Biol. 1992 Jul;118(1):139–148. doi: 10.1083/jcb.118.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L., Fannon A. M., Kwong P. D., Thompson A., Lehmann M. S., Grübel G., Legrand J. F., Als-Nielsen J., Colman D. R., Hendrickson W. A. Structural basis of cell-cell adhesion by cadherins. Nature. 1995 Mar 23;374(6520):327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Stahl N., Yancopoulos G. D. The alphas, betas, and kinases of cytokine receptor complexes. Cell. 1993 Aug 27;74(4):587–590. doi: 10.1016/0092-8674(93)90506-l. [DOI] [PubMed] [Google Scholar]
- Stöckli K. A., Lottspeich F., Sendtner M., Masiakowski P., Carroll P., Götz R., Lindholm D., Thoenen H. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989 Dec 21;342(6252):920–923. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- Ultsch M. H., Somers W., Kossiakoff A. A., de Vos A. M. The crystal structure of affinity-matured human growth hormone at 2 A resolution. J Mol Biol. 1994 Feb 11;236(1):286–299. doi: 10.1006/jmbi.1994.1135. [DOI] [PubMed] [Google Scholar]
- Ward L. D., Howlett G. J., Discolo G., Yasukawa K., Hammacher A., Moritz R. L., Simpson R. J. High affinity interleukin-6 receptor is a hexameric complex consisting of two molecules each of interleukin-6, interleukin-6 receptor, and gp-130. J Biol Chem. 1994 Sep 16;269(37):23286–23289. [PubMed] [Google Scholar]
- Weis W. I., Kahn R., Fourme R., Drickamer K., Hendrickson W. A. Structure of the calcium-dependent lectin domain from a rat mannose-binding protein determined by MAD phasing. Science. 1991 Dec 13;254(5038):1608–1615. doi: 10.1126/science.1721241. [DOI] [PubMed] [Google Scholar]
- Zurawski S. M., Vega F., Jr, Doyle E. L., Huyghe B., Flaherty K., McKay D. B., Zurawski G. Definition and spatial location of mouse interleukin-2 residues that interact with its heterotrimeric receptor. EMBO J. 1993 Dec 15;12(13):5113–5119. doi: 10.1002/j.1460-2075.1993.tb06206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos A. M., Ultsch M., Kossiakoff A. A. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992 Jan 17;255(5042):306–312. doi: 10.1126/science.1549776. [DOI] [PubMed] [Google Scholar]