Abstract
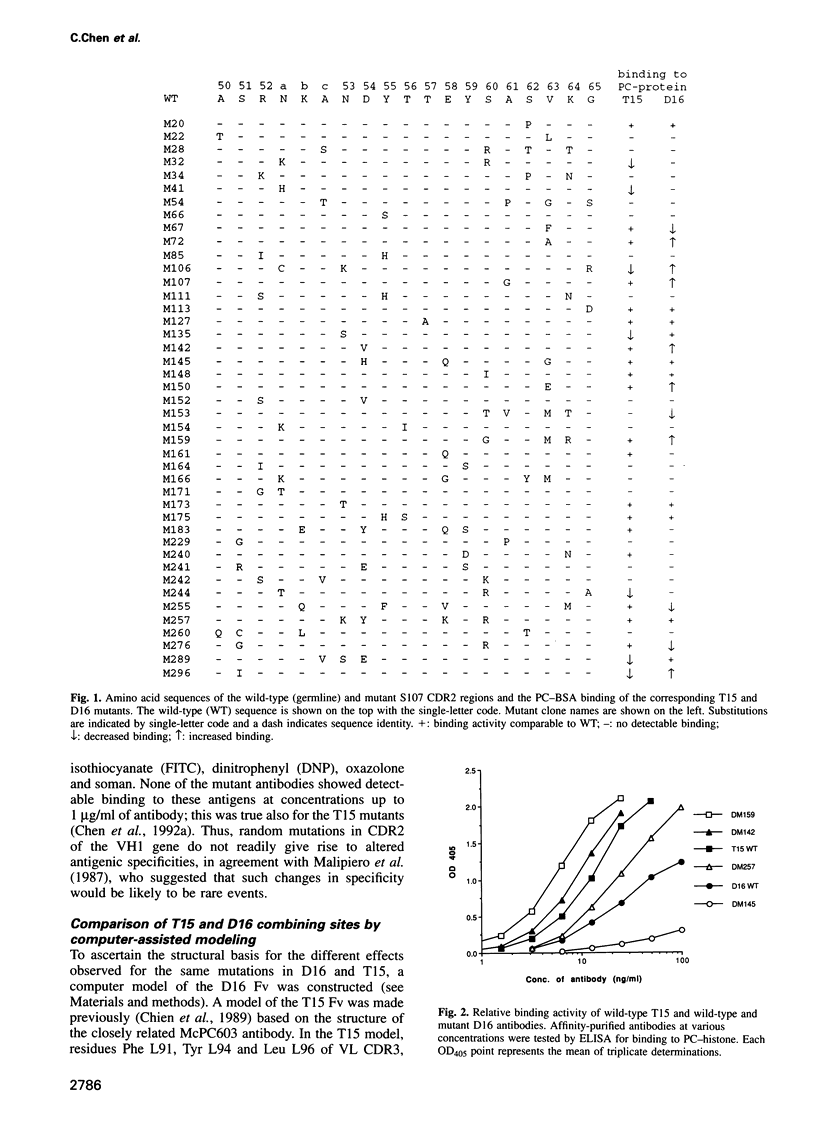
We examined the positive and negative effects of somatic mutation on antibody function using saturation mutagenesis in vitro to mimic the potential of the in vivo process to diversify antibodies. Identical mutations were introduced into the second complementarity determining region of two anti-phosphocholine antibodies, T15 and D16, which share the same germline VH gene sequence. T15 predominates in primary responses and does not undergo affinity maturation. D16 is representative of antibodies that co-dominate in memory responses and do undergo affinity maturation. We previously reported that > 50% of T15 mutants had decreased antigen binding capacity. To test if this high frequency of binding loss was unique to T15 or a consequence of random point mutations applicable to other combining sites, we analyzed the same mutations in D16. We show that D16 suffers a similar loss of function, indicating an equally high potential for B-cell wastage. However, only D16 displayed the capacity for somatic mutation to improve antigen binding, which should enhance its persistence in memory responses. Mutation of residues contacting the haptenic group, as determined by molecular modeling, did not improve binding. Instead, productive mutations occurred in residues that either contacted carrier protein or were distant from the antigen binding site, possibly increasing binding site flexibility through long-range effects. Targeting such residues for mutation should aid in the rational design of improved antibodies.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel M., Berek C. Somatic mutations in antibodies expressed by germinal centre B cells early after primary immunization. Int Immunol. 1990;2(9):813–819. doi: 10.1093/intimm/2.9.813. [DOI] [PubMed] [Google Scholar]
- Berek C., Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev. 1987 Apr;96:23–41. doi: 10.1111/j.1600-065x.1987.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Brown M., Stenzel-Poore M., Stevens S., Kondoleon S. K., Ng J., Bächinger H. P., Rittenberg M. B. Immunologic memory to phosphocholine keyhole limpet hemocyanin. Recurrent mutations in the lambda 1 light chain increase affinity for antigen. J Immunol. 1992 Jan 15;148(2):339–346. [PubMed] [Google Scholar]
- Bruderer U., Stenzel-Poore M. P., Bächinger H. P., Fellman J. H., Rittenberg M. B. Antibody combining site heterogeneity within the response to phosphocholine-keyhole limpet hemocyanin. Mol Immunol. 1989 Jan;26(1):63–71. doi: 10.1016/0161-5890(89)90021-7. [DOI] [PubMed] [Google Scholar]
- Chang S. P., Brown M., Rittenberg M. B. Immunologic memory to phosphorylcholine. II. PC-KLH induces two antibody populations that dominate different isotypes. J Immunol. 1982 Feb;128(2):702–706. [PubMed] [Google Scholar]
- Chen C., Bruderer U., Rittenberg M. B. The developmental patterns of B cell precursors distinguishing between environmental and nonenvironmental forms of phosphocholine. Cell Immunol. 1992 Sep;143(2):378–388. doi: 10.1016/0008-8749(92)90034-m. [DOI] [PubMed] [Google Scholar]
- Chen C., Martin T. M., Stevens S., Rittenberg M. B. Defective secretion of an immunoglobulin caused by mutations in the heavy chain complementarity determining region 2. J Exp Med. 1994 Aug 1;180(2):577–586. doi: 10.1084/jem.180.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Roberts V. A., Rittenberg M. B. Generation and analysis of random point mutations in an antibody CDR2 sequence: many mutated antibodies lose their ability to bind antigen. J Exp Med. 1992 Sep 1;176(3):855–866. doi: 10.1084/jem.176.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Stenzel-Poore M. P., Rittenberg M. B. Natural auto- and polyreactive antibodies differing from antigen-induced antibodies in the H chain CDR3. J Immunol. 1991 Oct 1;147(7):2359–2367. [PubMed] [Google Scholar]
- Chien N. C., Pollock R. R., Desaymard C., Scharff M. D. Point mutations cause the somatic diversification of IgM and IgG2a antiphosphorylcholine antibodies. J Exp Med. 1988 Mar 1;167(3):954–973. doi: 10.1084/jem.167.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien N. C., Roberts V. A., Giusti A. M., Scharff M. D., Getzoff E. D. Significant structural and functional change of an antigen-binding site by a distant amino acid substitution: proposal of a structural mechanism. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5532–5536. doi: 10.1073/pnas.86.14.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M. Canonical structures for the hypervariable regions of immunoglobulins. J Mol Biol. 1987 Aug 20;196(4):901–917. doi: 10.1016/0022-2836(87)90412-8. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Tramontano A., Levitt M., Smith-Gill S. J., Air G., Sheriff S., Padlan E. A., Davies D., Tulip W. R. Conformations of immunoglobulin hypervariable regions. Nature. 1989 Dec 21;342(6252):877–883. doi: 10.1038/342877a0. [DOI] [PubMed] [Google Scholar]
- Claflin J. L., Berry J. Genetics of the phosphocholine-specific antibody response to Streptococcus pneumoniae. Germ-line but not mutated T15 antibodies are dominantly selected. J Immunol. 1988 Dec 1;141(11):4012–4019. [PubMed] [Google Scholar]
- Claflin J. L., George J., Dell C., Berry J. Patterns of mutations and selection in antibodies to the phosphocholine-specific determinant in Proteus morganii. J Immunol. 1989 Nov 1;143(9):3054–3063. [PubMed] [Google Scholar]
- Claflin J. L., Lieberman R., Davie J. M. Clonal nature of the immune response to phosphorylcholine. II. Idiotypic specificity and binding characteristics of anti-phosphorylcholine antibodies. J Immunol. 1974 May;112(5):1747–1756. [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Sheriff S. Antibody-antigen complexes. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- Desaymard C., Giusti A. M., Scharff M. D. Rat anti-T15 monoclonal antibodies with specificity for VH- and VH-VL epitopes. Mol Immunol. 1984 Oct;21(10):961–967. doi: 10.1016/0161-5890(84)90154-8. [DOI] [PubMed] [Google Scholar]
- Eigenbrot C., Gonzalez T., Mayeda J., Carter P., Werther W., Hotaling T., Fox J., Kessler J. X-ray structures of fragments from binding and nonbinding versions of a humanized anti-CD18 antibody: structural indications of the key role of VH residues 59 to 65. Proteins. 1994 Jan;18(1):49–62. doi: 10.1002/prot.340180107. [DOI] [PubMed] [Google Scholar]
- French D. L., Laskov R., Scharff M. D. The role of somatic hypermutation in the generation of antibody diversity. Science. 1989 Jun 9;244(4909):1152–1157. doi: 10.1126/science.2658060. [DOI] [PubMed] [Google Scholar]
- Glockshuber R., Stadlmüller J., Plückthun A. Mapping and modification of an antibody hapten binding site: a site-directed mutagenesis study of McPC603. Biochemistry. 1991 Mar 26;30(12):3049–3054. doi: 10.1021/bi00226a010. [DOI] [PubMed] [Google Scholar]
- Herron J. N., He X. M., Ballard D. W., Blier P. R., Pace P. E., Bothwell A. L., Voss E. W., Jr, Edmundson A. B. An autoantibody to single-stranded DNA: comparison of the three-dimensional structures of the unliganded Fab and a deoxynucleotide-Fab complex. Proteins. 1991;11(3):159–175. doi: 10.1002/prot.340110302. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Black S. J., Tokuhisa T., Herzenberg L. A. Memory B cells at successive stages of differentiation. Affinity maturation and the role of IgD receptors. J Exp Med. 1980 May 1;151(5):1071–1087. doi: 10.1084/jem.151.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S., Bird R. E. Construction of single-chain Fv derivatives monoclonal antibodies and their production in Escherichia coli. Methods Enzymol. 1991;203:88–98. doi: 10.1016/0076-6879(91)03006-3. [DOI] [PubMed] [Google Scholar]
- Kluskens L., Lee W., Köhler H. Immune response to phosphorylcholine. I. Characterization of the epitope-specific antibody. Eur J Immunol. 1976 Jul;5(7):489–496. doi: 10.1002/eji.1830050712. [DOI] [PubMed] [Google Scholar]
- Lee W., Cosenza H., Köhler H. Clonal restriction of the immune response to phosphorylcholine. Nature. 1974 Jan 4;247(5435):55–57. doi: 10.1038/247055a0. [DOI] [PubMed] [Google Scholar]
- Levy S., Mendel E., Kon S., Avnur Z., Levy R. Mutational hot spots in Ig V region genes of human follicular lymphomas. J Exp Med. 1988 Aug 1;168(2):475–489. doi: 10.1084/jem.168.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malipiero U. V., Levy N. S., Gearhart P. J. Somatic mutation in anti-phosphorylcholine antibodies. Immunol Rev. 1987 Apr;96:59–74. doi: 10.1111/j.1600-065x.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Manser T., Parhami-Seren B., Margolies M. N., Gefter M. L. Somatically mutated forms of a major anti-p-azophenylarsonate antibody variable region with drastically reduced affinity for p-azophenylarsonate. By-products of an antigen-driven immune response? J Exp Med. 1987 Nov 1;166(5):1456–1463. doi: 10.1084/jem.166.5.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padlan E. A., Cohen G. H., Davies D. R. On the specificity of antibody/antigen interactions: phosphocholine binding to McPC603 and the correlation of three-dimensional structure and sequence data. Ann Inst Pasteur Immunol. 1985 Mar-Apr;136C(2):271–276. doi: 10.1016/s0769-2625(85)80058-1. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Crews S. T., Douglas R., Sorensen G., Johnson N., Nivera N., Gearhart P. J., Hood L. The generation of diversity in phosphorylcholine-binding antibodies. Adv Immunol. 1984;35:1–37. doi: 10.1016/s0065-2776(08)60572-6. [DOI] [PubMed] [Google Scholar]
- Pollock R. R., French D. L., Gefter M. L., Scharff M. D. Identification of mutant monoclonal antibodies with increased antigen binding. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2298–2302. doi: 10.1073/pnas.85.7.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintáns J., Cosenza H. Antibody response to phosphorylcholine in vitro. II. Analysis of T-dependent and T-independent responses. Eur J Immunol. 1976 Jun;6(6):399–405. doi: 10.1002/eji.1830060605. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Roberts V. A., Iverson B. L., Iverson S. A., Benkovic S. J., Lerner R. A., Getzoff E. D., Tainer J. A. Antibody remodeling: a general solution to the design of a metal-coordination site in an antibody binding pocket. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6654–6658. doi: 10.1073/pnas.87.17.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts V. A., Stewart J., Benkovic S. J., Getzoff E. D. Catalytic antibody model and mutagenesis implicate arginine in transition-state stabilization. J Mol Biol. 1994 Jan 21;235(3):1098–1116. doi: 10.1006/jmbi.1994.1060. [DOI] [PubMed] [Google Scholar]
- Rodwell J. D., Gearhart P. J., Karush F. Restriction in IgM expression. IV. Affinity analysis of monoclonal anti-phosphorylcholine antibodies. J Immunol. 1983 Jan;130(1):313–316. [PubMed] [Google Scholar]
- Rothstein T. L., Gefter M. L. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune sera. Mol Immunol. 1983 Feb;20(2):161–168. doi: 10.1016/0161-5890(83)90127-x. [DOI] [PubMed] [Google Scholar]
- Satow Y., Cohen G. H., Padlan E. A., Davies D. R. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7 A. J Mol Biol. 1986 Aug 20;190(4):593–604. doi: 10.1016/0022-2836(86)90245-7. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Kocks C., Rajewsky K., Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987 Mar 13;48(5):757–770. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore M. P., Bruderer U., Rittenberg M. B. The adaptive potential of the memory response: clonal recruitment and epitope recognition. Immunol Rev. 1988 Oct;105:113–136. doi: 10.1111/j.1600-065x.1988.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore M. P., Rittenberg M. B. Clonal diversity, somatic mutation, and immune memory to phosphocholine-keyhole limpet hemocyanin. J Immunol. 1989 Dec 15;143(12):4123–4133. [PubMed] [Google Scholar]
- Tramontano A., Chothia C., Lesk A. M. Framework residue 71 is a major determinant of the position and conformation of the second hypervariable region in the VH domains of immunoglobulins. J Mol Biol. 1990 Sep 5;215(1):175–182. doi: 10.1016/S0022-2836(05)80102-0. [DOI] [PubMed] [Google Scholar]




