Abstract
DNA double strand breaks are a particularly toxic form of DNA damage and the mammalian cell has evolved an intricate set of responses to repair this type of DNA lesion. A key early event in the DNA damage response (DDR) is ATM phosphorylation of the histone variant H2AX at serine 139 at the site of the DNA break. Phosphorylated S139 H2AX, or γH2AX, forms a docking site for binding of MDC1, leading to sustained recruitment of other DNA repair factors that mediate the repair of the DNA double strand break. Moreover, recruitment of MDC1 to the break site activates cell cycle checkpoints, protecting the cell from replication of damaged DNA templates. While the molecular events leading to DNA double strand break repair have been well described, the deactivating or homeostatic mechanisms following completion of repair remain largely unexplored. Recent publications by our laboratories and the Medema laboratory shed new light on this issue. Both publications showed that the Wild-type p53-Induced Phosphatase 1 (WIP1) directly dephosphorylates γH2AX. WIP1 migrates to the sites of irradiation-induced foci (IRIF), though at a delayed rate relative to MDC1 and mediates γH2AX dephosphorylation, presumably after DNA repair is complete. This prevents recruitment of other repair factors such as MDC1 and 53BP1 to the DNA damage sites and promotes the dissolution of IRIF. In addition, overexpression of WIP1 has a suppressive effect on DNA double strand break repair. Taken together, these reports further implicate WIP1 as a critical homeostatic regulator of the DDR.
Keywords: Wip1, PPM1D, γH2AX, MDC1, ATM, ATR, DNA double strand break repair
The DNA double strand break (DSB), in which both strands of the double helix are severed simultaneously, is one of the most serious of DNA lesions. DSBs can be generated endogenously as a result of collapsed or stalled replication forks, V(D)J recombination, immunoglobulin class switching, or meiotic recombination. DSBs are also caused by exogenous insults that include ionizing radiation (IR), radiomimetic drugs, or other chemical agents that induce stalled replication forks. DSBs, if not repaired, can lead to cell death, mutations and genomic instability which may contribute to tumorigenesis.1
To counteract the deleterious effects of DSBs and other DNA lesions, cells have evolved an elaborate DNA damage response (DDR).2 Following DNA damage, phosphatidylinositol 3-kinase-like protein kinases (PIKKs) such as ataxia telangiectasia mutated (ATM) and ataxia telangiectasia mutated and Rad3-related (ATR) play a pivotal role as transducer kinases in activation of the DDR by phosphorylating more than 700 target proteins.3 A third member of PIKK family, DNA-dependent protein kinase (DNA-PKcs), is activated by DSBs and initiates non-homologous end joining (NHEJ) for DSB repair.4
During activation of the DDR, H2AX is one of the earliest targets of the sensor PIKKs. H2AX is a variant of the histone H2A, one of the four major core histones in the nucleosome.5–7 Compared to other H2A variants, H2AX encodes a conserved and unique carboxyl terminal tail containing an SQ motif at codon 139. The Ser 139 residue of the tail can be phosphorylated by ATM, ATR and DNA-PKcs and is referred to as γH2AX. γH2AX phosphorylation can be visualized by immunofluorescence staining using an antibody specific to γH2AX.8 Exposure to IR induces discrete nuclear γH2AX foci within minutes, each of which is believed to represent a single DSB site. In contrast, UV irradiation induces a diffuse pan-nuclear γH2AX staining pattern instead of discrete nuclear foci.9
The role of γH2AX phosphorylation in DSB signaling is relatively well characterized. The DSB is initially recognized by the MRN (Mre11/Rad50/Nbs1) sensor complex. Binding of the MRN complex to the DSB ends recruits and activates ATM, which then phosphorylates adjacent H2AX molecules.5 The phosphorylated tail of γH2AX binds to the BRCT domain of MDC1 (mediator of DNA damage checkpoint 1) which in turn recruits additional activated ATM through its FHA domain to the DSB site.10,11 This stepwise process initiates from the DSB site and phosphorylated γH2AX can spread up to several megabases around the DSB site.12 Although initial γH2AX phosphorylation is MDC1-independent, MDC1 is critical for amplifying γH2AX phosphorylation by serving as a linking molecule between γH2AX and ATM.11 Aside from MDC1, other DDR factors such as 53BP1, BRCA1, and additional MRN complexes are recruited around the DSB. While not required for the recruitment of some DDR factors, γH2AX is required for sustained recruitment of DDR factors and the amplification of damage signaling.13
Once the DSB repair machinery is fully engaged around the break site and the DSB is repaired, γH2AX phosphorylation is no longer required and must be reversed so that the chromatin may return to a prestress state. Indeed, western blotting and immunofluorescence staining of γH2AX show that total levels of γH2AX as well as γH2AX foci intensity gradually decline. Such observations suggest the presence of phosphatases that target γH2AX. In fact, four serine/threonine phosphatases in human cells have been identified as γH2AX phosphatases: PP2A, PP4, PP6 and PP2Cγ.14–18 PP2A, PP4 and PP6 are closely related in sequence and belong to the phosphoprotein phosphatase (PPP) family, whereas PP2Cγ belongs to the metal-dependent protein phosphatase (PPM) family.19 The first identified γH2AX phosphatase was PP2A.14 PP2A is recruited to the damage site in an H2AX dependent manner and siRNA knockdown of PP2A(C), the catalytic subunit of PP2A, results in persistent camptothecin-induced γH2AX foci and inefficient DNA repair.14 The PP4 complex (PP4C, PP4R2 and PP4R3β), which associates with chromatin, also dephosphorylates chromatin-bound γH2AX. Depletion of PP4C, PP4R2 or PP4R3 in human cells increases γH2AX levels both before and after camptothecin treatment.15 Moreover, depletion of PP4 has no impact on basal γH2AX levels in ATR-deficient and non-dividing cells, suggesting that PP4 targets only ATR-mediated γH2AX phosphorylation during DNA replication. Another PP2A-like phosphatase, PP6, along with its regulatory subunit PP6R1, was also shown to dephosphorylate γH2AX.17 Knockdown of PP6c results in persistence of γH2AX and 53BP1 foci. As observed for other PP2A-like phosphatases, PP6 also plays a role in the recovery from the G2/M checkpoint. Finally, PP2Cγ, a member of the PP2C family, has been co-purified with the histone dimer H2A-H2B in a screen for factors required for H2A-H2B incorporation into chromatin.18 PP2Cγ may mediate H2AX recycling by dephosphorylating dissociated γH2AX-H2B and incorporating unphosphorylated H2AX-H2B back into the nucleosome.
Most recently, a fifth phosphatase has been shown to dephosphorylate γH2AX. Our laboratories and the Medema laboratory identified the wild-type p53-induced phosphatase (WIP1) as a novel γH2AX phosphatase and demonstrated that γH2AX dephosphorylation by WIP1 suppresses DSB repair and abrogates cell cycle checkpoints.20,21 WIP1 was originally discovered in a screen for p53 target genes in IR-treated cells.22 It is a member of PP2C family and also called PP2Cδ or PPM1D (Protein Phosphatase 1D magnesium-dependent). In contrast to the PPP family, PP2C activity is not inhibited by okadaic acid, but depends on Mg2+ or Mn2+ ions.22 Members of the PP2C family are monomeric proteins that do not require regulatory subunits for activity, though they encode additional C-terminal domains that may confer substrate specificity.19 The most common function of PP2C phosphatases is to regulate stress signaling. Like other PP2C members, WIP1 is also involved in stress regulation, especially the DDR pathway.23 WIP1 dephosphorylates proteins that are phosphorylated by the DNA damage-activated sensor kinases ATM, ATR and DNA-PK, such as Chk1, Chk2, p53, ATM, Mdm2 and Mdmx.24–28 Other targets of WIP1 include p38 MAP kinase and NFκB.29,30 Dephosphorylation of ATM/ATR/DNA-PK target proteins by WIP1 reduces cell cycle checkpoint activity and p53 stability.20,24 As a result, WIP1 abrogates cell cycle checkpoints and suppresses p53 activities. Thus, WIP1 has been proposed to deactivate the DDR by dephosphorylating multiple ATM/ ATR-activated proteins, including p53.23 Given the established importance of p53 in tumor suppression and the emerging evidence for the DDR as an early barrier to cancer progression,31,32 such WIP1 functions implicate it as a potential oncogene. WIP1 is amplified and/ or overexpressed in a variety of human tumors including breast adenocarcinoma, ovarian clear cell adenocarcinoma, neuroblastoma, pancreatic adenocarcinoma, gastric carcinoma and medulloblastoma.23 In in vitro transformation assays, WIP1 promotes transformation of primary rodent fibroblasts in cooperation with other oncogenes.33,34 Studies with WIP1 transgenic mice and knockout mice show that WIP1 overexpression may promote cancer progression, while reduction of WIP1 levels inhibits cancer formation and progression.35,36 Obviously, the mechanisms of WIP1 oncogenicity may be better understood by identifying its target proteins and characterizing the biological consequences of such dephosphorylation. It is likely that the discovery of the WIP1-H2AX interactions will be of importance in our understanding of DDR regulation as well as oncogenic regulatory pathways.
In the recently reported WIP1-H2AX studies, both groups showed conclusively that purified WIP1 could dephosphorylate γH2AX or a serine 139 phosphopeptide derived from H2AX in in vitro phosphatase assays (Table 1).20,21 Both groups also demonstrated co-immunoprecipation of WIP1 and H2AX in cellular lysates. Overexpression of WIP1 in cells was associated with greatly reduced levels of γH2AX after DNA damage, while treatment of cells with WIP1 siRNAs resulted in increased and prolonged γH2AX levels after various DNA damage scenarios, including ionizing radiation, ultraviolet radiation, hydroxyurea, doxorubicin and etoposide (Table 1). Irradiation of Wip1 null mice by the Donehower laboratory also resulted in enhanced levels of tissue γH2AX compared to wildtype mice. The effects of WIP1 on H2AX phosphorylation were not dependent on ATM, as these effects were observed in ATM-deficient cells and mice. In cellular immunolocalization assays, the Medema laboratory showed that WIP1 and γH2AX co-localized in the chromatin fractions of DNA damaged cells. Importantly, they also demonstrated that MDC1 and WIP1 co-migrated to laser-initiated DNA damage regions of cells, though WIP1 migrated at a delayed rate compared to MDC1. MDC1 binds directly to γH2AX in damage foci, so co-localization of WIP1 and γH2AX is assumed. Thus, the role of WIP1 in damage foci (IRIF) formation was investigated. In WIP1 overexpressing cells, γH2AX, MDC1 or 53BP1 foci formation after exposure to IR is robustly downregulated, whereas WIP1 depletion enhanced γH2AX foci formation and MDC1 and 53BP1 foci association. Finally, the Donehower/Lu laboratories utilized two assays to examine rates of DSB repair. These assays revealed that reduction of WIP1 enhanced γH2AX association with individual DSBs and enhanced the rates at which DSBs were repaired. In another assay, overexpression of WIP1 was shown to suppress homologous recombination (HR)- and non-homologous end joining (NHEJ)-mediated DSB repair, while WIP1 knockdown by either siRNA or WIP1 inhibitors resulted in enhanced measures of DSB repair. The combined results by both groups provide a strong argument that WIP1 is a key γH2AX phosphatase that plays an important role in homeostatic regulation of DSB repair in response to DNA damage (Table 1). Because WIP1 likely targets many elements of the DDR pathway, known (Chk1, Chk2, p53, Mdm2, Mdmx and ATM) and unknown, we cannot attribute γH2AX dephosphorylation solely to WIP1 activity. Moreover, the other aforementioned γH2AX phosphatases surely play a role as well. Rather, WIP1 dephosphorylation of γH2AX is likely to be one of many contributing factors that negatively modulate the DDR pathways and enable cellular homeostasis after DSB repair is complete.
Table 1.
Major findings of Macurek et al. (2010) and Moon et al. (2010)
| Both papers demonstrated that… |
| - WIP1 dephosphorylates pS139 H2AX phosphopeptide and intact γ-H2AX in vitro |
| - WIP1 and H2AX physically interact with each other |
| - WIP1 overexpression downregulates γ-H2AX levels in cells after DNA damage |
| - WIP1 knockdown increases and prolongs γ-H2AX after DNA damage |
| - WIP1 dephosphorylation of γ-H2AX is ATM-independent |
| - WIP1 overexpression inhibits damage-induced γ-H2AX and MDC1 foci formation |
| Macurek et al. demonstrated that… |
| - WIP1 is bound to chromatin and modestly mobile |
| - WIP1 localizes to damage foci after induction of DNA strand breaks |
| - WIP1 inhibits the G2/M checkpoint after damage |
| Moon et al. demonstrated that… |
| - Wip1 deficiency in mice increases both basal and IR-induced γ-H2AX levels |
| - WIP1 inhibits damage-induced migration of 53BP1 to DNA damage foci |
| - WIP1 suppresses HR- and NHEJ-mediated DSB repair |
The presence of multiple γH2AX kinases may give a clue as to why cells have multiple γH2AX phosphatases. Although all three PIKKs, ATM, ATR and DNA-PKcs, phosphorylate H2AX, they are activated by distinct mechanisms and seem to have distinct target subsets of H2AX. Previous studies suggested that in response to DSB, H2AX near the DSB is targeted by ATM and DNA-PKcs and that H2AX further away from the DSB is phosphorylated either by ATM in a MDC1-independent manner or by ATR after DSB resection. Intriguingly, Savic et al. suggested that γH2AX phosphorylation around the DSB can induce γH2AX phosphorylation even on other chromosomes surrounding the DSB.37,38 On the other hand, in response to replication stress, exposed single-stranded DNA recruits ATR in cooperation with RPA and ATRIP to mediate γH2AX phosphorylation.39 We discovered that WIP1 impairs IR-induced γH2AX phosphorylation in both ATM proficient and deficient cells, suggesting that WIP1 targets γH2AX generated by ATR and DNA-PKcs. Indeed, WIP1 suppresses both HR- and NHEJ-mediated DSB repair. The latter is dependent on DNA-PK function.40 We speculate that the shifted equilibrium of γH2AX/H2AX and aberrant DSB repair may contribute to dysregulation of immunoglobulin class switching recombination and immune defects observed in Wip1-deficient mice.41,42 We also demonstrated that basal levels of γH2AX are increased in WIP1-depleted cells or Wip1-deficient mice. This suggests that WIP1 could target spontaneously generated γH2AX during S-phase and that WIP1 may play an analogous role with PP4 in reversing γH2AX phosphorylation caused by replication stress. Both WIP1 and PP4 have been found to be overexpressed in human cancers.23,43 Possibly, premature dephosphorylation of γH2AX could facilitate DNA replication even in the presence of collapsed replication forks and increase mutations and genomic instability.
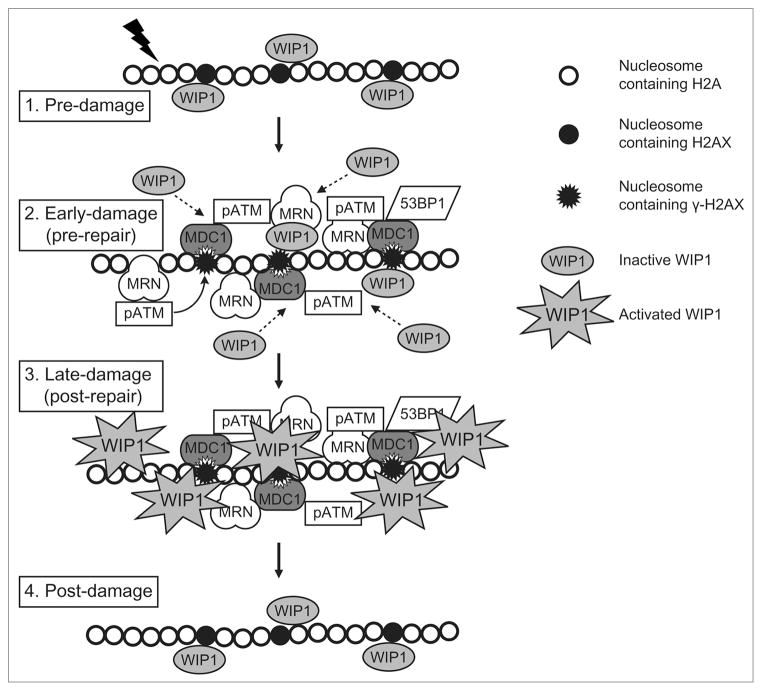
These results have allowed us to formulate a model shown in Figure 1 based on what is known about the role of γH2AX in formation of DNA damage foci. Following initiation of a double strand break, the primary sensor kinases for DSBs, such as ATM, phosphorylate the members of the MRN complex (Mre11, Rad50 and Nbs1) that binds to the DSB and recruits activated ATM, which phosphorylates chromatin-bound H2AX around the break to form γH2AX. γH2AX recruits MDC1, which is dependent on the serine 139 phosphorylated form of H2AX for binding. MDC1 then recruits additional repair factors that include 53BP1, other MRN complexes, and ATM, ensuring the spreading of the ATM-mediated DDR signal for several megabases along the chromatin from the site of the break. While WIP1 may migrate to the activated complex soon after it is formed, as the Medema group has shown, we hypothesize that it is kept relatively inactive until the DSB is repaired. Once the break is repaired, WIP1 is activated and dephosphorylates γH2AX, ATM, and other proteins within the damage complex, resulting in dissociation of key interacting proteins such as γH2AX and MDC1, and dissolution of the repair foci. This allows the successfully repaired cells to return to a normal prestress state.
Figure 1.
WIP1 reverses IR-induced foci (IRIF) formation. WIP1 is a nuclear protein, but some WIP1 molecules are chromatin-associated. We hypothesize that WIP1 is relatively inactive in non-damaged cells. In response to IR, the MRN complex recognizes the DSB end and recruits activated ATM that in turn phosphorylates H2AX and initiates a cascading recruitment of DDR factors including MDC1 and 53BP1. Additional WIP1 is also recruited to the IRIF, though at a slightly delayed rate compared to MDC1. WIP1 remains in its inactive state during these early stages of IRIF formation. Once the damage is successfully repaired, WIP1 may be activated so that it dephosphorylates γH2AX, resulting in destruction of H2AX-MDC1 interactions and other foci interactions, and dissolution of the IRIF. The chromatin returns to a normal prestress state as H2AX is dephosphorylated and WIP1 returns to its inactive state.
The above model raises new questions regarding WIP1 functions. For example, WIP1 is expressed basally at modest levels. In response to stress, Wip1 transcription is upregulated relatively rapidly in a p53-dependent manner.22 Yet its protein appears to be relatively inactive in dephosphorylating targets such as γH2AX until late in the DDR. Post-transcriptional or post-translational regulatory mechanisms likely keep WIP1 activity under control until DNA damage is fully repaired. Yet, how is WIP1 recruited to the repair complex and what proteins recruit it there? Which of those proteins in the DNA damage foci activate WIP1 after repair is complete? How is WIP1 modified to increase its activity? And after the DNA damage is repaired and the IRIF dissolved, how is WIP1 itself deactivated or degraded? We are optimistic that such questions will be answered in the near future and the answers will provide important new insights into how WIP1 homeo-statically regulates the DNA damage response.
Acknowledgments
This work was supported by grants to L.A.D. and X.L. by the National Cancer Institute and by a predoctoral training fellowship to T.-A.N. by the Department of Defense Breast Cancer Research Program.
References
- 1.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:5–7. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–6. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 4.Weterings E, Chen DJ. DNA-dependent protein kinase in nonhomologous end joining: a lock with multiple keys? J Cell Biol. 2007;179:183–6. doi: 10.1083/jcb.200705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stucki M, Jackson SP. gammaH2AX and MDC1: anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair (Amst) 2006;5:534–43. doi: 10.1016/j.dnarep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Fillingham J, Keogh MC, Krogan NJ. GammaH2AX and its role in DNA double-strand break repair. Biochem Cell Biol. 2006;84:568–77. doi: 10.1139/o06-072. [DOI] [PubMed] [Google Scholar]
- 7.Bonner WM, Redon CE, Dickey JS, Nakamura AJ, Sedelnikova OA, Solier S, Pommier Y. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–67. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 9.Marti TM, Hefner E, Feeney L, Natale V, Cleaver JE. phase after UV H2AX phosphorylation within the G1 irradiation depends on nucleotide excision repair and not DNA double-strand breaks. Proc Natl Acad Sci USA. 2006;103:9891–6. doi: 10.1073/pnas.0603779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart GS, Wang B, Bignell CR, Taylor AM, Elledge SJ. MDC1 is a mediator of the mammalian DNA damage checkpoint. Nature. 2003;421:961–6. doi: 10.1038/nature01446. [DOI] [PubMed] [Google Scholar]
- 11.Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell. 2005;123:1213–26. doi: 10.1016/j.cell.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–16. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol. 2003;5:675–9. doi: 10.1038/ncb1004. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell. 2005;20:801–9. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Chowdhury D, Xu X, Zhong X, Ahmed F, Zhong J, Liao J, et al. A PP4-phosphatase complex dephosphorylates gamma-H2AX generated during DNA replication. Mol Cell. 2008;31:33–46. doi: 10.1016/j.molcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakada S, Chen GI, Gingras AC, Durocher D. PP4 is a gamma H2AX phosphatase required for recovery from the DNA damage checkpoint. EMBO Rep. 2008;9:1019–26. doi: 10.1038/embor.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas P, Zhong J, Ye R, Moorhead GB, Xu X, Lees-Miller SP. Protein Phosphatase 6 Interacts with the DNA-Dependent Protein Kinase Catalytic Subunit and Dephosphorylates {gamma}-H2AX. Mol Cell Biol. 30:1368–81. doi: 10.1128/MCB.00741-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimura H, Takizawa N, Allemand E, Hori T, Iborra FJ, Nozaki N, et al. A novel histone exchange factor, protein phosphatase 2Cgamma, mediates the exchange and dephosphorylation of H2A-H2B. J Cell Biol. 2006;175:389–400. doi: 10.1083/jcb.200608001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–84. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Macurek L, Lindqvist A, Voets O, Kool J, Vos HR, Medema RH. Wip1 phosphatase is associated with chromatin and dephosphorylates gammaH2AX to promote checkpoint inhibition. Oncogene. 2010;29:2281–91. doi: 10.1038/onc.2009.501. [DOI] [PubMed] [Google Scholar]
- 21.Moon SH, Lin L, Zhang X, Nguyen TA, Darlington Y, Waldman AS, et al. Wildtype p53-induced phosphatase 1 dephosphorylates histone variant {gamma}-H2AX and suppresses DNA double strand break repair. J Biol Chem. 2010;285:12935–47. doi: 10.1074/jbc.M109.071696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiscella M, Zhang H, Fan S, Sakaguchi K, Shen S, Mercer WE, et al. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci USA. 1997;94:6048–53. doi: 10.1073/pnas.94.12.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu X, Nguyen TA, Moon SH, Darlington Y, Sommer M, Donehower LA. The type 2C phosphatase Wip1: an oncogenic regulator of tumor suppressor and DNA damage response pathways. Cancer Metastasis Rev. 2008;27:123–35. doi: 10.1007/s10555-008-9127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X, Nannenga B, Donehower LA. PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev. 2005;19:1162–74. doi: 10.1101/gad.1291305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimoto H, Onishi N, Kato N, Takekawa M, Xu XZ, Kosugi A, et al. Regulation of the antioncogenic Chk2 kinase by the oncogenic Wip1 phosphatase. Cell Death Differ. 2006;13:1170–80. doi: 10.1038/sj.cdd.4401801. [DOI] [PubMed] [Google Scholar]
- 26.Shreeram S, Demidov ON, Hee WK, Yamaguchi H, Onishi N, Kek C, et al. Wip1 phosphatase modulates ATM-dependent signaling pathways. Mol Cell. 2006;23:757–64. doi: 10.1016/j.molcel.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Lu X, Ma O, Nguyen TA, Jones SN, Oren M, Donehower LA. The Wip1 Phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell. 2007;12:342–54. doi: 10.1016/j.ccr.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Lin L, Guo H, Yang J, Jones SN, Jochemsen A, Lu X. Phosphorylation and degradation of MdmX is inhibited by Wip1 phosphatase in the DNA damage response. Cancer Res. 2009;69:7960–8. doi: 10.1158/0008-5472.CAN-09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takekawa M, Adachi M, Nakahata A, Nakayama I, Itoh F, Tsukuda H, et al. p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J. 2000;19:6517–26. doi: 10.1093/emboj/19.23.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chew J, Biswas S, Shreeram S, Humaidi M, Wong ET, Dhillion MK, et al. WIP1 phosphatase is a negative regulator of NFkappaB signalling. Nat Cell Biol. 2009;11:659–66. doi: 10.1038/ncb1873. [DOI] [PubMed] [Google Scholar]
- 31.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–70. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 32.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–5. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Yang Y, Peng Y, Austin RJ, van Eyndhoven WG, Nguyen KC, et al. Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat Genet. 2002;31:133–4. doi: 10.1038/ng888. [DOI] [PubMed] [Google Scholar]
- 34.Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA, et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet. 2002;31:210–5. doi: 10.1038/ng894. [DOI] [PubMed] [Google Scholar]
- 35.Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW, et al. Inactivation of the Wip1 phosphatase inhibits mammary tumori-genesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet. 2004;36:343–50. doi: 10.1038/ng1317. [DOI] [PubMed] [Google Scholar]
- 36.Nannenga B, Lu X, Dumble M, Van Maanen M, Nguyen TA, Sutton R, et al. Augmented cancer resistance and DNA damage response phenotypes in PPM1D null mice. Mol Carcinog. 2006;45:594–604. doi: 10.1002/mc.20195. [DOI] [PubMed] [Google Scholar]
- 37.Savic V, Yin B, Maas NL, Bredemeyer AL, Carpenter AC, Helmink BA, et al. Formation of dynamic gamma-H2AX domains along broken DNA strands is distinctly regulated by ATM and MDC1 and dependent upon H2AX densities in chromatin. Mol Cell. 2009;34:298–310. doi: 10.1016/j.molcel.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savic V, Sanborn KB, Orange JS, Bassing CH. Chipping away at gamma-H2AX foci. Cell Cycle. 2009;8:3285–90. doi: 10.4161/cc.8.20.9719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–62. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 40.Collis SJ, DeWeese TL, Jeggo PA, Parker AR. The life and death of DNA-PK. Oncogene. 2005;24:949–61. doi: 10.1038/sj.onc.1208332. [DOI] [PubMed] [Google Scholar]
- 41.Choi J, Nannenga B, Demidov ON, Bulavin DV, Cooney A, Brayton C, et al. Mice deficient for the wild-type p53-induced phosphatase gene (Wip1) exhibit defects in reproductive organs, immune function and cell cycle control. Mol Cell Biol. 2002;22:1094–105. doi: 10.1128/MCB.22.4.1094-1105.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schito ML, Demidov ON, Saito S, Ashwell JD, Appella E. Wip1 phosphatase-deficient mice exhibit defective T cell maturation due to sustained p53 activation. J Immunol. 2006;176:4818–25. doi: 10.4049/jimmunol.176.8.4818. [DOI] [PubMed] [Google Scholar]
- 43.Wang B, Zhao A, Sun L, Zhong X, Zhong J, Wang H, et al. Protein phosphatase PP4 is overexpressed in human breast and lung tumors. Cell Res. 2008;18:974–7. doi: 10.1038/cr.2008.274. [DOI] [PubMed] [Google Scholar]