Abstract
The unmet clinical need for myocardial repair after irreversible ischemic injury requires a better understanding of the biological properties of cardiac stem cells (CSCs). Using a primary culture of neonatal rat myocardial cells, we describe the formation and maturation of contracting cardiomyocyte colonies stemming from c-kit+, Sca+, or Isl1+ CSCs, which occurs in parallel to the hypertrophy of the major cardiac myocyte population. The contracting cardiomyocyte colonies (~1–2 colonies per 1 × 105 of myocardial cells) were identified starting from eighth day of culturing. At first, spontaneous weak, asynchronous, and arrhythmic contractions of the colonies at a rate of 2–3 beats/min were registered. Over time, the contractions of the colonies became more synchronous and frequent, with a contraction rate of 58–60 beats/min by the 30th day of culturing. The colonies were characterized by the CSCs subtype-specific pattern of growth and structure. The cells of the colonies were capable of spontaneous cardiomyogenic differentiation, demonstrating expression of both sarcomeric α-actinin and α-sarcomeric actin as well as the maturation of contractile machinery and typical Ca2+ responses to caffeine (5 mМ) and K+ (120 mМ). Electromechanical coupling, characterized by cardiac muscle-specific Ca2+-induced Ca2+ release, was evident at 3 weeks of culturing. Thus, the co-cultivation of CSCs with mature cardiac cells resulted in the formation of contracting cardiomyocyte colonies, resembling the characteristics of in vivo cardiomyogenesis. The proposed model can be used for the investigation of fundamental mechanisms underlying cardiomyogenic differentiation of CSCs as well as for drug testing and/or other applications.
Keywords: primary culture of neonatal myocardial cells, cardiac myocytes, resident cardiac stem cells, proliferation, differentiation, contracting cardiomyocyte colonies, electromechanical coupling, Ca2+-induced Ca2+ release
Introduction
Recent advances in the understanding of major biological characteristics of stem cells have stimulated the interest in potential therapeutic applications of stem cells, in particular for tissue and organ repair. Limited myocardial regeneration after irreversible ischemic injury results in the formation of post-infarct scarring, underlining the need for new cardiac regenerative therapies. The identification of several distinct types of resident cardiac stem cells (CSCs) in the adult human heart, including c-kit+,1 Isl1+,2 Sca1+,3 and cardiosphere-generating progenitors,4 has stimulated the development of new regenerative strategies based either on the site-specific delivery of exogenous CSCs or on the activation of host CSCs by certain stimuli (for review, see refs. 5 and 6 and references therein). Currently available techniques of CSCs isolation, sorting, and expansion can be used for generating significant amounts of immature cardiac progenitor cells in culture, both for research purposes and for potential use in regenerative medicine.7-11 However, the maturation of these progenitors into fully differentiated contracting cardiac myocytes is generally impeded by the lack of spontaneous differentiation of CSCs and cardiac progenitors in culture. The agonist-induced directed differentiation of various subsets of CSCs into cardiomyocytes has been unequivocally demonstrated in in vitro models. In particular, isolated and expanded CSCs belonging to certain subsets were differentiated into cardiomyocytes after chemical stimulation with 5′-azacytidine,2,11 oxytocin,12 and trichostatin A13 incubation in differentiating media containing dexamethasone7 and co-cultivation with neonatal10,14 and adult cardiac myocytes.15 Although significant strides have been made toward understanding the cardiomyogenic potential of CSCs, it should be noted that the proportion of CSCs amenable to differentiation into mature cardiac myocytes with contractile phenotype was found to be limited to 0.25–10% in the above studies,12,13,15,16 while the process of differentiation lasted for several weeks. In addition, the direct comparison of the cardiomyogenic properties between different CSC subsets is precluded by the variability of isolation and cultivation techniques. Therefore, it is important to develop a model of in vitro cardiomyogenesis enabling serial characterization of growth/differentiation of individual CSCs belonging to different types of resident CSCs (c-kit+, Sca+, and Isl1+), each subjected to the same isolation procedure and identical starting conditions. In the present work, we aimed to describe and characterize such a model, which is based on the demonstration of full cardiomyogenic differentiation of 3 major CSC subsets in a primary culture of neonatal rat myocardial cells, resulting in the formation of contracting cardiomyocyte colonies. After additional validation, we conclude that the proposed model could be used for the investigation of fundamental mechanisms underlying cardiomyogenic differentiation of CSCs as well as for the generation of 3-dimensional cardiomyocyte clusters potentially suitable as a substrate for cell therapy.
Results
Myocardial cell growth and proliferation in primary culture
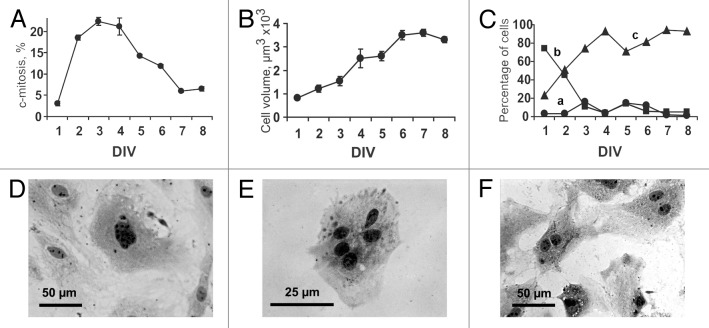
In comparison with the baseline, the total cell count in the myocardial cell culture increased by 1.4 ± 0.2-, 2.2 ± 0.9-, 4.2 ± 1.4-, and 4.8 ± 1.0-fold on the 2nd, 3rd, 4th, and 5th day in vitro (DIV), respectively. As evidenced by the accumulation of c-mitoses, the mitotic activity increased dramatically from DIV 2 to DIV 4 (Fig. 1A). During this period, the percentage of the cells arrested in the metaphase using colchicine averaged 20–23% per day. The percentage of mitotic cells significantly decreased after day 4 and averaged 6–7% on DIV 7–8. Therefore, nearly 70% of the cardiac cells entered mitotic division during DIV 2–5, while the mitotic index varied from 1.6 to 0.4% during the same period. The mean cell volume increased gradually over the first 6 DIV (Fig. 1B), followed by stabilization on DIV 7–8. In particular, the cell volume averaged 819 ± 68, 1532 ± 212, and 3246 ± 190 μm3 on DIV 1, 3, and 6, respectively. To estimate temporal changes in the number of cultured myocardial cells belonging to distinct populations, all of the cells were sub-classified into 3 broad categories according to their volume: (1) <250 μm3; (2) 250–1000 μm3; and (3) >1000 μm3. The first population comprised small cells with a mean cell volume of 140 ± 54 μm3, which corresponded well to the data on the dimensions of resident CSCs found in adult mouse hearts19 as well as to our data on the average volume of immunostained rat CSCs in culture (see “Results”, Structure of the contracting cardiomyocyte colonies). Over 8 d, the percentage of these cells within the total cell population did not exceeded 15% (Fig. 1C, a). The second cell population was characterized by larger cell volume (609 ± 231 μm3), potentially indicative of proliferating cardiac myocytes. The fraction volume of these cells decreased dramatically from 74% on DIV 1 to 4% on DIV 4, suggesting intensive division and subsequent development into mature cardiac myocytes (Fig. 1C, b). Large cardiac myocytes with a cell volume of >1000 μm3 were initially present in culture in a small proportion, but their percentage increased significantly from DIV 1 to DIV 4 (23 and 93%, respectively, Fig. 1C, c). On DIV 4, the cardiac myocytes with a volume of >1000 μm3 could be classified into 4 size categories: (1) 1000–2000 μm3 (30%); (2) 2000–4000 μm3 (48%); (3) 4000–8000 μm3 (15%); and (4) >8000 μm3 (7%).
Figure 1. Eight-day in vitro (DIV) growth of myocardial cells obtained from newborn rat heart. (A) Changes in the proportion of dividing cells (c-mitoses) over 8 d of culturing. (B) Changes in mean cell volume over 8 d of culturing. Values are expressed as the mean ± SD (C) Temporal changes in the number of cultured myocardial cells with different cell volume. (a) Population of small cells with mean cell volume <250 μm3; (b) Population of cells with mean cell volume of 250–1000 μm3; (c) Population of cells with mean cell volume >1000 μm3. (D–F) Polyploid and multinucleated cardiac myocytes. Hematoxylin staining. Digital camera Leica DFC300 FX, Trinocular Microscope H 605T (WPI), objective ×40. (D) Polyploid cardiac myocyte, DIV 8. (E) Cardiac myocyte with 5 nuclei, DIV 4. (F) Binucleated cardiac myocytes, DIV 6.
Physiological growth of the cardiac myocytes in culture resulted in the appearance of polyploids (Fig. 1D) and multinucleated, mostly binucleated, myocytes (Fig. 1E and F, respectively). Binucleated myocytes were detected starting from DIV 2 (~1%); the fraction volume of the binucleated cells increased to 9.5% on DIV 8. The fraction of cardiac myocytes with more than 2 nuclei varied from 0.2 to 0.4% during the entire observation period.
Formation of contracting cardiomyocyte colonies within the primary culture
Depending on the density of cell plating, 3 distinct patterns of spontaneous contraction were identified. First, a confluent monolayer of contracting myocytes with a contraction rate of 25–35 beats/min was observed after 7 d of culture when the cells were plated at a density of 5 × 104 cells/cm2 (Fig. 2A; Video S1). At a lower plating density (2 × 104 cells/cm2), 2 more patterns were observed: (1) spontaneous contractions at a rate of 30–50 beats/min of either individual mature myocytes or small groups of cardiac myocytes consisting of 2–3 cells were recorded for several days starting just after cell adhesion (Fig. 2B; Video S2); and (2) some of the plated cells yielded highly uniform growing colonies of different sizes (Figs. 2C and 3; Video S3). Rare microcolonies consisting of the cells capable of mitotic division were identified after 5 d of culturing. The incidence of colony formation was approximately 10 colonies per 100 000 plated cells, out of which only 1–2 colonies acquired the ability to contract. On the 8–10th DIV, the colonies started to generate rare weak contractions at a rate of 2–3 beats/min (Fig. 4A; Video S3). Over time, the contractions of the colonies became more synchronous and frequent. Visual confirmation of the progressive increase in the contraction rate of the same colony on the 18th, 25th, and 30th DIV (25, 46, and 58 beats/min, respectively) can be observed through the representative video recordings (Videos S4–6). The functional differentiation of the myocytes within the growing colonies and the structural organization of the contracting colonies are characterized in the following sections.
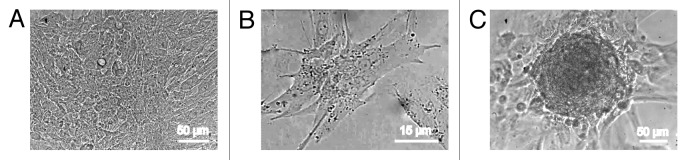
Figure 2. Three different patterns of spontaneously contracting structures in the primary culture of rat neonatal myocardial cells. (A) The confluent monolayer of contracting myocytes resulting from cell plating at a density of 5 × 104 cells/cm2 (contraction rate: 28 beats/min, DIV 11). (B) The contraction of individual mature cardiomyocytes (density of cell plating: 2 × 104 cells/cm2, contraction rate: 47 beats/min, DIV 3). (C) Contracting cardiomyocyte colony in the culture (density of cell plating: 2 × 104 cells/cm2, contraction rate: 10 beats/min, DIV 11). Digital camera Leica DFC300 FX. The images were obtained using an inverted microscope (PIM-III, WPI), objective ×40.
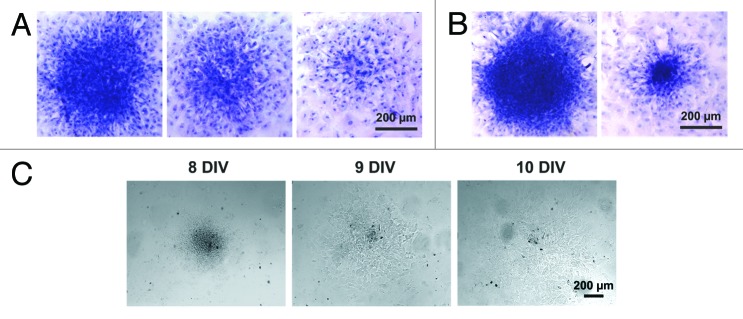
Figure 3. Structure of the cardiomyocyte colonies emerging in the primary culture of neonatal rat myocardial cells. (A) Colonies of different sizes identified on DIV 7. (B) Colonies of different sizes identified on DIV 8. Hematoxylin staining. Digital camera Leica DFC300 FX, objective ×10. (C) Dynamic changes in the size and shape of the living colony over the period of 3 DIV (DIV 8–10). Digital camera Leica DFC300 FX, objective ×10.
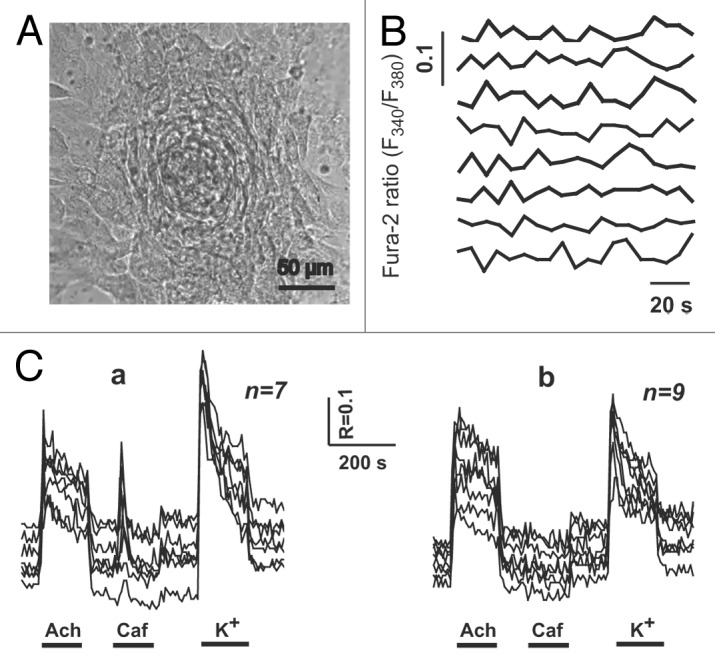
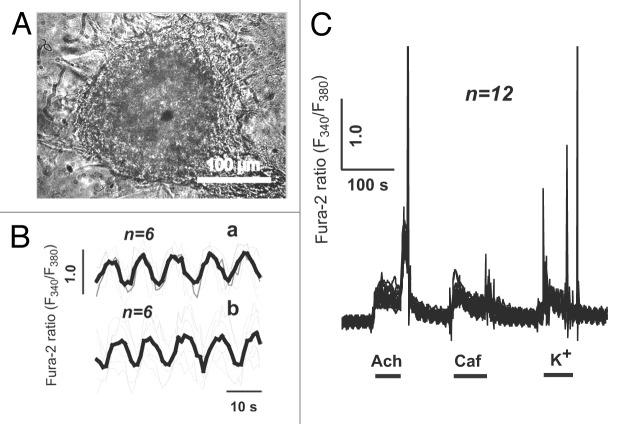
Figure 4. Spontaneous and agonist-induced calcium transients in the individual cells of the cardiomyocyte colony on DIV 10. (A) The contracting cardiomyocyte colony on the 10th day after plating of newborn rat myocardial cells. Contraction rate: 2–3 beats/min. Digital camera Leica DFC300 FX, inverted microscope PIM-III (WPI, USA), objective ×40. (B) Spontaneous [Ca2+]i fluctuations in the individual cardiomyocytes of the same colony. (C) Acetylcholine-, caffeine-, and KCl-induced (20 μM, 5 mM, and 120 mM, respectively) Ca2+ transients. (a) In the cells of the colony responsive to all applied stimuli (n = 7). (b) In the cells of the colony non-responsive to caffeine administration (n = 9).
Calcium transients
In the individual cells of the colony with a low contraction rate (2–3 beats/min) on the 10th DIV (Fig. 4A), spontaneous weak and asynchronous fluctuations in the intracellular Са2+ concentration ([Са2+]i) were registered (Fig. 4B). The maturity of sarcolemmal acetylcholine receptors as well as ryanodine receptors (RyR2) of the sarcoplasmic reticulum (SR) was tested by measuring agonist-induced Са2+ transients. According to the pattern of the agonist-induced Ca2+ response, 2 different types of the cells were identified within the colonies with a low contraction rate. The first cell type was characterized by a pronounced [Са2+]i increase in response to all of the applied stimuli (acetylcholine [Ach], caffeine [Caf], and K+; Fig. 4C, a), while the second cell type demonstrated a decreased (compared with the first cell type) magnitude of Ca2+ response to K+, which was associated with the lack of Ca2+ release after the stimulation of RyRs with Caf (Fig. 4C, b). Along with the spontaneous asynchronous fluctuations in [Са2+]i, the latter observation is indicative of the immaturity of RyRs and/or an insufficient amount of Ca2+ in the SR of the cells comprising second cell type. However, even slightly extending the duration of culturing (DIV 12–14) resulted in a more advanced functional differentiation of cardiac myocytes within the colonies. In particular, the spontaneous calcium transients were synchronous, intense, and uniform in all of the cells of the colony on DIV 12 (Fig. 5A). The rate of calcium peaks coincided with the contraction rate of the entire colony and averaged 22 beats/min. In contrast to the immature cells of the colonies with a low contraction rate, the individual cells of synchronously contracting colonies on DIV 12–14 demonstrated concurrent robust Ca2+ responses to the application of Ach, Caf, and K+ (Fig. 5C). Furthermore, spontaneous Са2+ transients were generated nearly synchronously in all of the cells of the colony, although a mild phase difference could be detected between 2 cell samples (Fig. 5B). The likely explanation for this observation could be the different distance between the cells of the colony and the pacemaker cell, which is presumably localized in the core of the colony. The uniform Ca2+ response to the application of Caf in the cells of the mature colonies provides evidence for the establishment of electromechanical coupling finalizing cardiomyogenic differentiation of the cells within the colonies. In addition, the cells of the mature colonies failed to demonstrate calcium transients in calcium-free medium (data not shown).
Figure 5. Spontaneous and agonist-induced calcium transients in the individual cells of the cardiomyocyte colony on DIV 12. (A) The contracting cardiomyocyte colony on the 12th day after plating of newborn rat myocardial cells. Contraction rate: 22 beats/min. Digital camera Leica DFC300 FX, inverted microscope PIM-III (WPI), objective ×40. (B) Spontaneous [Ca2+]i fluctuations inside individual cardiomyocytes of the colony. Mild phase difference is observed between 2 cell samples (upper and lower panel). (C) Acetylcholine-, caffeine-, and KCl-induced (20 μM, 5 mM, and 120 mM, respectively) Ca2+ transients in the cells of the colony.
Structure of the contracting cardiomyocyte colonies
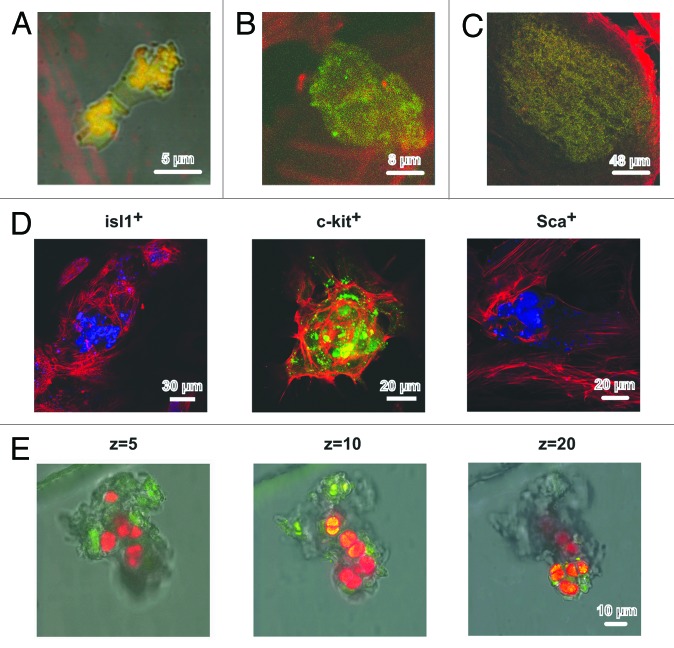
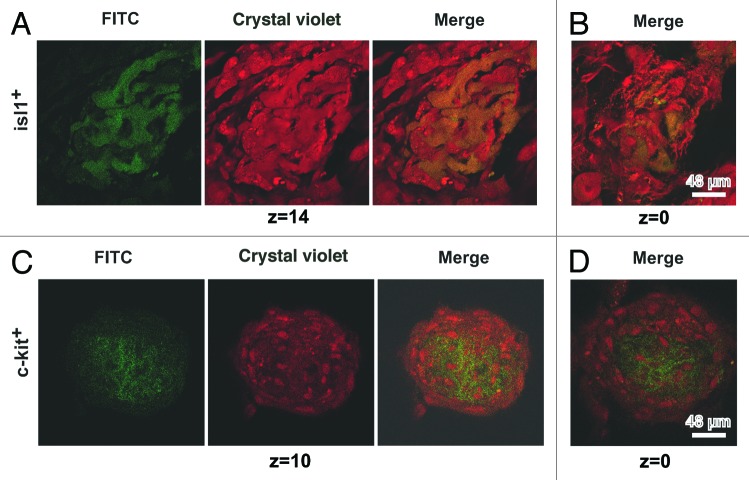
Time-lapse confocal imaging was used for additional characterization of the cardiomyocyte colonies. Five to seven days after plating, 3-dimensional colonies of different size derived from single CSCs of c-kit+, Isl1+, and Sca1+ subsets were found in the culture. At DIV 13, the dimensions of Isl1+ and c-kit+ CSCs-derived colonies were comparable with to those of contracting colonies (see “Calcium transients”). The vertical dimension of different colonies on DIV 10–17 varied from 13 to 97 μm. On days 11–13 of culture, the vertical dimensions of Isl1+ and c-kit+ CSC-derived colonies were comparable, while the dimensions of Sca-1+ colonies in z-axis were much lower, and they averaged 19–20 μm. However, the vertical dimension of Sca-1+ colonies reached 56–60 μm at DIV 17. The typical evolution of the Isl1+ CSC-derived colonies over the period from DIV 2 to DIV 11 is presented in Figure 6A–C. The median optical sections of the colonies clearly demonstrated that CSCs were localized in the core of the colony (Fig. 6D). In particular, Isl1+ cells (12th section out of 24) were tightly clustered in the center of the colony, having a vertical dimension of 36 μm (Fig. 6D, left panel). Mature cardiac myocytes were arranged peripherally and included both pre-existing myocytes and those differentiated from Isl1+ progenitors. The latter notion is supported by the fact that some actin-positive cardiac myocytes within the colony still co-expressed Isl1 antigen. The colonies of c-kit+ cells were characterized by a more diffuse distribution of CSCs (Fig. 6D, middle panel). The peripheral zone of the colony also contained actin-positive cardiac myocytes co-expressing c-kit. The Sca1+ CSC-derived colonies were flat (19 μm in z-axis) and contained a small amount of centrally nested CSCs (11th section out of 20) (Fig. 6D, right panel). Figure 6E displays a representative example of the spatial inhomogeneity of the c-kit+ CSC-derived colony in terms of cardiomyogenic differentiation. Cardiac myocytes expressing α-sarcomeric actin (red) were more prevalent in the upper part of the colony (Z = 20), while immature c-kit+ CSCs (green) tended to be localized in the basal part of the colony (Z = 5). The coexpression of c-kit+ and α-sarcomeric actin was evident in some small cells (~10 μm) residing in the core of the colony (Z = 10), which is indicative of the beginning of cardiomyogenic differentiation (Fig. 6E, middle panel). This finding supports the suggestion that these cells might represent a population of transit amplifying cells committed to the cardiomyocyte lineage. Figure 7A demonstrates the maturation of cardiac myocytes within the representative Isl1+ CSC-derived colony on DIV 13 (66 μm in z-axis). The middle part of the colony contained both Isl1+ cells (green, FITC) and cardiac myocytes (red, crystal violet) of comparable size. However, the Isl1+ cells were observed to be much smaller in the basal part of the colony (Fig. 7B), which suggests that the differentiation of Isl1+ CSCs associated with their enlargement is initiated in the upper portion of the colony. It seems that Isl1+ CSCs acquire the ability to differentiate after several rounds of division, which is corroborated by the observation that less mature Isl1+ colonies contain smaller Isl1+ CSCs (Fig. 7A cf. Fig. 6D). At the same DIV, c-kit+ CSC-derived colonies exhibited different architecture. A representative c-kit+ colony (24 μm in z-axis) is shown in Figure 7C and D. Both the basal and apical portions of the colony contained a significant amount of centrally localized undifferentiated c-kit+ CSCs (green) surrounded by mature cardiac myocytes (red) that were initially present in the culture. It follows, therefore, that in contrast to Isl1+ cells, c-kit+ CSCs demonstrated active proliferation without any sign of differentiation on DIV 13.
Figure 6. Structure of cardiomyocyte colonies grown in the primary culture of rat neonatal myocardial cells. (A–C) Different stages of development of the colonies stemming from Isl1+ CSCs. (A) Cell division, DIV 2. Isl1+ (FITC, green), GATA-4 (phycoerythrin, red). (B) Colony consisting of approximately 8 cells, DIV 11. Isl1+ (FITC, green), actin (rhodamine-phalloidin, red). (C) Large Isl1+ colony, DIV 11. Isl1+ (FITC, green), actin (rhodamine-phalloidin, red). (D) The optical sections of colonies formed by Isl1+, c-kit+, and Sca1+ CSCs on the 11th DIV. Isl1+ CSCs (Alexa 405, blue), Z = 12. c-kit+ CSCs (FITC, green), Z = 12. Sca1+ CSCs (Alexa 405, blue), Z = 11. Actin was stained using rhodamine-phalloidin (red). (E) Differentiation of c-kit+ CSCs inside the colony on the 13th DIV. Overlaid optical section of transmitted light and fluorescent images in 2 emitting wavelengths: 488 nm (FITC) and 543 nm (Alexa) in the bottom (Z = 5), in the middle (Z = 10), and the top (Z = 20) parts of the colony. c-kit+ expression was revealed by FITC-conjugated antibodies (green), and α-sarcomeric actinin was revealed by Alexa-conjugated antibodies (red). Confocal microscope, Leica TCS SP5 (Germany), objective ×63, oil.
Figure 7. Differentiation of Isl1+ and c-kit+ CSCs inside the colonies on the 13th DIV. The optical sections of colonies on 2 levels: (A) Isl1+ middle (Z = 14) and (B) bottom (Z = 0). (C) c-kit+ top (Z = 10) and (D) bottom (Z = 0). Confocal microscope, LEICA TCS SL, objective ×63, oil.
Discussion
The present work describes the formation and evolution of cardiomyocyte colonies stemming from Sca-1+, c-kit+, and Isl1+ CSCs in the primary culture of neonatal rat myocardial cells. Microcolony formation initiated soon after plating the myocardial cells, which was concomitant with intensive proliferation of pre-existing cardiac myocytes. The cessation of myocyte division with the subsequent transition to hypertrophy was accompanied by the gradual differentiation of the cells comprising the colonies. The colonies were characterized by CSC subset-specific 3-dimensional structures, with CSCs residing in the core, and cardiac-specific protein-expressing cells localized peripherally. Spontaneous functional cardiomyogenic differentiation of the cells within some but not all colonies resulted in coordinated contractile behavior, which was further verified by the presence of typical calcium transients, calcium-induced calcium release (CICR) processes, and electromechanical coupling. Cardiomyogenic differentiation of CSCs has been demonstrated convincingly using either chemical stimulation of purified and expanded CSCs with certain agonists3,11,12 or co-cultivation of CSCs with neonatal10 and adult15 cardiac myocytes. In the present study, an alternative approach was used; that is, the proliferation and differentiation of resident CSCs belonging to different subsets in a primary culture of neonatal rat myocardial cells were characterized. This approach offers the advantage of combining the characterization of pre-existing cardiac myocyte growth during the first several days of postnatal life and resident CSC proliferation/differentiation during a 30-d period.
Myocardial cell growth in primary culture
A better understanding of the mechanisms that govern the physiological regulation of cellular homeostasis in the mammalian heart during the first 7 d of postnatal life is crucial for the development of cardiac regeneration therapies. It is known that the proliferation of cardiac myocytes ceases after the third postnatal day, thereby preventing the efficient repair of myocardial injury in the adult heart.20,21 To further explore the proliferation of cardiac myocytes, we first characterized cell growth in a primary culture of cardiac cells obtained from newborn rats. The data indicated that the number of proliferating cells with a cell volume varying from 250 to 1000 μm3 decreased dramatically on day 4 of culturing. This process was determined to be accompanied by the intense growth of these cells, because the proportion of cells with volumes exceeding 1000 μm3 averaged 93% on the fourth DIV. Furthermore, the mean volume of the cultured cells increased progressively during culturing and averaged 819 ± 68, 1532 ± 212, and 3246 ± 190 μm3 on DIV 1, DIV 3, and DIV 6, respectively (Fig. 1B). It has been shown previously that the number of cardiac myocytes increases by 68% during first 3 d of postnatal development in the rat heart.20 This burst of proliferative activity is followed by its cessation, while the volume of cardiac myocytes was shown to increase 2.5-fold from the 3rd to 12th day of postnatal development (1416 ± 320 and 3533 ± 339 μm3, respectively).20 In our study, the mean cell volume increased 1.9-fold on DIV 3 and 4-fold on DIV 6 compared with baseline (Fig. 1B). It has been shown that the overwhelming majority of cardiac myocytes are mononucleated in the newborn rat until the age of 3 d.20 Starting from the fourth day after birth, the number of binucleated cardiac myocytes progressively increased, averaging 90% in 12-d-old rats. Notably, karyokinesis was preceded by intensive DNA synthesis, which did not result in mitotic division. Our data are consistent with these observations, showing similar temporal dynamics of these processes. It follows, therefore, that the process of cell growth in primary cultures of myocardial cells has much in common with cell dynamics in the neonatal heart. The time of transition of cardiac myocytes from hyperplasia to hypertrophy was comparable in these 2 models, as were the volumes of cardiac myocytes at the beginning of the hypertrophy phase. Taken together, these observations suggest that primary cultures of neonatal rat myocardial cells could be an adequate in vitro model to investigate the factors that block cardiomyocyte proliferation.
Structure of cardiomyocyte colonies
Considering the presence of small round-shaped cells (<250 μm3) in the cultures could potentially represent CSCs, we utilized immunocytochemical techniques to identify CSC subsets within the growing cardiomyocyte colonies. Consequently, Sca1+, c-kit+, and Isl1+ CSC-derived 3D-patterned colonies were identified and characterized. Significant variability in the time to onset of first contractions and the diameter and height of the colonies is suggestive of the major biological differences in the rate of proliferation/differentiation of colony-forming CSC subsets. In particular, it was found that Sca1-positive CSCs have a lower proliferation rate and, thus, produce small flat colonies incapable of contractions, at least during 25 d of culturing. Consistent with these findings, Sca1+ CSCs were previously shown to have lower proliferation rates and a reduced capacity for differentiation in comparison with other CSCs.22 C-kit+- and Isl1+ CSC-derived colonies had comparable dimensions but were different in terms of differentiation rates. While weak asynchronous contractions were registered in Isl1+ CSC-derived colonies as early as DIV 8, the c-kit+ CSC-derived colonies displayed first contractions by the 18th DIV. The delayed appearance of the contractile phenotype in c-kit+ CSC-derived colonies might be explained by an extended duration of the proliferation phase in c-kit+ CSCs. In contrast to more committed Isl1+ progenitors, c-kit+ cells are considered to be the only true CSCs;6 hence, c-kit+ cells require more cycles of division before differentiation is initiated. This idea is supported by the finding that c-kit+ CSC-derived colonies at the 13th DIV contained high numbers of centrally localized small c-kit+ CSCs (Fig. 7C and D), while Isl1+ CSC-derived colonies were composed of relatively large Isl1+ cardiac myocytes (Fig. 7A and B).
Cardiomyogenic differentiation of Isl1+ and c-kit+ CSCs within the colonies was confirmed by the expression of α-sarcomeric actin and sarcomeric α-actinin. Notably, the number of cardiomyocyte colonies grown in culture was quite low, averaging ~10 colonies per 100 000 cells. Only 1–2 colonies out of 10 acquired the ability to contract. Assuming that each colony is produced by a single CSC, this finding fits well with the available data on the number of CSCs in the heart. For example, there are only 500–600 Isl1+ CSCs in the heart of 1–5-d-old rats10 and 500–1000 and ~45 000 Sca+ and c-kit+ CSCs in the adult mouse heart, respectively.15,16 The major difference of the present study from previous works on cardiomyogenic differentiation of CSCs (e.g., Laugwitz and coworkers10) is that CSCs were cultured together with other myocardial cells, including cardiomyocytes, fibroblasts, endothelial cells, and vascular smooth muscle cells. Given that cell (de)differentiation could be induced by paracrine factors of cellular origin,23 direct coupling,15 or cytosolic24,25 and/or mitochondrial exchange,26 we hypothesize that the commitment of a CSC to a certain lineage might depend on the type of neighboring cell(s). If this assumption is true, the contact of a CSC with a mature cardiac myocyte would trigger its differentiation in the cardiomyogenic direction, while contacts with endothelial cells or vascular smooth muscle cells would commit a CSC to that respective lineage. This idea is supported by the findings on the ability of Isl1+ CSCs and especially Sca-1+ and c-kit+ CSCs to differentiate not only into cardiac myocytes, but also into vascular cells.7 Interestingly, owing to the relatively low density of culturing in our experiments, occasional CSC-derived colonies were most likely deprived of any contact with mature cells. This situation is analogous to the formation of clones derived from a single CSC, where all of the cells of the clone retain an undifferentiated state, albeit they express the same marker as the ancestor cell.6 However, the total number of colonies found in our experiments corresponds fairly well to the data on the amount of CSCs in the newborn heart (see above). The proportion of contracting colonies might reflect the number of CSC-derived colonies that were able to establish contact with neighboring mature cardiac myocytes. Further studies are needed to test this hypothesis.
Although the number of contracting cardiomyocyte colonies was found to be moderate, the number of mature cardiac myocytes produced from CSCs within the colonies was appreciable. At the advanced stages of culturing, mature colony-derived cardiac myocytes were emanating from the colonies, and some of the myocytes were integrated into the monolayer. Based on the data on the enhanced differentiation of cardiac myofibroblasts27 and CSCs28 in 3D culture systems, we suggest that the 3D structure of the cardiomyocyte colonies contributed to the improved cardiomyogenic yield observed in the present study.
Functional differentiation of cardiac myocytes within the colonies
Synchronization of individual cell contractions within the colony, as well as the progressive increase in the contraction rate over time, could be attributed to the maturation of both dihydropyridine receptors and RyRs, leading to the establishment of electromechanical coupling typical of fully differentiated cardiac myocytes.29 Therefore, caffeine-induced Са2+ release from the SR provides strong evidence for the final cardiomyogenic differentiation of the colony cells. Furthermore, synchronously contracting cells of the colonies demonstrated a coordinated response to caffeine in the Са2+-containing medium but failed to produce a Са2+ response in a Са2+-free environment. This provides additional evidence for the complete differentiation of colony cells into mature cardiac myocytes displaying typical CICR.
An increasing spatial association of dihydropyridine receptors and RyRs, due to the growth of the T-system, contributes to effective CICR, which ensures the contraction rate of the colonies approached 58–60 beats/min. It is generally accepted that CICR is not operative during the embryonic period in the mammalian heart, emerging as a mechanism of intracellular Са2+ regulation several weeks postnatally.29 Soon after birth, the contribution of Са2+ release through RyRs to the total intracellular Ca2+ rise during systole does not exceed 15%, while it averages 88% at 3 wk of age.30 In our model, it required 18–20 d for the colonies to mature from weakly contracting microcolonies to large 3D-patterned colonies demonstrating synchronous high-magnitude contractions of the entire colony. This period closely resembles the evolution of electromechanical coupling from predominantly sarcolemma-dependent in the neonatal mammalian heart to CICR-dependent at the age of 3 wk. A similar trend was also observed in cultured embryonic stem cells.31,32 However, while embryonic stem cell cultures can be used successfully to investigate prenatal heart development,33,34 the formation of contracting cardiomyocyte colonies is more relevant to postnatal cardiomyogenesis.
Limitations of the study
The present study has several methodological limitations. Although the registration of spontaneous and agonist-induced Са2+ transients convincingly demonstrated the functional differentiation of cardiac myocytes in the colonies, electrophysiological action potential recordings would also be valuable in verifying cardiomyocyte phenotypes. In addition, detailed information on the ratio between different cell types (cardiac myocytes, fibroblasts, endothelial, and vascular smooth muscle cells) in the primary culture would aid in the understanding of the mechanisms of colony formation. It also remains to be elucidated whether the process of contracting-colony formation in culture is relevant to in vivo cardiomyogenesis.
Conclusions
The present study demonstrated that culturing resident CSCs in a mixed culture of neonatal myocardial cells resulted in full cardiomyogenic differentiation of CSCs, which was associated with the formation of contracting cardiomyocyte colonies. Immunocytochemical staining of the cultured cells with antibodies against Sca1, c-kit, and Isl1 antigens showed that the immunophenotype of some of the cultured cells closely resembled the characteristics of previously described CSCs.3,7,10 In contrast to previously published studies on agonist-induced CSC differentiation, we observed spontaneous full cardiomyogenic differentiation of CSCs within the colonies, possibly due to co-cultivation with mature cardiac myocytes. The data obtained demonstrate that over the course of colony development, resident CSCs undergo mitotic division followed by a differentiation phase, resulting in the formation of a contracting colony composed of mature cardiomyocytes. It is likely that similar processes might take place in the in vivo setting, especially after various forms of cardiac injury (e.g., ischemia or mechanical overload) triggering the proliferation of CSCs. Moreover, it might be suggested that the process of contracting-colony formation in a primary culture not only simulates myocardial regeneration after injury, but also underlies myocardial self-renewal. In this regard, the formation of contracting colonies in the culture could be a valuable tool for investigating the mechanisms of CSC proliferation and differentiation as well as the modulation of CSC proliferation/differentiation with certain exogenous and endogenous factors. In addition, the proposed model could be used for electrophysiological studies aimed at investigating the mechanisms of cardiac pacemaker activity, preclinical testing of drugs with putative chronotropic or inotropic effects, and/or other applications.
Materials and Methods
Animals
Cardiac cell suspensions were prepared from 1–2-d-old Wistar rats. All of the experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the local ethics committee.
Isolation of cardiac cells
The hearts were enzymatically dissociated into a single cell suspension as previously described with modification.17 Briefly, the hearts were rapidly excised and rinsed in Ringer solution consisting of 146 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 11 mM glucose, and 10 mM HEPES at pH 7.4. The hearts were minced and incubated in the same solution with the addition of 1 mg/ml collagenase IA (Sigma-Aldrich) and 0.12% trypsin (FLUKA, Sigma-Aldrich) at 37 °C for 30 min. The suspensions thus obtained were left for 2–3 min for precipitation of undissociated tissue fragments. The supernatant was centrifuged at 400 × g for 10 min.
Cell culture
Cells were transferred to DMEM supplemented with 10% fetal calf serum (Biolot, Russia), 50 U/ml of penicillin, and 50 μg/ml of streptomycin (Biolot), followed by a 1 h preincubation in a glass petri dish for the elimination of non-myocytic cells. The cells were cultured on two 12 × 24-mm glass strips pre-coated with 0.1 mg/ml poly-D-lysine (Sigma-Aldrich) placed in 40-mm petri dishes (Medpolimer). The cells were plated at an initial density of 1 × 105 cells/ml. The incubation was performed in a CO2 incubator (Jouan) at 37 °C in humid air containing 5% СО2. The medium was changed 2 times per week.
Cell proliferation and volume
The proliferation of the cardiac cells was assessed by the quantification of mitotic cells in the colchicine-treated culture. The cells were cultured in 24-well plates (Costar). Colchicine (Serva), at a final concentration of 0.05 μg/ml, was added daily to 3 successive wells, followed by the determination of the proportion of mitotic cells on images obtained the succeeding day using an inverted microscope (PIM-III, WPI) and digital camera (DFC300 FX, Leica). The results were generated by averaging the data obtained in 5 individual experiments. The cell dimensions were determined using living cell populations (PIM-III, WPI). The cell length (D) and width (d) were determined using digital morphometry (VideoTest) on the images of the cells removed from the surface of the plate using a mixture of trypsin-versene (1:3). The cell volume (V) was calculated using the following formula: V = 3.14 / 6 × d2 × D. The cell dimensions were assessed in 3 individual experiments. The cell colonies formed in the culture were stained by hematoxylin (Sigma).
Intracellular calcium transients
For intracellular Ca2+ transient measurements, cells were incubated with membrane-permeable fura-2 (10 μM in Ringer solution for 1 h at 26 °C; Sigma-Aldrich). The cells were analyzed using the InCytIM2TM dual wavelength fluorescence imaging system (Intracellular Imaging Inc). Ca2+ transients were estimated as a ratio of fluorescence at 340/380 nm (F340/F380).18 Ringer solution containing 120 mМ KCl and 26 mМ NaCl was used for membrane depolarization. The cardiomyogenic differentiation of the cells within the growing colonies was additionally verified by the determination of acetylcholine- and ryanodine receptor agonist-induced Ca2+ responses. For this purpose, the cells were exposed to the following treatments: Ach (20 μM), Caf (5 mM), and hyperkalemic Ringer solution (120 mM KCl). Both Ach and Caf were purchased from Sigma-Aldrich.
Immunocytochemistry
Cultured cells and cell colonies were washed with PBS and fixed in 2.5% paraformaldehyde for 20 min at room temperature. Then, the cells were permeabilized with 0.25% Triton-X100 in PBS for 10 min. Two different protocols for immunostaining were used. In the first series, the immunostaining of the CSCs was performed using the following antibodies: mouse anti-c-kit monoclonal antibodies (Invitrogen, 5 μg/ml), mouse anti-Sca1 polyclonal antibodies (Abcam, 1:100), and rabbit anti-Isl1 monoclonal antibodies (Abcam, 1:100). Rabbit anti-GATA4 polyclonal antibodies (Abcam, 1:500) were used as a marker of early cardiomyogenic differentiation. The secondary antibodies were as follows: goat anti-mouse phycoerythrin-conjugated antibodies (Invitrogen, 1:100), goat anti-mouse FITC-conjugated antibodies (AbD Serotec, 1:100), and donkey anti-rabbit FITC-conjugated antibodies (AbD Serotec, 1:100). The cells were stained with crystal violet (2 mg/ml, Sigma-Aldrich) for 5 min. In the second series, the primary mouse anti-Isl1 (Abcam) and anti-Sca1 (Abcam) monoclonal antibodies were preliminary conjugated with Alexa 405 according to Zenon technology (Invitrogen) and then used for immunostaining at a 1:100 dilution.
Commercially available FITC-conjugated antibodies (Abcam, 1:100) were used for the detection of c-kit+ CSCs. Filamentous actin was detected using rhodamine-phalloidin (10 μg/ml, Sigma-Aldrich). Mouse anti-sarcomeric α actinin antibodies (Abcam) were used for confirmation of cardiodifferentiation of cells inside the colonies. The cell nuclei were stained with Hoechst (10 μg/ml, Molecular Probes). The cells were visualized by confocal microscopy with 40× and 63× oil objectives (Leica TCS SP5) or a 63× oil objective (Leica TCS SL). The optical sections were spaced 1.02 μm along the z-axis.
Statistical analysis
All of the data are expressed as the mean ± standard deviation. The statistical analyses were performed using the SPSS 13.0 software package (SPSS Inc Software). Significant differences between groups were evaluated using analysis of variance. P values ≤ 0.05 were considered significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to Dr M Galagudza for helpful suggestions during preparation of the manuscript. The study was supported by grants from Russian Foundation of Fundamental Research (RFFI No 09-04-00954 and 12-04-00941), Program of Presidium of Russian Academy of Sciences “Fundamental Sciences for Medicine” (2012, 2013).
Glossary
Abbreviations:
- CSCs
cardiac stem cells
- DIV
day in vitro
- RyR2
ryanodine receptors
- SR
sarcoplasmic reticulum
- Ach
acetylcholine
- Caf
caffeine
- CICR
calcium-induced calcium release
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27768
References
- 1.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ, Chien KR. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–7. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 3.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 5.Koudstaal S, Jansen Of Lorkeers SJ, Gaetani R, Gho JM, van Slochteren FJ, Sluijter JP, Doevendans PA, Ellison GM, Chamuleau SA. Concise review: heart regeneration and the role of cardiac stem cells. Stem Cells Transl Med. 2013;2:434–43. doi: 10.5966/sctm.2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/S0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto S, Kawaguchi N, Ellison GM, Matsuoka R, Shin’oka T, Kurosawa H. Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts. Stem Cells Dev. 2010;19:105–16. doi: 10.1089/scd.2009.0041. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi N, Smith AJ, Waring CD, Hasan MK, Miyamoto S, Matsuoka R, Ellison GM. c-kitpos GATA-4 high rat cardiac stem cells foster adult cardiomyocyte survival through IGF-1 paracrine signalling. PLoS One. 2010;5:e14297. doi: 10.1371/journal.pone.0014297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laugwitz K-L, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vliet P, Roccio M, Smits AM, van Oorschot AA, Metz CH, van Veen TA, Sluijter JP, Doevendans PA, Goumans MJ. Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth Heart J. 2008;16:163–9. doi: 10.1007/BF03086138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuura K, Wada H, Nagai T, Iijima Y, Minamino T, Sano M, Akazawa H, Molkentin JD, Kasanuki H, Komuro I. Cardiomyocytes fuse with surrounding noncardiomyocytes and reenter the cell cycle. J Cell Biol. 2004;167:351–63. doi: 10.1083/jcb.200312111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–41. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 15.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 16.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam ML, Bartoli M, Claycomb WC. The 21-day postnatal rat ventricular cardiac muscle cell in culture as an experimental model to study adult cardiomyocyte gene expression. Mol Cell Biochem. 2002;229:51–62. doi: 10.1023/A:1017999216277. [DOI] [PubMed] [Google Scholar]
- 18.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- 19.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28:1737–46. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- 21.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–80. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006;113:1451–63. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- 23.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–9. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: a novel mechanism for cell fate changes? Circ Res. 2005;96:1039–41. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 25.Acquistapace A, Bru T, Lesault PF, Figeac F, Coudert AE, le Coz O, Christov C, Baudin X, Auber F, Yiou R, et al. Human mesenchymal stem cells reprogram adult cardiomyocytes toward a progenitor-like state through partial cell fusion and mitochondria transfer. Stem Cells. 2011;29:812–24. doi: 10.1002/stem.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plotnikov EY, Khryapenkova TG, Vasileva AK, Marey MV, Galkina SI, Isaev NK, Sheval EV, Polyakov VY, Sukhikh GT, Zorov DB. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med. 2008;12(5A):1622–31. doi: 10.1111/j.1582-4934.2007.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poobalarahi F, Baicu CF, Bradshaw AD. Cardiac myofibroblasts differentiated in 3D culture exhibit distinct changes in collagen I production, processing, and matrix deposition. Am J Physiol Heart Circ Physiol. 2006;291:H2924–32. doi: 10.1152/ajpheart.00153.2006. [DOI] [PubMed] [Google Scholar]
- 28.Bartosh TJ, Wang Z, Rosales AA, Dimitrijevich SD, Roque RS. 3D-model of adult cardiac stem cells promotes cardiac differentiation and resistance to oxidative stress. J Cell Biochem. 2008;105:612–23. doi: 10.1002/jcb.21862. [DOI] [PubMed] [Google Scholar]
- 29.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 30.Escobar AL, Ribeiro-Costa R, Villalba-Galea C, Zoghbi ME, Pérez CG, Mejía-Alvarez R. Developmental changes of intracellular Ca2+ transients in beating rat hearts. Am J Physiol Heart Circ Physiol. 2004;286:H971–8. doi: 10.1152/ajpheart.00308.2003. [DOI] [PubMed] [Google Scholar]
- 31.Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91:189–201. doi: 10.1161/01.RES.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Lieu DK, Siu CW, Fu JD, Tse HF, Li RA. Facilitated maturation of Ca2+ handling properties of human embryonic stem cell-derived cardiomyocytes by calsequestrin expression. Am J Physiol Cell Physiol. 2009;297:C152–9. doi: 10.1152/ajpcell.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satin J, Kehat I, Caspi O, Huber I, Arbel G, Itzhaki I, Magyar J, Schroder EA, Perlman I, Gepstein L. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol. 2004;559:479–96. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Evans T. Embryonic stem cells as a model for cardiac development and disease. Drug Discov Today Dis Models. 2008;5:147–55. doi: 10.1016/j.ddmod.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.