Abstract
The circadian timing system orchestrates most of mammalian physiology and behavior in synchrony with the external light/dark cycle. This regulation is achieved through endogenous clocks present in virtually all body cells, where they control key cellular processes, including metabolism, transport, and the cell cycle. Consistently, it has been observed in preclinical cancer models that both the efficacy and toxicity of most chemotherapeutic drugs depend on their time of administration. To further explore the molecular basis underlying the link between the circadian timing system and the cellular response to anticancer drugs, we investigated the circadian transcriptome and CDK inhibitor toxicity in colon mucosa cells. We first show here that among 181 circadian transcripts, approximately 30% of them drive the cell cycle in the healthy mouse colon mucosa, with a majority peaking during the early resting phase. The identification of 26 mitotic genes within this cluster further indicated that the transcriptional coordination of mitosis by the circadian clock participates in the gating of cell division in this tissue. Subsequent selective siRNA-mediated silencing of these 26 targets revealed that low expression levels of the mitotic and anti-apoptotic gene Birc5/survivin significantly and specifically increased the sensitivity of colon epithelial cells to CDK inhibitors. By identifying Birc5/survivin as a potential determinant for the circadian modulation of CDK inhibitor toxicity, these data provide a mechanistic basis for the preclinical development of future CDK inhibitor-based chronotherapeutic strategies.
Keywords: colon mucosa, circadian clock, cell cycle, Birc5, cyclin-dependent kinase inhibitor, seliciclib
Introduction
Living organisms, from cyanobacteria to humans, adapt their cellular, physiological, and behavioral processes to the predictable alternation of days and nights. The resulting rhythms are driven by self-sustained endogenous clocks oscillating with a circadian (~24 h) periodicity, which all share common design principles based on a time delayed feedback mechanism.1 In mammals, circadian clocks are found in virtually every cell and are organized as a hierarchical and integrated system with a central pacemaker at the top, located in the suprachiasmatic nuclei (SCN) of the hypothalamus, that is reset every day by light, and which, in turn, coordinates local clocks present in the periphery.2 Although peripheral clocks oscillate autonomously, they require an intact SCN clock to keep their phase relationship through both neuronal and endocrine signaling pathways.3 At the molecular level, the core mechanism of mammalian clocks is composed of a set of clock genes (Clock, Bmal1, Per1, Per2, Cry1, Cry2, RORα, RORβ, RORγ, Rev-erbα, Rev-erbβ) that interact together through complex interlocked transcriptional/posttranslational feedback loops.4 Robustness and appropriate time delay to maintain the 24 h periodicity is provided by a network of additional modulatory loops and extensive posttranslational regulation of all core components. Circadian oscillations of core clock gene expression are adjusted with regard to their phase through input pathways and drive downstream rhythmic outputs directly or indirectly.
In the periphery, a circadian rhythm of cell division and cell proliferation has been observed in multiple mammalian tissues and organs, including liver, skin, hair follicles, bone marrow, and intestine, while clock mutant mice display an altered cellular proliferation phenotype5-11. This regulation is thought to occur via transcriptional or posttranslational control by core clock proteins of cell cycle components such as the G2/M kinase Wee1, the ATM and Chek2 checkpoint protein, and the cyclin-dependent kinase inhibitors p16 and p21.5,11-13 These links are increasingly recognized to be highly relevant to cancer progression and treatment. Indeed, accumulating clinical and epidemiological evidence supports the notion that circadian disruption caused, for instance, by shift work, light at night exposure, or chronic jetlag, may be a neglected risk factor of human cancer epidemiology, while cancer progression may, in turn, contribute to circadian misalignment.14,15 This hypothesis is further supported by animal experiments demonstrating that genetic or environmental disruption of the circadian timing system accelerates spontaneous or provoked tumorigenesis.16,17 Additionally and importantly, the toxicity and efficacy of most anticancer drugs have been found to vary significantly as a function of the time of administration in preclinical models.18,19 This has formed the ground of the anticancer chronotherapy paradigm, which is to treat cancer patients at an optimal time of the day in order to minimize drug toxicity while maximizing the desired effect. Clinical trials comparing the chronomodulated administration of the topoisomerase inhibitor irinotecan with conventional delivery have accordingly demonstrated that toxicity can be decreased up to 5-fold, while efficacy is increased 2-fold when using these unconventional schedules.18,19
Several mechanisms, including the circadian regulation of drug metabolism, transport and disposal, as well as the cell cycle have been proposed to be responsible for the chronotoxicity and chronoefficacy of chemotherapeutic drugs.18,19 The contribution of each of them is likely to vary depending on target tissues/organs, cancer type, drugs, genetics, age, sex, and circadian system disruption, including that which may result from the treatment itself.20 Because many anticancer drug targets are directly involved in the cell cycle, we focused on this process to identify genes that may play a role in the circadian modulation of the cellular response to these drugs. We selected the mouse colon mucosa, as a spontaneously proliferative tissue with an established circadian pattern of cell proliferation8,21 and which is often a target of anticancer drugs and CDK inhibitors, as a novel class of anticancer drugs currently under development for the treatment of malignancies as well as brain and kidney diseases.22 Following the analysis of the colon mucosa rhythmic transcriptome and subsequent screening of cell cycle genes whose expression levels determine cellular sensitivity to the CDK inhibitor seliciclib, we uncover the mitotic and anti-apoptotic gene birc5/survivin as a rhythmic cell cycle gene whose expression level determines the cellular sensitivity to CDK inhibitors.
Results
Therhythmictranscriptomeofcolonmucosacellsishighlyenrichedforcellcycletranscripts
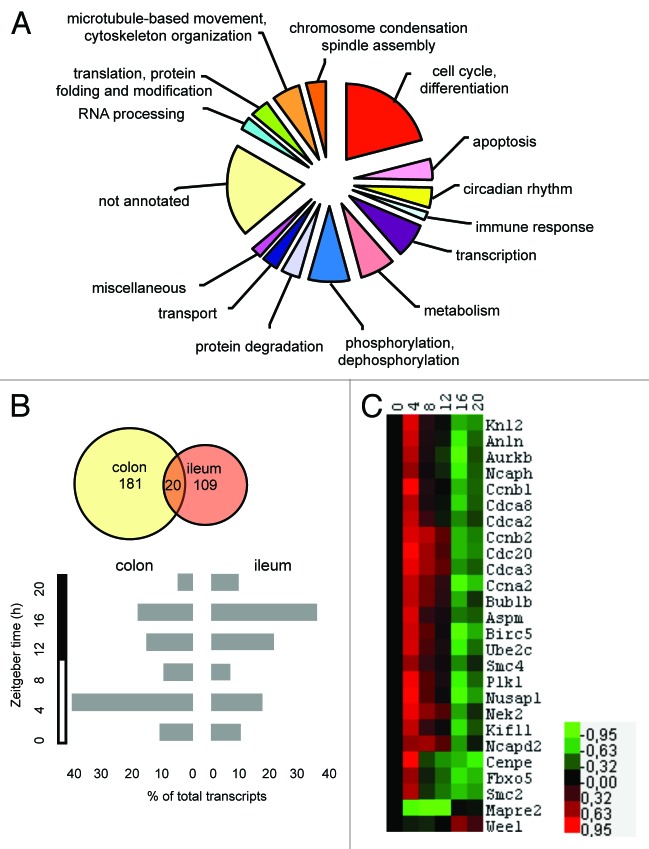
The colon mucosa epithelium is a classic example of tissue in which cells proliferate with a circadian periodicity in animals and humans.23 To get more insights into the mechanisms underlying the chronotoxicity of chemotherapeutic drugs in the colon mucosa we first performed a genome-wide analysis of circadian gene expression in adult mice maintained in a LD12:12 cycle. Proper entrainment of the colon mucosa molecular clock of these animals was confirmed by the profiling of clock and clock-controlled genes around the clock. All showed oscillation with a circadian period and the expected phase (FigS1; TableS1). Total mRNA were then analyzed using Affymetrix high-density microarrays, and following a stringent statistical analysis combining the SAM algorithm and a subsequent cosinor analysis, we identified 181 transcripts displaying a circadian gene expression pattern (TableS2). The annotation and functional categorization of this data set revealed a dramatic enrichment for genes related to cell cycle, apoptosis, spindle assembly, and microtubule organization, which together accounted for approximately 30% of all rhythmic transcripts identified in this screen (Fig.1A). As tissue specificity is a recognized hallmark of mammalian circadian gene expression in peripheral organs and tissues, we compared this data set with that from the distal ileum mucosa, which was sampled from the same animals. Following the SAM statistical analysis procedure, we found that the distal ileum mucosa transcriptome contained 109 rhythmic transcripts associated with processes consistent with the small intestine physiology, such as transport and metabolism. However, in contrast with the colon mucosa data set, no significantly enriched functional clusters emerged (TableS3). The overlap between the 2 data sets included 20 transcripts, among which 11 are known clock or clock-controlled genes, such as, for instance, Bmal1, Cry 2, Cirbp, and Dbp (Fig.1B, top; TableS4). The 9 remaining genes have not been previously associated with the circadian clock mechanism and may represent putative modulators of the core clock mechanism in the gastrointestinal tract.
Figure 1.
Genome-wide analysis of rhythmic gene expression in the mouse colon mucosa. (A) Functional categorization of the transcripts expressed rhythmically with a circadian period. (B) Overlap between the colon and ileum mucosa datasets (top) and comparison of the phase maps between these 2 tissues (bottom). (C) Heat map showing the clustering of the mitotic genes subset; numbers on the scale are Log2ratios relative to the ZT0 value.
The phase distribution analysis of the colon mucosa rhythmic transcriptome also revealed an unusual pattern, with nearly 60% of transcripts peaking during the light phase (ZT4–ZT8) (Fig.1B, bottom). This is in sharp contrast with the distribution observed in the ileum mucosa as well as in many other mouse peripheral tissues, in which a majority of transcripts peak during a larger time window centered around ZT8–16.10 Interestingly, virtually all the rhythmic transcripts related to the cell cycle, apoptosis, and cytoskeleton organization biological processes were clustered within the ZT4–ZT8 time window. To investigate further whether this was the result of the circadian coordination of particular cell cycle events, we analyzed in more detail the functional annotation of this subset of genes. We found that 26 out of 60 genes within this functional group were directly involved in the G2/M transition or specific steps of mitosis, strongly suggesting that cell division is gated by the circadian clock in this spontaneously proliferating tissue (Fig.1C; TableS5). This hypothesis is further supported by the observation that mRNA expression of the G2/M kinase Wee1 oscillated at the second harmonic of circadian rhythmicity in the colon mucosa of wild-type animals but not in that of clock-deficient Bmal1−/− animals (Fig.2; TableS1). We conclude from this analysis that progression of the cell cycle in the mouse colon mucosa through the G2/M-phase transition is temporally restricted to the early light phase around ZT4 and suggest that this occurs at least in part as a result of the CLOCK:BMAL1-dependent transcriptional regulation of the Wee1 gene.
Figure 2.
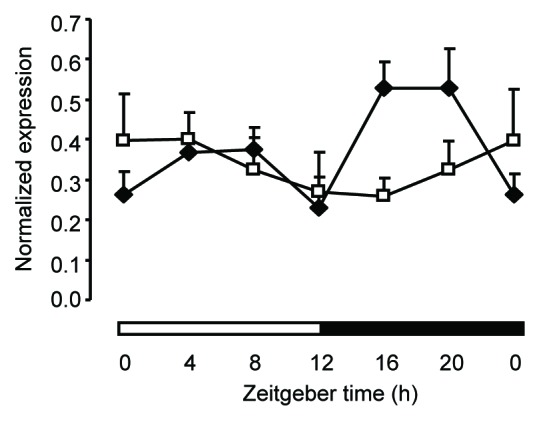
Wee1mRNA expression was measured every 4 h around the clock in the colon mucosa from wild-type (dark diamonds) and Bmal1−/− clock-deficient (open squares) mice. The black and white bars refer to the dark and light phases, respectively. The ZT0 time point is double plotted for visualization purposes. Oscillation at the second harmonic of circadian rhythmicity in wild-type mice was validated by the cosinor analysis (P < 0.01). Data are shown as mean + SEM, n = 3 for each time point.
Birc5expressionmodulatescoloncellssensitivitytotheCDKinhibitorseliciclib
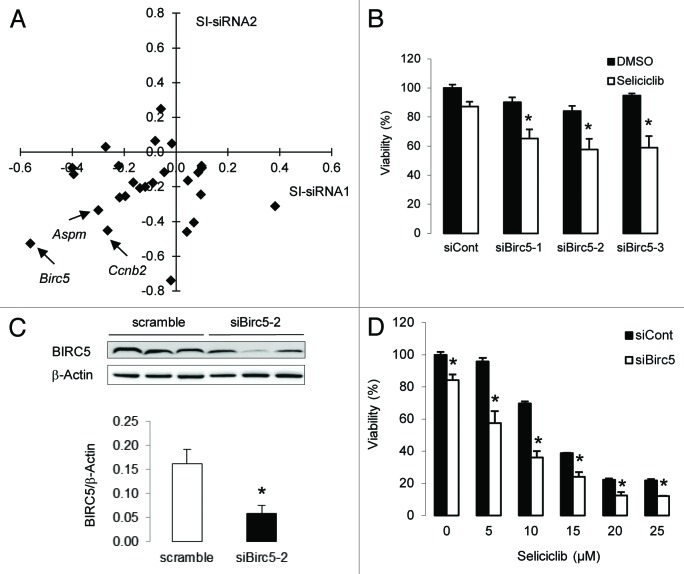
In an attempt to identify genes that may contribute to the chronotoxicity of CDK inhibitors, we reasoned that at least some of these genes would have to be both expressed with a circadian pattern and involved in the cell cycle process. To test this hypothesis, we selectively silenced each of the 26 M-pfhase genes identified in the microarray experiment in human HCT116 colon epithelial cells in the presence or absence of the seliciclib. HCT116 cells contain a functional circadian clock, and seliciclib is a CDK inhibitor currently under development, which has previously been shown to display a chronotoxicity profile in mice.24,25 Results obtained with 2 different siRNAs per target indicate that silencing of the mitotic and anti-apoptotic gene Birc5 (also known as survivin) most efficiently and consistently led to an increased sensitivity of HCT116 cells to the seliciclib treatment (Fig.3A). Silencing the abnormal spindle-like, microcephaly-associated (Aspm) and cyclin B2 (Ccnb2) mitotic genes also produced a similar effect, yet to a lower extent. To fully exclude off-target effects, the assay was repeated using a third anti Birc5 siRNA targeting a different region, and a similar result was obtained (Fig.3B). Figure3C shows that Birc5 silencing using the siBirc5-2 siRNA, which was used in all the follow-up experiments, resulted in a 64% reduction of BIRC5 protein levels as compared with the control scramble siRNA. Importantly, the magnitude of the combined effect of Birc5 silencing and selicilib treatment at 5 µM as compared with the effect produced by either treatment suggested a synergistic action. To further determine the range of this synergistic effect, we next performed a seliciclib dose-response curve. Results in Figure3D show that the synergistic effect of Birc5 silencing and seliciclib treatment was observed at the 5 µM concentration and to a lesser extent 10 µM but not at higher concentrations. The same effect was observed when using other CDK inhibitors, including alsterpaullone or the novel seliciclib derivative CR8, acting at a 30 times lower dose (Fig.4). To determine the specificity of this effect with regard to CDK inhibitors, we also tested 2 other classes of chemotherapeutic molecules, including the irinotecan derivative SN38, which targets topoisomerase 1, or the DNA damaging agent cisplatin. No effect similar to that of CDK inhibitors was observed with these compounds (Fig.4). We conclude that low Birc5 expression levels specifically sensitize HCT116 cells to CDK inhibitors.
Figure 3.
Identification of Birc5 as a potential determinant of seliciclib chronopharmacology. (A) HCT116 cells were treated with seliciclib (10 µM) in combination with siRNA targeting each of the 26 rhythmic mitotic genes identified in the microarray experiment. The correlation plot shows the effect on cell viability of 2 independent siRNA for every target (SI, sensitivity index). (B) Comparison of the effect of 3 siRNA targeting different sequences of the Birc5 mRNA on the viability of cells treated with seliciclib. (C) Expression of BIRC5 and β-Actin proteins in HCT116 cells treated with a scramble siRNA or the siRNA siBirc5-2 used in the follow-up experiments (upper panel, each lane represents an independent silencing experiment); quantification of BIRC 5 levels normalized to β-actin are shown in the bottom panel histogram; data are expressed as mean + SEM, n = 3. (D) Dose-response curve of seliciclib in the presence of a siRNA targeting Birc5 or a control siRNA. In (B and D), data are expressed relative to the siCont/DMSO control and as mean + SEM, n = 4–7. In (B–D) *denotes a P value < 0.05 with the Wilcoxon–Mann–Whitney test.
Figure 4.
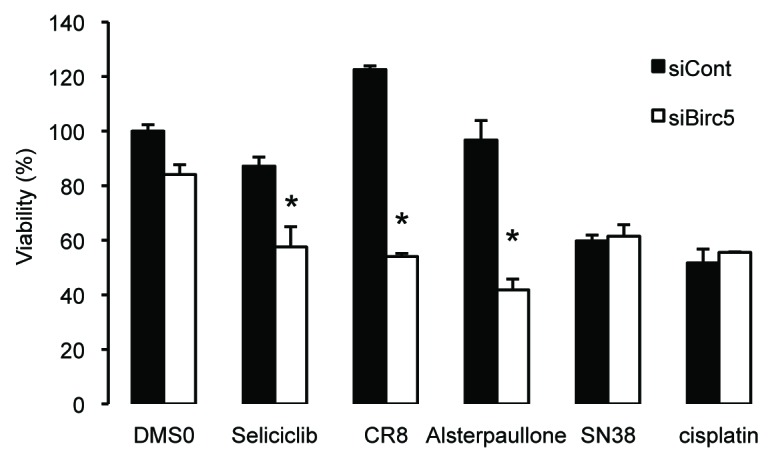
Birc5 silencing specifically sensitizes cells to CDK inhibitors. HCT116 cells were treated with a siRNA targeting Birc5 in combination with seliciclib (10 µM), CR8 (0.3 µM), Alsterpaullone (0.3 µM), irinotecan metabolite SN38 (5 µM), or cisplatin (3 µM). Data are expressed relative to the siCont/DMSO control and as mean+ SEM; n = 4; *denotes a P value < 0.5 with the Wilcoxon–Mann–Whitney test.
BIRC5oscillatesinhealthycolonmucosacells
To independently confirm the rhythmic expression profile of Birc5 at the mRNA and protein levels and determine precisely whether its phase correlates with the known chronotoxicity of seliciclib, we analyzed a separate set of mice around the clock with a 3 h time resolution. A significant 24 h period oscillation of the Birc5 mRNA was observed with a computed acrophase at ZT3:25 after light onset (cosinor P value < 0.001) (Fig.5A; TableS1). Western blotting analysis revealed a similar oscillation at the protein level, which closely mirrored that of the mRNA with peak and trough levels at ZT3–6 and ZT15–18, respectively (Fig.5B). This oscillation was specific to the colon, as no significant rhythm of the BIRC5 protein was observed in the ileum mucosa (Fig.S2). The BIRC5 profile strongly suggests that sensitivity of colon epithelial cells to seliciclib and other CDK inhibitors may be higher between ZT12–ZT21, when BIRC5 expression level is low.
Figure 5.
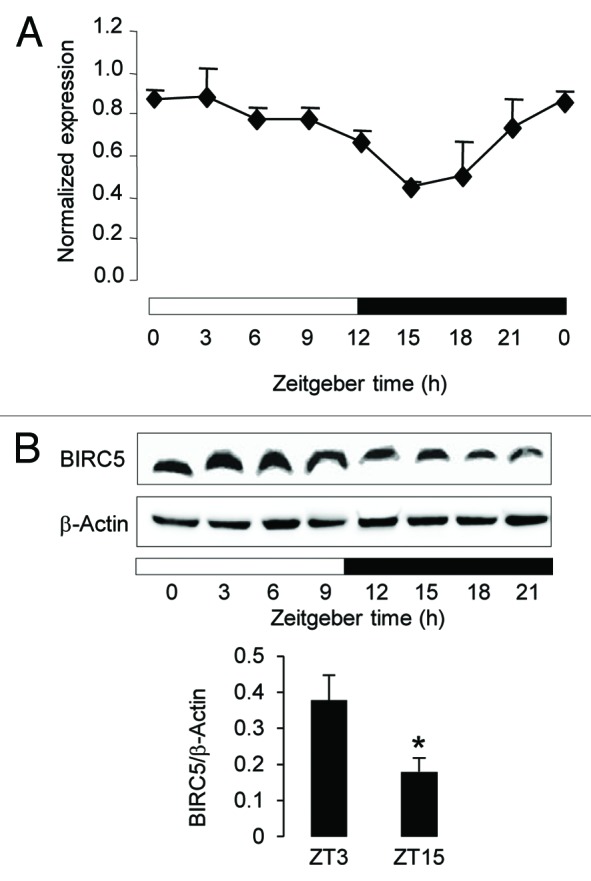
Circadian expression of Birc5 in the colon mucosa of B6D2F1 mice. (A) Birc5 mRNA expression was measured every 3 h around using qRT-PCR. Data were normalized using the constitutively expressed 36B4 mRNA. The ZT0 time point is double plotted for visualization purpose. Data are shown as mean + SEM, n = 3 for each time point. The circadian rhythmicity was validated by the cosinor analysis (P < 0.001) (B) BIRC5 protein expression was determined from ZT0 to ZT21 by Western Blotting analysis (upper panel). Peak (ZT3) and trough (ZT15) BIRC5 levels were determined in 3 independent samples and normalized to the β-actin levels (lower panel), data are shown as mean + SEM, *denotes a P value < 0.5 with the Wilcoxon–Mann–Whitney test. In (A and B), the black and white bars refer to the dark and light phases, respectively.
Discussion
Circadian rhythms of cell division have been observed for many years in several healthy rodent and human tissues including the oral and gastrointestinal mucosa.9,21,23 This may explain, at least in part, the chronopharmacological profile of chemotherapeutic drugs targeting specific cell cycle events as a result of a time of day-dependent toxicity toward healthy dividing cells.18,19 In this study, we have applied functional genomics and pharmacology approaches to the colon mucosa, a tissue in which healthy cells proliferate spontaneously with a circadian rhythm, in an attempt to identify putative molecular determinants of CDK inhibitors chronopharmacology.26 We focused our profiling analyses on mucosa cells instead of the whole tissue section, as reported previously, in order to enrich tissue samples with proliferating cells.27,28 This procedure identified cell cycle-related genes as the most prominent category of rhythmic transcripts. This dramatic enrichment for cell cycle genes was significantly higher than that previously reported for the colon, probably because only the mucosa was sampled in our study instead of the whole tissue.29 In support of this hypothesis, in a separate study, a 3-fold circadian variation was found for the mRNA expression of Abcc2, a protein involved in drug efflux in mouse ileum mucosa, while no rhythm was documented in ileum serosa.30 By analyzing the phase distribution of various subsets of rhythmic cell cycle transcripts, we also found that mitosis in this tissue is restricted to a very specific time window of the 24 h cycle that corresponds to the time when animals are resting. This finding was consistent with the antiphasic and BMAL1 dependent expression of the kinase Wee1, which inhibits entry into mitosis. Interestingly, while the Wee1 mRNA profile was markedly altered in the colon mucosa from clock-deficient Bmal1−/− mice, only subtle changes were seen for other mitotic genes, arguing against a direct regulation by the CLOCK:BMAL1 heterodimer for these genes (not shown). This strongly suggests that the Wee1-dependent coupling between the circadian clock and the cell cycle oscillators identified by Matsuo et al. in the regenerating liver5 might be more widespread than anticipated and apply to many cell types which require a circadian control of cell cycle progression. The rhythmic expression of other mitotic genes may then reflect a cell cycle-dependent adjustment to the clock-controlled expression of Wee1, rather than a direct regulation by CLOCK:BMAL1 of multiple cell cycle genes. Alternatively, their rhythmicity may be depending on post-transcriptional circadian regulation as recently suggested for a significant proportion of the genome.31 Collectively, these observations suggest that mitosis is gated by the circadian timing system in colon epithelial cells as in other cell types, possibly to protect cells from DNA damage resulting from exposure to food-borne chemicals during this critical cell cycle step.5,32 In support of this, cells lacking the circadian clock protein BMAL1 have a reduced sensitivity to DNA damaging anticancer drugs.33 It is therefore conceivable that, like other cells, colon mucosa cells are more susceptible to DNA damage during mitosis, and as a consequence both healthy and tumor cells are likely to respond to chemotherapy targeting mitotic proteins in a time of day dependent fashion in this tissue.
Experimental and clinical studies have shown that both the toxicity and the efficacy of 40 commonly used anticancer drugs are influenced by the time of administration.18,19 This time of day-dependent effect can be exploited for improving the therapeutic index of these drugs and has, accordingly, become the cornerstone for the implementation of chronomodulated delivery schedules to treat cancer patients.34 Increasing evidence indicates that the host circadian timing system plays a critical role in the chronopharmacology of chemotherapeutic agents, through its action on drug metabolism and disposal as well as the cell cycle, DNA repair, stress response, and apoptosis processes.18,19 The functional interaction between components of the clock and NFκB pathways may also additionally modulate in a circadian manner the effect of anticancer chemotherapy.35-37 Indeed, cancer cells undergo a NFκB-dependent metabolic adaptation, while tumor-promoting inflammation involving the NFκB pathway is a common feature of malignant disorders.38,39 In addition, compounds such as selenium have the potential to upregulate the BMAL1 core clock protein and thereby decrease the toxicity of cyclophosphamide in mice.40 This suggests that the concepts of anticancer chronotherapy could be advantageously extended to adjuvant drugs targeting the clock or cancer-associated processes such as inflammation.
One very common and important side effect of chemotherapeutic drugs results from their inability to discriminate rapidly dividing cancer cells from healthy proliferating cells, such as those from the bone marrow, the oral and gastrointestinal mucosae, as well as hair follicles. Because intestinal epithelial cells, and in particular those from the colon mucosa, exhibit a daily rhythm of cell division, it can be anticipated that chemotherapy schedules taking this variation into account may help to prevent potential side effects. Along this line, we have used the information gained from our microarray profiling experiment to investigate whether the expression level of specific mitotic genes could modulate the toxicity of the CDK inhibitor seliciclib. Seliciclib and its recent derivatives, such as CR8, are anticancer drug candidates currently under development to treat non-small cell lung cancer, nasopharyngeal cancer, and B-cell lymphomas, as well as non-malignant diseases including polycystic kidney disease.41,42 Seliciclib has also been shown to be effective for inhibiting human colon tumors in a xenograft model.43 We have previously shown that seliciclib displays a lethal toxicity that is significantly higher when administered at ZT19 as compared with ZT3.26 We therefore reasoned that the circadian oscillation of key cell cycle genes in the target tissues might contribute at least in part to the chronotoxicological profile of seliciclib. By focusing our siRNA screen to rhythmic mitotic genes, we identified the Birc5 gene as a candidate for the chronomodulation of seliciclib toxicity in colon epithelial cells. BIRC5 is a multifunctional nodal protein at the intersection between the cell cycle and cell death networks that is generally expressed at low levels in healthy tissues and overexpressed in virtually all tumors.44,45 The mitotic role of BIRC5 is to be a subunit of the chromosome passenger complex, which is required for proper chromosome segregation and cytokinesis, while its anti-apoptotic role is achieved through multiple interactions with cell death pathways.44 The use of Birc5 as a biomarker and a target for tumor diagnostic and treatment, respectively, has consequently become an attractive option for novel cancer therapeutic approaches.46 Indeed, small changes in its expression levels may lead to significant cell survival perturbation because of its hub protein function. This assumption is the rationale for the development of pharmacological inhibitors directed against the Birc5 mRNA, among which 2 of them, YM155 and LY2181308, are currently tested in phase II clinical trials.47 Here we provide evidence that: (1) the Birc5 gene is expressed with a significant circadian rhythm at the mRNA and protein levels in the colon mucosa, with the highest levels being observed between ZT0 and ZT3; and (2) siRNA-mediated silencing of Birc5 potentiates the toxicity of seliciclib at low doses in vitro. BIRC5 being a short-lived protein,48 its rhythmic expression pattern closely follows that of the mRNA and matches fairly well the known chronotoxicity profile of seliciclib, with the lowest toxicity at around ZT3. This predicts that exposure of colon epithelial cells to seliciclib when BIRC5 levels are high, that is during the early light phase, would decrease its toxicity in healthy dividing cells while allowing best efficacy as shown earlier.26 BIRC5 is phosphorylated by the CDK1–Cyclin B complex, and this is required for the correct localization of the chromosome passenger complex during mitosis.49 As CDK1 is selectively inhibited by seliciclib, we hypothesize that low BIRC5 expression levels combined with CDK1 inhibition result in a significant reduction of phosphorylated BIRC5 in treated cells, thereby increasing their sensitivity to the drug.50 This is supported by our observation that non-CDK inhibitor drugs do not synergize with Birc5 silencing to decrease cell proliferation. Because virtually all tumor cells overexpress BIRC5, innovative therapeutic strategies targeting Birc5 in humans may be improved by decreasing the exposure of healthy dividing cells during a specific time window corresponding to low expression levels, without losing therapeutic efficacy. Along this line, future therapies targeting Birc5 may benefit from a combined chronomodulated co-administration with seliciclib or its recent derivative CR8, which is active at up to 1000 lower doses.42,51
In summary, we have shown here that the colon mucosa displays a robust and extensive circadian regulation of the cell cycle transcriptional program. This work therefore extends the notion that the cell cycle is an important mechanism to be considered in anticancer drug chronopharmacology. The identification of BIRC5 as a link between the cell cycle, the circadian clock, and the cellular sensitivity to CDK inhibitors may also offer additional therapeutic opportunities for this promising molecular target.
MaterialsandMethods
Animals
Ten-wk-old B6D2F1 male mice were used for microarray profiling experiments. Clock-deficient Bmal1−/−male mice and their control littermates in the C57Bl/6J background were used at 8–12 weeks of age, before they develop arthropathy. Animals were housed in a 12 h light/12 h dark cycle (LD12:12), in a temperature- and humidity-controlled environment and fed ad libitum with chow diet. Zeitgeber time 0 (ZT0) referred to lights on. All experiments were conducted in compliance with the guidelines from CNRS and the local animal ethical committee for animal experiments.
DNAmicroarrays
Intestinal mucosa samples were collected and frozen in liquid nitrogen every 4 h for 24 h by scraping the luminal layer of dissected colon and ileum. Nine mice were used for each time point to generate 3 individual pools of 3 animals each. Total RNA was isolated with nucleospin RNA L kit from Macherey-Nagel (http://www.mn-net.com/tabid/1330/default.aspx). cDNA synthesis, biotin labeling of cRNA, and hybridization on Affymetrix GeneChip Murine Genome 430A 2.0 arrays (http://www.affymetrix.com/estore/browse/products.jsp?productId=131477#1_1) were performed according to the manufacturer’s recommendations at the Microarray Core Facility of the Institute of Research on Biotherapy, CHRU-INSERM-UM1 Montpellier, France (http://irb.chu-montpellier.fr/). To identify oscillating transcripts, data were processed in 2 steps. First, cell files were analyzed using the ChipInspector pipeline from Genomatix to detect transcripts displaying a differential expression during the time course. This software analyzes each probe individually and is based on the statistical analysis of microarray method.52 A probe coverage of 3 and a false discovery rate of 0% (colon) or 5% (ileum) were used. Time series for these transcripts were then subjected to a cosine wave fitting, using the cosinor method in order to identify oscillating transcripts with a 24 h period. Functional annotation and clustering was performed using DAVID.53 Microarray data are available at EBI ArrayExpress (http://www.ebi.ac.uk/microarray-as/ae/) under accession number E-MTAB-1576.
QuantitativeRT-PCR
Measurements were performed with a Light Cycler 1.5 (Roche Applied Science) using SYBR green I dye detection according to the manufacturer’s recommendations. cDNA, synthesized from 2–5 µg of total RNA using random primers and Superscript II (Invitrogen), was added to a reaction mixture (Faststart DNA SYBR Green I; www.roche-diagnostics.com) with appropriate primers at 0.5 μM each. Relative mRNA abundance was calculated using a standard curve method. Expression levels were normalized to the levels of the constitutively expressed 36B4 ribosomal protein mRNA. The sequence of the primer pairs used can be found in the Supplemental Material (TableS6).
CellcultureandsiRNA
HCT116 cells were obtained from ATCC and cultivated according to the supplier’s recommendations in Dulbecco modified Eagle medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum, 100 IU penicillin, 100 mg/ml streptomycin at 37 °C in 5% CO2. Cells were seeded at a density of 8000 cells/well in 96 multiwell plates and reverse transfected using the various Ambion silencer select siRNA (www.invitrogen.com) (TableS2) at a final concentration of 10 nM and lipofectamine (www.invitrogen.com). Two different siRNA per target were used. Cell viability was measured with the CellTiterGlow luminescent assay (www.promega.com) and a Centro LB960 luminometer (www.berthold.com). In the siRNA screen, the combined effect of seliciclib treatment and silencing was determined by calculating a sensitivity index for every targeted gene as follows: SI = Log2{[cell viabibility(siRNA+seliciclib)/cell viability(siRNA+DMSO)]/[average cell viabibility(siRNA+seliciclib) /average cell viability(siRNA+DMSO)]}. Seliciclib and CR8 were synthesized as described.54 Alsterpaullone, SN38 and cisplatin were from Sigma-Aldrich (www.sigmaaldrich.com). All drugs were dissolved in DMSO and used at the indicated concentrations.
Western blotting
HCT116 cells and mouse colon or ileum mucosae were frozen in liquid nitrogen and homogenized in cell lysis buffer from Cell Signaling (www.cellsignal.com). BIRC5 was detected in 30 µg of cytosolic proteins using standard immunoblotting procedures and a rabbit polyclonal anti-BIRC5 antibody from Abcam (ab24479) at a dilution of 1/500 (www.abcam.com). Loading was checked using a rabbit polyclonal anti-β-Actin antibody from Sigma-Aldrich (A2066) at a dilution of 1/300 (www.sigmaaldrich.com). Images were captured using a Fuji FX7 imager.
Statisticalanalyses
Circadian rhythms were tested, and their parameters computed with their respective 95% confidence intervals with the cosinor method using a script run with the R package Commander.55 Pairwise comparisons between control and treatment were tested using the Wilcoxon–Mann–Whitney non-parametric statistical test run with the R package Commander.55
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed
Acknowledgments
We thank Dr C.A. Bradfield, University of Wisconsin, Madison for kindly providing the Bmal1 knockout mice. Sophie Guérin is acknowledged for her expert technical assistance. This work was supported by CNRS, INSERM, University Nice Sophia-Antipolis, University Paris Sud, Hôpital Paul Brousse, ARC (grant N° 1032), the European commission through the FP6 projects Tempo LSHG-CT-2006-037543, and Crescendo LSHM-2005-18652, and the French National Agency for Funding of Research through the ERASYSBIO+ project C5SYS ANR-2009-SYSB-002-02 and the “Investments for the Future” LABEX SIGNALIFE program ANR-11-LABX-0028-01.
Glossary
Abbreviations:
- CDK
cyclin-dependent kinase
- qRT-PCR
quantitative real-time polymerase chain reaction
- SAM
statistical analysis of microarrays
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/27868
References
- 1.Doherty CJ, Kay SA. Circadian control of global gene expression patterns. Annu Rev Genet. 2010;44:419–44. doi: 10.1146/annurev-genet-102209-163432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–61. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- 3.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, et al. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–46. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowrey PL, Takahashi JS. Genetics of circadian rhythms in Mammalian model organisms. Adv Genet. 2011;74:175–230. doi: 10.1016/B978-0-12-387690-4.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–9. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 6.Spörl F, Korge S, Jürchott K, Wunderskirchner M, Schellenberg K, Heins S, Specht A, Stoll C, Klemz R, Maier B, et al. Krüppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc Natl Acad Sci U S A. 2012;109:10903–8. doi: 10.1073/pnas.1118641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granda TG, Liu XH, Smaaland R, Cermakian N, Filipski E, Sassone-Corsi P, Lévi F. Circadian regulation of cell cycle and apoptosis proteins in mouse bone marrow and tumor. FASEB J. 2005;19:304–6. doi: 10.1096/fj.04-2665fje. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy MF, Tutton PJ, Barkla DH. Comparison of the circadian variation in cell proliferation in normal and neoplastic colonic epithelial cells. Cancer Lett. 1985;28:169–75. doi: 10.1016/0304-3835(85)90072-2. [DOI] [PubMed] [Google Scholar]
- 9.Bjarnason GA, Jordan R. Circadian variation of cell proliferation and cell cycle protein expression in man: clinical implications. Prog Cell Cycle Res. 2000;4:193–206. doi: 10.1007/978-1-4615-4253-7_17. [DOI] [PubMed] [Google Scholar]
- 10.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A. 2007;104:3342–7. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gréchez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F. The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J Biol Chem. 2008;283:4535–42. doi: 10.1074/jbc.M705576200. [DOI] [PubMed] [Google Scholar]
- 12.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22:375–82. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Kowalska E, Ripperger JA, Hoegger DC, Bruegger P, Buch T, Birchler T, Mueller A, Albrecht U, Contaldo C, Brown SA. NONO couples the circadian clock to the cell cycle. Proc Natl Acad Sci U S A. 2013;110:1592–9. doi: 10.1073/pnas.1213317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Innominato PF, Focan C, Gorlia T, Moreau T, Garufi C, Waterhouse J, Giacchetti S, Coudert B, Iacobelli S, Genet D, et al. Chronotherapy Group of the European Organization for Research and Treament of Cancer Circadian rhythm in rest and activity: a biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res. 2009;69:4700–7. doi: 10.1158/0008-5472.CAN-08-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.monographs I. Painting, firefighting, and shiftwork. Eval Carcinog Risks Hum. 2010;98:9–764. [PMC free article] [PubMed] [Google Scholar]
- 16.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/S0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 17.Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Gréchez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–85. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 18.Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. 2007;47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- 19.Lévi F, Okyar A, Dulong S, Innominato PF, Clairambault J. Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol. 2010;50:377–421. doi: 10.1146/annurev.pharmtox.48.113006.094626. [DOI] [PubMed] [Google Scholar]
- 20.Ahowesso C, Li XM, Zampera S, Peteri-Brunbäck B, Dulong S, Beau J, Hossard V, Filipski E, Delaunay F, Claustrat B, et al. Sex and dosing-time dependencies in irinotecan-induced circadian disruption. Chronobiol Int. 2011;28:458–70. doi: 10.3109/07420528.2011.569043. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy MF, Tutton PJ, Barkla DH. Adrenergic factors regulating cell division in the colonic crypt epithelium during carcinogenesis and in colonic adenoma and adenocarcinoma. Br J Cancer. 1985;52:383–90. doi: 10.1038/bjc.1985.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–66. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 23.Marra G, Anti M, Percesepe A, Armelao F, Ficarelli R, Coco C, Rinelli A, Vecchio FM, D’Arcangelo E. Circadian variations of epithelial cell proliferation in human rectal crypts. Gastroenterology. 1994;106:982–7. doi: 10.1016/0016-5085(94)90757-9. [DOI] [PubMed] [Google Scholar]
- 24.Sherman H, Froy O. Expression of human beta-defensin 1 is regulated via c-Myc and the biological clock. Mol Immunol. 2008;45:3163–7. doi: 10.1016/j.molimm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Iurisci I, Filipski E, Sallam H, Harper F, Guettier C, Maire I, Hassan M, Iacobelli S, Lévi F. Liver circadian clock, a pharmacologic target of cyclin-dependent kinase inhibitor seliciclib. Chronobiol Int. 2009;26:1169–88. doi: 10.3109/07420520903209942. [DOI] [PubMed] [Google Scholar]
- 26.Iurisci I, Filipski E, Reinhardt J, Bach S, Gianella-Borradori A, Iacobelli S, Meijer L, Lévi F. Improved tumor control through circadian clock induction by Seliciclib, a cyclin-dependent kinase inhibitor. Cancer Res. 2006;66:10720–8. doi: 10.1158/0008-5472.CAN-06-2086. [DOI] [PubMed] [Google Scholar]
- 27.Hoogerwerf WA, Hellmich HL, Cornélissen G, Halberg F, Shahinian VB, Bostwick J, Savidge TC, Cassone VM. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. 2007;133:1250–60. doi: 10.1053/j.gastro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Sládek M, Rybová M, Jindráková Z, Zemanová Z, Polidarová L, Mrnka L, O’Neill J, Pácha J, Sumová A. Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology. 2007;133:1240–9. doi: 10.1053/j.gastro.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 29.Hoogerwerf WA, Sinha M, Conesa A, Luxon BA, Shahinian VB, Cornélissen G, Halberg F, Bostwick J, Timm J, Cassone VM. Transcriptional profiling of mRNA expression in the mouse distal colon. Gastroenterology. 2008;135:2019–29. doi: 10.1053/j.gastro.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okyar A, Piccolo E, Ahowesso C, Filipski E, Hossard V, Guettier C, La Sorda R, Tinari N, Iacobelli S, Lévi F. Strain- and sex-dependent circadian changes in abcc2 transporter expression: implications for irinotecan chronotolerance in mouse ileum. PLoS One. 2011;6:e20393. doi: 10.1371/journal.pone.0020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–54. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plikus MV, Vollmers C, de la Cruz D, Chaix A, Ramos R, Panda S, Chuong CM. Local circadian clock gates cell cycle progression of transient amplifying cells during regenerative hair cycling. Proc Natl Acad Sci U S A. 2013;110:E2106–15. doi: 10.1073/pnas.1215935110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khapre RV, Kondratova AA, Susova O, Kondratov RV. Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle. 2011;10:4162–9. doi: 10.4161/cc.10.23.18381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Innominato PF, Lévi FA, Bjarnason GA. Chronotherapy and the molecular clock: Clinical implications in oncology. Adv Drug Deliv Rev. 2010;62:979–1001. doi: 10.1016/j.addr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:12662–7. doi: 10.1073/pnas.1209965109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin II, Artemicheva NM, Deluca KA, Gudkov AV, et al. Core circadian protein CLOCK is a positive regulator of NFκB-mediated transcription. Proc Natl Acad Sci U S A. 2012;109:E2457–65. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellet MM, Zocchi L, Sassone-Corsi P. The RelB subunit of NFκB acts as a negative regulator of circadian gene expression. Cell Cycle. 2012;11:3304–11. doi: 10.4161/cc.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mauro C, Leow SC, Anso E, Rocha S, Thotakura AK, Tornatore L, Moretti M, De Smaele E, Beg AA, Tergaonkar V, et al. NFκB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol. 2011;13:1272–9. doi: 10.1038/ncb2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y, Spengler ML, Kuropatwinski KK, Comas-Soberats M, Jackson M, Chernov MV, Gleiberman AS, Fedtsova N, Rustum YM, Gudkov AV, et al. Selenium is a modulator of circadian clock that protects mice from the toxicity of a chemotherapeutic drug via upregulation of the core clock protein, BMAL1. Oncotarget. 2011;2:1279–90. doi: 10.18632/oncotarget.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Tourneau C, Faivre S, Laurence V, Delbaldo C, Vera K, Girre V, Chiao J, Armour S, Frame S, Green SR, et al. Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur J Cancer. 2010;46:3243–50. doi: 10.1016/j.ejca.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Bettayeb K, Oumata N, Echalier A, Ferandin Y, Endicott JA, Galons H, Meijer L. CR8, a potent and selective, roscovitine-derived inhibitor of cyclin-dependent kinases. Oncogene. 2008;27:5797–807. doi: 10.1038/onc.2008.191. [DOI] [PubMed] [Google Scholar]
- 43.Nutley BP, Raynaud FI, Wilson SC, Fischer PM, Hayes A, Goddard PM, McClue SJ, Jarman M, Lane DP, Workman P. Metabolism and pharmacokinetics of the cyclin-dependent kinase inhibitor R-roscovitine in the mouse. Mol Cancer Ther. 2005;4:125–39. [PubMed] [Google Scholar]
- 44.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 45.Altieri DC. New wirings in the survivin networks. Oncogene. 2008;27:6276–84. doi: 10.1038/onc.2008.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rödel F, Sprenger T, Kaina B, Liersch T, Rödel C, Fulda S, Hehlgans S. Survivin as a prognostic/predictive marker and molecular target in cancer therapy. Curr Med Chem. 2012;19:3679–88. doi: 10.2174/092986712801661040. [DOI] [PubMed] [Google Scholar]
- 47.Church DN, Talbot DC. Survivin in solid tumors: rationale for development of inhibitors. Curr Oncol Rep. 2012;14:120–8. doi: 10.1007/s11912-012-0215-2. [DOI] [PubMed] [Google Scholar]
- 48.Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci. 2000;113:4363–71. doi: 10.1242/jcs.113.23.4363. [DOI] [PubMed] [Google Scholar]
- 49.Tsukahara T, Tanno Y, Watanabe Y. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature. 2010;467:719–23. doi: 10.1038/nature09390. [DOI] [PubMed] [Google Scholar]
- 50.Meijer L, Bettayeb K, Galons H. (R)-Roscovitin (CYC202, Seliciclib). In: Smith PJ, Yue EW, eds. Inhibitors of Cyclin-dependentKinases as Anti-tumor Agents: CRC, 2006:187-225. [Google Scholar]
- 51.Guha M, Altieri DC. Survivin as a global target of intrinsic tumor suppression networks. Cell Cycle. 2009;8:2708–10. doi: 10.4161/cc.8.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 54.Oumata N, Ferandin Y, Meijer L, Galon H. Practical Synthesis of Roscovitine and CR8. Org Process Res Dev. 2009;13:641–4. doi: 10.1021/op800284k. [DOI] [Google Scholar]
- 55.Bingham C, Arbogast B, Guillaume GC, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.