Abstract
Modifications like asparagine deamidation, aspartate isomerization, methionine oxidation, and lysine glycation are typical degradations for recombinant antibodies. For the identification and functional evaluation of antibody critical quality attributes (CQAs) derived from chemical modifications in the complementary-determining regions (CDRs) and the conserved regions, an approach employing specific stress conditions, elevated temperatures, pH, oxidizing agents, and forced glycation with glucose incubation, was applied. The application of the specific stress conditions combined with ion exchange chromatography, proteolytic peptide mapping, quantitative liquid chromatography mass spectrometry, and functional evaluation by surface plasmon resonance analysis was adequate to identify and functionally assess chemical modification sites in the CDRs of a recombinant IgG1. LC-Met-4, LC-Asn-30/31, LC-Asn-92, HC-Met-100c, and HC Lys-33 were identified as potential CQAs. However, none of the assessed degradation products led to a complete loss of functionality if only one light or heavy chain of the native antibody was affected.
Keywords: protein degradation, deamidation, oxidation, glycation, recombinant antibodies, mass spectrometry, critical quality attributes, quality by design, developability
Introduction
Chemical modifications, including asparagine (Asn) deamidation, aspartate (Asp) isomerization, methionine/tryptophan (Met/Trp) oxidation, and non-enzymatic lysine (Lys) glycation, that occur in proteins have been extensively reviewed.1-17 Recombinant monoclonal antibodies (mAbs) are exposed to process and storage conditions that might influence the rate and extent of these modifications.18 Previous studies have shown that degradation of Asn and Asp residues in proteins can affect in vitro stability and in vivo biological functions.19-23 Five IgG1 mAbs have been reported to lose activity because of deamidation or isomerization in the complementary-determining regions (CDRs) of the heavy chain.24-28 In case of the recombinant IgG1 antibody trastuzumab (Herceptin®), the loss of potency is caused by the isomerization of heavy chain Asp-102 (CDR 3). Deamidation of the light chain Asn-30 (CDR 1) does not significantly affect trastuzumab potency.24 Two independent studies of other IgG1s reported the heavy chain Asn-55 (CDR 2) to be susceptible to deamidation in vivo25 and to exist in a stable succinimide form at mildly acidic pH.26 In independent investigations of different antibodies, the light chain Asp-32 (CDR 1), the light chain Asn-33 (CDR 1), the light chain Asp-56 (CDR 2), the heavy chain Asp-74, and the heavy chain Asp-99/101 (CDR 3) were found to form succinimide (Asu) or iso-Asp.27-29 In addition, Chelius et al. applied accelerated degradation conditions to identify four potential deamidation sites in the conserved regions of recombinant IgG1 mAbs.30
Oxidation of Met residues in the constant domains of recombinant IgG1 antibodies has been demonstrated to affect the interaction with protein A, the neonatal Fc receptor, and binding to the Fcγ receptors.31-33 So far, however, no susceptible Met residue within a CDR of recombinant IgG1 antibodies has been reported. In the case of trastuzumab, the heavy chain Met-107 (CDR 3) was found not to be susceptible to oxidation.34 Induction of Trp oxidation in the CDRs (heavy chain Trp-105; CDR 3) of a mAb by photooxidation resulted in a progressive loss of target binding and biological activity.35 In another case, the light chain Trp-32 (CDR 1) of a recombinant IgG1 was found to be susceptible to oxidation under real-time storage and elevated temperature conditions.36
Several IgG1s have been reported to be susceptible to Lys glycation in the CDRs of both the light and heavy chains. In three independent investigations, the light chain Lys-49 (CDR 2), the heavy chain Lys-65 (CDR 2), and the heavy chain Lys-98 (CDR 3) were found to represent accessible glycation sites.11,37,38 Moreover, Goetze et al. have analyzed the in vivo glycation rates of Lys residues in the conserved regions of recombinant mAbs.39
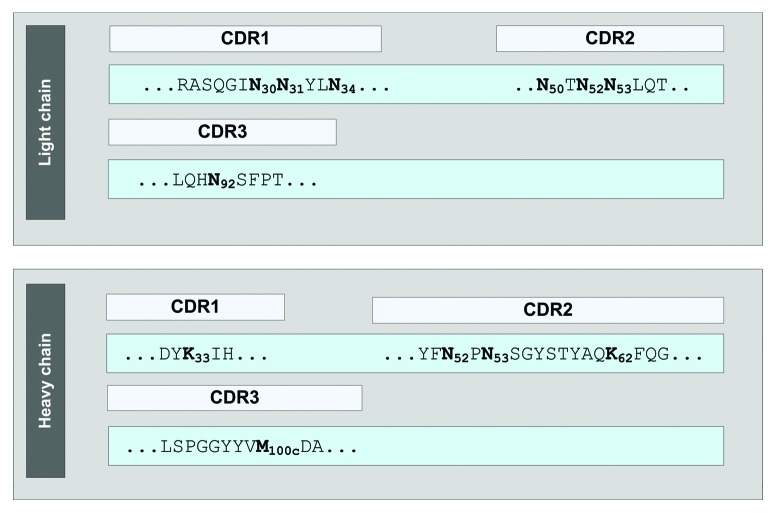
Thus, new developability concepts for the next generation of therapeutic proteins have recently been discussed.40-42 In the present study, an approach employing stress conditions (elevated temperatures, pH, oxidizing agents, and forced glycation with glucose incubation), ion exchange chromatography, and proteolytic peptide mapping combined with quantitative LC-MS for the induction, identification and quantification of Asn deamidation, Asp isomerization, Met oxidation, and Lys glycation was applied. This test system allowed us to identify light chain Met-4, Asn-30, Asn-31, Asn-92, heavy chain Lys-33, and Met-100c as potential chemical degradation sites in the variable region of a recombinant IgG1.
Results
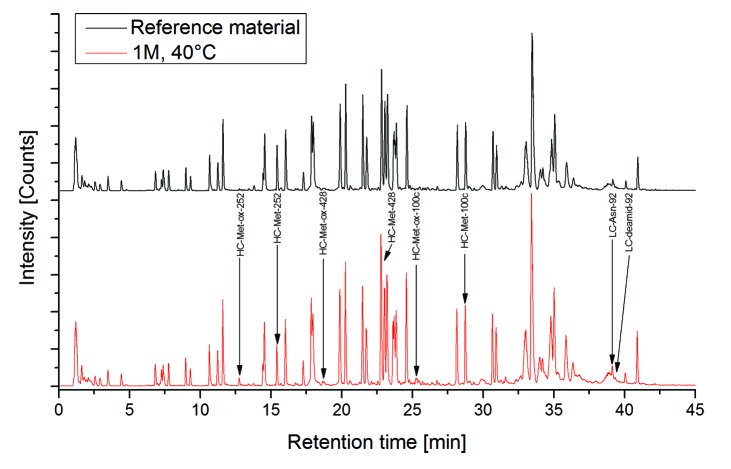
An approach employing different types of stress conditions was used to identify relevant chemical degradation sites in the CDRs of the recombinant mAb2 (Fig. 1). To initially assess potential sites for mAb2 degradation, we exposed mAb2 reference material to elevated temperature conditions (25 °C and 40 °C) for 1 mo (M). Following incubation of mAb2 at elevated temperatures, significant increases in acidic charge variants were observed by cation-exchange chromatography, whereas a decrease of the main mAb2 charge variant and basic charge variants could be detected (Fig. 2). In conclusion, the data suggest significant Asn deamidation leading to an increase in acidic variants and no prominent Asp isomerization occurrence. Next, the stressed samples were further analyzed by tryptic peptide mapping at pH 6.0 combined with quantitative UPLC-MS. The application of peptide mapping at mildly acidic conditions was selected to minimize artificial deamidation at peptide level.27 Figure 3 illustrates the total ion current (TIC) chromatograms of one stress sample (1 M at 40 °C) and the corresponding reference material (stored at -80 °C). Only slight differences in peak intensities of the tryptic peptides with retention times of 12–13 min, 16–17 min, 25–26 min, and 39 min could be observed. Subsequently, the extent of quantifiable Asn deamidation, Asp isomerization, and Met/Trp oxidation was determined by quantitative evaluation of specific ion current chromatograms of modified tryptic peptides and their unmodified parent peptides using the quantification software GRAMS/32. The Kabat amino acid numbering system was applied.43,44 The quantification results for those mAb2 amino acid residues that showed significant alterations in deamidation and oxidation are summarized in Table 1. Sequence determination of tryptic mAb2 peptides selected for quantitative evaluation was performed online by low-energy CID (exemplarily shown for the tryptic peptides containing LC-Asn-30/31, LC-Asn-92, LC-Met-4, HC-Lys-33, and HC-Met-100c, Supplementary files). For LC-Asn-30/31 (located in the CDR 1) we found moderately increased levels of Asu, Asp, and iso-Asp at both temperatures, whereas deamidation of LC-Asn-34 was not detectable. The LC-Asn-92 (located in the CDR 3) displayed a significant elevation in Asp formation upon application of temperature stress (6% to 17% after 1 M incubation at 40 °C); however, no formation of LC-Asu-92 and LC-iso-Asp-92 was detected by the applied separation system. In contrast, the Asu, Asp, and iso-Asp levels of LC-Asn-50/52/53 (located in the CDR 2) was not affected by the elevated temperature conditions and deamidation of HC-Asn-52/53 was not detectable. For the HC-Asn-384/389/390, located in the conserved region of recombinant IgG1 antibodies, no Asu formation was detected, but a moderate increase of Asp/iso-Asp formation was observed following incubation at 40 °C. These results are in agreement with previous studies on the evaluation of IgG1 conserved Asn residues in recombinant antibodies.27,30 No Asp isomerization to Asu or isoAsp was noticed for tryptic peptides located in the CDRs of mAb2. Consequently, the deamidation of multiple Asn residues upon temperature stress resulted in the direct formation of mAb2 acidic charge variants (Fig. 2).
Figure 1. Partial sequences of mAb2.
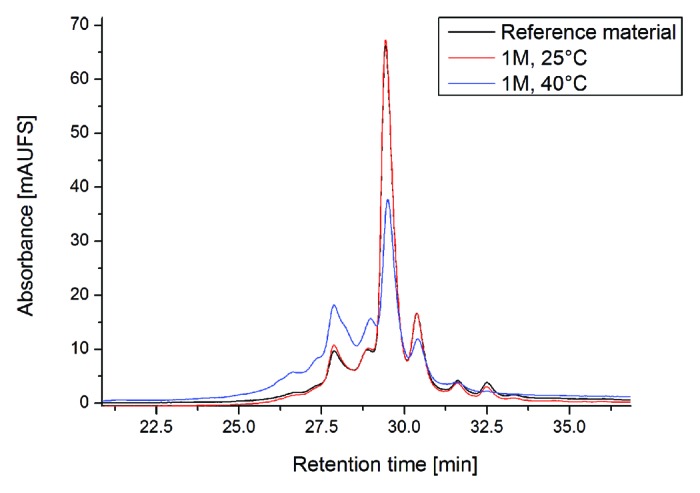
Figure 2. Cation-exchange chromatography of mAb2 reference material vs. temperature stressed material (stored at 25 °C and 40 °C; for 1 mo).
Figure 3. Total ion current chromatogram of reference material vs. temperature stressed sample (40 °C for 1 mo). Sequence determination of tryptic mAb2 peptides selected for quantification was performed online by low-energy CID (summarized in supportive information). Quantification results are summarized in Table 1.
Table 1. Assessment of potential degradation sites of mAb2 using accelerated temperature degradation conditions and quantitative UPLC-MS.
| Storage Duration/Temperature | RM/-80 °C | 1M/25 °C | 1M/40 °C | 1M/25 °C with Met |
1M/40 °C with Met |
|---|---|---|---|---|---|
| LC-Asn-30,31 | |||||
| LC-Asu-31 | 1.1 | 1.6 | 3.6 | 1.4 | 3.9 |
| LC-deamid-30 | 0.7 | 1.0 | 3.6 | 0.7 | 3.3 |
| LC-deamid-31 | 0.6 | 0.5 | 1.6 | 0.4 | 1.1 |
| LC-Asn-50,52,53 | |||||
| LC-Asu-50,52,53 | 0.3 | 0.4 | 0.4 | 0.4 | 0.9 |
| LC-deamid-50,52,53 | 0.6 | 0.9 | 0.9 | 0.6 | 0.4 |
| LC-Asn-92 | |||||
| LC-deamid-92 | 6.2 | 9.6 | 16.5 | 6.1 | 15.0 |
| HC-Asn-384,389,390 | |||||
| HC-deamid-384,389,390 | 1.0 | 1.3 | 2.6 | 1.9 | 1.9 |
| LC-Met-4 | |||||
| LC-Met-ox-4 | 2.7 | 2.7 | 3.2 | 3.0 | 2.8 |
| HC-Met-48 | |||||
| HC-Met-ox-48 | 3.8 | 2.5 | 2.5 | 3.2 | 3.1 |
| HC-Met-80 | |||||
| HC-Met-ox-80 | 3.2 | 2.8 | 3.0 | 3.4 | 2.9 |
| HC-Met-100c | |||||
| HC-Met-ox-100c | 4.2 | 6.2 | 12.3 | 4.1 | 4.1 |
| HC-Met-252 | |||||
| HC-Met-ox-252 | 3.7 | 5.5 | 14.0 | 4.4 | 4.4 |
| HC-Met-428 | |||||
| HC-Met-ox-428 | 2.3 | 2.6 | 4.9 | 2.3 | 2.5 |
| CEC | |||||
|---|---|---|---|---|---|
| % Acidic | 23.9 | 41.5 | 54.5 | 25.3 | 50.2 |
| % Main | 52.6 | 49.1 | 34.3 | 52.2 | 32.4 |
| % Basic | 14.8 | 5.8 | 6.8 | 14.4 | 11.5 |
| SEC | |||||
| % Fragment | < 0.1 | 0.3 | 0.4 | < 0.3 | 0.6 |
| % Monomer | 98.9 | 98.7 | 98.1 | 99.0 | 98.8 |
| % Aggregate | 1.1 | 1.3 | 1.5 | 0.9 | 0.6 |
| SPR | |||||
|---|---|---|---|---|---|
| % Target Binding | 100 (± 2) | 101 (± 2) | 89 (± 2) | 101 (± 2) | 91 (± 2) |
Relative quantification (in %) was conducted by specific ion current chromatogram analysis of tryptic peptides using the quantification software GRAMS/32TM. mAB2 charge variants were monitored by cation-exchange chromatography (CEC, without Carboxypeptidase B pre-treatment, selected batches vary in the content of heavy chain C-terminal lysine truncation). Formation of fragments and aggregates was monitored by size exclusion chromatography (SEC) and target binding activity was assessed by SPR-analysis. deamid, total Asp/iso-Asp content; RM, reference material.
The extent of oxidation at LC-Met-4, HC-Met-48, HC-Met-80, HC-Met-100c, HC-Met-252, and HC-Met-428 was also determined by selected ion current chromatogram analysis of the oxidized peptides (Table 1). We found that all of the Met residues of the reference material were slightly oxidized, albeit to different extents. Only HC-Met-100c (located in the CDR 3), HC-Met-252, and HC-Met-428 displayed a demonstrative elevation in Met oxidation upon application of temperature stress, suggesting that HC-Met-100c represents a potential degradation site in the CDR of mAb2. These results are in agreement with previous studies on the evaluation of IgG1 conserved Met residues (Met-252 and Met-428) in recombinant antibodies.31,32,34-36,45-50 No additional light or heavy chain Trp was found to be oxidized in the variable mAb2 region at detectable levels. Next, functional evaluation by surface plasmon resonance (SPR) technology indicated a connection between the increase of chemical degradation products and the loss of target binding activity (10% reduction after 1 M at 40 °C, Table 1). To initially evaluate if the loss in target binding activity was due to the oxidation at HC-Met-100c, we repeated the elevated temperature experiment after adding methionine to the formulation buffer. Although the addition of methionine did completely prohibit further oxidation of HC-Met-100c, the 10% loss of target binding activity was still detectable, which suggested that the deamidations at LC-Asn-30/31 or LC-Asn-92 were accountable for this effect.
In summary, LC-Asn-30/31, LC-Asn-92, and HC-Met-100c were identified as susceptible degradation site in the CDR of mAb2. The applied temperature stress conditions resulted in the simultaneous formation of deamidated and oxidized mAb2 variants. In addition, the relatively low differences of degraded variants in the reference vs. stressed material (at most 10%) complicated a detailed functional evaluation. To discriminate between deamidated and oxidized mAb2 variants more accurately, specific stress conditions (pH and oxidizing agent) were then developed.
To induce specific deamidation at LC-Asn-30/31 and LC-Asn-92, we exposed mAb2 reference material to elevated pH conditions (pH 8 and 9) for 3 and 7 d at 25 °C. As a consequence of this, significant increases in acidic charge variants were observed by cation-exchange chromatography, whereas a decrease of the main mAb2 charge variant could be detected (Table 2). Next, the stressed samples were analyzed by tryptic peptide mapping combined with quantitative UPLC-MS (Table 2). For LC-Asn-30/31, we found increased levels of deamidation, whereas Asu formation at LC-Asn-31 could not be detected at both pH conditions. The LC-Asn-92 displayed a significant increase in deamidation already at pH 8 conditions (from 8% to 25% after 7 d) suggesting again that LC-Asn-92 represents the most susceptible deamidation site in the CDRs of mAb2. In contrast to the temperature stress conditions, the degree of oxidation at HC-Met-100c, HC-Met-252, and HC-Met-428 was not significantly affected by the elevated pH conditions. Moreover, the structural integrity of the deamidated antibody was verified by size exclusion chromatography (SEC) where no significant fragment or aggregate formation was detected (Table 2). Next, functional evaluation by SPR indicated a connection between the increase of LC-Asn-92 or LC-Asn-30/31 deamidation and the loss of target binding activity (Table 2). To determine if the deamidation of LC-Asn-92 affected the mAb2 target binding activity, the respective IEC peak fraction (Prepeak 2) was isolated by cation-exchange chromatography (Fig. 4A, see material and methods for preparative cIEC conditions) and re-analyzed by SPR. The data obtained demonstrate that deamidation of LC-Asn-92 does mitigate the mAb2 target binding activity compared with the main IEC peak fraction (69% vs. 106%, Table 2). In summary, LC-Asn-92 represents the most susceptible deamidation site in the CDR of mAb2 and deamidation significantly decreases antigen binding.
Table 2. Identification and evaluation of mAb2 Asn deamidation sites using accelerated pH conditions and quantitative UPLC-MS.
| Storage Duration/Temperature | RM/-80 °C | 3d/25 °C/ pH8 | 7d/25 °C/ pH8 | 3d/25 °C/ pH9 | 7d/25 °C/ pH9 | CEC Mainpeak | CEC Prepeak2 |
|---|---|---|---|---|---|---|---|
| LC-Asn-30,31 | |||||||
| LC-Asu-31 | < 0.1 | 0.1 | < 0.1 | 0.1 | 0.1 | 1.4 | 1.8 |
| LC-deamid-30 | 1.1 | 2.6 | 5.6 | 4.5 | 11.5 | 1.0 | 1.6 |
| LC-deamid-31 | 0.7 | 2.6 | 7.2 | 4.6 | 11.0 | 0.2 | 1.3 |
| LC-Asn-50,52,53 | |||||||
| LC-Asu-50,52,53 | 0.6 | 0.7 | 0.8 | 0.8 | 1.0 | 0.8 | 1.3 |
| LC-deamid-50,52,53 | 0.5 | 0.6 | 0.7 | 0.7 | 0.9 | 0.9 | 0.8 |
| LC-Asn-92 | |||||||
| LC-deamid-92 | 7.5 | 12.7 | 24.9 | 14.4 | 27.1 | 0.5 | 41.4 |
| HC-Asn-384,389,390 | |||||||
| HC-deamid-384,389,390 | 1.6 | 3.5 | 9.2 | 9.4 | 26.0 | 1.6 | 0.6 |
| LC-Met-4 | |||||||
| LC-Met-ox-4 | 2.7 | 3.4 | 3.7 | 4.3 | 3.8 | 0.8 | 2.1 |
| HC-Met-48 | |||||||
| HC-Met-ox-48 | 2.7 | 2.8 | 3.2 | 3.5 | 3.4 | 1.8 | 1.8 |
| HC-Met-80 | |||||||
| HC-Met-ox-80 | 2.4 | 3.1 | 3.5 | 3.7 | 3.7 | 0.9 | 1.4 |
| HC-Met-100c | |||||||
| HC-Met-ox-100c | 2.6 | 3.9 | 4.5 | 5.1 | 4.9 | 3.0 | 6.4 |
| HC-Met-252 | |||||||
| HC-Met-ox-252 | 3.5 | 2.3 | 4.6 | 4.5 | 4.7 | 1.9 0.0 |
3.5 |
| HC-Met-428 | |||||||
| HC-Met-ox-428 | 2.0 | 3.2 | 3.8 | 3.4 | 3.7 | < 0.1 | 0.3 |
| CEC | |||||||
|---|---|---|---|---|---|---|---|
| % Acidic | 23.1 | 32.6 | 53.3 | 40.4 | 66.9 | 0.4 | 96.0 |
| % Main | 65.3 | 56.6 | 37.7 | 48.1 | 23.5 | 98.7 | 3.8 |
| % Basic | 11.6 | 10.8 | 9.0 | 11.5 | 9.6 | 0.9 | 0.2 |
| SEC | |||||||
|---|---|---|---|---|---|---|---|
| % Fragment | < 0.2 | < 0.3 | < 0.3 | < 0.3 | 0.4 | < 0.1 | < 0.1 |
| % Monomer | 98.9 | 98.7 | 98.7 | 98.3 | 98.0 | > 99.9 | > 99.8 |
| % Aggregate | 1.1 | 1.2 | 1.1 | 1.5 | 1.6 | < 0.1 | 0.1 |
| SPR | |||||||
|---|---|---|---|---|---|---|---|
| % Target Binding | 100 (± 2) | 90 (± 2) | 80 (± 1) | 89 (± 2) | 74 (± 1) | 106 (± 4) | 69 (± 2) |
Relative quantification (in %) was conducted by specific ion current chromatogram analysis of tryptic peptides using the quantification software GRAMS/32TM. mAb2 charge variants were monitored by cation-exchange chromatography (CEC). Formation of fragments and aggregates was monitored by size exclusion chromatography (SEC) and target binding activity was assessed by SPR-analysis. deamid, total Asp/iso-Asp content; RM, reference material.
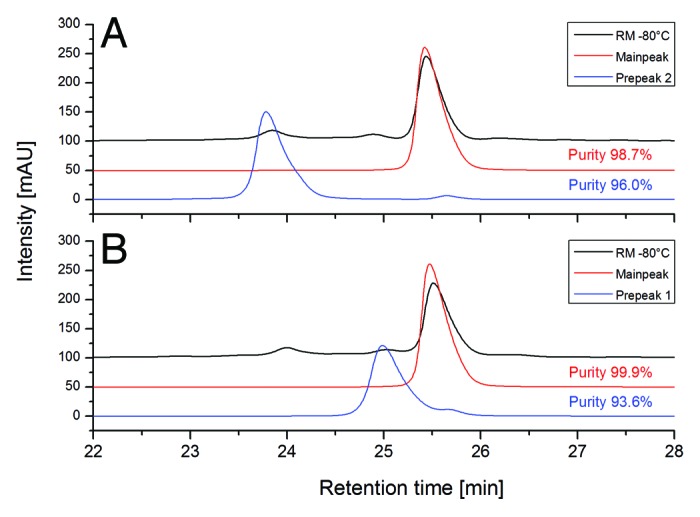
Figure 4. Purity analysis of cation-exchange chromatography (CEC) fractions (derived from a preparative CEC system) by analytical CEC. (A) Re-analysis of Prepeak 2 and main peak. (B) Re-analysis of Prepeak 1 and main peak.
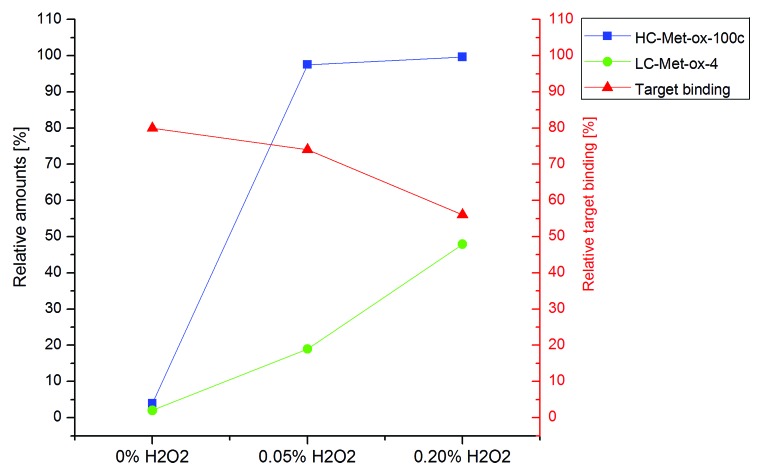
To induce specific oxidation at HC-Met-100c, we exposed mAb2 to different H2O2 concentrations for 24 h at 25 °C. No significant effect on mAb2 structural integrity was observed by SEC (Table 3). Next, the stressed samples were analyzed by tryptic peptide mapping combined with quantitative UPLC-MS (Table 3). We found increased levels of oxidation for LC-Met-4, whereas oxidations at HC-Met-48 and HC-Met-80 residues were not affected by the stress conditions. The HC-Met-100c displayed a significant elevation in oxidation already at 0.05% H2O2 (4% to ~100%) suggesting again that HC-Met-100c represents the most susceptible oxidation site in the CDRs of mAb2. In contrast to the temperature stress conditions, the degree of deamidation at LC-Asn-30/31 and LC-Asn-92 was not significantly affected by the applied oxidizing stress.
Table 3. Identification and evaluation of mAb2 Met oxidation sites using accelerated oxidation conditions and quantitative UPLC-MS.
| Storage Duration/Temperature | RM/-80 °C | 24h/25 °C/ w/o H2O2 | 24h/25 °C/0.05% H2O2 | 24h/25 °C/ 0.2% H2O2 |
|---|---|---|---|---|
| LC-Asn-30,31 | ||||
| LC-deamid-30 | 0.8 | 0.7 | 1.1 | 1.4 |
| LC-deamid-31 | 0.5 | 0.4 | 0.7 | 0.9 |
| LC-Asn-50,52,53 | ||||
| LC-Asu-50,52,53 | 0.4 | 0.3 | 0.4 | 0.5 |
| LC-deamid-50,52,53 | 0.7 | 0.3 | 0.7 | 0.8 |
| LC-Asn-92 | ||||
| LC-Asu-92 | 0.3 | 0.2 | 0.3 | 0.3 |
| LC-deamid-92 | 6.3 | 5.7 | 6.5 | 6.5 |
| HC-Asn-384,389,390 | ||||
| HC-deamid-384,389,390 | 1.3 | 1.8 | 2.2 | 1.3 |
| LC-Met-4 | ||||
| LC-Met-ox-4 | 3.3 | 2.0 | 19.0 | 47.9 |
| HC-Met-48 | ||||
| HC-Met-ox-48 | 1.8 | 1.9 | 1.8 | 1.8 |
| HC-Met-80 | ||||
| HC-Met-ox-80 | 1.9 | 2.1 | 2.0 | 1.9 |
| HC-Met-100c | ||||
| HC-Met-ox-100c | 3.3 | 3.9 | 97.5 | 99.4 |
| HC-Met-252 | ||||
| HC-Met-ox-252 | 3.0 | 3.3 | 87.5 | 99.0 |
| HC-Met-428 | ||||
| HC-Met-ox-428 | 1.6 | 1.8 | 56.2 | 80.4 |
| CEC | ||||
|---|---|---|---|---|
| % Acidic | 29.0 | 29.4 | n.q. | n.q. |
| % Main | 64.4 | 65.4 | n.q. | n.q. |
| % Basic | 6.6 | 5.2 | n.q. | n.q. |
| SEC | ||||
|---|---|---|---|---|
| % Fragment | 0.1 | 0.1 | 0.1 | 0.1 |
| % Monomer | 98.9 | 99.6 | 99.5 | 99.5 |
| % Aggregate | 1.1 | 0.3 | 0.4 | 0.4 |
| SPR | ||||
|---|---|---|---|---|
| % Target Binding | 100 (± 3) | 95 (± 3) | 74 (± 3) | 56 (± 3) |
Relative quantification (in %) was conducted by specific ion current chromatogram analysis of tryptic peptides using the quantification software GRAMS/32TM. mAb2 charge variants were monitored by cation-exchange chromatography (CEC). Formation of fragments and aggregates was monitored by size exclusion chromatography (SEC) and target binding activity was assessed by SPR-analysis. deamid, total Asp/iso-Asp; n.q. not quantifiable; RM, reference material.
Next, functional evaluation by SPR indicated a connection between the increase of HC-Met-100c or LC-Met-4 oxidation and the loss of target binding activity (Table 3). Since HC-Met-100c is fully oxidized at both H2O2 concentrations, the stepwise decreasing target binding activity is most likely a result of the increasing LC-Met-4 oxidation levels (Fig. 5). To verify if the HC-Met-100c or LC-Met-4 oxidation does affect the mAb2 target binding activity, we exposed mAb2 to lower H2O2 concentrations (0.003% to 0.02%). The obtained data indicate a correlation between increasing oxidation levels at LC-Met-4 and a loss of mAb2 target binding activity, whereas no correlation with HC-Met-100c oxidation values could be detected (Table 4). In conclusion, HC-Met-100c represents the most susceptible oxidation site in the CDR of mAb2, but oxidized HC-Met-100c does not affect antigen binding. In contrast, LC-Met-4 is less prone to oxidation, but this oxidation site might affect mAb2 target binding activity.
Figure 5. Correlation of HC-Met-100c and LC-Met-4 oxidation levels with mAb2 target binding activity.
Table 4. Identification and evaluation of mAb2 Met oxidation sites using refined oxidation conditions and quantitative UPLC-MS.
| Storage Duration/Temperature | RM/-80 °C | 24h/25 °C/ w/o H2O2 | 24h/25 °C/0.003% H2O2 | 24h/25 °C/0.009% H2O2 | 24h/25 °C/0.015% H2O2 | 24h/25 °C/0.02% H2O2 |
|---|---|---|---|---|---|---|
| LC-Met-4 | ||||||
| LC-Met-ox-4 | 3.0 | 3.9 | 4.2 | 5.3 | 7.9 | 11.2 |
| HC-Met-48 | ||||||
| HC-Met-ox-48 | 3.2 | 3.6 | 3.3 | 3.2 | 3.2 | 3.1 |
| HC-Met-80 | ||||||
| HC-Met-ox-80 | 2.9 | 3.1 | 3.1 | 3.1 | 3.2 | 3.2 |
| HC-Met-100c | ||||||
| HC-Met-ox-100c | 5.0 | 9.1 | 11.3 | 24.2 | 47.5 | 61.0 |
| HC-Met-252 | ||||||
| HC-Met-ox-252 | 5.6 | 12.4 | 13.4 | 20.7 | 37.2 | 49.9 |
| HC-Met-428 | ||||||
| HC-Met-ox-428 | 3.4 | 8.8 | 9.1 | 12.5 | 21.1 | 26.3 |
| CEC | ||||||
|---|---|---|---|---|---|---|
| % Acidic | 25.3 | 35.5 | 35.6 | 35.5 | 34.5 | 34.9 |
| % Main | 66.7 | 61.9 | 61.6 | 61.6 | 62.3 | 61.5 |
| % Basic | 8.0 | 2.7 | 2.8 | 2.9 | 3.2 | 3.5 |
| SEC | ||||||
|---|---|---|---|---|---|---|
| % Fragment | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| % Monomer | 98.9 | 98.9 | 98.9 | 98.9 | 98.9 | 98.9 |
| % Aggregate | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| SPR | ||||||
|---|---|---|---|---|---|---|
| % Target Binding | 100 (± 4) | 88 (± 4) | 88 (± 4) | 87 (± 4) | 86 (± 4) | 80 (± 4) |
Relative quantification (in %) was conducted by specific ion current chromatogram analysis of tryptic peptides using the quantification software GRAMS/32TM. mAb2 charge variants were monitored by cation-exchange chromatography (CEC). Formation of fragments and aggregates was monitored by size exclusion chromatography (SEC) and target binding activity was assessed by SPR-analysis. deamid, total Asp/iso-Asp content; RM, reference material.
In addition to the evaluation of potential mAb2 deamidation and oxidation sites, an approach employing forced glycation conditions (1 M glucose at 25 °C) was applied to identify susceptible Lys glycation sites in the CDRs. Incubation at 37 °C was also tested, but not evaluated further due to significant deamidation at LC-Asn-30/31 and LC-Asn-92, respectively (data not shown). Significant increases in acidic charge variants (especially Prepeak 1, Fig. 6) were observed by cation-exchange chromatography, and a decrease of the main mAb2 charge variant could be detected (Table 5). Next, the stressed samples were analyzed by tryptic peptide mapping combined with quantitative UPLC-MS (Table 5). For HC-Lys-62, we found a minimal increase in glycation levels, whereas HC-Lys-33 displayed a significant elevation in glycation formation (from 5% to 15%), suggesting that HC-Lys-33 represents the most susceptible glycation site in the CDRs of mAb2. In addition, no additional light or heavy chain Lys in the variable regions was found to be glycated at denotative levels (≥ 1%). In contrast to the temperature stress conditions, the degree of deamidation/oxidation at LC-Asn-30/31, LC-Asn-92, LC-Met-4, and HC-Met-100c was not significantly affected by the forced glycation conditions. The structural integrity of the glycated antibody was verified by SEC with no significant fragment or aggregate formation (Table 5). To verify if the HC-Lys-33 glycation does affect the mAb2 target binding activity, the respective IEC peak fraction was isolated from non-stressed material by cation-exchange chromatography (Prepeak 1, Fig. 4B) and analyzed by SPR. The obtained data demonstrate that glycation of HC-Lys-33 does moderately mitigate the mAb2 target binding activity compared with the main IEC peak fraction (89% vs. 119%, Table 5). In summary, HC-Lys-33 represents the most susceptible glycation site in the CDR and does moderately affect mAb2 antigen binding.
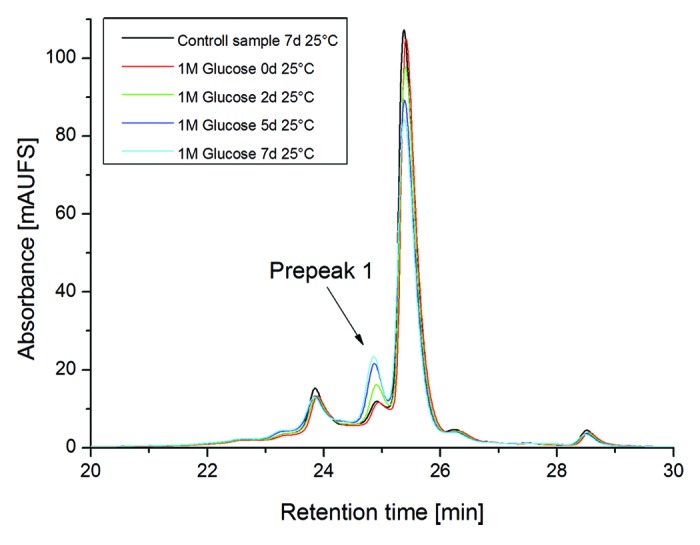
Figure 6. Cation-exchange chromatography of mAb2 reference material vs. glycated material (Forced glycation conditions: 1M Glucose for 3, 5 and 7 d at 25 °C).
Table 5. Identification and evaluation of mAb2 Lys glycation sites by forced glycation conditions and quantitative UPLC-MS.
| Storage Duration/Temperature | RM/-80 °C | 7d/25 °C w/o Glucose |
2d/25 °C 1M Glucose |
5d/25 °C 1M Glucose |
7d/25 °C 1M Glucose |
CEC Main Peak |
CEC Prepeak1 |
|---|---|---|---|---|---|---|---|
| LC-Asn-30,31 | |||||||
| LC-Asu-31 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 1.2 | 2.6 |
| LC-deamid-30 | 2.2 | 2.3 | 2.4 | 2.5 | 2.9 | 1.4 | 2.3 |
| LC-deamid-31 | 1.2 | 1.3 | 1.2 | 1.3 | 1.4 | 0.0 | 1.2 |
| LC-Asn-50,52,53 | |||||||
| LC-Asu-50,52,53 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.6 | 0.6 |
| LC-deamid-50,52,53 | 1.1 | 0.9 | 1.1 | 0.9 | 0.9 | 0.9 | 0.5 |
| LC-Asn-92 | |||||||
| LC-deamid-92 | 2.7 | 2.7 | 2.5 | 2.5 | 2.7 | 1.5 | 2.7 |
| HC-Asn-384,389,390 | |||||||
| HC-deamid-384,389,390 | 2.9 | 2.0 | 2.0 | 2.2 | 2.7 | < 0.1 | 5.5 |
| LC-Met-4 | |||||||
| LC-Met-ox-4 | 5.5 | 5.1 | 5.0 | 5.4 | 5.1 | 2.3 | 2.3 |
| HC-Met-48 | |||||||
| HC-Met-ox-48 | 5.2 | 4.9 | 4.8 | 5.4 | 4.8 | 4.0 | 2.0 |
| HC-Met-80 | |||||||
| HC-Met-ox-80 | 4.4 | 3.9 | 3.8 | 4.3 | 4.0 | 1.3 | 0.7 |
| HC-Met-100c | |||||||
| HC-Met-ox-100c | 5.9 | 5.5 | 5.5 | 5.8 | 5.5 | 3.9 | 3.9 |
| HC-Met-252 | |||||||
| HC-Met-ox-252 | 5.5 | 5.6 | 5.4 | 6.2 | 6.0 | 3.3 | 3.3 |
| HC-Met-428 | |||||||
| HC-Met-ox-428 | 4.0 | 3.9 | 3.7 | 4.3 | 4.6 | 2.2 | 1.5 |
| HC-Lys-33 | |||||||
| HC-Lys-glycated-33 | 5.3 | 5.2 | 9.5 | 13.5 | 14.9 | 0.21 | 24.4 |
| HC-Lys-62 | |||||||
| HC-Lys-glycated-62 | < 0.1 | < 0.1 | 0.8 | 1.4 | 1.7 | < 0.1 | < 0.1 |
| CEC | |||||||
|---|---|---|---|---|---|---|---|
| % Acidic | 23.1 | 29.0 | 32.0 | 36.0 | 37.8 | < 0.1 | 93.6 |
| % Main | 65.3 | 65.8 | 62.9 | 59.0 | 57.2 | > 99.9 | 6.4 |
| % Basic | 11.6 | 5.1 | 5.1 | 5.0 | 5.0 | < 0.1 | < 0.1 |
| SEC | |||||||
|---|---|---|---|---|---|---|---|
| % Fragment | < 0.2 | < 0.2 | < 0.2 | < 0.2 | < 0.2 | 0.08 | 0.25 |
| % Monomer | 98.9 | 98.9 | 99.0 | 99.0 | 99.1 | 99.7 | 99.6 |
| % Aggregate | 1.1 | 0.9 | 0.9 | 0.9 | 0.8 | 0.24 | 0.11 |
| SPR | |||||||
|---|---|---|---|---|---|---|---|
| % Target Binding | 100 (± 2) | n.a. | n.a. | n.a. | n.a. | 119 (± 4) | 89 (± 3) |
Relative quantification (in %) was conducted by specific ion current chromatogram analysis of tryptic peptides using the quantification software GRAMS/32TM. mAb2 charge variants were monitored by cation-exchange chromatography (CEC). Formation of fragments and aggregates was monitored by size exclusion chromatography (SEC) and target binding activity was assessed by SPR-analysis. deamid, total Asp/iso-Asp content; n.a., not applied; RM, reference material.
Discussion
For the identification and functional evaluation of CQAs derived from chemical amino acid modifications in the CDRs of mAbs, an approach employing specific stress conditions was applied. LC-Asn-30/31 (CDR1), LC-Asn-92 (CDR3), and HC-Met-100c (CDR3) were identified as susceptible mAb2 degradation sites in formulated drug substance by elevated temperature conditions. The applied temperature stress conditions, however, resulted in the simultaneous formation of deamidated and oxidized mAb2 variants at comparable low levels (specific increase of maximal 10% after 1 M at 40 °C). It should be noted that the quantitative evaluation of Asn deamidation (especially for LC-Asn-30/31 and HC-Asn-384,389,390) was only achieved by specific ion current chromatogram analysis of the relevant tryptic peptides and was not identifiable by TIC or LC-UV. Prolonged incubation times did not result in a more specific discrimination between deamidated and oxidized mAb2 variants, but did result in an increased loss of structural integrity. Several studies have evaluated Asn deamidation and Asp isomerization in recombinant antibodies using elevated temperature conditions.24,26-28 In the case of trastuzumab, LC-Asn-30 (CDR1) and HC-Asp-102 (CDR3) have been identified as the most vulnerable degradation sites.24 The application of elevated temperature stress on the mildly acidic formulated drug substance resulted in the simultaneous and almost equal formation of deamidated LC-Asn-30 and HC-isoAsp-102.24,27 In consequence, functional evaluation of both degradation variants could only be achieved after fractionation by cation-exchange chromatography.24 Thus, the use of accelerated temperature conditions enables the initial evaluation of chemical amino acid modifications in recombinant antibodies, but cannot facilitate the CQA assessment of multiple degradation sites with similar susceptibility.
By the application of elevated pH conditions, two Asn deamidation sites with different vulnerability (LC-Asn-92 > LC-Asn-30/31) were identified. For HC-Asn-53 (CDR2, see Fig. 1) no significant deamidation was observed. Two independent studies of other IgG1s reported the heavy chain Asn-55 (CDR 2) to be susceptible to deamidation in vivo25 and to exist in a stable succinimide form at mildly acidic pH.26 Prior work with model peptides demonstrated that XNS sequences undergo deamidation with the fastest kinetics at pH 7.4, 37 °C.51 In our study, a different susceptibility for mAb2 LC-Asn-92 and HC-Asn-53 was verified, suggesting that the differences in structural constraints could contribute to the different accessibility of antibody XNS motives.
Since only minor changes in oxidation and structural integrity were noticed, the approach appears to be suitable for the specific functional evaluation of susceptible Asn deamidation sites.
Functional evaluation of isolated acidic charge variants verified that deamidation of LC-Asn-92 in one light chain does mitigate, but does not completely abrogate, the mAb2 target binding activity. However, no clear effect of LC-Asn-30/31 deamidation on mAb2 target binding activity was detectable. These results are in agreement with a previous study on trastuzumab charge variants, in which deamidation of LC-Asn-30 (located in the CDR 1) was found to be less functional relevant compared with the pronounced inhibitory effect of HC-Asp-102 isomerization (located in the CDR 3) on antiproliferative activity.24
Using different H2O2 concentrations two accessible Met oxidation sites with different susceptibility (HC-Met-100c > LC-Met-4) were identified. Since no alterations in deamidation and structural integrity were noticed, the approach appears to be suitable for the specific evaluation of Met sites prone to oxidation. Shen et al. and Hensel et al. have evaluated the vulnerability of IgG1 HC-Met-100c and LC-Met-4 in two independent studies by the application of tert-butylhydroperoxide.34,36 In both studies, HC-Met-100c was found to be definitely less prone to oxidation compared with HC-Met-252 in the conserved region of recombinant IgG1s. In our study, a comparable susceptibility for mAb2 HC-Met-100c and HC-Met-252 was verified, suggesting that the differences in primary, and perhaps secondary, structural constraints could contribute to the increased accessibility. However, no correlation between oxidation levels at HC-Met-100c and mAb2 target binding activity was observed, demonstrating that Met oxidation in the CDR does not mitigate the antibody functionality per se. In contrast, LC-Met-4 is less prone to oxidation compared with HC-Met-100c and HC-Met-252, but oxidation at this site might affect mAb2 target binding activity either by a direct or an indirect effect. For a more detailed assessment LC-Met-4 and HC-Met-100c point mutations to leucine should be performed and analyzed for target binding activity.
In addition to the evaluation of potential mAb2 deamidation and oxidation sites, forced glycation conditions were utilized to identify susceptible Lys glycation sites in the CDRs.
Using this approach, two accessible Lys glycation sites with significant different susceptibility (HC-Lys-33 > HC-Lys-62) were identified. Since no changes in deamidation, oxidation, and structural integrity were noticed the approach appears to be suitable for the specific functional evaluation of Lys sites prone to glycation. Functional evaluation of isolated acidic charge variants verified that glycation of HC-Lys-33 in one heavy chain does moderately affect, but not abrogate, the mAb2 target binding activity. In previous studies, the LC-Lys-49 (CDR 2), the HC-Lys-65 (CDR 2), and the HC-Lys-98 (CDR 3) were found to represent accessible glycation sites of IgG1 mAbs.11,37,38 Glycation of HC-Lys-98 has no effect on antibody potency, whereas the functional consequence of glycation of LC-Lys-49 and HC-Lys-65 was not verified.
In summary, the application of specific stress conditions combined with ion exchange chromatography, proteolytic peptide mapping, quantitative LC-MS, and functional evaluation by SPR was adequate to identify and functionally assess chemical modification sites in the CDRs of a recombinant IgG1. The reported methodologies and work might also be of importance for other major classes of biopharmaceuticals such as Fc-fusion proteins, protein scaffolds, and bispecific antibodies.52-54 LC-Met-4, LC-Asn-30/31, LC-Asn-92, and HC Lys-33 were identified as potential mAb2 CQAs. However, none of the assessed degradation products led to a complete loss of functionality if only one light or heavy chain of the native antibody was affected. Similar results were also obtained for trastuzumab charge variants.24 However, the isomerization of one LC-Asp-92 in a human monoclonal IgG2 deactivated both antigen-binding regions.55 Hence, it is difficult to determine whether a chemical modification represents a true CQA in vivo. Accordingly, meaningful in vivo studies on the biological impact of specific degradation sites need to be conducted.56
Materials and Methods
Induction of mAb2 degradation products using forced stress degradation conditions
The recombinant IgG1 antibody mAb2 was expressed in a Chinese hamster ovary cell system. The antibody was manufactured at Roche Diagnostics, using standard cell culture and purification technology. mAb2 was formulated at a concentration of 25 mg/mL in a His-HCl buffer system (20 mM) at pH 5.5. To induce antibody Asn deamidation and Met oxidation the recombinant IgG1 antibody mAb2 was exposed to elevated temperatures (25 °C and 40 °C) with and without (10 mmol/L) Met in the formulation buffer for 1 mo. In addition, the antibody was then stressed by more specific stress model systems. To induce significant levels of Asn deamidation, the antibody was incubated at pH 8.0 and pH 9.0. Therefore, 10 mg of formulated antibody solution (800 µl) was diluted with 200 mM TRIS-HCl (pH 8.0 or pH 9.0) up to 2 ml followed by incubation for 7 d at 37 °C. Aliquots were taken after 3 and 5 d, respectively. Met Oxidation of mAb2 methionine residues was done in His-HCl buffer containing different concentrations of oxidizing agent H2O2 (ranging from 0.002% to 0.2% v/v) for 24 h at 25 °C. Glycation of Lys residues was achieved by diluting the formulated antibody with a glucose stock solution to a final concentration of 1 M glucose and incubated for 7 d at 25 °C. Aliquots were taken after 3 and 5 d.
Tryptic peptide mapping
For the detection and quantification of Asn deamidation, Asp isomerization and Met oxidation at peptide level, mAb2 was denatured in 0.2 M His-HCl, 8 M Gua-HCl, pH 6.0 by diluting 350 µg of mAb2 in a total volume of 300 µL. For reduction, 10 µl of 0.1 g/mL dithiothreitol (DTT) was added followed by incubation at 50 °C for 1 h. As a next step, the buffer solution was exchanged to a digestion buffer (0.02 M His-HCl, pH 6.0) using a NAP5®-gel filtration column (GE Healthcare, #17–0853–02). Subsequently, the NAP5®-eluate (500 µL) was mixed with 10 µL of a 0.25 mg/mL trypsin solution (Trypsin Proteomics grade, Roche, #03708985001) in 10 mM HCl and incubated at 37 °C for 18 h.27 For detection and quantification of glycated Lys residues within the molecule, mAb2 was digested with trypsin. mAb2 was first denatured in 0.4 M TRIS-HCl, 8 M Gua-HCl, at pH 8.5 by diluting 280 µg of oxidized mAb in a total volume of 300 µL. For reduction, 10 µl of 0.1 g/mL DTT were added and incubated at 50 °C for 1 h. After alkylation of free cysteines by adding 0.33 g/mL iodoacetic acid (IAA) and incubation at room temperature in the dark for 30 min, the buffer was exchanged to digestion buffer (0.1 M TRIS-HCl, pH 7.0) by application onto a NAP5®-gel filtration column. Subsequently, the NAP5®-eluate (500 µL) was mixed with 10 µL of a solution of 0.25 mg/mL trypsin (Trypsin Proteomics grade, Roche, Penzberg, Germany) in 10 mM HCl and incubated at 37 °C for 18 ± 2 h. The digest was stopped by adding 50 µl of a 10%-trifluoroacetic acid (TFA) solution.
Analysis of proteolytic peptides by liquid-chromatography mass-spectrometry
The tryptic peptide mixture was separated by RP-UPLC (ACQUITY) on a C18 column (BEH C18 1.7 µm 2.1 × 150 mm) and the eluate online analyzed with a LTQ Orbitrap Velos electrospray mass spectrometer (Thermo Scientific). The mobile phases consisted of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The chromatography was performed using a gradient from 1 to 35% solvent B in 45 min and finally from 35 to 80% solvent B in 3 min using a flow rate of 300 µL/min. UV absorption was measured at a wavelength of 220 nm. 3.5 µg digested protein was applied. UPLC-system and mass spectrometer were connected by PEEK-capillary tubes. Data acquisition was controlled by XCalibure software (Thermo Scientific, V2.1.0.1140). Parameters for MS detection were adjusted according to general experience available from peptide analysis of recombinant antibodies.
Data analysis for the quantification of deamidation/oxidation/glycation levels
Peptides of interest were identified manually by searching their m/z-values within the experimental mass spectrum. For the quantification, specific ion current (SIC) chromatograms of peptides of interest were generated on the basis of their monoisotopic mass and detected charge states using GRAMS AI software (Thermo Scientific, V8.0). Relative amounts of Asn deamidation and Met oxidation were calculated by manual integration of modified and unmodified peptide peaks. Glycation at Lys residues leads to a miscleaved glycated tryptic peptide. Therefore, the quantification of glycation levels was done by integration of the glycated conjoined peptide divided by the sum of the area of the two regularly cleaved non-glycated peptides. Taking into account that different peptide moieties have different ionization efficiency, only a relative quantification of glycation levels is attainable.
Identification of peptides by LC-MS/MS
MS/MS experiments were performed online on a Q-TOF SYNAPT G2 instrument (Waters) using the described chromatographic system. Peptides of interest were isolated on the basis of their mass and charge state and fragmentation induced by low-energy CID using helium as collision gas. The collision energy was adjusted according to stability and mass of the parent ion. Data acquisition was controlled by MassLynx software (Waters, V4.1 SCN870) using a manual acquisition mode. MS/MS-data were analyzed manually using MassLynx software for mass detection and data interpretation.
Cation exchange chromatography and fractionation of acidic charge variants
Initial characterization of mAb2 charge variants following incubation at elevated temperatures was executed by generic cation-exchange chromatography (CEC) for IgG1 antibodies without Carboxypeptidase B (CpB) pre-treatment.
Subsequent CEC was performed to monitor mAb2 charge variants using a ProPac WCX-10 analytical cation exchange column (4.0 × 250 mm; Dionex Softron GmbH, #054993). A step gradient using 20 mM MES, pH 6.2 as solvent A and 20 mM MES, 750 mM NaCl, pH 6.2 as solvent B at 1.0 mL/min was applied. Chromatographic separation was executed on an Ultimate3000 HPLC-system (Dionex Softron GmbH) using UV detection at 280 nm. 50 µg mAb2 pre-treated with CpB (0.5 mg/mL, Roche Diagnostics GmbH, #1010323300) for 30 min at 37 °C was injected for the chromatographic analysis. In addition, CEC fractionation was performed to collect mAb2 charge variants, for characterization purposes, using a ProPac WCX-10 Semiprep cation exchange column (9 × 250 mm, Dionex Softron GmbH, #063474). A step gradient using 20 mM MES, 10mM methionine, pH 6.2 as solvent A and 20 mM MES, 750 mM NaCl, 10mM methionine, pH 6.2 as solvent B at 5.0 mL/min and room temperature was applied. The following elution steps were applied during the separation: start concentration 5.0% B, gradient 1: 3 column volumes (CVs) to target concentration 12.2% B, gradient 2: 1.1 CVs to target concentration 12.3% B, gradient 3: 5 CVs to target concentration 20.0% B. Chromatographic separation was executed on a Äkta Explorer 10S equipped with UV detection at 280 nm and a Fra-950 fraction collector (GE Healthcare, #18–1145–05 and 18–6083–00). Briefly, 3 mg mAb2 was digested in a total volume of 2 ml with CpB at a final concentration of 1.5 mg/ml. Before loading onto the column, the sample was further incubated at 10 °C for 4 h. The specific peaks were collected in 1 ml fractions. The collected fractions from 20 fraction cycles were first concentrated by Amicon Ultra 30 kDa filter devices (Millipore, #UFC903024) to ~2.5 ml, followed by an buffer exchange to 20mM Histidin/His-HCl, 240mM sucrose, 0.02% Polysorbate20, 10 mM Methionin, pH 5.5 using PD10 columns (GE Healthcare, #11–0033–99). The purity of the collected fractions was verified by analytical SEC and analytical CEC (samples were directly loaded onto the column without additional CpB digestion).
Size exclusion chromatography
SEC was performed using a TSK-Gel G3000SWXL column (7.8 × 300 mm, 5 µm particle size; Tosoh Bioscience, #08541). An isocratic elution using 200 mM KH2PO4, 250 mM KCl, pH 7.0 at 0.5 mL/min as solvent was used for chromatographic separation on an Ultimate3000 HPLC-system (Dionex Softron GmbH) equipped with UV detection at 280 nm. 150 µg of mAb2 was injected for the chromatographic analysis. Relative quantification was performed by manual integration and comparison of peak areas.
Analysis of target binding by surface plasmon resonance
The interaction between the stressed or non-stressed mAb2 and the specific target protein was measured by SPR using a Biacore T100/T200 instrument (GE Healthcare). To evaluate mAb interaction to its specific target, a relative active concentration assay was performed. The specific target protein was immobilized onto a Biacore CM5-biosensor chip (GE Healthcare) via amine coupling to reach a high coupling density (about 4000 RU). The assay was performed at room temperature with HBS-P+ buffer (GE Healthcare) as running and dilution buffer. Five nM of native or stressed mAb2 samples were injected at a flow rate of 30 µL/min at room temperature. Association time was 30 s, followed by a 30 s dissociation phase. Regeneration of the chip surface was reached by a 30 s injection of 10 mM NaOH. Evaluation of SPR-data was performed by comparison of the response of samples and reference material two seconds before the end of the association phase. Activity of reference material was set to 100%.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
We are indebted to all members of the Laboratories in Penzberg and Basel for valuable discussions.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/27876
References
- 1.Yang H, Zubarev RA. Mass spectrometric analysis of asparagine deamidation and aspartate isomerization in polypeptides. Electrophoresis. 2010;31:1764–72. doi: 10.1002/elps.201000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vlasak J, Ionescu R. Heterogeneity of monoclonal antibodies revealed by charge-sensitive methods. Curr Pharm Biotechnol. 2008;9:468–81. doi: 10.2174/138920108786786402. [DOI] [PubMed] [Google Scholar]
- 3.Wakankar AA, Borchardt RT. Formulation considerations for proteins susceptible to asparagine deamidation and aspartate isomerization. J Pharm Sci. 2006;95:2321–36. doi: 10.1002/jps.20740. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu T, Matsuoka Y, Shirasawa T. Biological significance of isoaspartate and its repair system. Biol Pharm Bull. 2005;28:1590–6. doi: 10.1248/bpb.28.1590. [DOI] [PubMed] [Google Scholar]
- 5.Lowenson J, Clarke S. Does the chemical instability of aspartyl and asparaginyl residues in proteins contribute to erythrocyte aging? The role of protein carboxyl methylation reactions. Blood Cells. 1988;14:103–18. [PubMed] [Google Scholar]
- 6.Harris RJ. Heterogeneity of recombinant antibodies: linking structure to function. Dev Biol (Basel) 2005;122:117–27. [PubMed] [Google Scholar]
- 7.Hovorka S, Schöneich C. Oxidative degradation of pharmaceuticals: theory, mechanisms and inhibition. J Pharm Sci. 2001;90:253–69. doi: 10.1002/1520-6017(200103)90:3<253::AID-JPS1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 8.Ji JA, Zhang B, Cheng W, Wang YJ. Methionine, tryptophan, and histidine oxidation in a model protein, PTH: mechanisms and stabilization. J Pharm Sci. 2009;98:4485–500. doi: 10.1002/jps.21746. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Schöneich C, Borchardt RT. Chemical instability of protein pharmaceuticals: Mechanisms of oxidation and strategies for stabilization. Biotechnol Bioeng. 1995;48:490–500. doi: 10.1002/bit.260480511. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TH, Burnier J, Meng W. The kinetics of relaxin oxidation by hydrogen peroxide. Pharm Res. 1993;10:1563–71. doi: 10.1023/A:1018908316698. [DOI] [PubMed] [Google Scholar]
- 11.Quan C, Alcala E, Petkovska I, Matthews D, Canova-Davis E, Taticek R, Ma S. A study in glycation of a therapeutic recombinant humanized monoclonal antibody: where it is, how it got there, and how it affects charge-based behavior. Anal Biochem. 2008;373:179–91. doi: 10.1016/j.ab.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Fischer S, Hoernschemeyer J, Mahler HC. Glycation during storage and administration of monoclonal antibody formulations. Eur J Pharm Biopharm. 2008;70:42–50. doi: 10.1016/j.ejpb.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Brady LJ, Martinez T, Balland A. Characterization of nonenzymatic glycation on a monoclonal antibody. Anal Chem. 2007;79:9403–13. doi: 10.1021/ac7017469. [DOI] [PubMed] [Google Scholar]
- 14.Pace AL, Wong RL, Zhang YT, Kao YH, Wang YJ. Asparagine deamidation dependence on buffer type, pH, and temperature. J Pharm Sci. 2013;102:1712–23. doi: 10.1002/jps.23529. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Yip H, Katta V. Identification of isomerization and racemization of aspartate in the Asp-Asp motifs of a therapeutic protein. Anal Biochem. 2011;410:234–43. doi: 10.1016/j.ab.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Beck A, Carter PJ, Gerber HP, Lugovskoy AA, Wurch T, Junutula JR, et al. 8 (th) Annual European Antibody Congress 2012: November 27-28, 2012, Geneva, Switzerland. MAbs 2013; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck A, Wagner-Rousset E, Ayoub D, Van Dorsselaer A, Sanglier-Cianférani S. Characterization of therapeutic antibodies and related products. Anal Chem. 2013;85:715–36. doi: 10.1021/ac3032355. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Singh S, Zeng DL, King K, Nema S. Antibody structure, instability, and formulation. J Pharm Sci. 2007;96:1–26. doi: 10.1002/jps.20727. [DOI] [PubMed] [Google Scholar]
- 19.Flatmark T, Sletten K. Multiple forms of cytochrome c in the rat. Precursor-product relationship between the main component Cy I and the minor components Cy II and Cy 3 in vivo. J Biol Chem. 1968;243:1623–9. [PubMed] [Google Scholar]
- 20.Harding JJ, Beswick HT, Ajiboye R, Huby R, Blakytny R, Rixon KC. Non-enzymic post-translational modification of proteins in aging. A review. Mech Ageing Dev. 1989;50:7–16. doi: 10.1016/0047-6374(89)90054-7. [DOI] [PubMed] [Google Scholar]
- 21.Cacia J, Keck R, Presta LG, Frenz J. Isomerization of an aspartic acid residue in the complementarity-determining regions of a recombinant antibody to human IgE: identification and effect on binding affinity. Biochemistry. 1996;35:1897–903. doi: 10.1021/bi951526c. [DOI] [PubMed] [Google Scholar]
- 22.Paborji M, Pochopin NL, Coppola WP, Bogardus JB. Chemical and physical stability of chimeric L6, a mouse-human monoclonal antibody. Pharm Res. 1994;11:764–71. doi: 10.1023/A:1018948901599. [DOI] [PubMed] [Google Scholar]
- 23.Kroon DJ, Baldwin-Ferro A, Lalan P. Identification of sites of degradation in a therapeutic monoclonal antibody by peptide mapping. Pharm Res. 1992;9:1386–93. doi: 10.1023/A:1015894409623. [DOI] [PubMed] [Google Scholar]
- 24.Harris RJ, Kabakoff B, Macchi FD, Shen FJ, Kwong M, Andya JD, Shire SJ, Bjork N, Totpal K, Chen AB. Identification of multiple sources of charge heterogeneity in a recombinant antibody. J Chromatogr B Biomed Sci Appl. 2001;752:233–45. doi: 10.1016/S0378-4347(00)00548-X. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Lu J, Wroblewski VJ, Beals JM, Riggin RM. In vivo deamidation characterization of monoclonal antibody by LC/MS/MS. Anal Chem. 2005;77:1432–9. doi: 10.1021/ac0494174. [DOI] [PubMed] [Google Scholar]
- 26.Yan B, Steen S, Hambly D, Valliere-Douglass J, Vanden Bos T, Smallwood S, Yates Z, Arroll T, Han Y, Gadgil H, et al. Succinimide formation at Asn 55 in the complementarity determining region of a recombinant monoclonal antibody IgG1 heavy chain. J Pharm Sci. 2009;98:3509–21. doi: 10.1002/jps.21655. [DOI] [PubMed] [Google Scholar]
- 27.Diepold K, Bomans K, Wiedmann M, Zimmermann B, Petzold A, Schlothauer T, Mueller R, Moritz B, Stracke JO, Mølhøj M, et al. Simultaneous assessment of Asp isomerization and Asn deamidation in recombinant antibodies by LC-MS following incubation at elevated temperatures. PLoS One. 2012;7:e30295. doi: 10.1371/journal.pone.0030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlasak J, Bussat MC, Wang S, Wagner-Rousset E, Schaefer M, Klinguer-Hamour C, Kirchmeier M, Corvaïa N, Ionescu R, Beck A. Identification and characterization of asparagine deamidation in the light chain CDR1 of a humanized IgG1 antibody. Anal Biochem. 2009;392:145–54. doi: 10.1016/j.ab.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 29.Sreedhara A, Cordoba A, Zhu Q, Kwong J, Liu J. Characterization of the isomerization products of aspartate residues at two different sites in a monoclonal antibody. Pharm Res. 2012;29:187–97. doi: 10.1007/s11095-011-0534-2. [DOI] [PubMed] [Google Scholar]
- 30.Chelius D, Rehder DS, Bondarenko PV. Identification and characterization of deamidation sites in the conserved regions of human immunoglobulin gamma antibodies. Anal Chem. 2005;77:6004–11. doi: 10.1021/ac050672d. [DOI] [PubMed] [Google Scholar]
- 31.Bertolotti-Ciarlet A, Wang W, Lownes R, Pristatsky P, Fang Y, McKelvey T, Li Y, Li Y, Drummond J, Prueksaritanont T, et al. Impact of methionine oxidation on the binding of human IgG1 to Fc Rn and Fc gamma receptors. Mol Immunol. 2009;46:1878–82. doi: 10.1016/j.molimm.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Loew C, Knoblich C, Fichtl J, Alt N, Diepold K, Bulau P, Goldbach P, Adler M, Mahler HC, Grauschopf U. Analytical protein a chromatography as a quantitative tool for the screening of methionine oxidation in monoclonal antibodies. J Pharm Sci. 2012;101:4248–57. doi: 10.1002/jps.23286. [DOI] [PubMed] [Google Scholar]
- 33.Schlothauer T, Rueger P, Stracke JO, Hertenberger H, Fingas F, Kling L, Emrich T, Drabner G, Seeber S, Auer J, et al. Analytical FcRn affinity chromatography for functional characterization of monoclonal antibodies. MAbs. 2013;5:576–86. doi: 10.4161/mabs.24981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen JF, Kwong MY, Keck RG, Harris RJ. The application of tertbutylhydroperoxide oxidation to study sites of potential methionine oxidation in a recombinant antibody. In: Techniques in Protein Chemistry VII Academic Press Inc 1996:275-84. [Google Scholar]
- 35.Wei Z, Feng J, Lin HY, Mullapudi S, Bishop E, Tous GI, Casas-Finet J, Hakki F, Strouse R, Schenerman MA. Identification of a single tryptophan residue as critical for binding activity in a humanized monoclonal antibody against respiratory syncytial virus. Anal Chem. 2007;79:2797–805. doi: 10.1021/ac062311j. [DOI] [PubMed] [Google Scholar]
- 36.Hensel M, Steurer R, Fichtl J, Elger C, Wedekind F, Petzold A, Schlothauer T, Molhoj M, Reusch D, Bulau P. Identification of potential sites for tryptophan oxidation in recombinant antibodies using tert-butylhydroperoxide and quantitative LC-MS. PLoS One. 2011;6:e17708. doi: 10.1371/journal.pone.0017708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller AK, Hambly DM, Kerwin BA, Treuheit MJ, Gadgil HS. Characterization of site-specific glycation during process development of a human therapeutic monoclonal antibody. J Pharm Sci. 2011;100:2543–50. doi: 10.1002/jps.22504. [DOI] [PubMed] [Google Scholar]
- 38.Zhang B, Yang Y, Yuk I, Pai R, McKay P, Eigenbrot C, Dennis M, Katta V, Francissen KC. Unveiling a glycation hot spot in a recombinant humanized monoclonal antibody. Anal Chem. 2008;80:2379–90. doi: 10.1021/ac701810q. [DOI] [PubMed] [Google Scholar]
- 39.Goetze AM, Liu YD, Arroll T, Chu L, Flynn GC. Rates and impact of human antibody glycation in vivo. Glycobiology. 2012;22:221–34. doi: 10.1093/glycob/cwr141. [DOI] [PubMed] [Google Scholar]
- 40.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10:345–52. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 41.Chennamsetty N, Voynov V, Kayser V, Helk B, Trout BL. Design of therapeutic proteins with enhanced stability. Proc Natl Acad Sci U S A. 2009;106:11937–42. doi: 10.1073/pnas.0904191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Xu W, Dukleska S, Benchaar S, Mengisen S, Antochshuk V, Cheung J, Mann L, Babadjanova Z, Rowand J, et al. Developability studies before initiation of process development: improving manufacturability of monoclonal antibodies. MAbs. 2013;5:787–94. doi: 10.4161/mabs.25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson G, Wu TT. Kabat database and its applications: 30 years after the first variability plot. Nucleic Acids Res. 2000;28:214–8. doi: 10.1093/nar/28.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kabat EA, Wu TT, Perry H, Gottesman K, Foeller C. Sequences of proteins of immunological interest. NIH Publication 1991; No. 91-3242. [Google Scholar]
- 45.Chumsae C, Gaza-Bulseco G, Sun J, Liu H. Comparison of methionine oxidation in thermal stability and chemically stressed samples of a fully human monoclonal antibody. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850:285–94. doi: 10.1016/j.jchromb.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 46.Houde D, Kauppinen P, Mhatre R, Lyubarskaya Y. Determination of protein oxidation by mass spectrometry and method transfer to quality control. J Chromatogr A. 2006;1123:189–98. doi: 10.1016/j.chroma.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 47.Lam XM, Yang JY, Cleland JL. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody HER2. J Pharm Sci. 1997;86:1250–5. doi: 10.1021/js970143s. [DOI] [PubMed] [Google Scholar]
- 48.Liu D, Ren D, Huang H, Dankberg J, Rosenfeld R, Cocco MJ, Li L, Brems DN, Remmele RL., Jr. Structure and stability changes of human IgG1 Fc as a consequence of methionine oxidation. Biochemistry. 2008;47:5088–100. doi: 10.1021/bi702238b. [DOI] [PubMed] [Google Scholar]
- 49.Pan H, Chen K, Chu L, Kinderman F, Apostol I, Huang G. Methionine oxidation in human IgG2 Fc decreases binding affinities to protein A and FcRn. Protein Sci. 2009;18:424–33. doi: 10.1002/pro.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Timm V, Gruber P, Wasiliu M, Lindhofer H, Chelius D. Identification and characterization of oxidation and deamidation sites in monoclonal rat/mouse hybrid antibodies. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:777–84. doi: 10.1016/j.jchromb.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 51.Robinson NE, Robinson AB. Molecular clocks. Proc Natl Acad Sci U S A. 2001;98:944–9. doi: 10.1073/pnas.98.3.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beck A, Reichert JM. Therapeutic Fc-fusion proteins and peptides as successful alternatives to antibodies. MAbs. 2011;3:415–6. doi: 10.4161/mabs.3.5.17334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein C, Sustmann C, Thomas M, Stubenrauch K, Croasdale R, Schanzer J, Brinkmann U, Kettenberger H, Regula JT, Schaefer W. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs. 2012;4:653–63. doi: 10.4161/mabs.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wurch T, Pierré A, Depil S. Novel protein scaffolds as emerging therapeutic proteins: from discovery to clinical proof-of-concept. Trends Biotechnol. 2012;30:575–82. doi: 10.1016/j.tibtech.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Rehder DS, Chelius D, McAuley A, Dillon TM, Xiao G, Crouse-Zeineddini J, Vardanyan L, Perico N, Mukku V, Brems DN, et al. Isomerization of a single aspartyl residue of anti-epidermal growth factor receptor immunoglobulin gamma2 antibody highlights the role avidity plays in antibody activity. Biochemistry. 2008;47:2518–30. doi: 10.1021/bi7018223. [DOI] [PubMed] [Google Scholar]
- 56.Goetze AM, Schenauer MR, Flynn GC. Assessing monoclonal antibody product quality attribute criticality through clinical studies. MAbs. 2010;2:500–7. doi: 10.4161/mabs.2.5.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.