Abstract
A sensitive and selective method was developed to quantitate allopregnanolone and its 5β isomer pregnanolone in human plasma using liquid chromatography-differential mobility separation combined with MS/MS detection. The method employed a simple liquid-liquid extraction of 100 μL plasma with hexane/ethyl acetate. After extraction, the sample was derivatized using a quaternary aminooxy reagent. Separation of allopregnanolone, pregnanolone and their 3β epimers (epiallopregnanolone and epipregnanolone) was achieved using a Phenomenex Kinetex C18 2.1×100 mm 2.6 μm column. A linear calibration curve was obtained over the concentration range from 10 pg/mL to 25,000 pg/mL and the inter- and intra-day accuracy of the QC samples were between 90-110% with the inter- and intra-day precision less than 10%. The lower limit of quantitation is 50 fg (157 amol) on column for both allopregnanolone and pregnanolone which is 100 fold less than the underivatized compounds. The recovery is above 95% and the extracted samples are stable for at least 6 days when stored at 4°C. Plasma samples from normal, pregnant and postpartum women were analyzed using this method.
Keywords: allopregnanolone, pregnanolone, epiallopregnanolone, epipregnanolone, keto derivatization, differential mobility mass spectrometry, LC-MS/MS
Introduction
There is a growing interest in the therapeutic potential of GABAergic neuroactive steroid compounds for neuropsychiatric disorders. The 3α metabolites of progesterone, testosterone, deoxycortisol and androstenedione have been shown to have potent anxiolytic, analgesic, antiseizure, and neuroprotective effects in animal models [1]. The most studied of these compounds has been allopregnanolone [2, 3, 4]. However, understanding of the physiological role of this compound has been limited by the difficulty of reliably measuring it in biological samples. While radioimmunoassay (RIA) provides good sensitivity with lower limit of quantitation (LLOQ) at 15-25 pg [5, 6], it lacks specificity due to nonspecific reaction and cross reactivity. Currently the analyses of allopregnanolone in biological samples are typically performed using gas chromatography-mass spectrometry (GC-MS) [7-12]. The reported LLOQ is in the range between 100 fg to 200 pg. Though GC-MS provides better selectivity than RIA, it also requires some labor intensive sample extraction steps.
Liquid chromatography-mass spectrometry (LC-MS) is becoming increasingly popular in steroid analysis due to its specificity, versatility and ability to measure multiple components [13-22]. The first challenge in LC-MS analysis of allopregnanolone and pregnanolone is the poor ionization efficiency of these compounds. The ionization efficiency of neutral steroids is relatively low for ionization methods commonly employed in LC-MS [14, 15], e.g. electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). The second challenge is the presence of numerous isobaric interferences in biological samples. As demonstrated in this paper, the interferences cannot be eliminated even with more specific detection i.e. multiple reaction monitoring (MRM), where both parent and product ion masses are monitored. Currently only a few papers are available on the quantitative analysis of allopregnanolone using LC-MS. Higashi et al. [17, 22] developed a LC-ESI-MS/MS method to analyze allopregnanolone, epiallopregnanolone and 5-alpha dihydroprogesterone in rat brain and serum. Samples were extracted and purified using solid phase extraction (SPE) and derivatized with permanently charged 2-hydrazino-1-methylpyridine (HMP) synthesized in their own laboratory. The reported LLOQ was 1.25 pg in both brain and plasma samples. Liu et al [14] reported a semi-quantitative detection of some neutral neurosteroids and neurosteroid sulphates from rat brain samples by nano LC-ESI-MS after C18 and ion exchange SPE cleanup of the samples. The limit of detection for pregnanolone was reported to be 500 fg after derivatization with hydroxyammonium chloride. Unlike steroid sulphates which can be analyzed directly by LC-MS without derivatization [14, 23], derivatization has been necessary to enhance ionization efficiency of neutral steroids. In addition, extensive sample cleanup has been included in these LC-MS methods to remove interferences from the biological matrix.
Due to low level of neurosteroids in blood and brain, there is a demand for a more sensitive, specific and simple analytical method feasible for routine analysis. Here we present a quantitative LC-MS/MS method to detect 50 fg level of both allopregnanolone and pregnanolone in human plasma. A simple liquid-liquid extraction was used followed by derivatization with a commercially available, permanently charged quaternary aminooxy (QAO) reagent [24] to enhance compound ionization efficiency. Differential mobility spectrometry (DMS) was implemented in the method to increase selectivity. The method was applied to the analysis of plasma samples from normal, pregnant and postpartum women.
Experimental section
Chemicals and reagents
Allopregnanolone (5α-pregnan-3α-ol-20-one), pregnanolone (5β-pregnan-3α-ol-20-one), epiallopregnanolone (5α-pregnan-3β-ol-20-one) and epipregnanolone (5β-pregnan-3β-ol-20-one) were purchased from Steraloids Inc. (Newport, RI, USA). Allopregnanolone-d4 (17, 21, 21, 21-d4, purity 96-98%) was purchased from Cambridge Isotope Laboratories Inc. (Andover, MA, USA). HPLC grade methanol and acetonitrile were obtained from Caledon Laboratory Ltd. (Georgetown, Ontario, Canada). HPLC grade ethyl acetate and hexane were obtained from Sigma-Aldrich. Ammonium formate was from Aldrich and formic acid (~98%) was from Fluka. O-(3-trimethylammoniumpropyl) hydroxylamine bromide (Amplifex™ Keto reagent) was obtained from SCIEX (Foster City, CA). Milli-Q water (18.2 MΩ) was used for all solution preparations.
Double charcoal stripped human plasma was purchased from Bioreclamation LLC (NY, USA). Plasma samples were obtained from healthy pregnant (during weeks 39-40) and non-pregnant (during early follicular phase) subjects as previously described [25] with the additional inclusion of plasma samples collected at 6 weeks postpartum. The samples were stored at −80°C prior to analysis.
Standard solution, calibration and quality control sample preparation
Stock solution of 1 mg/mL allopregnanolone, allopregnanolone-d4, pregnanolone, epiallopregnanolone and epipregnanolone were prepared in methanol. Mixed standard solution of allopregnanolone and pregnanolone was prepared at 5000 ng/mL in methanol and diluted to 1250, 500, 250, 125, 50, 25, 10, 5.0, 2.5, 1.25, 0.50, 0.25 ng/mL in methanol. Several double charcoal stripped blank plasma lots were tested for the presence of endogenous analytes and the plasma lot with the lowest endogenous concentration was used to prepare plasma standards and quality control (QC) samples by spiking 20 μL of above methanol standards into 980 μL of blank plasma. The resulting plasma standards spanned a concentration range from 5 pg/mL to 25 ng/mL. Low (LQC), medium (MQC) and high (HQC) quality control samples were prepared at 50, 500, 5000 pg/mL, respectively, using the same lot of stripped plasma. Allopregnanolone-d4 was used as the internal standard (IS) and the working solution of allopregnanolone-d4 was prepared at 10 ng/mL in 70% methanol in water.
Sample preparation
20 μL of allopregnanolone-d4 working solution was added to 100 μL of each plasma sample, standard and QC, and the samples were then extracted with 600 μL of 1:1 (v:v) mixed solvent of ethyl acetate and hexane. After extraction, 500 μL of the upper organic layer was transferred to a clean vial and evaporated to dryness using speed vacuum concentrator at 35 °C for about 20 minutes. 50 μL of Amplifex™ Keto working solution was then added to the vial and allowed to react at room temperature for 1 hour. Afterwards, each sample was diluted by adding 150 μL of 70 % methanol in water and 10 μL of the final sample was injected onto the LC system.
Same extraction procedure was used for For direct analysis without derivatization. Dried sample extract from ethyl acetate / hexane was reconstituted in 200 μl 70% methanol in water, and 20 μL was injected onto the LC system.
The concentration of the internal standard was 2 ng/mL in plasma and 1 ng/mL in sample extract for both derivatized and underivatized samples.
LC-Differential Mobility-MS/MS conditions
Samples were analyzed using a Shimadzu Promenence LC 20 system coupled with an AB SCIEX Triple Quad™ 6500 mass spectrometer equipped with and without the differential ion mobility device (SelexION™, AB SCIEX). The temperature of the auto sampler was set at 4 °C. The chromatographic separation was performed on a Phenomenex Kinetex C18 2.1×100 mm, 2.6 μm column at 40 °C using the following mobile phases: water / acetonitrile 90/10 (V/V) with 5mM ammonium formate and 0.1% formic acid as mobile phase A and water / acetonitrile 10/90 (V/V) with 5mM ammonium formate and 0.1% formic acid as mobile phase B. The flow rate was set at 0.5 mL/min.
For underivatized samples, the following gradient was used: 0–0.2min 10% B, 0.2–1.5 min 10–30% B, 1.5–8 min 30–75% B, 8–12 min 100% B, 12–16 min 10% B. Three MRM transitions at 301.2→135.1 (CE=24, CXP=17), 301.2→105.1 (CE=52, CXP=12) and 301.2→91.1 (CXP=70, CXP=10) were monitored for allopregnanolone and pregnanolone. The MRM transition at 305.3→135.1 (CE=24, CXP=17) was monitored for allopregnanolone-d4.
For derivatized samples, the gradient was changed to: 0–0.2min 10% B, 0.2–3 min 10–35% B, 3–6 min 35–45% B, 6–9 min 100% B, 9–12 min 10% B. Two MRM transitions at 433.3→374.4 (CE=34, CXP =15) and 433.3→126.1 (CE=50, CXP=15) were monitored for derivatized allopregnanolone and pregnanolone. MRM transitions at 437.3→378.4 (CE=34, CXP=15) or 437.3→130.2 (CE=50, CXP=50) were monitored for derivatized allopregnanolone-d4.
Source position and source parameters were optimized using post column T-infusion of allopregnanolone at mobile phase flow rate of 0.5 mL/min. Curtain gas (CUR) was set at 20 psi, collision gas (CAD) at 10, ion spray voltage (IS) at 3500 V, source temperature (TEM) at 600 °C, declustering potential (DP) at 80V, ion source gas 1 (GS1) and gas 2 (GS2) were both set at 60 psi. The optimal source position and source parameters were found to be the same for derivatized and underivatized samples.
The DMS device can be easily installed or uninstalled in between the ion source and orifice plate of the AB SCIEX Triple Quad™ 6500 instrument without breaking the vacuum. When the DMS device is installed, data can be acquired with the device either turned on or off (also known as transparent mode of operation). Optimization of DMS parameters was performed using T-infusion of derivatized allopregnanolone at mobile phase flow rate of 0.5 mL/min. The following DMS setting gave the best compromise between sensitivity and selectivity: DMS temperature (DT) at “low” (150 °C), resolution (DR) at “off”, separation voltage (SV) at 3700 V, DMS offset (DMO) at −3 V and compensation voltage (COV) at 7 V.
Data analysis
Data analysis was performed using MultiQuant 2.1 with SignalFinder™ algorithm (AB Sciex). Calibration curve was obtained using area ratio of analyte and internal standard against analyte concentration. Linear regression and weighting of 1/x2 was used for calibration curve. Accuracy and precision were calculated by the software.
Results and Discussion
LC-MS/MS analysis of underivatized allopregnanolone
A full scan Q1 mass spectrum was acquired for allopregnanolone using ESI and APCI (Fig. S1 A and B in Electronic Supplementary Material), and demonstrated that ESI is slightly more sensitive than APCI towards this compound. In both modes [M+H-H2O]+ is more intense than [M+H]+, and this is consistent with the observation of Ma et al in their LC-APCI/ESI-MS analysis of allopregnanolone [26]. At collision energy less than 20 eV, the product ion scan of [M+H-H2O]+ (Fig. S1 C in Electronic Supplementary Material) showed one predominant fragment ion at 283.4 resulting from the further loss of H2O. As the collision energy increased, a broad range of fragment ions with m/z about 14 Da apart appeared corresponding to the characteristic ring fragments of the steroids [27]. Fragment 135, 105 and 91 are the most sensitive product ions with almost equal intensity at their corresponding CE and CXP optimum and therefore were monitored as product ions in our MRM analysis.
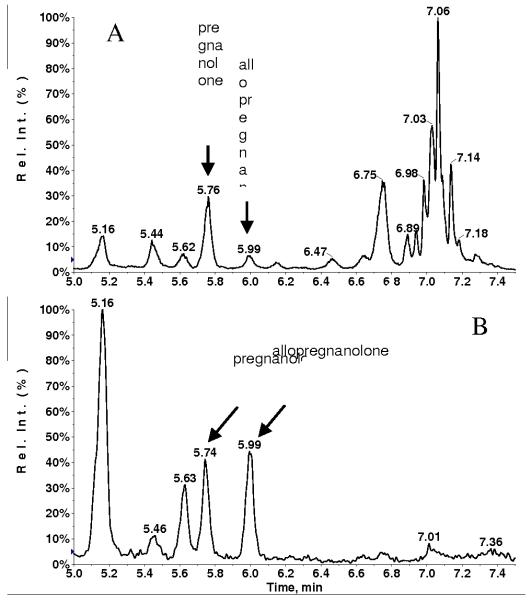
Fig. 1 displays the overlaid MRM chromatograms for 3 extracted samples using positive ESI: a blank, a blank containing IS, and a plasma sample spiked with 500 pg/mL allopregnanolone (LLOQ level). Numerous intense interference peaks were observed due to the presence of isobaric compounds present in the plasma matrix. Using the LC gradient described above, allopregnanolone was adequately separated from the interference peaks, and the retention time of allopregnanolone was found to be 7.03 min. The LLOQ for underivatized allopregnanolone in plasma sample is 5 pg on column with a signal-to-noise (S/N) of ~ 10.
Fig. 1.
XIC (MRM transition 301→135) of extracted blank (red), blank with internal standard (green) and 500 ng/mL spiked allopregnanolone (blue) plasma sample.
LC-MS/MS analysis of derivatized allopregnanolone and pregnanolone
Introduction of a charged functional group to a molecule by derivatization has been reported to greatly enhance the sensitivity in ESI mode due to the pre-formation of an ionic species [15, 19, 20, 28, 29]. Recently a novel, permanently charged quaternary aminooxy (QAO) reagent: O-(3 trimethylammoniumpropyl) hydroxylamine bromide, was used to improve the sensitivity of LC-MS analysis for testosterone using positive ESI [24]. This QAO reagent reacts with the carbonyl group to form an oxime bond and introduces a trimethyl ammonium (−NMe3+) charged moiety to the compound. The sensitivity enhancement was reported to be 80 fold for testosterone.
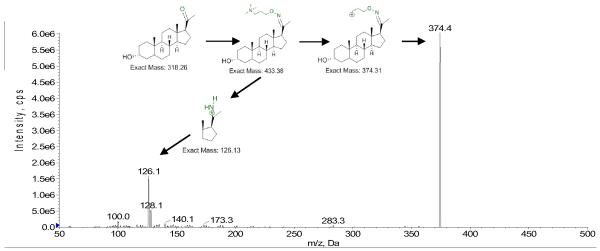
Derivatization of allopregnanolone with this QAO reagent resulted in the addition of 115 mass unit at C-20 position. After derivatization, two major product ions were observed for allopregnanolone when subjected to fragmentation by MS/MS, Fig. 2. At lower collision energy values, the fragment ion at m/z 374.4 (m/z 378.3 for IS), resulting from the loss of neutral trimethylamine (CE optimum at 34), is predominant. As the collision energy is increased, an additional fragment ion at m/z 126.1 (m/z 130.1 for IS) is generated (CE optimum at 50). Though the intensity of the fragment ion at m/z 126.1 is much lower than that at m/z 374.4 (about 8 fold), it is a more specific fragment ion to monitor for the detection of allopregnanolone because it contains part of the structure of both allopregnanolone and the derivatization reagent, whereas the fragment ion at m/z 374.4 contains the entire structure of allopregnanolone and part of the derivatization reagent (Scheme inserted in Fig. 2).
Fig. 2.
Product ion scan of derivatized allopregnanolone.
Upon derivatization of allopregnanolone with the QAO reagent, the most sensitive MRM transition (433.3 → 374.4) was measured to be more than 2000 times greater in peak area as compared to the most sensitive MRM transition for underivatized allopregnanolone. The secondary MRM transition (433.3 → 126.1) demonstrated an increase in peak area of about 300 fold.
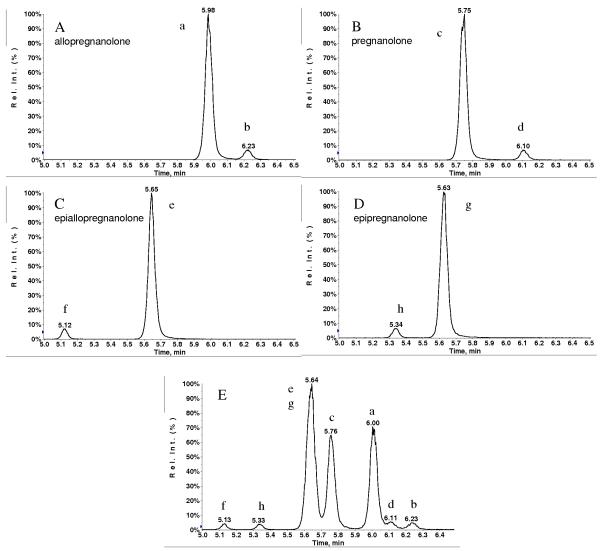
It has been shown previously [7,17, 21, 24] that derivatization of a keto group at C-3 position of the steroid backbone can lead to the formation of geometric cis- and trans-isomers which can be separated chromatographically as twin peaks of almost equal intensity, but derivatization of the keto group at C-20 position usually leads to one single peak. Upon derivatization of allopregnanolone and pregnanolone with the QAO reagent at C-20 position, the formation of both cis- and trans-isomers was observed; however one isomer was formed preferentially, accounting for approximately 95% of the total derivatization products. The two isomers were well separated under our LC condition, as demonstrated in Fig. 3 A and B. We postulate that the peak with higher intensity is likely the trans-isomer, since formation of the cis-isomer would be unfavorable due to the steric hindrance from the bulky ring structure [30]. Only the larger of the two chromatographic peaks, representing 95% of the total derivatization products, was used for quantitation.
Fig. 3.
MRM (433.3→ 126.1) chromatograms of individual (A-D) and mixture (E) of allopregnanolone, pregnanolone, epiallopregnanolone and epipregnanolone after derivatization: trans- (a) and cis- (b) allopregnanolone, trans- (c) and cis- (d) pregnanolone, trans- (e) and cis- (f) epiallopregnanolone, trans- (g) and cis- (h) epipregnanolone.
Epiallopregnanolone and epipregnanolone are 3β isomers of allopregnanolone and pregnanolone respectively, differing only in the orientation of a single OH bond. These epimers have the potential to interfere with the analysis of allopregnanolone and pregnanolone, since all of these compounds have common precursor ion masses and fragmentation products, and will therefore share the same MRM transitions. To prevent any potential interference, it is therefore critical to ensure that all of these isomers are chromatographically separated. Derivatization of epiallopregnanolone and epipregnanolone was performed, and these were analyzed individually by LC-MS/MS to identify the chromatographic peaks corresponding to each of the compounds. Derivatization of these two epimers resulted in the formation of their corresponding cis- and trans-isomers, as shown in Fig. 3 C and D. It is interesting to note that for these 3β isomers, the larger chromatographic peak (presumed to be the derivatized trans-isomer, for the reasons outlined above) elutes later, whereas in the case of allopregnanolone and pregnanolone, the larger chromatographic peak elutes first, indicating trans-isomers are more polar than the cis-isomers for 3α isomers. Fig. 3 E displays the chromatogram of a mixture containing derivatized allopregnanolone, pregnanolone, epiallopregnanolone and epipregnanolone. Under our chromatographic conditions, both epiallopregnanolone and epipregnanolone eluted earlier than allopregnanolone and pregnanolone, and therefore did not interfere with the quantitation of the latter two compounds.
To evaluate method performance, calibration standards and QC samples were prepared by spiking allopregnanolone and pregnanolone into ‘blank’ plasma matrix. Due to the high sensitivity of the method, it was impossible for us to obtain a plasma matrix which contained no detectable endogenous allopregnanolone and pregnanolone. Among the different lots of blank plasma we tested, the lowest endogenous plasma concentration was approximately 1–2 pg/mL for both compounds, corresponding to 20–40% of the peak area of the 5 pg/mL plasma calibration standard. Although the method demonstrated sufficient sensitivity to enable accurate quantitation of allopregnanolone (accuracy 97.2 ±3.7 (%) and 98.5 ±2.6 (%) for MRM transition 433.3→374.4 and 433.3→126.1 respectively, n=5) and pregnanolone (accuracy 99.0 ±8.5 (%) for MRM transition 433.3→126.1, n=5) at 5 pg/mL, we have chosen to use 10 pg/mL as the lowest point of the calibration curve to ensure that the signal in our plasma blank (due to the presence of endogenous allopregnanolone and pregnanolone) did not exceed 20% of LLOQ in accordance with guidelines on the bioanalytical methods [31]. This corresponds to 50 fg on column for both compounds, which is 100 fold improvement to the LLOQ obtained without derivatization (5 pg).
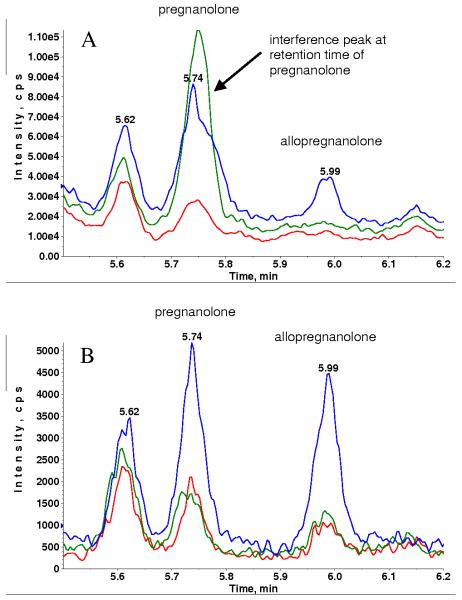
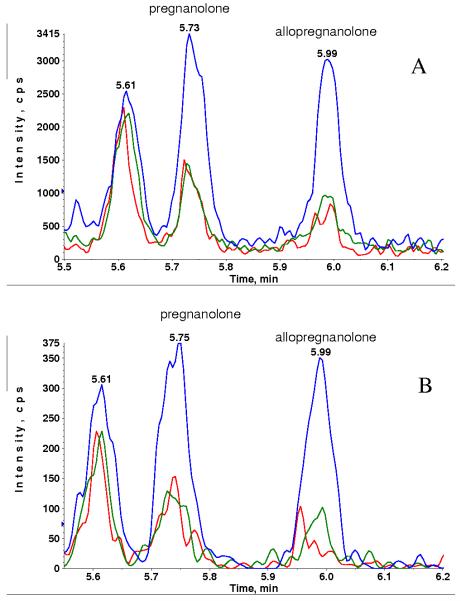
Fig. 4 is the overlaid chromatograms of extracted plasma blank, blank with internal stand and plasma standard containing 5 pg/mL of allopregnanolone (retention time = 5.99 min) and pregnanolone (retention time = 5.74 min) for MRM transitions 433.3 → 374.4 (Fig. 4 A) and 433.3 → 126.1 (Fig. 4 B). The plasma blank demonstrated low, but measureable, endogenous levels of allopregnanolone and pregnanolone, which are estimated to be 1–2 pg/mL. Although the signal was significantly higher for MRM transition 433.3 → 374.4, the background was also higher (~2e4 cps), resulting in S/N ~ 15 for allopregnanolone at 5 pg/mL. Despite the lower signal for MRM transition 433.3 → 126.1, the background was also much lower (~500 cps), resulting in a slightly better S/N ~ 25 for allopregnanolone.
Fig. 4.
XIC of MRM transition 433.3→374.4 (A) and MRM transition 433.3→ 126.1 (B) for extracted blank plasma (red), blank with internal standard (green) and spiked plasma standard at 5 pg/mL (blue) without DMS.
For pregnanolone, blank with internal standard showed 5 times more signal than blank without internal standard at MRM transition 433.3 →374.4 but this was not observed for MRM transition 433.3 →126.1 indicating the presence of an interference at MRM 433.3 →374.4. We found that this interference was mostly observed in blanks or lower concentration standards and QC samples. Though we have not yet understood the exact cause of the interference, it appeared to be introduced during sample extraction because a repeat extraction of the same sample did not necessarily show the same interference.
Table 1 presents a summary of the method performance. Statistics are presented for calibration curves, and for intra- and inter-day QC accuracy and precision, for both compounds. Linear calibration was obtained from 10 pg/mL to 25,000 pg/mL for both compounds, using a weighting of 1/x2. Due to the presence of the aforementioned interference for pregnanolone in the MRM transition 433.3 →374.4, only the MRM transition 433.3 →126.1 was used for quantitation of pregnanolone. Good accuracy and precision were observed for both calibration curve and QC samples, and none of the QC samples failed (outside accuracy acceptance criteria of ± 15% [31]) in these analysis.
Table 1.
Calibration curve and QC intra- and inter- day accuracy and precision of derivatized allopregnanolone and pregnanolone without DMS
| Compound | MRM transition | Calibration curve 10-25,000 pg/mL |
||
|---|---|---|---|---|
| Slope | Intercept | r 2 | ||
| Allopregnanolone | 433.3→374.4 | 0.34483 | 0.00109 | 0.9977 |
|
| ||||
| 433.3→126.1 | 0.05140 | 0.00017 | 0.9985 | |
|
| ||||
| Pregnanolone | 433.3→374.4 | Interference | ||
|
| ||||
| 433.3→126.1 | 0.05078 | 0.00030 | 0.9994 | |
|
| ||||
|
QC intra-day accuracy (%) (n=4)
|
||||
| LQC ( 50 pg/mL) |
MQC ( 500 pg/mL) |
HQC ( 5000 pg/mL) |
||
|
| ||||
| Allopregnanolone | 433.3→374.4 | 108.3 ±2.8 | 106.3 ±2.4 | 97.3 ±3.0 |
|
| ||||
| 433.3→126.1 | 106.1 ±1.1 | 104.4 ±1.4 | 97.9 ±3.0 | |
|
| ||||
| Pregnanolone | 433.3→374.4 | Interference | ||
|
| ||||
| 433.3→126.1 | 103.2 ±3.3 | 104.3 ±1.3 | 98.9 ±1.3 | |
|
| ||||
|
QC inter-day accuracy (%) (n=8)
|
||||
| LQC ( 50 pg/mL) |
MQC ( 500 pg/mL) |
HQC ( 5000 pg/mL) |
||
|
| ||||
| Allopregnanolone | 433.3→374.4 | 106.9 ±3.6 | 105.2 ±2.7 | 96.8 ±2.3 |
|
| ||||
| 433.3→126.1 | 107.3 ±2.6 | 102.9 ±2.3 | 97.4 ±2.3 | |
|
| ||||
| Pregnanolone | 433.3→374.4 | Interference | ||
|
| ||||
| 433.3→126.1 | 104.0 ±2.4 | 100.6 ±3.1 | 96.9 ±2.8 | |
The recovery of this method is above 95% for both allopregnanolone and pregnanolone and the extracted samples were stable for at least 6 days when stored at 4 °C.
LC-differential mobility-MS/MS analysis of derivatized allopregnanolone and pregnanolone
The principle and operation of differential mobility spectrometry (DMS) have been discussed in detail by Schneider and Krylov et al [32-34]. DMS is a form of ion mobility spectrometry that can be used to separate ions based on the difference between their ion mobility coefficients in high and low electric fields. When combined with tandem mass spectrometry, DMS provides additional selectivity by pre-separating ions of similar mass which otherwise cannot be resolved by LC-MS, and it can also enhance the quality of mass analysis by reducing chemical noise. In the work described here ion mobility separations were performed using the SelexION™ differential mobility device interfaced to the AB SCIEX Triple Quad™ 6500 mass spectrometer. Ions generated in the ion source are transported through the DMS device toward the orifice of the mass spectrometer by a transport gas. A RF voltage called the separation voltage (SV) is applied across the ion transport channel, perpendicular to the direction of the transport gas flow. Due to the difference in ion mobility coefficients in high and low electric field, ions migrate toward the walls and leave the flight path. Their trajectory is corrected by a counterbalancing DC voltage called compensation voltage (COV). By selecting certain combination of SV and COV, only ions with a particular differential mobility can pass through DMS device, therefore enabling the removal of interferences and reduction of background noise.
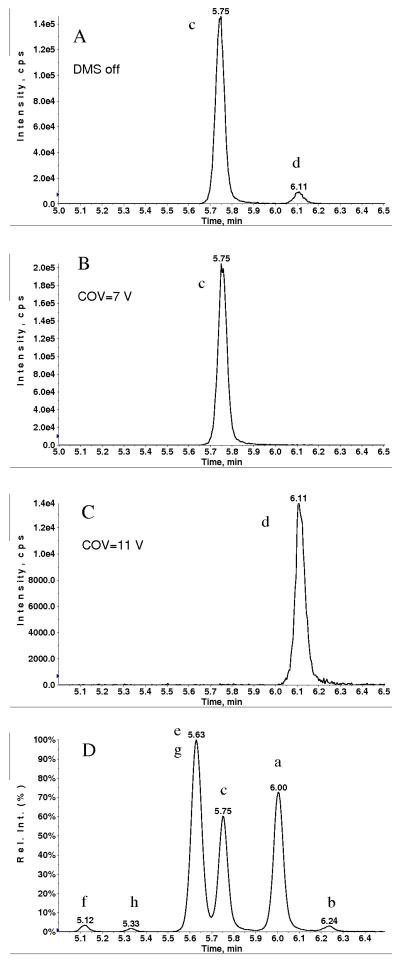
Optimization of the DMS parameters for derivatized allopregnanolone and pregnanolone was performed by setting the separation voltage (SV) to a fixed value of 3700V, while increasing the compensation voltage (COV) across a broad voltage range using a step-size of 0.5V. The optimum COV value, producing a maximum in signal intensity, was recorded for each compound. As mentioned earlier, derivatization resulted in the formation of cis- and trans-isomers for both allopregnanolone and pregnanolone (Fig. 3). It was interesting to note that the optimum COV value for both the cis- and trans-isomers of allopregnanolone was 7.0 V, therefore these could not be separated by the DMS device. However, the cis- and trans-isomers of pregnanolone exhibited different optimum COV values, so these isomers could be separated by the DMS device (Fig. 5 B, C). The COV maximum for the predominant and early-eluting isomer (trans-pregnanolone) was 7.0V (Fig. 5 B), while the COV maximum for the minor and later-eluting isomer (cis-pregnanolone) was 11.0V (Fig. 5 C). Therefore, by setting COV at 7.0 V, it was possible to prevent transmission of cis-pregnanolone through the DMS device, resulting in the removal of the chromatographic peak at 6.11 minutes (Fig. 5 D and Fig. 3 E). This was a fortunate outcome, since the peak at 6.11 minutes interfered slightly with the allopregnanolone peak at 5.99 minutes, so removal of the former peak was desirable, especially for samples with much higher concentration of pregnanolone than allopregnanolone.
Fig. 5.
MRM (433.3→ 126.1) chromatogram of derivatized pregnanolone (A) DMS off (transparent mode) (B) COV= 7V, SV=3700 (C) COV=11 V, SV=3700 and (D) MRM chromatogram of mixture of allopregnanolone, pregnanolone, epiallopregnanolone and epipregnanolone at COV=7 V, SV=3700: trans- (a) and cis- (b) allopregnanolone, trans-pregnanolone (c), trans- (e) and cis- (f) epiallopregnanolone, trans- (g) and cis- (h) epipregnanolone.
As mentioned in the experimental section, when DMS is installed, data can be acquired with DMS device turned on or off. In DMS off mode (also known as the transparent mode of operation), DMS parameters are not set to their optimum as in DMS on mode. Therefore, signal acquired in DMS off mode (Fig. 5 A) is generally bit lower than that in DMS on mode (Fig. 5 B and C).
Fig. 6 is the overlaid chromatograms of extracted blank, blank with internal standard and plasma standard with 5 pg/mL allopregnanolone (retention time = 5.99 min) and pregnanolone (retention time = 5.75 min) at COV= 7 V and SV= 3700 V for MRM transitions 433.3 → 374.4 (Fig. 6 A) and 433.3 → 126.1 (Fig. 6 B). For MRM transition 433.3→374.4 (Fig. 6 A), the background was reduced from ~ 2 e4 cps without DMS (comparison between Fig. 4 A (without DMS) and Fig. 6 A (with DMS)) to below 500 cps with DMS, which is about 40 times reduction of the background. Furthermore, the use of the DMS device completely eliminated the previously observed interference for pregnanolone in the plasma blank containing internal standard (comparison between Fig. 4 A (without DMS) and Fig. 6 A (with DMS)). Despite the loss of absolute signal of about 10 times due to transmission losses through DMS device [32], similar or slightly better S/N was obtained for allopregnanolone (~20) due to the dramatic reduction in chemical background. For MRM transition 433.3→126.1 (Fig. 6 B), the background was reduced to below 50 cps from ~ 500 cps (Fig. 4 B) by using DMS and the S/N obtained was ~30 for both compounds.
Fig. 6.
XIC of MRM transition 433.3→374.4 (A) and MRM transition 433.3→ 126.1 (B) for extracted blank plasma (red), blank with internal standard (green) and spiked plasma standard at 5 pg/mL (blue) with DMS at COV = 7 V and SV = 3700 V.
For allopregnanolone, the accuracy at LLOQ of 10 pg/mL for MRM transition 433.3→374.4 and 433.3→126.1 is 99.5 ±5.5 (%) and 101.9 ±7.6 (%) (n=6) respectively. For pregnanolone, the accuracy at LLOQ of 10 pg/mL for MRM transition 433.3→374.4 and 433.3→126.1 is 99.1 ±7.3 (%) and 99.3 ±7.7 (%) (n=6) respectively. Table 2 presents a summary of the method performance, employing the DMS device. Statistics are presented for calibration curves, and for intra- and inter-day QC accuracy and precision for both compounds. Linear calibration was obtained from 10 pg/mL to 25,000 pg/mL for both compounds, using a weighting of 1/x2. Good accuracy and precision were observed for both calibration curve and QC samples, and none of the QC samples failed (outside accuracy acceptance criteria of ± 15% [31]) in these analysis.
Table 2.
Calibration curve and QC intra- and inter- day accuracy and precision of derivatized allopregnanolone and pregnanolone with DMS
| Compound | MRM transition | Calibration curve 10-25,000 pg/mL |
||
|---|---|---|---|---|
| Slope | Intercept | r 2 | ||
| Allopregnanolone | 433.3→374.4 | 0.27881 | 0.00096 | 0.9980 |
|
| ||||
| 433.3→126.1 | 0.05460 | 0.00015 | 0.9969 | |
|
| ||||
| Pregnanolone | 433.3→374.4 | 0.31453 | 0.00158 | 0.9965 |
|
| ||||
| 433.3→126.1 | 0.03995 | 0.00019 | 0.9940 | |
|
| ||||
|
QC intra-day accuracy (%) (n=4)
|
||||
| LQC ( 50 pg/mL) |
MQC ( 500 pg/mL) |
HQC ( 5000 pg/mL) |
||
|
| ||||
| Allopregnanolone | 433.3→374.4 | 105.0 ±1.9 | 103.5 ±1.3 | 99.4 ±0.3 |
|
| ||||
| 433.3→126.1 | 105.0 ±2.8 | 103.3 ±1.3 | 98.9 ±1.3 | |
|
| ||||
| Pregnanolone | 433.3→374.4 | 106.6 ±2.5 | 104.4 ±1.1 | 98.7 ±1.8 |
|
| ||||
| 433.3→126.1 | 106.4 ±2.5 | 104.6 ±1.3 | 99.4 ±1.4 | |
|
| ||||
|
QC inter-day accuracy (%) (n=8)
|
||||
| LQC ( 50 pg/mL) |
MQC ( 500 pg/mL) |
HQC ( 5000 pg/mL) |
||
|
| ||||
| Allopregnanolone | 433.3→374.4 | 105.5 ±1.7 | 103.4 ±1.4 | 99.4 ±0.5 |
|
| ||||
| 433.3→126.1 | 104.7 ±2.6 | 103.3 ±1.0 | 98.3 ±1.0 | |
|
| ||||
| Pregnanolone | 433.3→374.4 | 106.0 ±2.8 | 104.6 ±1.6 | 98.0 ±1.5 |
|
| ||||
| 433.3→126.1 | 105.1 ±4.1 | 103.5 ±2.6 | 99.2 ±1.1 | |
In summary, we found that there are several advantages of using differential mobility spectrometry (DMS) in the development of this LC-MS/MS method: reduction of the chemical background, removal of interference for pregnanolone at MRM transition 433.3→374.4, and removal of the derivatized cis-pregnanolone. The S/N for both allopregnanolone and pregnanolone at 5 pg/mL was similar or slightly better if DMS was used. The estimated limit of detection is about 5 fg on column (1 pg/mL in plasma) with S/N about 3~5 for both compounds with or without DMS.
Analysis of plasma samples
13 plasma samples were analysed using the method described above. The samples were collected from 3 different groups of healthy females, including: (i) five samples from women in the early follicular phase of their menstrual cycle, (ii) four samples from women at weeks 39-40 of pregnancy, and (iii) four samples collected at 6 weeks postpartum. Table 3 lists the measured concentrations of allopregnanolone and pregnanolone from the sample analyses performed both with and without DMS. The concentrations measured for allopregnanolone and pregnanolone agreed very well using MRM transition 433.3→374.4 and 433.3→126.1 with DMS, and using MRM transition 433.3→126.1 without DMS. However, when MRM transition 433.3→374.4 was used without DMS, three of the samples (N118, P5 and P11) showed much higher calculated pregnanolone concentration. This phenomenon was observed mostly in samples with lower pregnanolone concentration which is consistent with our observation of an interference during the analysis of blank and lower concentration standards and QC samples. This highlights the utility of DMS for eliminating unanticipated interferences in real samples. Figure 7 displays the chromatograms for sample P5 at MRM transition 433.3→374.4, both without and with the use of DMS. It can be seen that the relative peak height for pregnanolone is larger when DMS is not employed due to the presence of the interference. This resulted in the overestimation of pregnanolone concentration when DMS was not used.
Table 3.
Allopregnanolone and pregnanolone concentration in human plasma samples with and without DMS
| Sample name |
Allopregnanolone concentration, pg/mL | Pregnanolone concentration, pg/mL | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 433.3→374.4 | 433.3→126.1 | 433.3→374.4 | 433.3→126.1 | |||||
|
| ||||||||
| DMS | No DMS | DMS | No DMS | DMS | No DMS | DMS | No DMS | |
| Follicular | ||||||||
| N102 | 65.8 | 68.3 | 62.5 | 65.8 | 43.4 | 45.2 | 42.4 | 42.8 |
| N106 | 184.1 | 195.1 | 188.3 | 186.7 | 101.6 | 102.2 | 99.0 | 91.3 |
| N107 | 212.8 | 224.4 | 210.1 | 217.7 | 78.7 | 76.6 | 71.6 | 74.4 |
| N117 | 60.0 | 61.5 | 58.0 | 58.7 | 25.6 | 29.7 | 27.1 | 27.6 |
| N118 | 25.8 | 28.0 | 26.9 | 25.3 | 14.8 | 45.3** | 16.7 | 15.3 |
| Postpartum | ||||||||
| P5 | 14.4 | 14.7 | 15.9 | 14.3 | 11.1 | 80.0** | 11.0 | 12.1 |
| P8 | 10.0 | 11.4 | 9.5* | 10.7 | 6.0* | 7.9* | 6.7* | 5.0* |
| P10 | 31.3 | 30.2 | 31.6 | 30.5 | 54.8 | 52.6 | 55.0 | 50.9 |
| P11 | 5.7* | 5.4* | 5.6* | 4.6* | 8.0* | 21.2** | 7.6* | 5.4* |
| Pregnant | ||||||||
| Preg 6 | 8253.4 | 8308.0 | 8174.0 | 8495.5 | 5262.9 | 5139.5 | 4964.8 | 5055.3 |
| Preg 11 | 15470.5 | 15677.5 | 16031.8 | 15980.3 | 8354.4 | 7955.6 | 8122.1 | 7938.3 |
| Preg 12 | 4250.1 | 4263.7 | 4235.3 | 4282.6 | 2753.9 | 2765.2 | 2589.4 | 2594.5 |
| Preg 23 | 7930.1 | 7747.6 | 7778.2 | 7939.9 | 6282.9 | 6032.4 | 6078.2 | 6062.4 |
Value below LLOQ.
Higher calculated concentrations for pregnanolone due to interference at MEM 433.3→374.4 without DMS
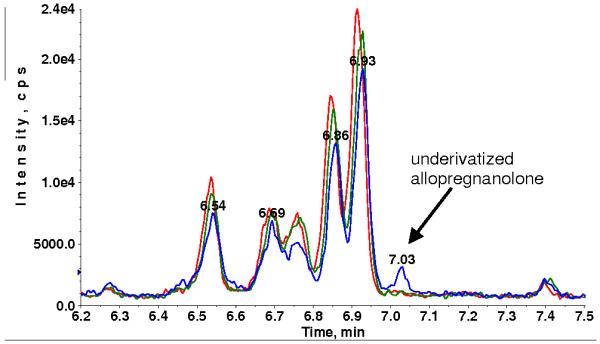
Fig. 7.
XIC of MRM transition 433.3→374.4 for sample P5 without (A) and with DMS (B).
The measured concentrations of allopregnanolone and pregnanolone were clustered in relatively narrow ranges for each of the study groups. The lowest concentrations for both allopregnanolone and pregnanolone were observed in the postpartum women with an average concentration of 15.4 pg/mL (4.6 - 31.6 pg/mL) for allopregnanolone and 21.0 pg/mL (5.4 – 55.0 pg/mL) for pregnanolone. The average concentration in non-perinatal women was 111.3 pg/mL (25.3 – 224.4 pg/mL) for allopregnanolone and 54.0 pg/mL (14.8 – 102.2 pg/mL) for pregnanolone. As expected, the highest concentrations were observed in pregnant women with average values of 9.0 ng/mL (4.2 – 16.0 ng/mL) for allopregnanolone and 5.5 ng/mL (2.6 – 8.3 ng/mL) for pregnanolone. These values agree with some of the literature values obtained in pregnant [35-37] and non-pregnant [38-39] women. Lower levels were observed in our postpartum group as compared to the values reported by Evans et al. [35] and they need to be confirmed in a larger number of samples.
To verify that our method did not suffer from ion suppression of the analytes – a phenomenon whereby co-elution of matrix components with the analytes results in a suppression of the ionization of the analytes, in the mass spectrometer ion source – we have performed an ion-suppression test using post-column T-infusion. An extracted plasma blank was injected onto the LC-MS/MS system; meanwhile a mixture of 12.5 ng/mL of allopregnanolone and pregnanolone in 70% methanol was T-infused into the LC flow post-column. The chromatographic region where the analytes eluted (5.5 – 6.5 minutes) was free from any suppression of the ion signal for allopregnanolone and pregnanolone (Fig. S2A in Electronic Supplementary Material). Since the blank plasma sample was charcoal-stripped, it is possible that the matrix is not representative of real samples. Therefore, we also evaluated ion suppression upon injecting postpartum sample P11 which has the lowest concentration (below LLOQ) of allopregnanolone and pregnanolone. The ion suppression pattern with this sample injection did not differ from the blank injection, and no ion-suppression was observed at the region where the analytes eluted (Fig. S2 B in Electronic Supplementary Material).
Conclusion
The sample preparation and LC-MS/MS method presented here provided a sensitive and selective quantitative analysis for allopregnanolone and its isomer pregnanolone in plasma samples. The sensitivity and selectivity of the method was enhanced through (i) the use of a keto-specific derivatization reagent, designed to boost sensitivity for mass spectrometric detection, and (ii) the use of differential mobility spectrometry (DMS) prior to MS/MS analysis, to enhance the selectivity of the method by removing interfering species and chemical noise. Derivatization was performed with a permanently charged quaternary amine reagent, which significantly increased the ionization efficiency of both compounds in positive electrospray ionization (ESI) mode. A LLOQ of 5 pg/mL (25 fg on column) can be achieved with blank matrix free of endogenous analytes. The method is simple, quick and amenable to automation for high throughput routine analysis. We recommend that MRM transition 433.3→126.1 be used for both compounds on instruments without DMS due to interference at MRM transition 433.3→374.4 for pregnanolone. For instruments with DMS, if target LLOQ is less than 10 pg/mL MRM transition 433.3→374.4 is a better choice than 433.3→126.1 due to its higher sensitivity. In addition, sample dilution and injection volume in this method can also be adjusted if more sensitivity is required.
Supplementary Material
Acknowledgments
The authors greatly acknowledge the helpful discussions with Dr. Suya Liu and Dr. Bradley B. Schneider and support from UL1 TR000457-06 from the National Center for Advancing Translational Sciences, National Institutes of Health and a NARSAD award (MA).
Footnotes
Note: Unless otherwise noted in our product literature, AB SCIEX products are For Research Use Only. Not for use in Diagnostics Procedures. For more information refer to www.absciex.com/product. The trademarks mentioned herein are the property of AB Sciex Pte. Ltd. or their respective owners. AB SCIEX™ is being used under license.
Contributor Information
Wen Jin, AB SCIEX, 71 Four Valley Drive, Concord, Ontario, L4K 4V8, Canada.
Michael Jarvis, AB SCIEX, 71 Four Valley Drive, Concord, Ontario, L4K 4V8, Canada.
Michal Star-Weinstock, AB SCIEX, 500 Old Connecticut Path, Framingham, MA 01701, USA.
Margaret Altemus, Weill Medical College, Cornell University, Box 244, 1300 York Avenue, New York, NY 10065, USA.
References
- [1].Belelli D, Lambert JJ. Neurosteroids: Endogenous regulators of the GABAA receptor. Nature Reviews: Neuroscience. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- [2].Pinna G. Kalinin Vladimir., editor. Neurosteriod Biosynthesis Upregulation: A Novel Promising Therapy for Anxiety Disorders and PTSD. Anxiety Disorders. 2011 Prof. ISBN: 978-953-307-592-1, InTech, http://www.intechopen.com/
- [3].Shannon EE, Porcu P, Purdy RH, Grant KA. Characterization of the discriminative stimulus effects of the neuroactive steroid pregnanolone in DBA/2J and C57BL/6J inbred mice. J Pharmacology and Experimental Therapeutics. 2005;314(2):675–685. doi: 10.1124/jpet.104.082644. [DOI] [PubMed] [Google Scholar]
- [4].Irwin RW, Wang JM, Chen S, Brinton RD. Neuroregenerative mechanisms of allopregnanolone in Alzheimer’s disease. Front. Endocrinol. 2012;2(117):1–14. doi: 10.3389/fendo.2011.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bicίková M, Lapcίk O, Hampl R, Stárka L, Knuppen R, Haupt O, Dibbelt L. A novel radioimmunoassay of allopregnanolone. Steroids. 1995;60(2):210–213. doi: 10.1016/0039-128x(94)00039-f. [DOI] [PubMed] [Google Scholar]
- [6].Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age and endocrine influences. Journal of Clinical Endocrinology and Metabolism. 1998;83(6):2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- [7].Cheney DL, Uzunov D, Costa E, Guidotti Gas chromatographic-mass fragmentographic quantitation of 3α-hydroxy-5α-pregnan-20-one (allopregnanolone) and its precursors in blood and brain of adrenalectomized and castrated rats. The Journal of Neuroscience. 1995;15(6):4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Porcu P, O’Buckley TK, Alward SE, Marx CE, Shampine LJ, Girdler SS, Morrow AL. Simultaneous quantification of GABAergic 3α, 5α/3α, 5β neuroactive steroids in human and rat serum. Steroids. 2009;74(4-5):463–473. doi: 10.1016/j.steroids.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vallée M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroid in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/ mass spectrometry. Anal. Biochem. 2000;287(1):153–166. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- [10].Liere P, Akwa Y, Weill_engerer S, Eychenne B, Pianos A, Robel P, Sjövall J, Schumacher M, Baulieu EE. Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry. J. Chromatogr B Biomed Sci. Appl. 2000;739(2):301–312. doi: 10.1016/s0378-4347(99)00563-0. [DOI] [PubMed] [Google Scholar]
- [11].Kim YS, Zhang H, Kim HY. Profiling neurosteroids in cerebrospinal fluids and plasma by gas chromatography/eletron capture negative chemical ionization mass spectrometry. Anal. Biochem. 2000;277(2):187–195. doi: 10.1006/abio.1999.4384. [DOI] [PubMed] [Google Scholar]
- [12].Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang YQ, Karu K, Griffiths WJ. Analysis of neurosterols and neurosteroids by mass spectrometry. Biochimie. 2007;89:182–191. doi: 10.1016/j.biochi.2006.10.008. [DOI] [PubMed] [Google Scholar]
- [14].Liu S, Sjövall J, Griffiths WJ. Neurosteroids in rat brain: extraction, isolation and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal. Chem. 2003;75(21):5835–5846. doi: 10.1021/ac0346297. [DOI] [PubMed] [Google Scholar]
- [15].Higashi T, Shimada K. Derivatization of neutral steroids to enhance their detection characteristics in liquid chromatography-mass spectrometry. Anal. Bioanal Chem. 2004;378(4):875–882. doi: 10.1007/s00216-003-2252-z. [DOI] [PubMed] [Google Scholar]
- [16].Santa T, Al-Dirbashi O, Fukushima T. Derivatization reagents in liquid chromatography/electrospray ionization tandem mass spectrometry for biomedical analysis. Drug Discov Ther. 2007;1(2):108–118. [PubMed] [Google Scholar]
- [17].Higashi T, Nagahama A, Otomi N, Shimada K. Studies on neurosteroids XIX. Development and validation of liquid chromatography-tandem mass spectrometric method for determination of 5alpha-reduced pregnane-type neurosteroids in rat brain and serum. J. Chromatogr B. 2007;848(2):188–199. doi: 10.1016/j.jchromb.2006.10.036. [DOI] [PubMed] [Google Scholar]
- [18].Mitamura K, Shimada K. Derivatization in liquid chromatography/mass spectrometric analysis of neurosteroids. Chromatography. 2001;22(1):11–15. [PubMed] [Google Scholar]
- [19].Higashi T, Yamauchi A, Shimada K. 2-Hydrazino-1-methylpyrindine: a highly sensitive derivatization reagent for oxosteroids in liquid chromatography-electrospray ionization-mass spectrometry. J. Chromatogr. B. 2005;825:214–222. doi: 10.1016/j.jchromb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- [20].Higashi T, Tadashi N, Noriko H, Kazutake S. Alternative procedure for charged derivatization to enhance detection response of steroids in electrospray ionization-MS. Chem. Pharm. Bull. 2007;55(4):662–665. doi: 10.1248/cpb.55.662. [DOI] [PubMed] [Google Scholar]
- [21].Higashi T, Takido N, Shimada K. Detection and characterization of 20-oxosteroids in rat brains using LC-electron capture APCI-MS after derivatization with 2-nitro-4-trifluoromethylphenylhydrazine. Analyst. 2003;128(2):130–133. doi: 10.1039/b212375d. [DOI] [PubMed] [Google Scholar]
- [22].Mukai Y, Higashi T, Nagura Y, Shimada K. Studies on neutrosteroids XXV. Influence of a 5 α-reductase inhibitor, finasteride, on rat brain neurosteroid levels and metabolism. Biol. Pharm. Bull. 2008;31(9):1646–1650. doi: 10.1248/bpb.31.1646. [DOI] [PubMed] [Google Scholar]
- [23].Griffiths WJ, Liu S, Yang Y, Purdy RH, Sjövall J. Nano-electrospray tandem mass spectrometry for the analysis of neurosteroids sulphates. Rapid Commun. Mass Sprectrom. 1999;13:1595–1610. doi: 10.1002/(SICI)1097-0231(19990815)13:15<1595::AID-RCM681>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- [24].Star-Weinstock M, Williamson BL, Dey S, Pillai S, Purkayastha S. Anal Chem. 2012;84:9310–9317. doi: 10.1021/ac302036r. [DOI] [PubMed] [Google Scholar]
- [25].Altemus M, Fong J, Yang R, Damast S, Luine V, Ferguson D, Jacobson K. Changes in cerebrospinal fluid neurochemistry during pregnancy. Biological Psychiatry. 2004;56:386–392. doi: 10.1016/j.biopsych.2004.06.002. [DOI] [PubMed] [Google Scholar]
- [26].Ma YC, Kim HY. Determination of steroids by liquid chromatography/mass spectrometery. J. Am Soc Mass Sprectrom. 1997;8:1010–1020. [Google Scholar]
- [27].Liu S, Sjövall J, Griffiths WJ. Analysis of oxosteroids by nano-electrospray mass spectrometry of their oximes. Rapid Commun. Mass Spectrom. 2000;14:390–400. doi: 10.1002/(SICI)1097-0231(20000331)14:6<390::AID-RCM882>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- [28].Griffiths WJ, Liu S, Alveliu G, Sjövall J. Derivatization for the characterization of neutral oxosteroids by electrospray and matrix-assisted laser desorption/ionization tandem mass spectrometry: the Girard P derivative. Rapid Commun. Mass Spectrom. 2003;17:924–935. doi: 10.1002/rcm.1002. [DOI] [PubMed] [Google Scholar]
- [29].Griffiths WJ, Wang Y. Analysis of oxysterols by electrospray tandem mass spectrometry. J. Am Soc. Mass Spectrom. 2006;17:341–362. doi: 10.1016/j.jasms.2005.10.012. [DOI] [PubMed] [Google Scholar]
- [30].Mosher MD, Meisenbach S. Syn and Anti isomer preference in oximes: an undergraduate organic chemistry experiment. Chem. Educator. 2002;7:356–358. [Google Scholar]
- [31].Guideline on Bioanalytical Method Validation. European Medicines Agency; 2011. http://www.ema.europa.eu/docs. [DOI] [PubMed] [Google Scholar]
- [32].Schneider BB, Covey T, Coy SL, Krylov EV, Nazarov E. Planar differential mobility spectrometer as a pre-filter for atmospheric pressure ionization mass spectrometry. Int. J. Mass Spectrom. 2010;298:45–54. doi: 10.1016/j.ijms.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schneider BB, Covey T, Coy SL, Krylov EV, Nazarov E. Control of chemical effects in the separation process of a differential mobility mass spectrometer system. Eur. J. Mass Spectrom. 2010;16:57–71. doi: 10.1255/ejms.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Krylov EV, Nazarov EG, Miller RA. Differential mobility spectrometer: Model of operation. Int. J. Mass Spectrom. 2007;266(1-3):76–85. [Google Scholar]
- [35].Evans SEG, Ross LE, Sellers EM, Purdy RH, Romach M. 3α-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecological Endocrinology. 2005;21(5):268–279. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- [36].Hill M, Cibula D, Havíková, Kancheva L, Fait T, Kancheva R, Pařízek A, Stárka L. J. Steriod Biochem. Mol. Biol. 2007;105:166–175. doi: 10.1016/j.jsbmb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- [37].Kancheva R, Hill M, Cibula D, Včeláková H, Kancheva L, Vrbíková J, Fait T, Pařízek, Stárka Relationships of circulating pregnanolone isomers and their polar conjugates to the status of sex, menstrual cycle, and pregnancy. J. Endocrinology. 2007;195:67–78. doi: 10.1677/JOE-06-0192. [DOI] [PubMed] [Google Scholar]
- [38].Murphy BEP, Abbott FV, Allison CM, Watts C, Ghadirian AM. Elevated levels of some neuroactive progesterone metabolites, particularly isopregnanolone, in women with chronic fatigue syndrome. Psychoneuroendocrinology. 2004;29:245–268. doi: 10.1016/s0306-4530(03)00026-x. [DOI] [PubMed] [Google Scholar]
- [39].Bicikova M, Putz Z, Hill M, Hampl R, Dibbelt L, Tallova J, Starka L. Serum levels of neurosteriod allopregnanolone in patients with premenstrual syndrome and patients after thyroidectomy. Endocrine Regulations. 1998;32:87–92. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.