Abstract
BDNF, acting in the mesolimbic dopamine reward pathway, promotes susceptibility to stress; however, the mechanisms controlling its release remain unknown. We report that phasic optogenetic activation of this pathway increases BDNF levels in nucleus accumbens (NAc) of socially stressed mice, but not stress-naïve mice. This stress gating of BDNF signaling is mediated by CRF acting in NAc. These results unravel a stress-context detecting function of the brain’s mesolimbic circuit.
Brain derived neurotrophic factor (BDNF) plays numerous important functions in the developing and mature brain1, 2. Among these is an essential role in the mesolimbic dopamine (DA) reward pathway in mediating responses to severe social stress3. BDNF activity is upregulated by chronic social defeat stress in NAc4, 5, a key component of the mesolimbic pathway that receives projections from ventral tegmental area (VTA) DA neurons6, 7, 8. BDNF deletion selectively in this VTA-NAc pathway of adult mice reduces susceptibility to the deleterious effects of stress and induces antidepressant-like responses at the molecular, cellular, and behavioral levels4, 5. Conversely, micro-injection of BDNF into NAc increases stress susceptibility5. However, the regulatory mechanisms controlling BDNF release from VTA terminals in NAc are poorly understood.
BDNF release from neuronal processes depends on neural activity1, 9. VTA DA neurons display two types of firing patterns in vivo, single-spike, tonic firing and high frequency, phasic firing6, 10, 11. Phasic firing of VTA DA neurons is essential for reward behaviors10, 12. Previous work found that chronic social defeat stress increases the firing rate of VTA DA neurons in susceptible mice, a phenomenon that is absent in resilient animals5,11. Furthermore, optogenetically mimicking this increased phasic firing in the VTA-NAc pathway of subthreshold-stress exposed mice induces depression-like behavioral abnormalities, such as reduced social interaction13. Here, utilizing the same in vivo model, we combined retrograde viral vectors (PRV-Cre) expressing Cre-recombinase and Cre-inducible channelrhodopsin (AAV-DIO-ChR2-eYFP) to specifically express ChR2 in VTA neurons projecting to NAc to explore the relationship of firing patterns and BDNF regulation in this pathway.
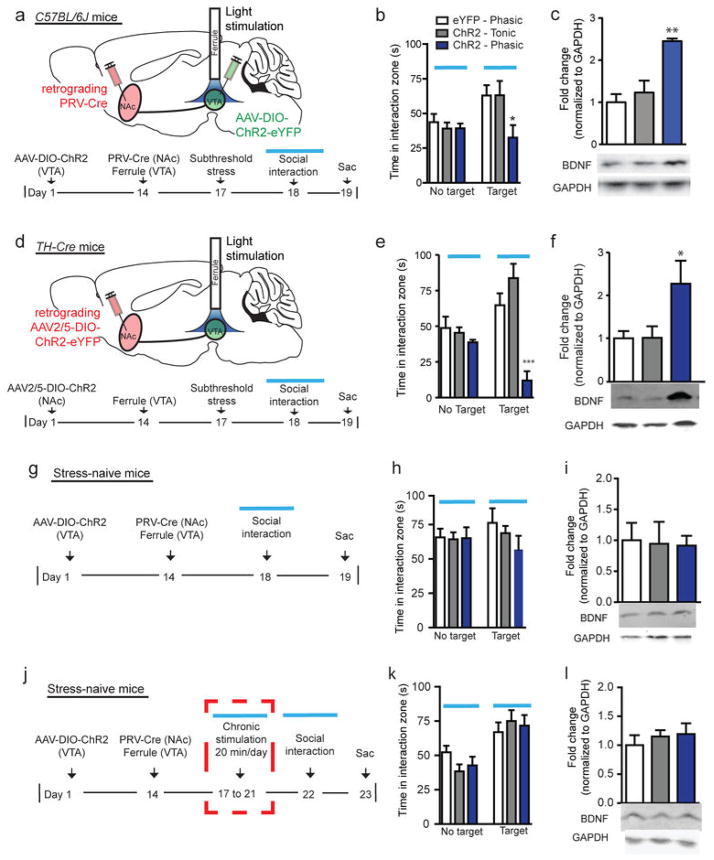
To specifically express ChR2 in VTA neurons that project to NAc (VTA-NAc neurons), we injected PRV-Cre into NAc (Supplementary Fig. 1) and ChR2 into VTA (Supplementary Fig. 2). Optical activation of VTA cell bodies in this pathway with a phasic but not tonic stimulation pattern, following a well-established subthreshold social stress5, 13, rapidly induced reduced social interaction (Fig. 1a–b; Supplementary Fig. 3). Consistent with the behavioral data, phasic but not tonic stimulation significantly increased BDNF protein levels in NAc 24 hr after the optic stimulation and social interaction test (Fig. 1c), the time point when BDNF was previously seen to be upregulated by chronic social defeat4, 5. In contrast, subthreshold social stress without stimulation had no effect on either social behavior13 (Supplementary Fig. 4a) or BDNF levels (Supplementary Fig. 4b) compared to stress-naïve control. To selectively target VTA DA neurons that project to NAc, we injected the retrograde traveling AAV2/5-DIO-ChR2 into NAc of tyrosine hydroylase (TH)-Cre transgenic mice (Fig. 1d). Validation confirmed the viability of using AAV2/5-DIO-ChR2 to express functional ChR2 in VTA DA neurons projecting to NAc (Supplementary Fig. 5). Consistent with our initial findings, we found that phasic activation of VTA DA neurons that project to NAc induces social avoidance (Fig. 1e) and, further, a significant increase in BDNF protein levels in NAc 24 hr following stimulation (Fig. 1f). These findings demonstrate a firing-pattern dependent regulation of BDNF in the VTA-NAc circuit, mediated specifically by DA neurons. Furthermore, this DA neuron-dominated effect on BDNF regulation in this pathway is consistent with our previous observation that the vast majority (95%) of ChR2-expressing VTA-NAc neurons are TH+13.
Figure 1.
Phasic activation of the VTA-NAc pathway increases BDNF in NAc of socially stressed mice, but not of stress-naïve mice. (a) Schematic of PRV-Cre infused into NAc, AAV-DIO-ChR2-eYFP infused into VTA, and ferrule implantation into VTA. Below, experimental timeline detailing subthreshold social defeat stress. (b) Phasic stimulation with AAV-DIO-ChR2-eYFP of VTA-NAc cells decreases social interaction (F2,20=4.56, *P<0.05, n=7–9). No Target denotes the absence, and Target the presence, of a social target (CD1 mouse) during behavior. Blue bars represent blue light activation during social interaction test. (c) Phasic but not tonic stimulation of VTA-NAc cells increases BDNF levels in NAc 24 hr post-stimulation (F2,19=14.55, **P<0.01, n=7–8). (d) Schematic of AAV2/5-DIO-ChR2-EYFP into NAc and ferrule implantation into VTA of TH-Cre mice. Below, experimental timeline. (e) Phasic stimulation with AAV2/5-DIO-ChR2-EYFP of VTA DA neurons that project to NAc induces social avoidance (F2,15=19.35, ***P<0.001, n=6). (f) Phasic but not tonic stimulation of VTA DA neurons projecting to NAc increases BDNF levels in NAc 24 hr post-stimulation (F2,15=3.69, *P<0.05, n=6). (g) Experimental timeline of naïve animals. (h) Phasic stimulation of VTA-NAc cells does not induce social avoidance in stress-naïve animals (F2,18=1.12, P=0.35, n=6–8). (i) Phasic stimulation does not increase BDNF levels in the absence of subthreshold social defeat stress (F2,18=0.03, P=0.97, n=6–8). (j) Experimental timeline of naïve animals with repeated optical stimulation. (k) Five days of repeated stimulation for 20 min of VTA-NAc cells does not induce social avoidance in stress-naïve mice (F2,24=0.30, P=0.74, n=8–10). (l) Five days of repeated stimulation for 20 min of VTA-NAc cells does not change BDNF levels in the absence of subthreshold defeat stress (F2,21=0.38, P=0.69, n=8). All data presented as mean ± s.e.m.
Mesolimbic DA neurons respond to rewarding as well as aversive stimuli, and can mediate divergent behavioral outputs5, 6, 8, 11, 14, 15, 16. This suggests a possible context-specific role of this circuit in encoding behaviors. Thus, we next tested if the phasic stimulation-induced increase in BDNF levels in NAc was contextually dependent on stress. We repeated our first experiments in stress-naïve mice (Fig. 1g). Consistent with previous work13, optical stimulation of VTA-NAc neurons of naïve mice had no effect on social interaction behavior (Fig. 1h). Such stimulation also produced no change in BDNF levels in NAc (Fig. 1i), suggesting that phasic stimulation alone is not sufficient to induce this adaptation and its behavioral sequelae. These data highlight that the context of a stress combined with phasic firing of mesolimbic DA neurons is critical for these pathological adaptations. We next determined if repeated stimulation of these neurons (20 min each day for 5 days) is sufficient to produce an effect (Fig. 1j). After 5 days of repeated stimulation, we found no changes in social interaction (Fig. 1k) as well as no upregulation of BDNF levels in NAc (Fig. 1l). These findings demonstrate that both contextual stress and phasic activation of the VTA-NAc pathway are required to induce BDNF signaling in NAc.
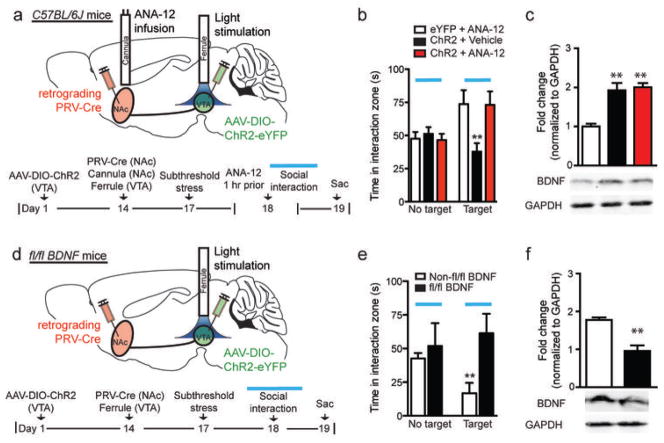
To determine if increased BDNF levels in NAc play a causal role in the optogenetically-induced avoidance behavior, we blocked the BDNF receptor TrkB using the TrkB antagonist ANA-12 (1 μg)17, administered into NAc 1 hr prior to the optical stimulation and social interaction test (Fig. 2a). The TrkB antagonist completely blocked the ability of phasic stimulation to induce social avoidance (Fig. 2b), without affecting elevated levels of BDNF (Fig. 2c). Furthermore, we performed similar experiments in BDNFfl/fl mice4 (Fig. 2d), where BDNF was deleted selectively in the VTA-NAc pathway of adult mice, and saw blockade of the optically-induced avoidance behavior (Fig. 2e) as well as a loss of increased BDNF levels in NAc (Fig. 2f). Collectively, these results suggest that firing pattern-dependent regulation of BDNF in the VTA-NAc circuit is functionally important in mediating social avoidance behavior.
Figure 2.
BDNF is necessary for the optogenetically induced susceptible phenotype. (a) Schematic and timeline of VTA-NAc neurons with cannula implantation into NAc for infusion of the TrkB inhibitor, ANA-12, 1 hr prior to behavior test with optical stimulation. (b) Intra-NAc ANA-12 infusions eliminates social avoidance with phasic stimulation (F2,23=5.59, **P<0.01, n=6–10), but (c) does not prevent the increase in BDNF levels in NAc (F2,22=16.18, **P<0.01, n=8–9). (d) Schematic and timeline showing targeting of VTA-NAc cells in fl/fl BDNF and control animals. (e) Phasic stimulation of VTA-NAc cells does not induce social avoidance in fl/fl BDNF mice (unpaired t-test, t11=2.9, P<0.01; n=6–7) and (f) does not increase BDNF in NAc (unpaired t-test, t9=4.78, **P<0.01; n=5–6).
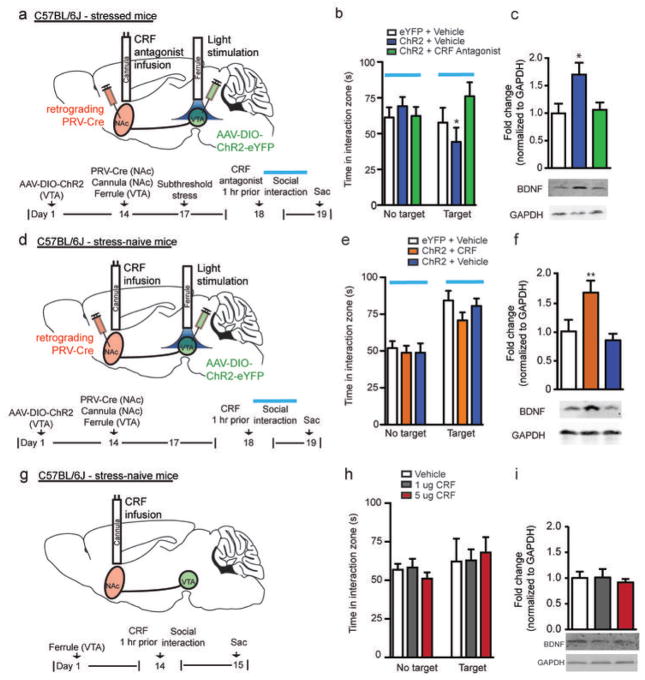
We next explored the molecular basis by which stress gates the VTA-NAc circuit. Recent work has shown that the stress-induced neuropeptide corticotrophin releasing factor (CRF) is functional both pre- and postsynaptically in NAc18, and that increased CRF signaling in NAc increases motivation for cued rewards19. Based on these findings, we investigated if CRF is the contextual signal that is required for phasic stimulation of VTA-NAc neurons to induce BDNF and social avoidance. Following the same subthreshold stress paradigm used earlier (Fig. 1), we infused a CRF receptor antagonist (alpha-helical CRF, 1 μg), which blocks both CRF receptors 1 and 2, into NAc 1 hr prior to phasic stimulation and behavioral testing (Fig. 3a). Infusion of the CRF antagonist into NAc effectively blocked the phasic-firing induced social avoidance, while animals that received intra-NAc infusion of vehicle with phasic stimulation of VTA exhibited the expected avoidance behavior (Fig. 3b). Furthermore, animals that received intra-NAc infusions of the CRF antagonist and phasic stimulation did not show changes in BDNF levels in NAc (Fig. 3c). Additionally, animals infused with AAV-DIO-eYFP into VTA that received intra-NAc infusions of CRF antagonist exhibited no changes in behavior (Supplementary Fig. 6a) or BDNF levels (Supplementary Fig. 6b). We next investigated whether intra-NAc infusion of CRF (1 μg) with phasic stimulation of the VTA-NAc pathway modulates BDNF levels in the absence of stress (Fig. 3d). Using stress-naïve animals, phasic, but not tonic, stimulation caused a significant increase in NAc BDNF levels in the CRF-infused mice compared to vehicle-infused controls, but CRF infusion had no effect on social behavior in these social stress-naïve mice (Fig. 3e–f and Supplementary Fig. 7a–b). Additionally, in the absence of stress or optical stimulation, animals that received CRF infusion alone (1 or 5 μg) exhibited no changes in behavior (Fig. 3g–h) or BDNF levels (Fig. 3i) compared to vehicle infusion. Together, these findings implicate CRF, acting in NAc, in gating the ability of phasic firing, in the context of stress, to increase BDNF signaling in NAc (Supplementary Fig. 8a).
Figure 3.
CRF is required for the phasic firing induced increase in BDNF in NAc in the context of stress. (a) Timeline of animals that received intra-NAc CRF antagonist infusions. (b) Intra-NAc CRF antagonist (alpha-helical CRF) infusion eliminates social avoidance seen with subthreshold stress plus phasic stimulation of VTA-NAc neurons (F2,21=3.99, *P<0.05, n=6–9) and (c) blocks the expected BDNF increase in NAc (F2,18=4.66, *P<0.05, n=6–8). (d) Timeline of stress-naïve animals that received intra-NAc CRF infusions. (e) Intra-NAc CRF infusions in stress-naïve animals does not alter social interaction behavior (F2,23=0.58, P=0.57, n=8–9). (f) Phasic stimulation alone induces an increase in NAc BDNF levels in intra-NAc CRF infused, stress-naïve mice (F2,19=5.88, *P<0.01, n=6–8). (g) Timeline of stress and stimulation-naïve animals that received intra-NAc CRF infusions. (h) Intra-NAc CRF infusions in stress and stimulation-naïve animals does not alter social interaction behavior (F5,12=0.082, P=0.922, n=5). (i) Intra-NAc CRF infusions of 1 or 5 μg does not alter NAc BDNF levels (F2,12=0.186, P=0.833, n=5).
Similar to conventional neurotransmitters, BNDF release from axons and dendrites depends on neuronal activity1, 9. In this in vivo study, we demonstrate that neither acute nor 5 days of repeated phasic activation of VTA-NAc neurons induces a significant increase in NAc BDNF levels of stress-naïve mice. Further, BDNF upregulation is blocked in socially stressed and phasically activated animals given an intra-NAc infusion of CRF antagonist. In contrast, the same phasic activation of this pathway induces upregulation of BDNF in NAc of mice subjected to subthreshold social stress, which in unstimulated animals has no effect on BDNF levels. This induction of BDNF signaling in NAc is absent in mice that lack BDNF selectively in VTA-NAc neurons. These results show that phasic firing alone is not sufficient to induce BDNF upregulation in this circuit, and suggest that mesolimbic DA neurons have a stress context-detecting function, which is mediated by CRF acting in NAc (Supplementary Fig. 8b). These data unravel a gating function of the brain’s mesolimbic reward circuitry in producing selective responses to environmental stimuli20.
ONLINE METHODS
Experimental subjects
Male 7–8 week old C57BL/6J mice, TH-Cre mice7, 10, floxed (fl/fl) BDNF mice (B6129PF2/J background)4, B6129PF2/J (control for floxed BDNF mice), and 6-month old CD1 retired breeders4, 5, 11, 13 were kept at 22–25°C in a 12-hr light/dark cycle and fed ad libitum. Some of the differences in stress responses are likely due to background differences. All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committees at Icahn School of Medicine at Mount Sinai.
Viral vectors and stereotaxic surgeries for optogenetic approaches
A doublefloxed (DIO) Cre-dependent adeno-associated virus (AAV) vector expressing channelrhodopsin-2 (ChR2) fused with enhanced yellow fluorescent protein (eYFP) (AAV-DIO-ChR2–eYFP), a replication-defective version of the retrograde travelling pseudorabies virus expressing Cre (PRV–Cre), and a doublefloxed (DIO) retrograde traveling Cre-dependent AAV2/5 vector expressing ChR2 fused with eYFP (AAV2/5-DIO-ChR2-eYFP) were used in this study for optical activation of the VTA-NAc pathway13. AAV-DIO-ChR2 was purchased from University of North Carolina Vector Core, PRV-Cre was engineered by an author laboratory (JFM), and AAV2/5-DIO-ChR2-eYFP was purchased from University of Pennsylvania Vector Core. For surgeries, mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg), positioned in a small-animal stereotaxic instrument (Kopf Instruments, Tujunga, California) and the skull surface was exposed. Thirty-three gauge syringe needles (Hamilton, Reno, Nevada) were used to bilaterally infuse 0.5 μl of AAV-DIO-ChR2-eYFP or AAV-DIO-eYFP at a rate of 0.1 μl/min into VTA (bregma coordinates: anteroposterior, −3.2; mediolateral, −0.5; dorsoventral, −4.6; 7° angle). 0.5 μl of retrograde PRV-Cre or AAV2/5-DIO-ChR2-eYFP was bilaterally infused into NAc (bregma coordinates: anteroposterior, +1.5; mediolateral, +1.6; dorsoventral, −4.4; 10° angle) at a rate of 0.1 μl/min. Optic fibers (ferrules) were implanted into VTA (bregma coordinates: anteroposterior, −3.2; mediolateral, −0.5; dorsoventral, −4.4; 7° angle). For drug infusions, a 26-gauge guide cannula, 3.6 mm in length from the cannula base, was implanted bilaterally into NAc (bregma coordinates: anteroposterior, +1.5; mediolateral, 0; anteroposterior, −3.9; 0° angle. Mice were allowed four days of recovery before drug infusions and stimulation.
Microinjections
One hr prior to the social interaction test, mice received an intra-NAc infusion of a TrkB antagonist (ANA-12, 1 μg)17, CRF antagonist (alpha-helical CRF, 1 μg), CRF (1 μg)18, or vehicle. Drugs were infused through an injector cannula for a total volume of 0.3 μl per hemisphere at a continuous rate of 0.1 μl per min using a micro-infusion pump (Harvard Apparatus). Injector cannulae were removed 2 min after infusions were complete and animals were allowed to sit undisturbed for 1 hr prior to the social interaction test. All drugs were purchased from Sigma.
Blue light stimulation
Animals received either tonic or phasic stimulation of the VTA-NAc pathway during the social interaction test (2.5 min) or chronically (20 min) for five days prior to the behavior test. Animals received either ChR2 (473 nm) or eYFP infusions into VTA and were given either a low-frequency tonic (0.5 Hz) or high-frequency phasic (20 Hz) stimulation, exposing cells to 5 spikes over each 10-sec epoch as we previously used12, 13. These protocols were established based on ex vivo studies that showed VTA DA neurons exhibit increased firing in susceptible animals5 and in vivo studies that showed an increase in overall firing as well as burst firing in VTA DA neurons in susceptible animals11.
Subthreshold social defeat stress paradigm
This paradigm is a well-established protocol, which has been successfully used to detect the mechanisms that promote a susceptible, depression-like, phenotype5, 13. Otherwise, these mechanisms are difficult to explore under maximal conditions such as the chronic (10-day) social defeat paradigm. To measure the molecular and cellular factors that increase social avoidance behavior, we adapted this subthreshold defeat protocol. Under these conditions, male C57BL/6J mice were exposed to a novel CD1 male aggressor for 5 min, followed by 15 min rest in the home cage. Exposure to the CD1 aggressor occurs 2 times with 15 min intervals between each exposure. Twenty-four hr later, mice are assessed using the social interaction test described below. This subthreshold defeat protocol does not induce social avoidance in stress-naïve mice, but makes the mice more sensitive to further stress.
Social interaction test
A social interaction test was performed on day following the subthreshold defeat paradigm as described in our previous work5, 13. For this test, an open field arena is divided into an interaction zone and two opposing corner zones. A mesh-plastic target box is placed into the interaction zone. A test mouse is allowed to roam around the open field arena for 2.5 min with no social target (CD1 mouse) in the mesh box (denoted as No Target in Social Interaction Figures). Following this, a novel CD1 mouse is placed in a metal mesh-plastic target box in the interaction zone (denoted as Target in Social Interaction Figures) and the test mouse is placed back into the open arena for another 2.5 min. Utilizing the Ethovision tracking software program, the times spent in the interaction zone and corner zones are measured during phasic or tonic optical stimulation, 2.5 min each with or without social target. Mice that spend significantly less time interacting with the social target and a significantly higher amount of time in the corner zones are determined as having social avoidance behavior4, 5. This social interaction test also provides a measurement of distance travelled as a locomotor activity calculation.
Immunohistochemistry
For immunofluorescence experiments, tissue sections were fixed in 4% PBS-buffered paraformaldehyde. Perfused brains were kept in 10% formalin at 4°C for 24 hr, then transferred to 30% sucrose for 24hr, and finally were transferred to PBS and sliced into coronal sections using a vibrating blade microtome (Leica Microsystems, model VT1000S). The tissue was blocked in PBS-T (0.3% Triton X-100) including 2% BSA (Sigma) and then exposed overnight to antibodies against GFP (Abcam, chicken, 1:1000) and tyrosine hydroxylase (Pel-Freez, rabbit, 1:1000). The antibodies were labeled with goat anti-chicken Alexa 488 and goat anti-rabbit Alexa 633 (Invitrogen, 1:1000), respectively. Tissue was mounted with antifade solution, including DAPI (VectaShield; Vector Laboratories). Sections were imaged on a LSM 710 confocal (Zeiss).
Western blotting
Bilateral 14-gauge NAc punches were obtained 1 mm coronal NAc sections from mice 24 hr post social interaction test with optical stimulation. Punches were then sonicated (Cole Parmer, Vernon Hills, Illinois, USA) in 30 μl of homogenization buffer containing 320 mM sucrose, 5 nM Hepes buffer, 1% SDS, phosphatase inhibitor cocktails I and II (Sigma, St. Louis, MO, USA), and protease inhibitors (Roche, Basel, Switzerland). The concentration of protein was determined using a DC protein assay (Bio-Rad, Hercules, CA) and 30 μg of total protein was loaded onto a 18% gradient Tris-HCl polyacrylamide gel for electrophoresis fractionation (Bio-Rad, Hercules, CA). Samples were then transferred onto a nitrocellulose membrane and then blocked in Odyssey® blocking buffer (Li-Cor, Lincoln, NE, USA) for one hr for Li-Cor analysis. After blocking, the same membrane was incubated in 4°C overnight with either antibodies against BDNF (1:5,000, Santa Cruz sc546), detecting truncated BDNF, or GAPDH (1:10,000, cell signaling 2118) in Odyssey® blocking buffer. Following thorough washing with TBST, blots were incubated for 1 hr at room temperature with IRDye® secondary antibodies (1:10,000; Li-Cor, Lincoln, NE, USA) in Odyssey® blocking buffer. Blots were then scanned using Odyssey® Infrared Imaging System (Li-Cor, Lincoln, NE, USA) and quantified using ImageJ (NIH, Bethesda, Maryland, USA).
Statistics
Student’s t-tests were used for analysis of two experimental groups. One-way ANOVA was used for analysis of three or more experimental groups. Main and interaction effects were considered significant at p < 0.05. All data are expressed as mean ± SEM.
Supplementary Material
References
- 1.Park H, Poo MM. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 2.Nestler EJ, et al. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 3.Nestler EJ, Carlezon WA., Jr Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Berton O, et al. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan V, et al. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Grace AA, et al. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Lobo MK, et al. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lammel S, et al. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuda N, et al. J Neurosci. 2009;29:14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai HC, et al. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao JL, et al. J Neurosci. 2010;30:16453–16458. doi: 10.1523/JNEUROSCI.3177-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo JW, et al. Science. 2012;338:124–128. doi: 10.1126/science.1222265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhury D, et al. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koob GF, et al. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Tye KM, et al. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wanat MJ, Bonci A, Phillips PE. Nat Neurosci. 2013;16:383–385. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cazorla M, et al. J Clin Invest. 2011;121:1846–1857. doi: 10.1172/JCI43992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemos JC, et al. Nature. 2013;490:402–406. [Google Scholar]
- 19.Pecina S, Schulkin J, Berridge KC. BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnitzer MJ. Nature. 2002;416:683. doi: 10.1038/416683a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.