Abstract
A defining characteristic of psychopathy is the willingness to intentionally commit moral transgressions against others without guilt or remorse. Despite this ‘moral insensitivity’, the behavioral and neural correlates of moral decision-making in psychopathy have not been well studied. To address this issue, the authors used functional magnetic resonance imaging (fMRI) to record hemodynamic activity in 72 incarcerated male adults, stratified into psychopathic (N = 16) and nonpsychopathic (N = 16) groups based on scores from the Hare Psychopathy Checklist-Revised, while they made decisions regarding the ‘severity of moral violations’ of pictures that did or did not depict moral situations. Consistent with hypotheses, an analysis of brain activity during the evaluation of pictures depicting moral violations in psychopaths vs. nonpsychopaths showed atypical activity in several regions involved in moral decision-making. This included reduced moral/non-moral picture distinctions in the ventromedial prefrontal cortex and anterior temporal cortex in psychopaths relative to nonpsychopaths. In a separate analysis, the association between severity of moral violation ratings and brain activity across participants was compared in psychopaths versus nonpsychopaths. Results revealed a positive association between amygdala activity and severity ratings that was greater in nonpsychopaths than psychopaths, and a negative association between posterior temporal activity and severity ratings that was greater in psychopaths than nonpsychopaths. These results reveal potential neural underpinnings of moral insensitivity in psychopathy and are discussed with reference to neurobiological models of morality and psychopathy.
Keywords: Morality, psychopathy, fMRI, medial prefrontal cortex, anterior temporal cortex, amygdala
Psychopathy is a disorder defined by a cluster of interpersonal, affective and behavioral characteristics including impulsivity, grandiosity, callousness and lack of empathy (Cleckley, 1976; Hare, 1998). A core feature of psychopathy is early emerging, severe and persistent antisocial behaviors, many of which are often described as ‘immoral’ (e.g. committing acts of violence against others). Psychopaths also show a profound lack of guilt or remorse for their antisocial actions. The societal cost of psychopathy is high, in large part due to immoral (and often criminal) acts committed by individuals with the disorder, and because psychopaths are frequently incarcerated for their immoral actions. Thus, understanding the factors that contribute to immoral behavior in psychopathy would have significant benefit for society and the psychopath.
A unique characteristic of psychopathy is the willingness to commit moral transgressions despite being able to indicate their ‘wrongness’. As such, psychopaths are unlikely to demonstrate clear cognitive deficits when reasoning about moral decisions, such as whether a person should keep money found in a lost wallet (Glenn et al., 2009; Cima et al., 2010). The psychopath is just as likely as the nonpsychopath to say that the money should be returned, even if this bears no relation to what the psychopath would actually do when faced with this scenario. However, psychopaths may show subtler deficits in moral reasoning. Blair (1995; 1997) found that adult psychopaths and children with psychopathic tendencies had greater difficulty than nonpsychopaths distinguishing between moral violations (hitting another person) and conventional violations (dressing in opposite-sex clothes). Thus, some behavioral evidence of deficits in moral reasoning in psychopathy has been identified. It would also be beneficial, however, to study moral reasoning in a manner that is not dependent on verbal responses, since this may elicit more reliable findings.
One way to address this issue is to use functional neuroimaging techniques to record brain activity in psychopaths and nonpsychopaths during moral decision-making. Although the neural correlates of moral decision-making have been established in healthy populations (Greene & Haidt, 2002; Moll, de Oliveira-Souza, Krueger, & Grafman, 2005), the functional integrity of the ‘moral brain’ in psychopathy has not been well studied. Functional imaging can provide information about potential neurobiological abnormalities associated with moral decision-making in psychopaths, even if their behavioral responses appear ‘normal’. Although the neural correlates underlying moral decision-making in psychopathy have not been well studied, functional imaging studies have demonstrated that adult psychopaths show atypical brain activity during emotional processing, which is critically involved in moral judgment (Young & Koenigs, 2007). For example, psychopaths show decreased activity in limbic brain regions such as the amygdala, ventromedial prefrontal cortex, anterior cingulate, and anterior temporal cortex (Birbaumer, Veit, Lotze et al., 2005; Kiehl, Smith, Hare et al., 2001; Veit, Flor, Erb et al., 2002; Muller, Sommer, Dohnel, Weber, Schmidt-Wilcke, & Hajak, 2008a; but see Muller, Sommer, Wagner et al., 2003) and increased lateral prefrontal activity (Kiehl et al., 2001; Muller et al., 2003; see also Gordon, Baird, & End, 2004) in response to emotional stimuli such as unpleasant words or pictures, compared to nonpsychopaths. These findings have contributed to the ‘paralimbic hypothesis’ of psychopathy (Kiehl, 2006), which proposes that multiple regions within and adjacent to the limbic system are dysfunctional, tending to be underreactive to emotional and other salient stimuli (e.g. moral violations). Several of these regions have been implicated in moral decision-making in healthy populations, including the ventromedial prefrontal cortex, anterior temporal cortex, and amygdala (Greene & Haidt, 2002; Moll et al., 2005; Raine & Yang, 2007).
Blair (2007) proposed that psychopathy-related dysfunction in two of these regions, the ventromedial prefrontal cortex and the amygdala, contributes to impaired moral socialization beginning at an early age. The proposal is based on the importance of stimulus-reinforcement associations in moral socialization (learning that certain behaviors are harmful to others and should be avoided) and the role of the amygdala and ventromedial prefrontal cortex in these processes (the former in valence representation, i.e. ‘good’ or ‘bad’, the latter in outcome expectancy). In other words, dysfunction within these regions makes psychopaths less sensitive to the aversive consequences of moral transgressions and less likely to avoid committing them. Consistent with this hypothesis, lesions to the ventromedial prefrontal cortex lead to impaired moral reasoning (Koenigs et al., 2007; Ciaramelli, Muccioli, Ladavas, & di Pellegrino, 2007), and psychopathic behavior (particularly when the damage occurs early in life, see case studies described in Anderson et al., 1999). Glenn, Raine, and Schug (2009), using functional magnetic resonance imaging (fMRI), studied psychopathy in a community sample and found that psychopathy scores were negatively correlated with ventromedial prefrontal and amygdala activity during the evaluation of complex moral dilemmas.
The goal of the current study was to use fMRI to record hemodynamic activity from incarcerated psychopaths and nonpsychopathic offenders during moral decision making. Since the prevalence of psychopathy is higher in prison compared to community settings (Hare, 2003), we were able to recruit a relatively large sample of individuals with high psychopathy scores. Such large samples have been rare in previous neuroimaging studies of psychopathy. Participants were scanned using fMRI while they viewed unpleasant pictures that did or did not depict moral violations (e.g. an act of violence vs. an argument; a hand breaking into a house vs. a mutilated hand), and rated the ‘severity of moral violation’ present in the pictures. The primary hypothesis was that psychopaths, relative to nonpsychopaths, would show reduced activity in brain regions known to be involved in processing morally-salient stimuli while viewing pictures depicting moral violations. These included the ventromedial prefrontal cortex and anterior and posterior temporal cortex, brain regions that may represent the roles of emotional responses, evaluating social cues, and theory of mind in moral decision making (Greene & Haidt, 2002). While psychopaths do not show broad impairments in all of these forms of processing (e.g. theory of mind; Richell, Mitchell, Newman, Leonard, Baron-Cohen, & Blair, 2003), we predicted they would be less likely than nonpsychopaths to engage them when evaluating moral violations.
In addition to examining brain activity during the viewing of moral pictures, a parametric modulation analysis was conducted on the violation severity ratings given by participants. This analysis investigated whether increased activity in hypothesized brain regions during picture viewing was associated with higher (positive modulation) or lower (negative modulation) severity ratings, and whether this pattern differed across nonpsychopaths and psychopaths. In prior research with healthy controls we have reported positive modulatory effects in the amygdala and ventromedial prefrontal cortex (Harenski et al., 2008), which may represent an association between emotional responses to moral violations and perceived moral violation severity. Given the hypothesized dysfunction in these regions in psychopathy (Blair, 2007), the hypothesis was that nonpsychopaths would show a positive association between amygdala and ventromedial prefrontal activity and violation severity ratings, whereas psychopaths would not.
Method
Participants
Seventy two adult male volunteer participants were recruited from a medium-security North American prison. Additional participants who volunteered for the study but met exclusion criteria were not included (N = 39). Exclusion criteria were: age younger than 18 or older than 55, non-fluency in English, reading level lower than 4th grade, IQ score lower than 80, history of seizures, prior head injury with loss of consciousness > 30 minutes, current Diagnostic and Statistical Manual of Mental Disorders (4th ed.; American Psychiatric Association, 1994) Axis I diagnosis, lifetime history of a psychotic disorder or psychotic disorder in a first-degree relative, or current alcohol or drug use. Thirteen additional participants who met study inclusion criteria were not included in the current study due to excessive motion during scanning (> 6mm, N = 8), poor behavioral performance (missing many ratings, N = 3), or equipment malfunction (N = 2).
Assignment to psychopath and nonpsychopath groups was based on scores from the Psychopathy Checklist-Revised (PCL-R; Hare, 2003). The PCL-R is a reliable and valid instrument for the assessment of psychopathy in incarcerated populations (Hare, 1980, 1996; Hart & Hare, 1989; Fulero, 1996). The PCL-R is comprised of 20 items, each scored on a 3-point scale (0, 1, or 2), that measure the personality and behavior characteristics of psychopathy. PCL-R scores range from 0–40. PCL-R assessments were performed by a research assistant or postdoctoral researcher (trained and supervised by K.K.), and involved a semi-structured interview covering school adjustment, employment, relationships, family, and criminal activity, in addition to a review of the participant’s institutional records. Twenty percent of all PCL-Rs were double rated by a postdoctoral researcher with extensive training (C.H. or M.S.; inter-rater reliability = .925). Participants with scores of 30 or above (N = 16) and 29 or below (N = 56) were classified as psychopaths and nonpsychopaths, respectively, in line with recommended cutoff scores (Hare, 2003). Of the 56 nonpsychopaths, 16 with the lowest PCL-R scores were selected to match the psychopaths on age, IQ (Vocabulary and Matrix Reasoning subtests of the Wechsler Adult Intelligence Scale (WAIS; Wechsler, 1997) were used to estimate IQ (Ryan, Lopez, & Werth, 1999)), substance use history (via a modified version of the Addiction Severity Inventory, McLellan, Kushner, & Metzger, 1992), and ethnicity (Table 1). These 16 participants were matched with the psychopaths on all variables except substance use history (hallucinogen use) and IQ; both significantly lower in the nonpsychopaths. To achieve matching, the two highest-scoring individuals of the lowest scorers, one who had no substance abuse history and one who had low IQ (< 90), were removed from the sample and replaced with the two next-lowest scoring individuals that had substantial substance use history and average IQ (> 100). After this procedure the 16 psychopaths and 16 nonpsychopaths were matched on all variables in Table 1. The PCL-R scores of nonpsychopaths ranged from 7 – 18. Overall, 32 participants were included in the group analysis that compared brain activity in psychopaths and nonpsychopaths during moral decision-making. All 72 participants were included in a supplemental correlation analyses between brain activity during moral decision making and PCL-R scores.
Table 1.
Comparisons between Psychopaths and Nonpsychopaths on Demographic, Cognitive, PCL-R, and Substance Use measures*
| Nonpsychopaths (n = 16) | Psychopaths (n = 16) | Statistic (t) | p value | |
|---|---|---|---|---|
|
Demographic | ||||
| Age | 34.8 (10.95) | 33.3 (8.44) | 0.43 | 0.67 |
| Ethnicity | 0.00† | 1.00 | ||
| Caucasian | N = 6 | N = 6 | ||
| Non-Caucasian¥ | N = 10 | N = 10 | ||
| Handedness Score‡ | 43.4 (53.90) | 56.9 (32.6) | 0.83 | 0.42 |
|
Cognitive | ||||
| IQ | 98.3 (14.23) | 104.8 (10.84) | 1.2 | 0.24 |
|
Psychopathy | ||||
| PCL-R Total | 13.3 (3.06) | 31.8 (2.54) | 18.68 | < .001 |
| Factor 1 | 3.8 (1.72) | 11.4 (2.06) | 11.26 | < .001 |
| Factor 2 | 7.82 (3.45) | 16.9 (1.86) | 9.28 | < .001 |
|
Substance Use (Past)§ | ||||
| Total Years used | ||||
| Alcohol | 12.5 (11.48) | 11.6 (10.2) | 0.22 | 0.83 |
| Cannabis | 11.3 (12.5) | 9.4 (8.5) | 0.49 | 0.63 |
| Cocaine | 4.7 (8.84) | 7.1 (8.4) | 0.75 | 0.46 |
| Methamphetamine | 1.4 (2.71) | 3.6 (6.66) | 1.16 | 0.26 |
| Heroin | 0.3 (1.25) | 0.9 (1.59) | 1.19 | 0.24 |
| Hallucinogens | 1.8 (3.14) | 2.9 (4.48) | 0.81 | 0.43 |
Data are given as Mean (SD) unless otherwise noted.
χ2 statistic.
Nonpsychopaths: 7 Hispanic, 2 African American, 1 Hispanic/American Indian. Psychopaths: 6 Hispanic, 2 African American, 2 American Indian
Information was not available for one psychopathic participant.
Information was not available for two psychopathic participants.
All 72 participants completed the Structured Clinical Interview for DSM Disorders (SCID), administered by a trained research assistant or postdoctoral researcher. All participants except one nonpsychopath met criteria for a past substance use disorder. In addition, one psychopathic participant and two nonpsychopathic participants met criteria for a single past major depressive episode, and another nonpsychopathic participant met criteria for past panic disorder (during childhood). No other Axis I disorders were present.
Participants were paid $1/hr. for participation, a rate commensurate to pay for work assignments at the facility. All participants provided written informed consent and the study was conducted in accordance with institutional ethical standards.
Stimuli and Task
Three picture sets (25 moral, 25 non-moral, 25 neutral) were selected primarily from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1995), and supplemented with pictures from media sources. All moral pictures depicted unpleasant social scenes indicating a moral violation (e.g. a hand breaking into a house, a person attacking another person). Non-moral pictures depicted unpleasant social scenes without moral content (e.g. a mutilated hand, two individuals arguing). Neutral pictures depicted affectively neutral social scenes without moral content (e.g. a hand being fingerprinted, two individuals having a conversation). The full picture set can be viewed at www.mrn.org/mrt_stimuli. Moral and non-moral pictures were a subset of those used in Harenski and Hamann (2006), and were matched on emotional arousal and social complexity based on the ratings of three separate groups of healthy participants: a pilot study conducted by the first author, Harenski & Hamann (2006), and Harenski et al. (2010). Neutral pictures were matched to moral and non-moral pictures on social complexity.
Participants were informed that they would see a series of pictures depicting people and events. For each picture, they were instructed to determine whether it represented a moral violation (i.e., an action or attitude that the participant considered to be morally wrong) and, for pictures that did contain a moral violation, to rate the severity on a 1–5 scale, with 5 representing the highest violation severity. For other pictures that the participant deemed not to contain a moral violation, they were instructed to give a rating of 1. Emphasis was placed on asking the participants to make ratings based on their own moral values, not what others or society would think was a moral violation. During fMRI scanning, participants completed five practice trials to ensure they understood how to perform the task. In each trial, a picture was displayed for six seconds, while the participant determined whether it represented a moral violation. Next, a rating scale was shown. The rating scale was displayed in continuous presentation format, such that a red bar began at ‘1’ (none) and progressed to ‘5’ (severe) over a period of 4 seconds (see Figure 1). The participant pressed a button to stop the bar when it reached the rating they wished to give. This rating format was chosen for simplicity (needing to press only one button rather than several different buttons). Next, a 4-second rest period occurred during which a black screen with a white fixation cross was displayed. Moral, non-moral, and neutral picture trials were presented in a randomized order, and interspersed with ‘null’ fixation trials of the same duration as picture trials. The randomization of the null trials created variable rest periods (14, 24, or 34 seconds when a picture trial was followed by 1, 2, or 3 null trials, respectively) which induced jitter. The 100 total trials (25 moral, 25 non-moral, 25 neutral, and 25 null) were presented across two separate runs. Images were rear-projected into the scanner using an LCD projector, controlled by a PC computer. Tasks were designed and presented and responses were recorded using Presentation (version 10.78, http://nbs.neuro-bs.com).
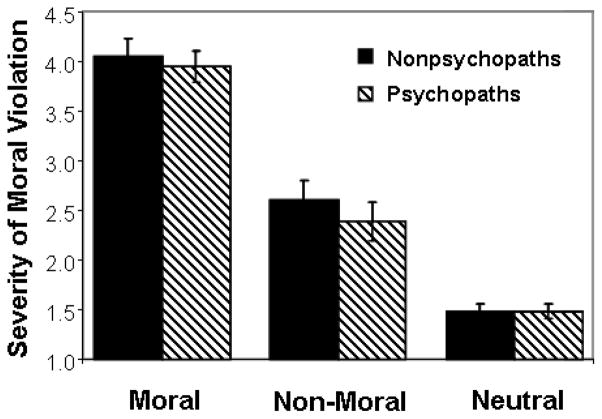
Figure 1.
Severity of moral violation ratings by condition in psychopaths and nonpsychopaths. Bars = standard error.
The continuous presentation format of the rating scale could affect the ratings of individuals who did not fully attend to the stimuli. In other words, a higher rating could be given because the participant was slow to respond rather than because they intended to give a high violation severity rating. To address this issue, responses were not accepted after the bar reached ‘5’. If a participant was indeed not paying attention during the task, they should have many ‘missed’ ratings. Participants who had multiple missed ratings (more than 5 out of the 75 pictures) were excluded from analysis (N = 3).
MRI Data Acquisition and Analysis
MR images were collected with a mobile Siemens 1.5T Avanto with advanced SQ gradients (max slew rate 200T/m/s (346 T/m/s vector summation, rise time 200us) equipped with a 12 element head coil. The EPI gradient-echo pulse sequence (TR/TE 2000/39 ms, flip angle 90°, FOV 24 × 24 cm, 64 × 64 matrix, 3.4 by 3.4 mm in plane resolution, 5 mm slice thickness, 30 slices) effectively covers the entire brain (150 mm) in 2.0 seconds. Head motion was limited using padding and restraint. Any participant with head motion greater than 6mm was excluded from analysis.
Functional images were analyzed using Statistical Parametric Mapping software (SPM5). Images were realigned using INRIAlign – a motion correction algorithm unbiased by local signal changes (Freire & Mangin, 2001; Freire, Roche, & Mangin, 2002). For each participant, the realignment parameters (3 translation; 3 rotations) were entered as covariates of no interest in the statistical model to regress variance due to movement. Functional images were spatially normalized to the MNI template via a 9-parameter affine transformation followed by smoothing with basis functions to account for nonlinear differences (Ashburner & Friston, 1999), and smoothed (8 mm FWHM). High frequency noise was removed using a low pass filter (cutoff–128s). Images were normalized to a mean of 100 (arbitrary units) to compensate for intensity variations across runs (note this is not the ‘proportional’ scaling procedure that can result in artifactual deactivations when global effects are correlated with the local BOLD signal - see Desjardins, Kiehl, & Liddle, 2001). Picture presentations (moral, non-moral, neutral) and the rating period were modeled as separate events. The primary event of interest, picture presentation, was modeled with the standard hemodynamic response function with a six second duration. Functional images were computed for each participant that represented hemodynamic responses associated with viewing moral, non-moral, or neutral pictures. Group differences in moral relative to non-moral and neutral picture viewing were analyzed using a 2 Group (Nonpsychopath/Psychopath) × 3 Condition (Moral/Non-Moral/Neutral) ‘flexible factorial’ ANOVA in SPM5, which includes between and within-participant effects.
We also analyzed hemodynamic responses associated with individual ‘severity of moral violation’ ratings. This was accomplished using the parametric modulation analysis in SPM5, in which the participant’s ratings of each picture were entered as covariates in the first-level analysis. A functional image was computed for each participant that represented the average association between brain activity and severity ratings across all pictures. Neutral pictures were not included in this analysis, since nearly all pictures were rated ‘1’ on severity by all participants1. This analysis determined whether increased activity in any brain regions during picture viewing was associated with higher (positive modulation) or lower (negative modulation) violation severity ratings. One-sample t-tests were conducted in each group to assess whether significant positive or negative modulatory effects were present in any brain regions. To determine whether modulatory effects differed across nonpsychopaths and psychopaths, a between-group ANOVA was conducted on the parametrically modulated images.
For both analyses, four regions of interest were defined using results from the same task from 28 healthy, non-incarcerated participants (Harenski, Antonenko, Shane, & Kiehl, 2008): ventromedial prefrontal cortex (BA 10/11), bilateral amygdala, right anterior temporal cortex (BA 21), and bilateral posterior temporal cortex (BA 39)2. Ten-mm radius spheres were defined around center coordinates derived from the activation peaks in each region, and corrected with a family-wise error (FWE) threshold of p < .05 using small volume correction in SPM5. Whole-brain analyses were also conducted to explore whether additional regions showed differential effects in psychopaths and nonpsychopaths. These analyses were thresholded at p < .001, uncorrected, with a cluster threshold ≥ 567 mm3 (21 contiguous voxels). The threshold was determined based on Monte Carlo simulation using the AlphaSim program written by D. Ward in AFNI software (http://afni.nimh.nih.gov/). For the parametric modulation analysis, we used a slightly more lenient threshold of p < .001, uncorrected, cluster threshold ≥ 135 mm3 (5 contiguous voxels), because this analysis is sensitive to individual moral judgments for each picture, and the effects are more subtle than the effects of moral vs. non-moral picture viewing, as previously demonstrated in non-antisocial participants (Harenski et al., 2008).
Activations were overlaid on a representative high-resolution structural T1-weighted image from a single subject from the SPM5 canonical image set, coregistered to Montreal Neurological Institute (MNI) space. All coordinates are reported in MNI space.
Results
Severity of Moral Violation Ratings
A Group (Nonpsychopath/Psychopath) × Condition (Moral/Non-Moral/Neutral) ANOVA was used to assess group differences in online ‘severity of moral violation’ ratings. A main effect of Condition (F(2,90) = 134.11, p < .00001) indicated that psychopaths and nonpsychopaths rated moral pictures significantly higher on violation severity than non-moral (p < .00001) and neutral (p < .00001) pictures (Figure 1). Non-moral pictures were also rated significantly higher on violation severity than neutral pictures (p < .00001). This latter result is consistent with our prior research in non-antisocial populations (Harenski et al., 2008) and may reflect the fact that participants occasionally over-interpret what is represented by the non-moral pictures (e.g. if someone is in distress, another person must have caused it). No main effect of Group (F(1,90) = 0.67, p = .42) nor Group × Condition interaction (F(2,90) = 0.26, p = .77) was present. Thus, psychopaths and nonpsychopaths were similarly able to identify moral violations and rate their severity.
Brain Activity during Moral Picture Viewing
The Group (Nonpsychopath/Psychopath) × Condition (Moral/Non-Moral/Neutral) analysis revealed an interaction in the anterior temporal cortex (BA 21) and ventromedial prefrontal cortex (BA 10). As can be seen in Figure 2, this result indicated that nonpsychopaths showed a significant moral greater than non-moral and neutral picture distinction in these regions, whereas psychopaths did not. Psychopaths did not show any moral greater than non-moral or neutral activations that were significantly different than nonpsychopaths. No significant group differences in the anterior temporal cortex or ventromedial prefrontal cortex were present in the individual conditions (moral, non-moral, neutral). Other regions showing increased activity during moral relative to non-moral and neutral picture viewing across groups are listed in Table 2.
Figure 2.
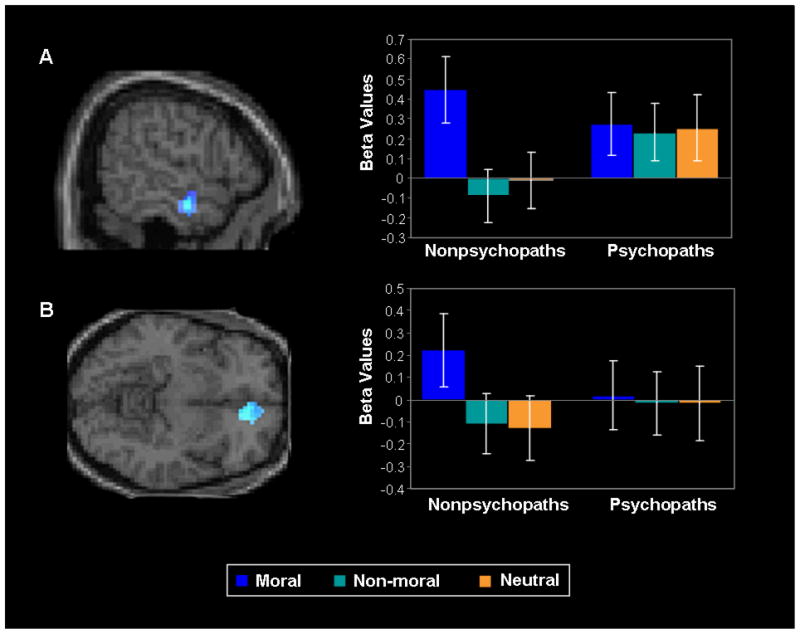
Interaction in A) anterior temporal cortex (BA 21; x = 57, y = −9, z = −18, F = 17.16, p = .000082 FWE corrected) and B) ventromedial prefrontal cortex (BA 10; x = 12, y = 39, z = −9, F = 6.54, p = .066 FDR corrected) revealing increased activity during moral vs. non-moral and neutral picture viewing in nonpsychopaths but not psychopaths. Bars = standard error.
Table 2.
Brain Regions Showing Differential Activity During Moral Picture Viewing in Nonpsychopaths and Psychopaths
| Region (BA) | x | y | z | F value | SVC | Cluster volume, mm3 |
|---|---|---|---|---|---|---|
| Group × Condition Interaction | ||||||
| *R. Middle Temporal Gyrus (21) | 57 | −9 | −18 | 17.16 | .000082 | 918 |
| *R. Medial Frontal Gyrus (10) | 12 | 39 | −9 | 6.54 | .066† | 1809 |
| Main Effect of Condition (N = 32) | ||||||
| Moral > Nonmoral | t value | |||||
| *L. Superior Temporal Gyrus (39) | −48 | −72 | 15 | 6.82 | .00000044 | 2349 |
| *R. Middle Temporal Gyrus (21) | 57 | −6 | −18 | 5.56 | .0000017 | 1701 |
| *R. Medial Frontal Gyrus (11) | 9 | 54 | −12 | 5.16 | .00035 | 1944 |
| *R. Superior Temporal Gyrus (39) | 48 | −63 | 27 | 5.07 | .00039 | 2592 |
| *L. Middle Temporal Gyrus (21) | −60 | −9 | −21 | 3.70 | .007 | 540 |
| L. Precuneus (19/7) | −27 | −77 | 43 | 7.63 | 71469 | |
| L. Parahippocampal Gyrus (35) | −24 | −27 | −9 | 7.19 | 9099 | |
| L. Superior Frontal Gyrus (8) | −21 | 26 | 46 | 5.97 | 28280 | |
| R. Cerebellum | 18 | −33 | −16 | 5.36 | 7047 | |
| R. Cerebellum | 45 | −54 | −28 | 4.00 | 1188 | |
| L. Middle Frontal Gyrus (10) | −36 | 49 | −5 | 4.72 | 945 | |
| R. Middle Frontal Gyrus (9/10) | 36 | 36 | 26 | 4.58 | 2079 | |
| R. Putamen | 21 | 12 | 5 | 4.50 | 648 | |
| R. Middle Frontal Gyrus (6/8) | 30 | 14 | 55 | 4.49 | 1701 | |
| L. Inferior Temporal Gyrus (21) | −59 | −7 | −17 | 4.28 | 783 | |
| Nonmoral > Moral | ||||||
| No differences | ||||||
| Moral > Neutral | ||||||
| *L. Superior Temporal Gyrus (39) | −45 | −69 | 18 | 5.21 | .00011 | 2295 |
| *R. Middle Temporal Gyrus (21) | 57 | −6 | −21 | 5.02 | .00016 | 1269 |
| *R. Superior Temporal Gyrus/Inferior | ||||||
| Parietal Cortex (39/40) | 56 | −63 | 39 | 4.96 | .00029 | 2592 |
| *L. Middle Temporal Gyrus (21) | −60 | −12 | −18 | 2.78 | .042‡ | 351 |
| *L. Amygdala | −15 | −6 | −21 | 3.37 | .042 | 243 |
| *R. Parahippocampal | ||||||
| Gyrus/Amygdala | 33 | −9 | −21 | 3.22 | .060 | 1971 |
| *R. Medial Frontal Gyrus (10) | 12 | 51 | −6 | 3.05 | .086 | 1701 |
| L. Superior Parietal Cortex/Posterior | ||||||
| Cingulate (7/31) | −18 | −72 | 57 | 7.08 | 39663 | |
| L. Parahippocampal Gyrus (19) | −36 | −48 | −6 | 5.74 | 3915 | |
| R. Parahippocampal Gyrus (27) | 27 | −30 | −9 | 4.85 | 1566 | |
| L. Superior Frontal Gyrus (8/9) | −3 | 54 | 33 | 5.60 | 40419 | |
| L. Inferior Frontal Gyrus (47) | −33 | 18 | −21 | 5.22 | 2025 | |
| R. Inferior Frontal Gyrus (47) | 48 | 30 | −6 | 4.94 | 1431 | |
| L. Cerebellum | −6 | −54 | −21 | 4.90 | 864 | |
| L. Cerebellum | −15 | −33 | −24 | 3.96 | 864 | |
| R. Hypothalamus | 3 | −3 | −12 | 4.56 | 3240 | |
| Neutral > Moral | ||||||
| L. Lingual Gyrus (17) | −15 | −87 | −3 | 3.83 | 837 | |
| Nonmoral > Neutral | ||||||
| R. Inferior Temporal Gyrus (19) | 51 | −57 | −3 | 4.35 | 1755 | |
| L. Middle Frontal Gyrus (11) | −42 | 42 | −12 | 4.00 | 810 | |
| L. Superior Temporal Gyrus (38) | −45 | 18 | −24 | 3.79 | 621 | |
| Neutral > Nonmoral | ||||||
| *L. Superior Temporal Gyrus (39) | −51 | −75 | 18 | 3.40 | .039 | 1134 |
| L. Parahippocampal Gyrus (37) | −24 | −45 | −12 | 4.62 | 1836 | |
| R. Cerebellum | 24 | −45 | −15 | 4.50 | 1296 | |
| L. Posterior Cingulate (30) | −18 | −60 | 9 | 4.15 | 621 | |
| R. Middle Frontal Gyrus (9) | 33 | 42 | 30 | 4.12 | 729 | |
| R. Cuneus (17) | 3 | −81 | 9 | 3.58 | 1404 | |
| Main Effect of Group | ||||||
| Nonpsychopaths > Psychopaths | ||||||
| No differences | ||||||
| Psychopaths > Nonpsychopaths | ||||||
| R. Middle Temporal Gyrus (19) | 36 | −72 | 18 | 4.41 | 621 | |
BA: Brodmann Area. SVC: Family-wise small volume corrected values listed for regions of interest. Other regions listed are significant at p < .001, uncorrected, cluster size ≥ 567mm3. Asterisks denote regions of interest.
FDR corrected statistic, p = .156 FWE
FDR corrected statistic, p = .117 FWE
Brain Regions Modulating ‘Severity of Moral Violation’ Ratings
A parametric modulation analysis was used in which the severity of moral violation ratings for each picture were entered as individual regressors. This analysis reflects the perceived violation severity of each picture based on the individual’s own ratings, providing a measure of within-participant moral sensitivity. One sample t-tests conducted for each group separately revealed a significant positive modulation in amygdala in nonpsychopaths, indicating that increased activity in the right amygdala during picture viewing was associated with higher severity ratings. This effect was not present in the psychopaths, and the between-group difference was marginally significant (Table 3, Figure 3a). Psychopaths showed a significant negative modulation in the right posterior temporal cortex (BA 39), indicating that increased activity in this region during picture viewing was associated with lower violation severity ratings. This effect was not present in the nonpsychopaths, and the between-group difference was significant (Table 3, Figure 3b). For other regions showing modulation effects, see Table 3.
Table 3.
Brain Regions Associated With Severity of Moral Violation Ratings
| Region (BA) | x | y | z | F value | SVC | Cluster volume, mm3 |
|---|---|---|---|---|---|---|
| Nonpsychopaths > Psychopaths | ||||||
| *R. Amygdala | 33 | 0 | −21 | 9.43 | .099 | 1107 |
| R. Superior Temporal Gyrus (39) | 57 | −51 | 27 | 9.83 | 135 | |
| t value | ||||||
| Positive modulation in Nonpsychopaths | ||||||
| *R. Amygdala | 33 | 0 | −21 | 4.31 | .014 | 1971 |
| Positive modulation in Psychopaths | ||||||
| No significant modulatory effects | ||||||
| Negative modulation in Nonpsychopaths | ||||||
| No significant modulatory effects | ||||||
| Negative modulation in Psychopaths | ||||||
| *R. Superior Temporal Gyrus (39) | 51 | −60 | 27 | 3.77 | .035 | 1782 |
| R. Middle Frontal Gyrus (8) | 45 | 9 | 42 | 5.83 | 405 | |
| L. Superior Frontal Gyrus (8) | −3 | 18 | 60 | 3.91 | 162 | |
BA: Brodmann Area. SVC: Family-wise small volume corrected values listed for regions of interest. Other regions are significant at p < .001, uncorrected, cluster size ≥ 135mm3. Asterisks denote regions of interest. Although the superior temporal gyrus (BA 39) was a region of interest, the activated region in the nonpsychopath vs. psychopaths comparison did not pass the SVC threshold because it was slightly anterior to the ROI derived from healthy controls.
Figure 3.
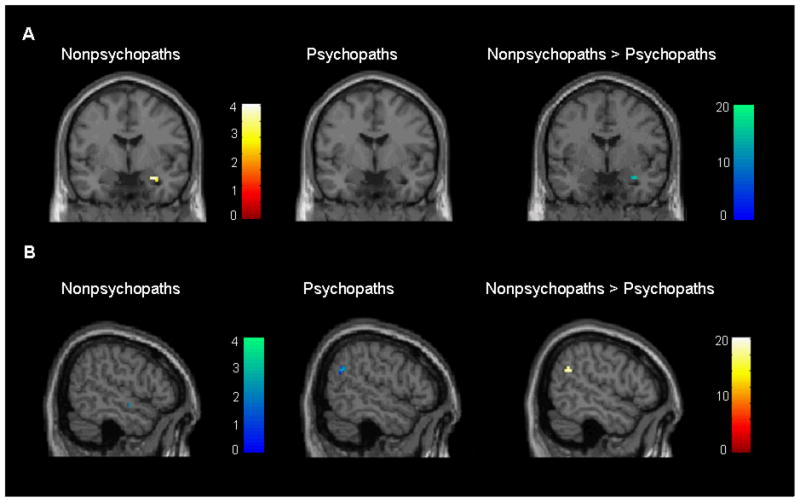
A) Positive association between right amygdala activity and violation severity ratings present in nonsychopaths (N = 16; x = 33, y = 0, z = −21, t = 4.31, p = .014 FWE corrected), absent in psychopaths (N = 16), and greater in nonpsychopaths than psychopaths (x = 33, y = 0, z = −21; F = 9.43, p = .099 FWE corrected). B) Negative association between right posterior temporal activity (BA 39) and violation severity ratings absent in nonpsychopaths (N = 16), present in psychopaths (N = 16, x = 51, y = −60, z = 27, t = 3.77, p = .035 FWE corrected), and greater in nonpsychopaths than psychopaths (x = 57, y = −51, z = 27; F = 9.83, p < .001, uncorrected). Although both the amygdala and posterior temporal activity – severity rating associations are greater in nonpsychopaths vs. psychopaths, the individual group results illustrate that the latter is due to the negative association in psychopaths (rather than a positive association in nonpsychopaths, as is the case with the amygdala).
Correlation analysis between PCL-R scores and brain activity during moral picture viewing
The between-group differences that were present in the ventromedial prefrontal cortex fell below the family-wise correction threshold (Table 2). However, a supplemental correlation analysis in all 72 participants revealed a significant negative correlation between ventromedial prefrontal activity and PCL-R scores during moral relative to non-moral (t = 3.44, p = .035 FWE corrected) and neutral (t = 3.36, p = .041 FWE corrected) picture viewing (see Figure S1.
Two regions that showed differences between nonpsychopaths and psychopaths, the ventromedial prefrontal cortex (greater moral vs. non-moral and moral vs. neutral activity in nonpsychopaths relative to psychopaths) and right amygdala (greater positive association between amygdala activity and violation severity ratings in nonpsychopaths relative to psychopaths), have been implicated in affective deficits in psychopathy (Blair et al., 2006; Kiehl et al., 2001, 2006). To investigate whether effects in these regions were related to affective traits of psychopathy, correlation analyses were performed between brain activity and the two factor and four facet scales of the PCL-R3. Factor 1 represents interpersonal (Facet 1, e.g., conning/manipulation) and affective (Facet 2, e.g., low empathy) characteristics of psychopathy. Factor 2 represents lifestyle (Facet 3, e.g., irresponsibility) and antisocial (Facet 4, e.g., criminal versatility) characteristics. In the moral vs. non-moral contrast, Factor 2 scores were negatively correlated with ventromedial prefrontal activity (t = 3.83, p = .01 FWE corrected). However, this correlation was not present in the moral vs. neutral contrast. Ventromedial activity was not significantly correlated with Factor 1 or any Facet scores in either contrast. In the parametric modulation analysis, Facet 2 scores were negatively correlated with the modulatory effect in the right amygdala (t = 3.79, p = .019 FWE corrected). This indicates that participants with lower Facet 2 scores had a stronger association between amygdala activity and violation severity ratings. No significant correlations with the Factor scores, or other Facet scores, were present in this region.
Discussion
This study explored whether psychopaths differ from nonpsychopaths in neural systems underlying moral decision making. Consistent with hypotheses and prior studies, nonpsychopaths showed increased activity in the anterior temporal cortex and ventromedial prefrontal cortex during moral relative to non-moral and neutral picture viewing, whereas psychopaths did not. Nonpsychopaths showed a positive association between moral violation severity ratings and amygdala activity that was not present in psychopaths. Psychopaths showed a negative association between moral violation severity ratings and posterior temporal activity that was not present in nonpsychopaths. These results demonstrate neural abnormalities in moral picture processing in psychopaths, and indicate that psychopaths utilize different brain regions when making moral decisions than do nonpsychopaths.
Psychopaths showed reduced moral vs. non-moral and neutral picture distinctions in the anterior temporal cortex relative to nonpsychopaths. Whereas nonpsychopaths showed increased activity during moral relative to non-moral and neutral picture viewing, psychopaths showed nearly identical activity across all conditions. These results can be integrated with studies showing psychopathy-related reductions in gray matter (de Oliveira-Souza, Hare, Bramati et al., 2007; Muller, Ganbauer, Sommer et al., 2008b) and hemodynamic activity (Kiehl, Smith, Mendrek, Forster, Hare, & Liddle, 2004; Muller et al, 2008a) in this region. The current results extend these findings to demonstrate a functional abnormality during the evaluation of moral stimuli. The anterior temporal cortex has been engaged in prior moral decision making studies (though less consistently than the medial prefrontal cortex; Greene & Haidt, 2002; Moll et al., 2005). Heekeren, Wartenburger, Schmidt, Prehn, Schwintowski, & Vrillinger (2005) found that anterior temporal activity in response to morally-salient statements such as ‘A gave B a bloody nose’, or ‘A never paid the money back’ was significantly reduced when the statements described bodily harm. The authors suggested that the presence of bodily harm leads to reduced processing depth, restricting the generation of semantic and emotional context associated with moral processing. Thus, one interpretation of the finding that nonpsychopaths clearly distinguished the moral vs. non-moral pictures within the anterior temporal cortex, whereas psychopaths did not, is that nonpsychopaths showed increased processing depth when viewing moral relative to non-moral and neutral pictures. More specifically, while psychopaths recognized morally-salient stimuli as such (based on their ratings), this recognition was not as fully instantiated in the ‘moral brain’ as it was in nonpsychopaths.
Although theory and research regarding the functions of the anterior temporal cortex have focused on general conceptual processing (e.g. semantic memory; see Rogers, Hocking, Noppeney et al., 2005), recent research has indicated a specific role in social conceptual processing (Zahn, Moll, Krueger, Huey, Garrido, & Grafman, 2007; Zahn, Moll, Paiva et al., 2009). Ermer, Guerin, Cosmides, Tooby, and Miller (2006) reported increased anterior temporal activity when participants evaluated statements describing social contracts (e.g. ‘If you use the library, then you must pay the fee’), and representing potential violations of those contracts (e.g. ‘John did not pay a fee’). The location of the anterior temporal activity observed in the preset study overlaps closely with that reported by Ermer et al. (2006). Moral violations can be viewed as violations of social contracts that individuals have with each other and society (e.g., if a person is going to drink alcohol, they must not drive). Being able to reason about these types of contracts is fundamental for social order and getting along with others. The current results demonstrate that nonpsychopaths made a clear moral/non-moral picture distinction, which could indicate that they made these inferences when evaluating moral pictures but not non-moral pictures. Psychopaths did not show this distinction between moral and non-moral pictures.
Psychopaths also showed reduced moral vs. non-moral and neutral picture distinctions in the ventromedial prefrontal cortex. This region has been consistently implicated in moral decision-making in healthy populations (Greene & Haidt, 2002; Moll et al., 2005; Raine & Yang, 2007), and may support the integration of emotional responses with moral decision making. Koenigs et al. (2007) found that patients with damage to the ventromedial prefrontal cortex showed impaired reasoning about moral dilemmas, particularly those with an affective component that involved consideration of harm caused to others. De Oliveira-Souza et al. (2007) found that structural ventromedial prefrontal deficits in psychopathy were associated with low empathy. One possibility is that psychopaths lack an affective and/or empathic response to moral pictures (many of which depicted individuals in distress). The group difference in ventromedial prefrontal activity is consistent with studies demonstrating ventromedial prefrontal dysfunction in psychopathy during the performance of emotion-based tasks (Birbaumer et al., 2005, Veit et al., 2002; Kiehl et al., 2001). It is also consistent with the hypothesis that ventromedial prefrontal dysfunction contributes to moral insensitivity in psychopathy (Blair, 2007), and the results of Glenn et al. (2009), who reported a negative correlation between psychopathy scores and ventromedial prefrontal activity during the evaluation of complex moral dilemmas. It should be emphasized, however, that we observed a negative correlation between ventromedial prefrontal activity and Factor 2 (but not Factor 1) scores during moral picture viewing. This result is less consistent with an emotion-based interpretation and may be related to the psychopath’s antisocial tendencies.
The results of the parametric modulation analysis provide stronger evidence that psychopaths had reduced emotional responses during moral decision-making. Nonpsychopaths, but not psychopaths, showed a positive association between amygdala activity during picture viewing and moral violation severity ratings. In the complete sample, this association was correlated with the Facet 2 (affective) scale of the PCL-R. Our prior work has shown an amygdala-severity rating association in non-incarcerated participants (Harenski et al., 2008). Given the established role of the amygdala in emotion processing (Phan, Wager, Taylor, & Liberzon, 2005), this result may indicate that nonpsychopaths utilized affective cues from the pictures to guide their severity ratings, whereas psychopaths did not. Although it is unknown whether psychopaths focused more or less on certain features of moral pictures that did nonpsychopaths (e.g. facial expressions, symbols of moral violations such as a pointed gun, background contextual features of scenes, etc.), future studies could explore this possibility using eyetracking or memory testing. It should be noted that the group difference in the amygdala occurred only at a trend level, thus should be considered preliminary and caution should be taken in generalizing to non-antisocial populations.
Psychopaths showed a negative modulation of severity of moral violation ratings in the posterior temporal cortex (BA 39), meaning that increased activity during moral picture viewing was associated with lower severity ratings. This effect was not present in the nonpsychopaths. This region, often referred to as the temporo-parietal junction, is one of the better understood regions regarding its involvement in moral decision-making. The role of the temporo-parietal junction in theory of mind processing is well established (Gallagher & Frith, 2003; Saxe & Kanwisher, 2003), and a study found increased activity in this region when participants determined whether moral violations were intentional rather than accidental (Young et al., 2007). While psychopaths did not show theory of mind deficits relative to nonpsychopaths in a previous study (Richell et al., 2003), it was the psychopath’s ability to undertake theory of mind processing, rather than their tendency to undertake such processing, that was evaluated. Psychopaths have intact theory of mind processing but invoke it within different contexts than nonpsychopaths. For example, psychopaths may utilize theory of mind to reinterpret the moral salience of pictures. Some participants commented after scanning that they did not rate certain ‘moral’ pictures (e.g. an individual pointing a gun at another person) high on violation severity because they did not know the intentions of the individuals, or the context of the interaction (e.g. whether the individual was acting in self defense). It is possible that psychopaths invoked this type of reasoning more than nonpsychopaths, though we did not investigate this in the present study. Also, psychopaths did not rate moral pictures lower in violation severity than nonpsychopaths overall. But when they did rate pictures low, the lower rating was associated with increased temporo-parietal junction activity, indicating a unique recruitment of this region by psychopaths but not nonpsychopaths.
The parametric modulation analysis cannot determine whether associations between brain activity and violation severity ratings are causal. In other words, associations between posterior temporal or amygdala activity and severity ratings may occur because the type of processing associated with these regions influenced the subsequent rating, or they may occur because the rating influenced subsequent brain activity. This is an important distinction because it has been debated whether, for example, emotional responses influence moral judgments or moral judgments influence emotional responses (Huebner, Dwyer, & Hauser, 2008). If the positive association between amygdala activity and violation severity ratings that was absent in psychopaths does represent a reduced emotional response in psychopaths, this could be because psychopaths did not utilize emotional responses to guide their severity ratings, or because higher severity ratings did not enhance their emotional responses to the pictures.
It would be desirable to support the psychopaths’ neural abnormalities during moral decision-making with converging behavioral deficits. Unfortunately, this is often difficult, as psychopaths generally do not show abnormalities in moral reasoning (Glenn et al., 2009; Cima et al., 2010). In fact, a hallmark characteristic of psychopathy is that psychopaths commit moral violations despite being aware of their ‘wrongness’. Further, psychopaths are often skilled at giving the ‘right’ answers to interviewers and are unlikely to demonstrate cognitive deficits when evaluating moral scenarios. Instead, behavioral deficits in moral reasoning in psychopaths are likely to be subtle and only uncovered using moral tasks that are tailored to specific types of reasoning. Current investigations exploring a wide variety of moral reasoning skills in psychopaths are ongoing in our laboratory.
A potential alternative explanation of our findings is that they represent increased task difficulty in the moral condition. If the moral pictures require more effort to rate than the non-moral or neutral pictures, psychopaths may have exerted less effort on these pictures, resulting in decreased hemodynamic activity. It is plausible that the moral condition would require more effort, since the violation severity rating is unique to the moral condition, rarely occurring in the other conditions since no violation is present. To investigate this, in a prior pilot study with 24 healthy controls (described in Harenski et al., 2010) we tested participants on the same task outside the MRI scanner, with the response format changed to a 5-point Likert scale, to investigate reaction time across moral, non-moral, and neutral conditions. We found that RT was longer for moral and non-moral relative to neutral trials, but did not significantly differ between moral and non-moral trials (p = .75). This suggests the non-moral trials were as demanding as the moral trials (perhaps because it takes additional time to determine that an unpleasant picture does not contain a moral violation). Although we did not record reaction time in the present study (the continuous 1–5 scale precludes the recording of meaningful reaction time), we can think of no reason to suspect that psychopaths exerted less effort on the moral but not the equally effortful non-moral trials. Since we did not observe group differences related to the non-moral condition, it is unlikely that the results can be explained by group differences in effort.
Several limitations of this study should be noted. First, all participants were from an antisocial/incarcerated population. We did not include non-antisocial participants because our prior studies in these participants were conducted on a 3T (vs. 1.5T) MRI scanner, precluding direct comparisons. To ensure that our results could be generalized to non-antisocial populations, we used a statistical correction that included the center coordinate from regions activated by non-antisocial participants in a prior study using the same task (Harenski et al., 2008). If activations in regions of interest did not fall within 10mm of the center of regions activated by non-antisocial populations, they were not significant. Overall, the results of moral relative to non-moral picture viewing in the present group of nonpsychopaths were highly similar to those obtained with non-antisocial participants (Figure S2). This study is the first to present imaging results from incarcerated populations on a morality task, and although we observed many similarities with non-incarcerated populations, it will be important to directly compare these populations in future studies. Second, the individuals in the present study were diverse in ethnicity. While the PCL-R has been well-validated in Caucasian individuals, it has been less studied in other groups such as Hispanic individuals, which were roughly equal in representation to Caucasian participants. One study found that the PCL-R provides a reliable and valid measure of psychopathy in Hispanic populations (Sullivan, Abramowitz, Lopez, & Kosson, 2006). Third, most participants had a history of substance abuse. Although we ensured that prior use did not significantly differ across groups, it would be beneficial to compare substance abusing and non-substance abusing groups on the current task. Finally, the present study cannot determine whether neural abnormalities precede the development of psychopathic traits related to moral insensitivity.
In summary, psychopaths showed several differences in brain activity associated with moral decision-making relative to nonpsychopaths: 1) Reduced moral versus non-moral/neutral picture distinctions in the anterior temporal cortex and ventromedial prefrontal cortex relative to nonpsychopaths, 2) Lack of a positive association between amygdala activity and severity of moral violation ratings that was present in nonpsychopaths, 3) A negative association between posterior temporal activity and severity of moral violation ratings that was absent in nonpsychopaths. These results may represent neurobiological markers of moral insensitivity in psychopathy.
Supplementary Material
Acknowledgments
This research was supported by a grant (R01 MH070539; Kiehl) from the National Institute of Mental Health. Carla L. Harenski was supported by a Ruth L. Kirchstein National Research Service Award (1F32MH081469).
Footnotes
Portions of this data were presented at the annual meeting of the Society for Neuroscience (November, 2008), and the biennial meeting of the Society for the Scientific Study of Psychopathy (April, 2009).
We included both the moral and non-moral pictures in this analysis, because there was more variability in ratings for the non-moral relative to the neutral pictures. Based on the results (Figure 1), both nonpsychopaths and psychopaths sometimes rated non-moral pictures on violation severity, indicating that they inferred a moral violation was present. The critical aspect of this analysis is that it accounts for what the participant deems to be a moral violation, rather than what the experimenters pre-assigned to the moral and non-moral conditions. Thus we felt the analysis would be more representative of the participant’s moral judgments with the non-moral condition included.
Although right anterior temporal and right amygdala activity were not reported in Harenski et al. (2008), activity in these regions occurred at a lower statistical threshold than the one that was utilized (p < .005 vs. p < .001 uncorrected for the ATC during moral picture viewing, and p < .01 vs. p < .005 for the amygdala in the parametric modulation analysis).
The full correlation results are available upon request from the corresponding author. Because 4 participants did not have valid Facet 4 scores (2 items omitted), correlations with facet scores and hemodynamic activity had 68 participants.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/abn.
References
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, Flor H. Deficient fear conditioning in psychopathy: A functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Moral reasoning in the child with psychopathic tendencies. Personality and Individual Differences. 1997;26:477–485. [Google Scholar]
- Blair RJR. A cognitive developmental approach to morality: investigating the psychopath. Cognition. 1995;57:1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Mitchell D, Blair K. The psychopath: Emotion and the brain. Blackwell Publishing; 2005. [Google Scholar]
- Ciaramelli E, Muccioli M, Ladavas E, di Pellegrino G. Selective deficit in personal moral judgment following damage to the ventromedial prefrontal cortex. Social, Cognitive, and Affective Neuroscience. 2007;2:84–92. doi: 10.1093/scan/nsm001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima M, Tonnaer F, Hauser M. Psychopaths know right from wrong but don’t care. Social, Cognitive, and Affective Neuroscience. 2010;5:59–67. doi: 10.1093/scan/nsp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleckley H. The mask of sanity. St. Louis: Mosby; 1976. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Ignacio FA, Tovar-Moll F, Moll J. Psychopathy as a disorder of the moral brain: Fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1203–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional MRI analyses. Neuroimage. 2001;13:751–758. doi: 10.1006/nimg.2000.0719. [DOI] [PubMed] [Google Scholar]
- Edens JF, Marcus DK, Lilienfeld SO, Poythress NG. Psychopathic, not psychopath: Taxometric evidence for the dimensional structure of psychopathy. Journal of Abnormal Psychology. 2006;115:131–144. doi: 10.1037/0021-843X.115.1.131. [DOI] [PubMed] [Google Scholar]
- Guay JP, Ruscio J, Knight RA, Hare RD. A taxometric analysis of the latent structure of psychopathy: Evidence for dimensionality. Journal of Abnormal Psychology. 2007;116:701–716. doi: 10.1037/0021-843X.116.4.701. [DOI] [PubMed] [Google Scholar]
- Ermer E, Guerin S, Cosmides L, Tooby T, Miller MB. Social Neuroscience. 2006;1:196–219. doi: 10.1080/17470910600989771. [DOI] [PubMed] [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Transactions on Medical Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Fulero S. Review of the Hare-Psychopathy Checklist-Revised. In: Conoley JC, Impara JC, editors. 12th Mental Measurements Yearbook. Lincoln: Buros Institute; 1996. pp. 453–454. [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Molecular Psychiatry. 2009;14:5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA, Young L, Hauser M. Increased DLPFC activity during moral decision making in psychopathy. Molecular Science. 2009;14:909–11. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A. Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry. 2004;56:516–521. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Greene JD, Haidt J. How (and where) does moral judgment work? Trends in Cognitive Sciences. 2002;6:517–523. doi: 10.1016/s1364-6613(02)02011-9. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised. Toronto: Multi-Health Systems; 2003. [Google Scholar]
- Hare RD. Psychopaths and their nature: Implications for the mental health and criminal justice systems. In: Millon T, Simonsen E, Biket-Smith M, Davis RD, editors. Psychopathy: Antisocial, criminal and violent Behavior. New York: Guilford Press; 1998. [Google Scholar]
- Hare RD. Psychopathy – A clinical construct whose time has come. Criminal Justice and Behavior. 1996;23:24–54. [Google Scholar]
- Hare RD. A research scale for the assessment of psychopathy in criminal populations. Personality and Individual Differences. 1980;1:111–119. [Google Scholar]
- Harenski CL, Antonenko O, Shane MS, Kiehl KA. Gender differences in neural mechanisms underlying moral sensitivity. Social, Cognitive, and Affective Neuroscience. 2008;3:313–321. doi: 10.1093/scan/nsn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Hart SD, Hare RD. Discriminant validity of the Psychopathy Checklist in a forensic psychiatric population. Psychological Assessment. 1989;1:211–218. [Google Scholar]
- Heekeren HR, Wartenburger I, Schmidt H, Prehn K, Schwintowski HP, Villringer A. Influence of bodily harm on neural correlates of semantic and moral decision making. Neuroimage. 2005;24:887–897. doi: 10.1016/j.neuroimage.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Huebner B, Dwyer S, Hauser MD. The role of emotion in moral psychology. Trends in Cognitive Science. 2008;13:1–6. doi: 10.1016/j.tics.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Mendrek A, Forster BB, Hare RD, Liddle PF. Temporal lobe abnormalities in semantic processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Psychiatry Research-Neuroimaging. 2004;130:297–312. doi: 10.1016/j.pscychresns.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, Liddle PF. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio A. Damage to the prefrontal cortex increases utilitarian moral judgments. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS) Bethesda, MD: National Institute of Mental Health Center for the Study of Emotion and Attention; 1995. [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. The neural basis of human moral cognition. Nature Reviews Neuroscience. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- Muller JL, Sommer M, Dohnel K, Weber T, Schmidt-Wilcke &, Hajak G. Disturbed prefrontal and temporal brain function during emotion and cognition interaction in criminal psychopathy. Behavioral Sciences and the Law. 2008a;26:131–150. doi: 10.1002/bsl.796. [DOI] [PubMed] [Google Scholar]
- Muller JL, Ganbauer M, Sommer M, Dohnel K, Weber T, Schmidt-Wilcke &, Hajak G. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatry Research: Neuroimaging. 2008b;163:213–222. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Muller JL, Sommer M, Wagner V, Lange K, Taschler H, Roder CH, Schuierer G, Klein HE, Hajak G. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: Evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biological Psychiatry. 2003;54:152–162. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Social, Cognitive, and Affective Neuroscience. 2006;1:203–213. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richell RA, Mitchell DGV, Newman C, Leonard A, Baron-Cohen S, Blair RJR. Theory of mind and psychopathy: can psychopathic individuals read the ‘language of the eyes’? Neuropsychologia. 2003;41:523–526. doi: 10.1016/s0028-3932(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Rogers T, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini M, Patterson K, Price CJ. Anterior temporal cortex and semantic memory: Reconciling findings from neuropsychology and functional imaging. Cognitive, Affective, and Behavioral Neuroscience. 2006;6:201–213. doi: 10.3758/cabn.6.3.201. [DOI] [PubMed] [Google Scholar]
- Ryan JJ, Lopez SJ, Werth TR. Development and preliminary validation of a Satz-Mogel short form of the Wais-III in a sample of persons with substance abuse disorders. International Journal of Neuroscience. 1999;98:131–140. doi: 10.3109/00207459908994796. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Sullivan ES, Abramowitz CS, Lopez M, Kosson DS. Reliability and construct validity of the Psychopathy Checklist – Revised for Latino, European American, and African American male inmates. Psychological Assessment. 2006;18:382–392. doi: 10.1037/1040-3590.18.4.382. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotze M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;3283:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. New York: Psychological Corporation; 1997. [Google Scholar]
- Young L, Cushman F, Hauser M, Saxe R. The neural basis of the interaction between theory of mind and moral judgment. Proceedings of the National Academy of Sciences. 2007;104:8235–8240. doi: 10.1073/pnas.0701408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Koenigs M. Investigating emotion in moral cognition: a review of evidence from functional neuroimaging and neuropsychology. British Medical Bulletin. 2007;84:69–79. doi: 10.1093/bmb/ldm031. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Paiva M, Garrido G, Krueger F, Huey E, Grafman J. The neural basis of human social values: Evidence from functional MRI. Cerebral Cortex. 2009;19:276–283. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proceedings of the National Academy of Sciences USA. 2007;104:6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.