Abstract
Bacteria use quorum sensing to probe and respond to population densities in their external environment. The detection of quorum signaling molecules causes a virulence response in many pathogenic bacteria. Blocking this signaling pathway, without interfering with critical metabolic functions, would produce compounds that can disarm pathogens without killing them. By not blocking growth per se, this therapeutic approach would have a lower associated risk for the development of bacterial resistance. Modified forms of l-methionine can yield analogues of the essential methyl donor, S-adenosyl-l-methionine (AdoMet), by serving as substrates for AdoMet synthetase [Zano, S., et al. (2013) Arch. Biochem. Biophys. 536, 64]. The AdoMet analogues examined here were chosen for their putative inability to serve as precursors for the synthesis of the acylhomoserine lactone class of quorum sensing molecules. We now show that these AdoMet analogues can still function as methyl donors, for methylation of both DNA and catechol-based neurotransmitters. The rates of methyl transfer for several of these altered AdoMet analogues are comparable to those observed with unmodified AdoMet. Additional refinement of these structures is expected to produce lead compounds to be tested as selective therapeutic agents against infections by a broad range of pathogenic Gram-negative bacteria.
Single-cell organisms can behave as an organized community through a variety of different communication mechanisms.1 Such behavior can be beneficial as a defense mechanism, such as in biofilm formation,2,3 or as an offensive mechanism for success in a highly competitive environment.4 The generation of different signaling molecules, produced from the enzyme-catalyzed diversion of essential metabolic intermediates, can lead to the activation of unique sets of genes in response to different external stimuli. One of these essential metabolites, S-adenosyl-l-methionine (AdoMet), serves as the precursor for two structurally distinct classes of signaling molecules that are used as quorum sensors by a variety of bacterial species.
AdoMet is a highly versatile metabolite that participates in a wide variety of group transfer reactions, including the transfer of methyl, aminopropyl, and adenosyl groups,5 as well as a variety of radical generation reactions.6 In particular, AdoMet functions as a donor in the methylation reactions of DNA, RNA, and proteins as well as the methylation of small molecules such as phospholipids and various neurotransmitters.5,7 In addition to these essential roles in mammalian metabolism, AdoMet is also the precursor for the production of two different classes of quorum sensing molecules, acylhomoserine lactones (AHLs) and furanosyl borate diesters, in certain species of bacteria.8 These compounds regulate the expression of a large number of bacterial genes, including those associated with virulence in human pathogenic organisms.9 Many of these quorum-responsive genes produce virulence factors that cause disease pathology, as well as the components required for assembly of the polysaccharide matrix of biofilms that can protect microbes against antibiotics.3 Preventing the production of quorum sensing molecules would block an important signaling pathway that leads to virulence in many Gram-negative bacteria.10 However, any interference with the essential roles of AdoMet would be detrimental to a mammalian host and would also provide strong selective pressure for resistance by the targeted bacteria. The challenge is to block bacterial signaling without affecting mammalian metabolism and ideally to have a minimal effect on bacterial metabolism. This should be possible, as the biosynthesis of acylhomoserine lactone (AHL) quorum signals uses portions of AdoMet (the amino and carboxyl groups of the methionine moiety) that are not directly required for most other group transfer reactions from AdoMet.
Several methionine analogues that can function as effective substrates for AdoMet synthetases from Gram-negative bacterial human pathogens have been identified.11 The AdoMet analogue products from these enzyme-catalyzed transformations have modified functional groups that should effectively block them from serving as precursors for the AHL class of quorum sensing molecules, but to be viable starting points for the development of antivirulence drugs, these AdoMet analogues must still be capable of participating in the diverse group transfer reactions that are essential both for microbial and for mammalian metabolism. This work examines the ability of these AdoMet analogues to function as methyl donors for two divergent targets: the methylation of a carbon atom in bacterial DNA and the small molecule methylation of an oxygen atom in a specific class of mammalian neurotransmitters.
Experimental Procedures
Materials
pUC19 DNA was acquired from New England BioLabs (NEB) and propagated in the Escherichia coli XL-1 Blue strain under ampicillin selection. Plasmids were purified using a miniprep plasmid extraction kit (Qiagen). DNA CpG methyltransferase (M.SssI) and the restriction enzymes used for DNA mapping were also purchased from NEB. Methionine analogues were either purchased or synthesized as described previously11 and were used as freshly prepared stock solutions adjusted to neutral pH. Catechol O-methyltransferase (COMTase) was purchased from Calzyme Laboratories Inc., while (−)-epinephrine (+)-bitartrate, S-(5′-adenosyl)-l-methionine (AdoMet), and S-(5′-adenosyl)-l-homocysteine (AdoHcy) were obtained from Sigma-Aldrich. All of the other reagents were of the highest purity available.
Enzyme Purification
The metK genes of Neisseria meningitidis and Campylobacter jejuni were cloned into E. coli BL21(DE3) expression cells. The S-adenosyl-l-methionine synthetases (AdoMet synthetases, MetK) were purified using our previously published protocol.11 Briefly, E. coli BL21(DE3) cells containing cloned metK genes were grown in LB medium containing ampicillin, induced with 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) at 28 °C, and then grown to a high cell density. Each of these His-tagged enzymes was purified by nickel affinity chromatography using a linear imidazole gradient, followed by anion exchange chromatography and elution with a linear KCl gradient. Purified MetK enzymes from each source were concentrated and either examined immediately or stored at −80 °C until they were used in a glycerol-containing storage buffer.11
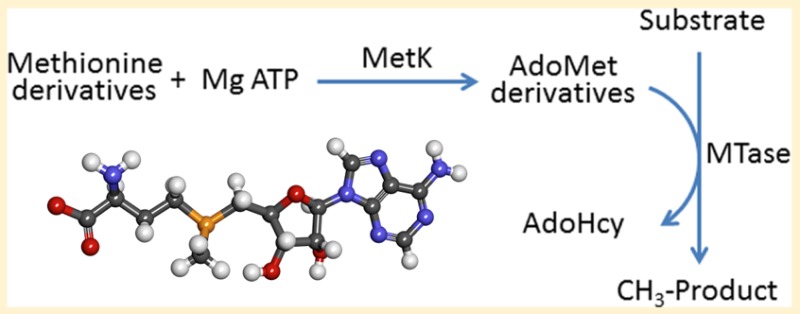
Synthesis of AdoMet and AdoMet Analogues
AdoMet and its derivatives were synthesized in vitro by the AdoMet synthetase-catalyzed reaction that uses ATP to activate methionine. Varying concentrations of l-methionine or methionine analogues were incubated with MgATP and AdoMet synthetase (0.4 mg/mL) in a buffer containing 20 mM Tris-HCl (pH 7.6) and 15 mM MgCl2. AdoMet and its analogues were quantified by liquid chromatography using a Waters high-performance liquid chromatography (HPLC) system. A calibration curve was generated by using AdoMet concentrations from 12.5 to 600 μM, and the amounts of AdoMet or AdoMet analogues produced from the AdoMet synthetase reaction were determined after the appropriate dilutions. This enzyme-catalyzed reaction was conducted for an extended time period to obtain the maximal levels of each AdoMet analogue used in the subsequent methyl transfer reactions. The synthetic reaction was quenched by spin filtration to remove the enzyme, and the resulting mixture was used as the substrate for the DNA and catechol methylation reactions.
DNA Protection Assay
The DNA protection assay was designed to determine the efficiency of DNA methylation by AdoMet and AdoMet analogues. The pUC19 plasmid was linearized with HindIII, to eliminate the effect of different plasmid conformations on restriction enzyme accessibility and facilitate site mapping during the DNA protection assay. Initial optimization of this assay was conducted by varying the enzyme concentration (2–4 units of M.SssI), the concentration of purchased AdoMet (up to 320 μM), and the reaction time (from 30 to 60 min). Once optimized, the AdoMet synthesis reaction described above was used to produce either AdoMet or AdoMet analogues. After the reaction had been quenched and the AdoMet products quantitated, 100 μM AdoMet or AdoMet esters (or the maximal amount obtained for the lower-yielding N-derivatized AdoMet products) were added to approximately 1 μg of linearized pUC19 DNA. The methylation reaction was then initiated by the addition of 4 units of DNA methyltransferase, M.SssI, in a buffer containing 10 mM Tris-HCl (pH 7.9), 50 mM NaCl, 10 mM MgCl2, and 1 mM DTT in a 10 μL reaction volume at 37 °C. M.SssI selectively generates 5-methylcytosine at CpG sequences. The extent of DNA CpG methylation was determined at various times by restriction digestion with 10 units of HpaII, a restriction endonuclease that is not capable of cleaving DNA at its CCGG recognition site if the central CpG is methylated. Digestion of the methylated DNA with MspI served as a control, because this enzyme cleaves at the same restriction site as HpaII but is not sensitive to DNA methylation at the internal cytosine. Restriction digestions were conducted according to the NEB protocols. The restriction fragments obtained after these reactions were analyzed by electrophoresis on 1.5% agarose gels in TAE buffer, with the bands visualized as negative images from ethidium bromide fluorescence staining. The degree of protection of pUC19 DNA afforded by methylation with the various AdoMet analogues was analyzed using gel digitizing software (UN-SCAN-IT Gel version 6.1) to compare the relative amount of fully methylated DNA to the total amount of DNA present.
Catechol Methylation Assay
The formation of AdoHcy during the methylation of epinephrine by COMTase, using the AdoMet or AdoMet analogues synthesized as described above, was monitored by an HPLC-based end point assay. Methylation reaction mixtures contained epinephrine (2.5 mM), COMTase (0.2 mg/mL), and AdoMet or AdoMet ester analogues (100 μM from the AdoMet synthetase reaction) at 37 °C in a buffer containing 50 mM Tris-HCl (pH 8.0), 0.5 mM MgCl2, and 0.25 mM dithiothreitol. At different times, 20 μL samples from these reaction mixtures were injected into a reversed phase C18 column (particle size of 5 μm, 150 mm × 4.6 mm, Thermo Fisher) equilibrated with 100 mM potassium phosphate buffer (pH 7.2). The AdoHcy product was separated from the reactants by using a linear gradient of 40% acetonitrile in a gradient HPLC system (Waters dual pumps 515, Waters Corp.) and quantified by its absorbance at 254 nm using an absorbance detector (Waters Corp.).
Results
Examination of DNA Methylation by AdoMet
MetK catalyzes the adenylation of methionine to produce the primary biological methyl donor, AdoMet. The MetK reaction was optimized to yield a final concentration of AdoMet sufficient for complete protection of 1 μg of pUC19 DNA by M.SssI methyltransferase (see below). To achieve this optimization, the amounts of l-methionine and MgATP substrates were varied, along with the reaction time. A gel-based assay was designed to examine the ability of newly synthesized AdoMet and AdoMet analogues to function as donors of methyl groups to DNA. Without methylation to protect the linearized pUC19 DNA, treatment with HpaII restriction endonuclease results in the expected cleavage at 14 different sites, leading to fragments that range in size from 26 to 501 bp. Fully methylated DNA is protected against cleavage and yields a single band at 2.69 kb, while partially methylated DNA gives bands between 0.5 and 2.69 kb depending on the extent of methylation. Complete protection of the pUC19 DNA was attained by reacting 4 mM l-methionine and MgATP for 60 min (Figure 1, lane 5) using the more active C. jejuni form of AdoMet synthetase.11
Figure 1.
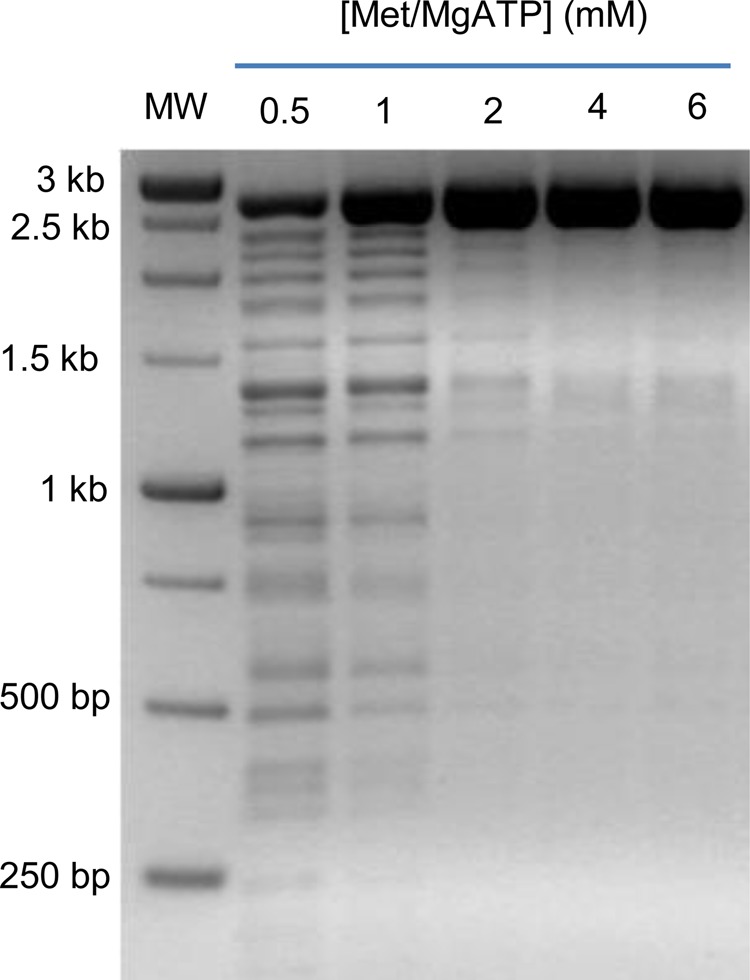
Optimization of AdoMet production catalyzed by AdoMet synthetase. The products of a coupled reaction are shown, where AdoMet synthetase produces AdoMet, which is then used by M.SssI to methylate substrate DNA (linearized pUC19). The concentrations of substrates l-methionine and MgATP were each increased from 0.5 to 6 mM for the 60 min reaction time, followed by restriction digestion with HpaII and gel electrophoresis, shown as a negative image of an ethidium-stained agarose gel, to determine the extent of DNA methylation achieved with each substrate level.
Optimization of the DNA Methyltransferase Assay
To measure the ability of AdoMet analogues to serve as DNA methyl donors, a sensitive assay for DNA methylation is needed. Methylation of linearized pUC19 DNA was optimized by varying the level of the M.SssI methyltransferase, the AdoMet concentration, and the reaction time for DNA methylation (Figure 2). Virtually complete methylation of pUC19 DNA was achieved, as evidenced by the failure of HpaII to cleave the DNA, by using 320 μM AdoMet and 4 units of M.SssI, with the reaction conducted for at least 1 h in a buffer containing 10 mM Tris-HCl (pH 7.9), 50 mM NaCl, 1 mM DTT, and 10 mM MgCl2 (Figure 2, lane 7).
Figure 2.
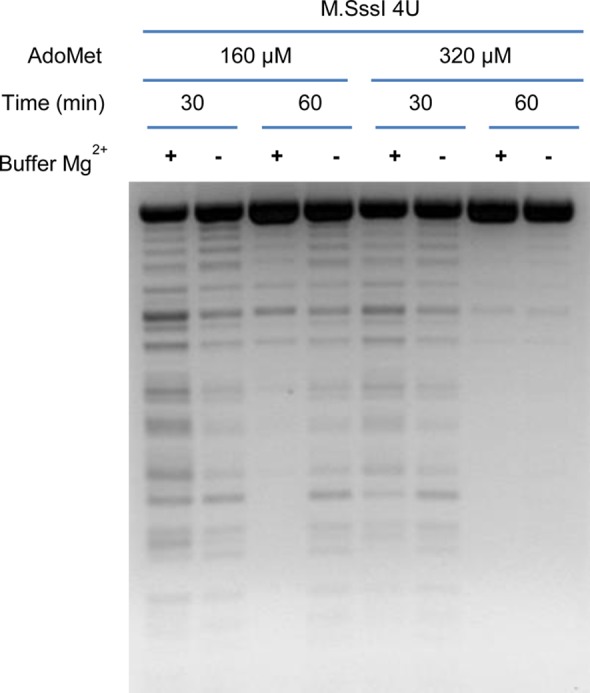
Restriction endonuclease analysis of pUC19 DNA after methylation. DNA was incubated for 30 or 60 min with either 160 or 320 μM AdoMet at a fixed amount of M.SssI DNA methyltransferase in the presence or absence of added MgCl2, followed by restriction digestion with HpaII.
An additional concern during assay development was a report that the M.SssI DNA methyltransferase activity becomes less processive and has a decreased affinity for its DNA substrate in the presence of higher levels of Mg2+.12 Therefore, the effect of Mg2+ ions on the DNA protection assay was evaluated under our optimized assay conditions. M.SssI methylates the pUC19 DNA substrate very efficiently in the absence of added Mg2+, but somewhat less efficiently at higher Mg2+ levels (Figure 2, buffer – and buffer + lanes, respectively). Therefore, to eliminate any interference, the MgCl2 concentration required for optimal MetK-catalyzed synthesis of AdoMet was diluted to ∼1 mM when AdoMet or AdoMet analogue products were examined as potential methyl donors in the DNA methylation reactions.
DNA Methylation Capability of AdoMet Analogues
The AdoMet synthetases (MetKs) purified from several Gram-negative bacterial pathogens can use different l-methionine derivatives as substrates, converting them to the corresponding AdoMet analogues.11 To evaluate the methyl donor ability of these AdoMet analogues, MetKs were used to generate these product analogues, which were then tested as potential substrates for the DNA methyltransferase, M.SssI. The MetK from C. jejuni has the highest overall catalytic efficiency among the enzymes from this family that have been studied11 and was used initially to generate the AdoMet analogues.
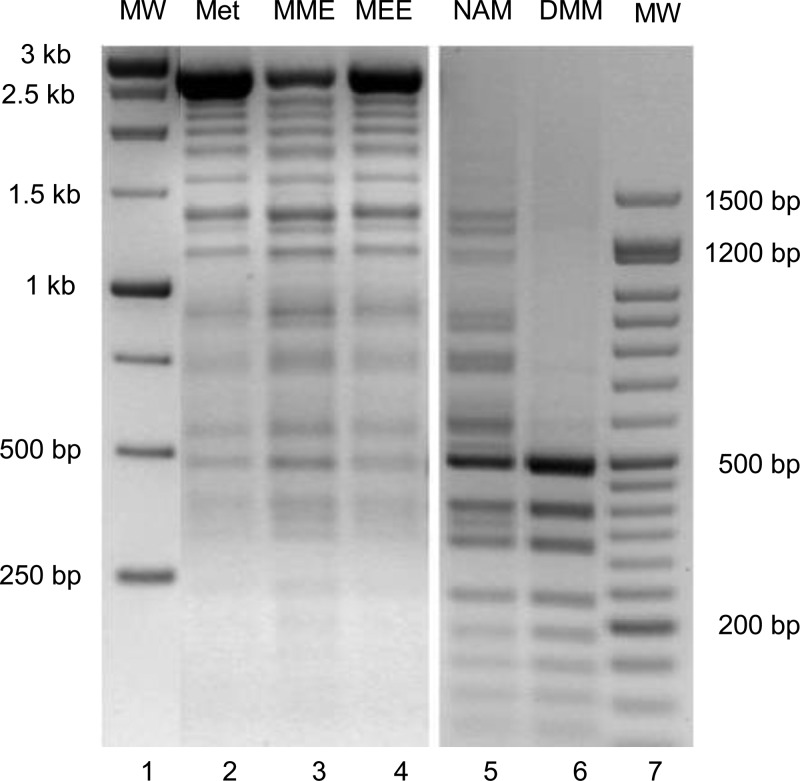
The various methionine esters each led to AdoMet products that were examined at 100 μM and found to serve as donors of methyl groups to DNA. l-Methionine and l-methionine ethyl ester (MEE) are each excellent substrates for C. jejuni MetK, and each supported significant DNA methylation, as demonstrated by the substantial protection from cleavage by the restriction endonuclease HpaII, with a very low abundance of bands below 500 bp (Figure 3, lanes 2 and 4); the great majority of DNA is found as the full-length, completely methylated linear substrate (2.69 kb). Control digestion of the fully methylated DNA with MspI showed the expected total cleavage, because MspI nuclease recognizes the same sites as HpaII but is not affected by CpG methylation (not shown, but similar to the results seen in Figure 3, lane 6). The methyl ester of methionine (MME) is a somewhat poorer substrate for MetK and also led to a smaller fraction of totally methylated DNA (Figure 3, lane 3).
Figure 3.
DNA methylation by M.SssI using different AdoMet analogues produced by the AdoMet synthetase-catalyzed activation of methionine derivatives. The methionine substrates included l-methionine (Met), l-methionine methyl (MME) and ethyl (MEE) esters produced by C. jejuni AdoMet synthetase and tested at 100 ± 5 μM, and N-acetyl-l-methionine (NAM) and N,N-dimethylmethionine (DMM) produced by N. meningitidis AdoMet synthetase and tested at ∼10 and <5 μM, respectively. The methylation of 1 μg of linearized pUC19 DNA was conducted for 1 h at 37 °C, followed by digestion with HpaII and gel electrophoresis.
The N-derivatized methionine analogues, N-acetyl-l-methionine (NAM) and N,N-dimethyl-l-methionine (DMM), are very poor substrates for the MetKs, and the limited amount of AdoMet analogue products obtained from these reactions catalyzed by C. jejuni MetK was not sufficient to provide any detectable level of DNA methylation. The MetK from N. meningitidis uses N-acetyl-l-methionine as a substrate with the highest efficiency of members of this enzyme family.11 Switching to this enzyme form and extending the reaction time to 2 h resulted in a greater production of the N-acetylated AdoMet analogue (∼10 μM) that was sufficient to cause partial methylation of DNA from this donor (Figure 3, lane 5). The extremely poor N,N-dimethyl-l-methionine MetK substrate yielded low levels of the corresponding AdoMet analogue (<5 μM), and this level of product did not lead to any significant amount of DNA protection (Figure 3, lane 6).
Efficiency of DNA Methylation
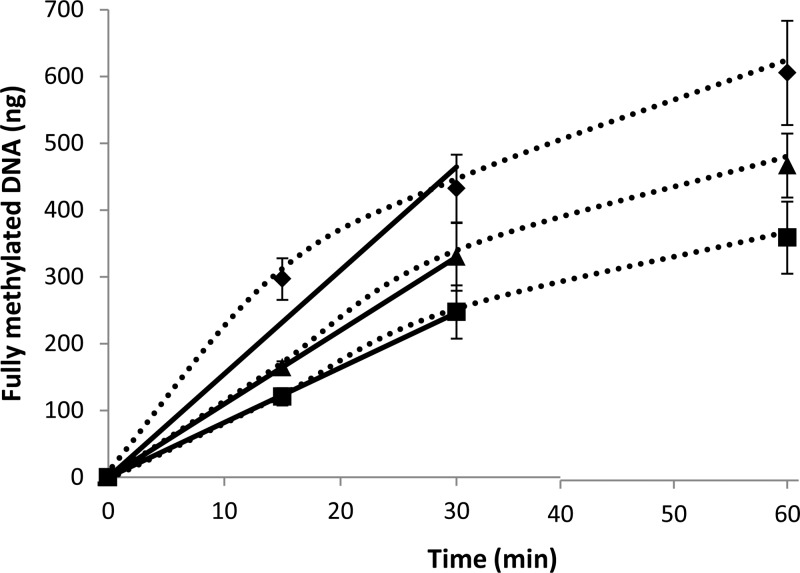
As described above, the AdoMet derivative of l-methionine ethyl ester showed a level of protection of pUC19 DNA under the experimental condition tested that was nearly the same as that achieved by AdoMet (Figure 3, lanes 2 and 4). To more accurately assess the methyl donor efficiency of the AdoMet ester analogues, the M.SssI DNA methyltransferase reaction was followed over different times using a fixed level (100 μM) of the in situ-generated AdoMet analogues. A parallel reaction with AdoMet formation from methionine served as the benchmark for comparison. A slightly lower rate of DNA protection was achieved with the AdoMet ethyl ester, with an initial rate of methylated DNA production (11 ng/min) that was ∼70% of that achieved with AdoMet as a methyl donor (Figure 4). The rate of DNA methylation with the AdoMet methyl ester (8 ng/min) was approximately half of that obtained with AdoMet under these reaction conditions. Similar differences in the methyl transfer efficiency of each AdoMet analogue were observed when the amount of fully methylated DNA produced was compared when the reaction time was extended to 60 min (Figure 4).
Figure 4.
Efficiency of DNA methylation with AdoMet analogues determined at a fixed level (100 μM) of each substrate at different time intervals at 37 °C. The amount of fully methylated DNA was determined by the total pixel count of the largest band that represents the fully protected pUC19 DNA using AdoMet (◆), AdoMet ethyl ester (▲), or AdoMet methyl ester (■) as the methyl donor.
Catechol Methylation by AdoMet Analogues
While some of the AdoMet analogues have now been shown to function proficiently as donors of methyl to DNA, it is not clear that they can effectively replace AdoMet with other methyltransferases having unrelated substrates. To assess the range of ability of the AdoMet analogues to serve as methyl donors, we sought to test an additional methyltransferase that is only distantly related to M.SssI. Catechol O-methyltransferase (COMTase) is in the same general methyltransferase fold (class I) as M.SssI,7 but the amino acid sequences of the two proteins are only <10% identical. In addition, COMTase is a mammalian enzyme that catalyzes the methylation of a small molecule substrate and targets an oxygen atom as the methyl acceptor rather than the carbon atom target of M.SssI.
First, a new assay was needed to follow this methyl transfer reaction. Because of the similar absorption spectra of the substrates and products in the COMTase-catalyzed reaction, and also the failure to develop an efficient enzyme-coupled assay, it became necessary to separate the reactants to measure the rates of the catechol methylation reaction. The rate of epinephrine methylation was examined by measuring the production of S-adenosyl-l-homocysteine (AdoHcy) in an HPLC-based assay. The separation of ATP, AdoMet, and AdoHcy is dependent on the pH of the mobile phase (data not shown), with the best separation achieved in phosphate buffer at pH 7.2. Under these conditions, the ATP substrate for AdoMet production eluted at 2.7 min, while AdoMet and AdoHcy eluted around 4 and 5.6 min, respectively (Figure 5A).
Figure 5.
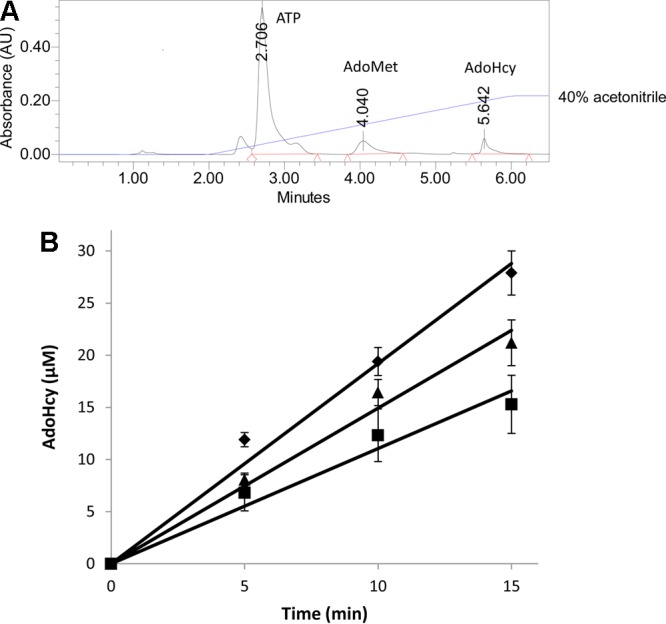
Methylation of epinephrine by COMTase using different AdoMet analogues. (A) HPLC separation of the catechol O-methyltransferase reaction mixture to allow quantitation of the S-adenosylhomocysteine (AdoHcy) product. (B) Time course for the production of S-adenosylhomocysteine during the methylation of epinephrine using AdoMet (◆), AdoMet methyl ester (■), or AdoMet ethyl ester (▲) as the methyl donor.
To determine the relative methylation efficiencies of the different AdoMet analogues, catechol O-methylation was performed by using the same amount of AdoMet and its analogues (100 ± 5 μM) that was used for DNA methylation, with the reaction time course monitored from 0 to 15 min. The initial velocity of the methyl transfer reaction was calculated by plotting the AdoHcy or AdoHcy analogue concentration (determined by HPLC peak area) versus time (Figure 5B). As was observed with DNA methylation, AdoMet ethyl ester is nearly as efficient as a methyl donor as AdoMet for the methylation of epinephrine, with an initial rate that is ∼80% of that achieved with AdoMet. The AdoMet methyl ester is also a viable donor of methyl to this catechol, with a rate that is ∼60% of that achieved by AdoMet under these reaction conditions (Figure 5B). Evidence of the AdoHcy product was also observed when the AdoMet analogue obtained from N-acetyl-l-methionine was used as the methyl donor. However, the low levels of this AdoMet analogue made it hard to reliably determine the concentration of the AdoHcy product and therefore difficult to measure the rate of catechol methylation.
Discussion
Production of Quorum Signal Molecules
The production of acylhomoserine lactones (AHLs), which function as quorum sensing molecules, diverts the essential metabolite AdoMet for use as a precursor. The enzyme-catalyzed reaction for AHL production requires a free carboxyl group in the methionine moiety of AdoMet for lactone formation and a free methionine amino group for acylation.13 Derivatizing either of these functional groups would interfere with AHL formation, effectively blocking quorum signaling. We have recently shown that methionine derivatives with modified carboxyl or amino groups can still function as viable substrates for AdoMet synthetases from various Gram-negative pathogens, leading to the production of modified AdoMet analogues.11 The next critical question that needed to be addressed was whether the AdoMet analogues produced in these reactions can still participate in each of the critical roles that are required both in bacterial and in mammalian metabolism.
DNA Methylation
The methylation of DNA is an essential step that controls the regulation of gene expression in metazoa.14 DNA methylation is less critical for gene regulation in bacteria but plays important roles in chromosome replication,15 mismatch repair,16 and restriction–modification systems.17 Several AdoMet analogues, produced from methionine analogues that were derivatized at either the α-amino group or the α-carboxyl group and incapable of supporting AHL biosynthesis, have been shown here to function as methyl donors for DNA methylation. In particular, the AdoMet ester derivatives support efficient DNA methylation, at rates that are within a factor of 2 of that achieved with unmodified AdoMet. N-Derivatized AdoMet analogues were also examined, but while these derivatives can also serve as donors for DNA methylation, the levels of methylated DNA produced were quite low. The low level of production of methyl-protected DNA with the N-derivatized AdoMet analogues is certainly related to the low levels of these compounds obtained from the MetK-catalyzed synthesis. Extending the MetK reaction time to 3 h for N,N-dimethyl-l-methionine in the presence of N. meningitidis AdoMet synthetase led to the production of <5 μM AdoMet analogue product. Treating the DNA substrate with this 20-fold lower level of the AdoMet analogue produces barely detectable DNA methylation products. Further optimization of AdoMet analogue synthesis is certainly possible, but any methionine derivatives would be administered as prodrugs that must be converted in vivo into methyl donor molecules. For these alternative N-derivatized methionine analogue substrates to block quorum signal production, overcoming competition from the endogenous methionine and AdoMet produced by these pathogenic organisms would be difficult to achieve. Success with this approach will require modification of the most potent alternative MetK substrates into stable derivatives that retain their capacity to serve as methyl donors.
Small Molecule Methylation
The methylation of catechol-type neurotransmitters is the initial step toward their subsequent degradation, a process needed to modulate signal levels. As with DNA methylation, AdoMet functions as the physiological methyl donor to initiate this breakdown. AdoMet analogues designed to block quorum signaling must still be capable of participating in these essential mammalian metabolic functions. The AdoMet derivatives that were functional methyl donors for DNA methylation were also found to be excellent substrates for the methylation of epinephrine. In fact, the relative rates of DNA methylation with the ethyl and methyl ester derivatives of AdoMet were nearly identical to those achieved for catechol methylation when the rates were examined relative to that achieved with AdoMet.
Conclusions
The results from these new studies establish that AdoMet analogues, produced by MetK from methionine derivatives and impaired for AHL quorum signal biosynthesis, can participate in one of their primary metabolic roles by functioning as effective methyl donors to DNA and the small molecule epinephrine.
Acknowledgments
Dr. Stephen Zano was responsible for the purification of the AdoMet synthetases that were used to produce AdoMet analogues from the l-methionine derivatives.
The authors declare no competing financial interest.
This work is supported by a grant to R.E.V. and R.M.B. from the National Institutes of Health (AI098702).
Funding Statement
National Institutes of Health, United States
References
- Williams P.; Winzer K.; Chan W. C.; Camara M. (2007) Look who’s talking: Communication and quorum sensing in the bacterial world. Philos. Trans. R. Soc., B 362, 1119–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. G.; Parsek M. R.; Pearson J. P.; Iglewski B. H.; Costerton J. W.; Greenberg E. P. (1998) The Involvement of Cell-to-Cell Signals in the Development of a Bacterial Biofilm. Science 280, 295–298. [DOI] [PubMed] [Google Scholar]
- Dickschat J. S. (2010) Quorum sensing and bacterial biofilms. Nat. Prod. Rep. 27, 343–369. [DOI] [PubMed] [Google Scholar]
- Henke J. M.; Bassler B. L. (2004) Bacterial Social Engagements. Trends Cell Biol. 14, 648–656. [DOI] [PubMed] [Google Scholar]
- Fontecave M.; Atta M.; Mulliez E. (2004) S-Adenosylmethionine: Nothing goes to waste. Trends Biochem. Sci. 29, 243–249. [DOI] [PubMed] [Google Scholar]
- Marsh E. N.; Patterson D. P.; Li L. (2010) Adenosyl Radical: Reagent and Catalyst in Enzyme Reactions. ChemBioChem 11, 604–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert H. L.; Blumenthal R. M.; Cheng X. (2003) Many paths to methyltransfer: A chronicle of convergence. Trends Biochem. Sci. 28, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.; Perez L. J.; Ng W. L.; Sempere R.; Bassler B. L. (2011) Mechanism of Vibrio cholerae Autoinducer-1 Biosynthesis. ACS Chem. Biol. 6, 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway W. R.; Hodgkinson J. T.; Bowden S. D.; Welch M.; Spring D. R. (2011) Quorum Sensing in Gram-Negative Bacteria: Small-Molecule Modulation of AHL and Al-2 Quorum Sensing Pathways. Chem. Rev. 111, 28–67. [DOI] [PubMed] [Google Scholar]
- Rasmussen T. B.; Givskov M. (2006) Quorum-sensing inhibitors as anti-pathogenic drugs. Int. J. Med. Microbiol. 296, 149–161. [DOI] [PubMed] [Google Scholar]
- Zano S.; Bhansali P.; Luniwal A.; Viola R. E. (2013) Alternative Substrates Selective for S-adenosylmethionine Synthetases from Pathogenic Bacteria. Arch. Biochem. Biophys. 536, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K.; Sillke J.; Gramatikoff K.; Schaffner W. (1994) The CpG-specific methylase SssI has topoisomerase activity in the presence of Mg2+. Nucleic Acids Res. 22, 5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. T.; Minogue T. D.; Val D. L.; von Bodman S. B.; Churchill M. E. (2002) Structural Basis and Specificity of Acyl-Homoserine Lactone Signal Production in Bacterial Quorum Sensing. Mol. Cell 9, 685–694. [DOI] [PubMed] [Google Scholar]
- Smith Z. D.; Meissner A. (2013) DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 14, 204–220. [DOI] [PubMed] [Google Scholar]
- Collier J. (2009) Epigenetic regulation of the bacterial cell cycle. Curr. Opin. Microbiol. 12, 722–729. [DOI] [PubMed] [Google Scholar]
- Marinus M. G.; Casadesus J. (2009) Roles of DNA adenine methylation in host-pathogen interactions: Mismatch repair, transcriptional regulation and more. FEMS Microbiol. Rev. 33, 488–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A.; Dryden D. T. F.; Raleigh E. A.; Wilson G. G.; Murray N. E. (2014) Highlights of the DNA cutters: A short history of the restriction enzymes. Nucleic Acids Res. 42, 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]