Abstract
Obesity, metabolic syndrome, and type 2 diabetes (T2D) are related disorders with widespread deleterious effects throughout the body. One important target of damage is the brain. Persons with metabolic disorders are at significantly increased risk for cognitive decline and the development of vascular dementia and Alzheimer’s disease. Our review of available evidence from epidemiological, clinical, and basic research suggests that neural dysfunction from T2D-related disease results from several underlying mechanisms, including metabolic, inflammatory, vascular and oxidative changes. The relationships between T2D and neural dysfunction are regulated by several modifiers. We emphasize two such modifiers, the genetic risk factor apolipoprotein E and an age-related endocrine change, low testosterone. Both factors are independent risk factors for Alzheimer’s disease that may also cooperatively regulate pathologic interactions between T2D and dementia. Continued elucidation of the links between metabolic disorders and neural dysfunction promises to foster the development of effective therapeutic strategies.
Keywords: Alzheimer’s disease, β-amyloid, apolipoprotein E, diabetes, inflammation, obesity, testosterone, type 2 diabetes, interactions
Introduction
The related conditions obesity, metabolic syndrome, and type 2 diabetes (T2D) have significant independent and combined effects on metabolic, inflammatory, and other pathways, which in turn have wide ranging deleterious effects on numerous organ systems including the cardiovascular and endocrine systems (1). A rapidly accumulating literature also identifies the nervous system as a target of obesity, metabolic syndrome, and T2D. We discuss evidence that this collection of metabolic disorders results in increased risks for both cognitive decline and dementia. There remain numerous unresolved issues, including the relative importance of several different degenerative mechanisms to the observed neuropathologies and whether observed impairments represent a continuum of a single degenerative process or a collection of separate pathologies that are differentially expressed.
Among the many harmful neural effects of T2D and its precursor conditions is an increased risk for Alzheimer’s disease (AD). The most common form of dementia, AD has a high and rapidly growing prevalence in the aging population. In the US alone, the number of persons afflicted with AD is estimated to be 5.1 million, a figure that is anticipated to rise to approximately 7.7 million by 2030 (2). Given that obesity and T2D also exhibit high prevalence with increasing trajectories, the interaction of these diseases poses a serious health threat. Thus, while we broadly discuss the range of neural effects of T2D on the brain, we will emphasize the impact of metabolic disorders on AD.
Metabolic dysfunction increases risk of cognitive impairment
Cognitive function is adversely affected by the prediabetic risk factors central obesity and metabolic syndrome. Longitudinal studies indicate worsening performance on measures of global cognitive function and some, but not all, specific abilities including working memory in persons with metabolic syndrome (3, 4). In some populations, there is evidence that women with metabolic syndrome may be particularly vulnerable to cognitive decline (5). In the absence of T2D, impairment in glucose regulation and increased serum insulin can lead to mild cognitive impairment (MCI), suggesting that lack of glycemic control may contribute to the observed cognitive decline (6, 7). Another key factor contributing to worsening of cognitive abilities in aging populations is obesity. Central obesity is associated with impairment in various aspects of cognitive functioning (8, 9). This relationship may be most important during middle age (8) and diminish during advanced age (10).
Like obesity and metabolic syndrome, T2D is also associated with significant impairments in various aspects of cognitive functioning. For example, a recent fMRI study showed that T2D patients have altered spontaneous neuronal activity in several brain regions that correlated with poorer cognitive performances (11). Other imaging studies show increased brain atrophy in individuals with T2D associated with cognitive impairments (12, 13). Additional factors such as ethnicity and smoking habits can increase the association between diabetes and cognitive impairment (14, 15). Notably, the rate of cognitive decline in persons with T2D is generally rather slow (16), although a subset of patients show rapid decline (17). Recent evidence suggests that a key factor underlying accelerated cognitive decline among people with T2D is depression. People with diabetes that score highest on depression indices also show the poorest performance on a range of cognitive tasks (18). Unclear is whether the observed variation in rates of cognitive decline reflect differential vulnerability of some patients to dementia.
Metabolic dysfunction increases risk of dementia
Obesity, metabolic syndrome, and T2D not only contribute to impaired cognitive function, but also increase the risk of AD. In the past several years, a wealth of data has shown that persons with T2D and features of metabolic syndrome are at significantly increased risk for development of dementias including AD (19, 20). The magnitude of the risk is a matter of debate. For example, one longitudinal study showed that people with T2D have more than a two-fold increase in risk for developing AD as compared to those without T2D (21). However, other studies indicate a more modest relationship between T2D and AD or even the lack of an association (22). A recent meta-analysis of longitudinal studies suggests that the relative risk for AD is approximately 1.5-fold higher among persons with T2D (23). As with cognitive decline, the increased risk of dementia associated with T2D appears to be affected by age. A longitudinal study showed that central obesity in middle-aged people increased the risk for dementia independent of diabetes (24). In the aged population, obesity and T2D appear to be only weakly related to AD risk (25).
The association between T2D and its precursor conditions is not limited to AD. In fact, T2D appears to be more strongly associated with increased risk of vascular dementia (VaD) than AD. Some studies suggest that the relationship between T2D and dementia risk largely reflects promotion of VaD and stroke-related dementia rather than AD (26). Findings from a meta-analysis suggest that T2D patients have a 2.5-fold increase in risk for VaD, a level significantly higher than the risk for AD (23). Imaging studies reveal that T2D is associated with increased levels of lacunar infarcts (27) and white matter hyperintensities (28). The presence and duration of hyperglycemia and T2D contribute to increases in brain atrophy and lacunar infarcts as compared to non-T2D patients (29). Together, clinical and epidemiological findings demonstrate that T2D is associated with increased risks for cognitive decline and dementia. Insights into the pathways underlying these effects are provided by studies in animal models.
T2D exacerbates neuropathology in animal models of Alzheimer’s disease
If T2D contributes pathological mechanisms with AD, then one may expect some crossover between pathologies in animal models of T2D and AD. Although it remains to be determined to what extent AD animal models exhibit evidence of T2D-like metabolic changes, several recent studies have demonstrated (i) AD-like neuropathology in animal models of T2D and (ii) accelerated AD-like neuropathology in rodent models of AD following experimental induction of T2D-like metabolic changes by dietary manipulations. For example, the BBZDR/Wor rat model of T2D exhibits several neural changes consistent with AD pathology including neuron loss, dystrophic neurites, increased levels of β-amyloid (Aβ) and tau hyperphosphorylation, and decreased expression of insulin and IGF-1 receptors (30). Tau hyperphosphorylation is also observed in the OLETF rat model (31) and db/db mouse model of T2D (32). Tau phosphorylation (33) and impaired cognitive performance are seen in rats with insulin-dependent diabetes induced using streptozotocin (STZ), a toxin that results in β-cell atrophy (34). Recent work shows that STZ in mice causes impaired insulin signaling that results in reduced insulin degrading enzyme (IDE) expression in brain with elevated Aβ, and increased tau phosphorylation (35). IDE is a downstream target of insulin signaling and is upregulated via the insulin-PI3K-AKT phosphorylation pathway, forming a negative feedback mechanism (36). Therefore, impaired insulin signaling may result in decreased IDE levels due to reduced AKT activation. Since IDE is also established as a significant contributor to enzymatic Aβ degradation (37, 38), a decrease in IDE levels would lead to reduced Aβ clearance and subsequent increased Aβ accumulation in the brain. Similarly, in AD mouse models, insulin resistance induced in Tg2576 AD transgenic mice by a high fat diet results in increased Aβ accumulation and decreased IDE (39). APP/PS1 mice exposed to sucrose-supplemented drinking water exhibit glucose intolerance, elevated insulin levels, Aβ deposition, and behavioral deficits (40). Thus, findings from experimental paradigms link T2D-like metabolic changes with promotion of AD-like neuropathology.
Several pathways have been independently suggested as linking mechanisms between T2D and AD such as hyperinsulinemia, inflammation, vascular factors, and oxidative stress. However, a combination of two or more of these pathways may be working together to connect the two disorders. In this following section, we consider the major mechanisms implicated in both T2D and AD and how these pathways contribute to the progression of AD from T2D.
Pathways linking T2D and AD: Dysregulation of insulin and glucose
Dysregulation of insulin and glucose are key characteristics of diabetes. Insulin resistance and impaired insulin signaling also have been linked to increases in AD pathology (41). Insulin and insulin receptor levels in brain decrease with normal aging (42). Moreover, insulin receptor expression in the brain decreases further with AD (43). IDE, a zinc-binding metalloprotease whose substrates include both insulin and Aβ, may contribute to the interactions between T2D and AD. In transgenic AD mice fed a high fat diet, deficient insulin signaling correlated with decreased IDE levels and increased Aβ levels (36, 39). Partial loss-of-function mutations in IDE are capable of inducing T2D and impairing degradation of Aβ (44). Although the mechanism(s) underlying the T2D effect is unclear, one possibility is that the loss of IDE function promotes hyperinsulinemia, which in the long-term may contribute to insulin resistance and impaired glucose tolerance. Pharmacological inhibition of IDE reduces the degradation of insulin, islet amyloid peptide (45), and Aβ (46). Interestingly, IDE has a higher affinity for insulin as a substrate than Aβ (47). Thus, one mechanistic hypothesis for the role of T2D in AD risk is that the hyperinsulinemia characteristic of T2D results in reduced degradation of Aβ by IDE, leading to Aβ accumulation. In the STZ model, diminished insulin signaling due to insulin deficiency may lead to downregulation of IDE levels, similarly leading to increased accumulation of Aβ and elevated AD risk.
Interestingly, treatment of T2D may improve neural function and/or slow AD pathogenesis. For example, improved diabetes control is associated with a slowing in cognitive decline (48). Moreover, common T2D medications such as rosiglitazone and metformin may decrease AD-related cognitive decline and Aβ levels (49, 50). Initial results of a clinical trial of intranasal insulin therapy in early AD and MCI patients indicate slowing of cognitive decline (51). Studies in animal models are consistent with clinical observations. In 3xTg-AD mouse model of AD, pioglitazone improved learning and plasticity and decreased Aβ and tau pathologies (52). Similarly, liraglutide reduced Aβ plaques and glial activation in APPswe/PS1dE9 model of AD (53). Still unclear is whether the primary mechanisms of reducing AD-related pathologies by these drugs involve glycemic control and decreasing insulin resistance or other effects on adipose tissue (54), body weight (55) and levels of proinflammatory cytokines (56).
Despite the strong association between hyperinsulinemia, hyperglycemia and AD pathogenesis, other compelling findings argue against a primary mechanistic role of metabolic factors in AD. For example, diet-induced obesity in 3xTg-AD mice increases Aβ burden and impairs behavior, although in female mice this acceleration of AD-like pathology occurred in the absence of significant changes in fasting levels of glucose and insulin (57). Consistent with these data is the observation that induction of insulin resistance and hyperinsulinemia via a mutated insulin receptor failed to significantly accelerate the rates of Aβ accumulation and cognitive decline in a transgenic AD mouse model (58). Similarly, knockout of insulin receptor substrate 2 in AD transgenic mice predictably yields significant metabolic dysfunction but reduces rather than accelerates AD-related pathologies (59, 60). Thus, obesity-related factors other than insulin resistance may be central to the mechanism by which obesity, metabolic syndrome, and T2D increase AD risk. One such factor is inflammation.
Pathways linking T2D and AD: Inflammation
Pro-inflammatory pathways may also contribute to interactions between T2D and AD. It is well established that central obesity, metabolic syndrome and diabetes all involve chronic systemic inflammation (61). Increased levels of several pro-inflammatory cytokines are observed in T2D (62) and several anti-inflammatory drugs have been shown to reduce this effect (63). Among persons with metabolic syndrome, those with relatively higher inflammation are more likely to develop cognitive impairment than those with low inflammation (64).
Obesity causes an increase in inflammatory cytokines not only in adipose tissue (65) but also in the nervous system. In animal models, diet-induced obesity induces an increase in inflammatory responses in many brain regions, including cerebral cortex (66) and hypothalamus (67). Recent studies from our laboratory demonstrate higher levels of the pro-inflammatory cytokines TNF-α and IL-1β in the cortex of mice maintained on high fat diet fed as well as in primary mixed glial cultures generated from these mice. We also show that the increase in proinflammatory factors seen both in the central and the peripheral nervous system reduces neuronal health (unpublished data).
In AD, inflammatory pathways have been widely hypothesized to directly contribute to disease initiation and progression (68). A classic neuropathological characteristic of AD brain is the abundant presence of activated astrocytes and microglia (69), the neural cell types most responsible for inflammatory responses in brain. Elevated levels of pro-inflammatory cytokines are observed in AD (70) as well as in transgenic models of AD (71, 72). Consistent with a primary role of inflammation in AD are the results of recent genome-wide association studies in which several genes linked with AD function in innate immunity (73). Unclear is whether the same genetic polymorphisms contribute to T2D. Although the literature remains undecided, there is evidence from observational studies that use of non-steroidal anti-inflammatory drugs (NSAIDs) may decrease the risk for developing AD (74). Given the role established pro-inflammatory profiles of obesity, metabolic syndrome, and T2D and the presumed role of these pathways in AD pathogenesis, inflammation is likely a key mechanism contributing to the interactions across the diseases.
Pathways linking T2D and AD: Other factors
Among the other possible mechanisms that contribute to the relationship between T2D and AD are those involving vascular risk factors, lipoprotein receptors, and oxidative stress. Vascular risk factors include hypertension, cerebrovascular diseases, and hypercholesterolemia. Studies have shown that the presence of a combination of these vascular factors promote the development of AD and AD-related neuropathology (30, 75). Defects in brain vasculature and blood-brain barrier are also seen in AD patients (76) suggesting that vascular factors in the nervous system are important in AD pathogenesis. Lipoprotein receptors and lipoprotein receptor-related protein-1 (LRP-1) are another set of factors involved in metabolic syndrome and AD. Lipoprotein receptors and LRP-1 aid in Aβ clearance from liver as well as brain (77, 78). LRP-1 is also involved in intracellular cholesterol and fatty acid storage (79) while LRP-6, has been shown to regulate body weight and glucose homeostasis (80). Pathways regulating LRP-1 have been shown to improve Aβ-induced learning and memory impairments in rats (81). Oxidative stress pathways play key roles in several pathological disorders including T2D and AD. One such pathway is advanced glycation resulting in the production of advanced glycation end products (AGEs) (82). In addition to AGEs, the receptor for advanced glycation end products (RAGE) has been identified to be a ligand for Aβ fibrils (83, 84) and may be involved in the neurotoxic effects of Aβ in neurons and microglia (85, 86). Further, RAGE regulates the accumulation and transport of Aβ across the blood-brain barrier (87). RAGE is known to be up-regulated in both T2D and AD (85, 88). Hence RAGE acts as a progression factor that exacerbates the immune and inflammatory pathways leading to cellular dysfunction (89), which in turn may facilitate interactions between T2D and AD.
Modifiers of T2D and AD relationship: Apolipoprotein E
The relationship between T2D and AD appears to be significantly influenced by several factors. One modulator is apolipoprotein E (ApoE). The ApoE ε4 allele is the most significant genetic risk factor for late-onset AD, the risk of AD increasing with the number of ApoE ε4 alleles present (90). ApoE functions in lipid transport and lipoprotein metabolism (91) and regulates several important neuronal actions including neuronal repair, synaptogenesis, nerve growth, and development (91). The severity of AD pathology is influenced by ApoE genotype as indicated by studies showing that the presence of ApoE ε4 alleles increases both the rate and amount of Aβ deposition (92).
The risk for AD in T2D cases is increased in ApoE ε4 carriers (20, 93). Further, the presence of ApoE ε4 in T2D cases with AD is associated with increased neurofibrillary tangles, amyloid plaques, and cerebral amyloid angiopathy (94). Recent clinical findings show that persons with ApoE ε4 have higher levels of lipid-depleted Aβ, an effect that is worsened by consumption of a high-fat, high glycemic index diet (95). ApoE ε4 carriers also have lower levels of insulin degrading enzyme, which may affect both insulin signaling and Aβ clearance in T2D and AD cases (96). In AD mice with sucrose-induced insulin resistance, ApoE levels are increased 2.5 fold, perhaps contributing to Aβ accumulation and increased AD pathology (40). The levels of insulin in cerebrospinal fluid and plasma appear to be lower in AD patients with an ApoE ε4 allele compared with patients with no ε4 allele (97). Insulin administration has been found to be more effective on aspects of memory and Aβ pathology in AD patients who were ApoE ε4 null compared with those who were ApoE ε4 carriers (98, 99). Taken together these studies suggest that ApoE genotype acts as a positive regulator of the T2D/AD relationship.
Modifiers of T2D and AD relationship: Low testosterone
Normal age-related depletion of testosterone in men, commonly referred to as andropause, results in a constellation of symptoms that reflect dysfunction and vulnerability to disease in androgen-responsive tissues including brain, muscle, bone, and adipose tissues (100). In the past several years, research from several groups including ours has identified andropause as a significant risk factor for AD (101). Men with low levels of testosterone in either blood (102, 103) or brain (104, 105) are at increased risk for developing AD. Importantly, low testosterone precedes both the cognitive (106) and neuropathological (104, 105) diagnoses of AD, suggesting that it is a contributing factor to rather than a result of the disease.
An established and rapidly growing body of epidemiological and clinical evidence indicates strong associations between low testosterone levels in men, T2D, and metabolic syndrome. Several studies have found a correlation between low testosterone and insulin resistance in men suggesting a role of low testosterone in insulin resistance (107, 108). Longitudinal studies have shown that low testosterone precedes metabolic syndrome, appearing 5-10 years prior to the development of metabolic and cardiovascular symptoms (109, 110). Since metabolic syndrome is often a precursor to development of T2D, it is not surprising that men with T2D have significantly lower levels of total and free testosterone in comparison to age-matched, controls with no diabetes (107). Testosterone therapy used for the treatment of androgen deficiency reduces features of T2D and metabolic syndrome, including insulin resistance, adiposity, and total cholesterol (111, 112) while improving glycemic control (113). On the other hand, the use of androgen deprivation therapy for treatment of prostate cancer indicates that testosterone depletion can increase the incidence and prevalence of T2D (114, 115) and metabolic syndrome (116). Further, androgen deprivation therapy has been found to lower insulin sensitivity and glycemic control, and increase insulin and cholesterol levels (117). Thus, available evidence indicates that T2D lowers testosterone levels in men and, conversely, that low testosterone increases indices of T2D.
Interestingly, there is compelling evidence of a significant relationship between androgens and ApoE genotype. In humans, circulating levels of testosterone are lower in men with at least one ApoE ε4 allele (118). In animal studies, Raber and colleagues have shown that androgens antagonize behavioral deficits in ApoE ε4 mice (119). Depletion of endogenous testosterone following castration in ApoE ε4 mice results in behavioral impairments in some but not all tasks (120). Similarly, inhibition of AR function by pharmacological and genetic approaches results in behavioral impairments in ApoE ε4 mice but not ApoE ε3 mice (119, 121). Further, lower levels of AR have been observed in both male and female ApoE ε4 mice, although it is unclear if ApoE ε4 is directly affecting AR levels or otherwise interfering with androgen binding to AR (119). Thus, ApoE ε4 genotype is associated with both lower testosterone levels and attenuation of neural androgen actions, effects that are predicted to magnify interactions effects of low testosterone, T2D and AD.
Conclusion
In summary, an extensive set of findings from epidemiological, clinical, and animal models have identified a complex set of interactions wherein T2D and its precursor conditions obesity and metabolic syndrome exert deleterious effects on the brain. The primary negative outcomes of these metabolic disorders are cognitive decline and increased risk for dementias of the vascular and Alzheimer’s types. Although cognitive decline is a component of all dementias, we suggest that the neural outcomes of T2D do not reflect a single condition but rather are manifestations of a range of pathologies. Numerous mechanisms are hypothesized to contribute to observed neuropathologies, including metabolic, inflammatory, vascular, and oxidative changes (Fig. 1). The magnitude and perhaps the form of neural injury are likely influenced by a set of modifiers. Specifically, we focused on age-related testosterone depletion in men and the Apo E ε4 allele as independent and interactive risk factors for AD and the promotion of AD pathogenesis by T2D.
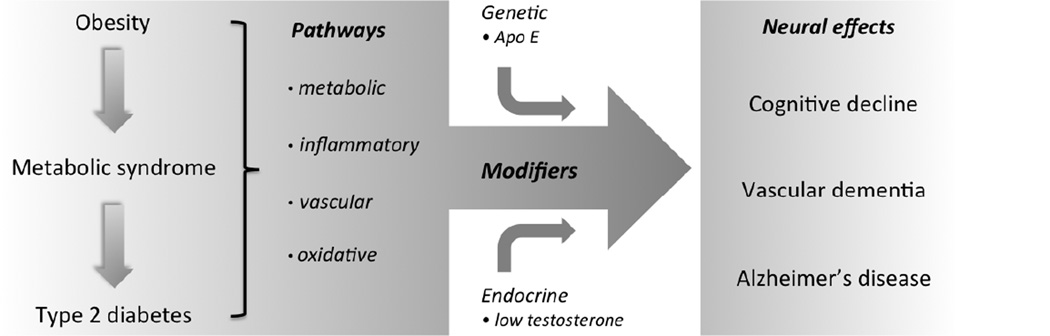
Figure 1.
Obesity and metabolic syndrome are precursor disorders to type 2 diabetes. All three conditions independently and interactively activate a range of metabolic, inflammatory, and oxidative changes that contribute to deleterious effects on the brain. The damaging effects of metabolic disorders are influenced by several modifying factors, including endocrine changes such as low testosterone and genetic factors such as the apolipoprotein E (Apo E) ε4 allele. In response to these damaging pathways, the brain exhibits cognitive decline and increased risk to Alzheimer’s and vascular dementias.
The multi-faceted and interactive nature of the associations between T2D and neural dysfunction and disease is daunting in its apparent complexity but encouraging in terms of potential therapies. In addition to reducing neural damage by conventional T2D-related approaches, promising strategies include specific interventions that target implicated pathways and modifying factors (Fig. 1). One example is testosterone-based therapy including the use of novel selective androgen receptor modulators, which are predicted to favorably affect both T2Dand AD-specific pathways as well as their interactions.
Acknowledgment
This work was supported by NIH grant AG34103 (CJP).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Anusha Jayaraman and Christian J. Pike declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Anusha Jayaraman, 3715 McClintock Avenue, Davis School of Gerontology, University of Southern California, Los Angeles, CA 90089-0191 USA, ajayaram@usc.edu, (213) 740-8244.
Christian J. Pike, 3715 McClintock Avenue, Davis School of Gerontology, University of Southern California, Los Angeles, CA 90089-0191 USA, cjpike@usc.edu, (213) 740-4205.
References
- 1.Zitzmann M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat Rev Endocrinol. 2009;5:673–681. doi: 10.1038/nrendo.2009.212. [DOI] [PubMed] [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 3.Raffaitin C, Feart C, Le Goff M, et al. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76:518–525. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K, Haan M, Blackwell T, et al. Metabolic syndrome and cognitive decline in elderly Latinos: findings from the Sacramento Area Latino Study of Aging study. J Am Geriatr Soc. 2007;55:758–762. doi: 10.1111/j.1532-5415.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 5.McEvoy LK, Laughlin GA, Barrett-Connor E, et al. Metabolic syndrome and 16-year cognitive decline in community-dwelling older adults. Ann Epidemiol. 2012;22:310–317. doi: 10.1016/j.annepidem.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaffe K, Blackwell T, Whitmer RA, et al. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging. 2006;10:293–295. [PubMed] [Google Scholar]
- 7.Stolk RP, Breteler MM, Ott A, et al. Insulin and cognitive function in an elderly population. The Rotterdam Study. Diabetes Care. 1997;20:792–795. doi: 10.2337/diacare.20.5.792. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Beiser A, Elias MF, et al. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 9.Gunstad J, Lhotsky A, Wendell CR, et al. Longitudinal examination of obesity and cognitive function: results from the Baltimore longitudinal study of aging. Neuroepidemiology. 2010;34:222–229. doi: 10.1159/000297742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berg E, Biessels GJ, de Craen AJ, et al. The metabolic syndrome is associated with decelerated cognitive decline in the oldest old. Neurology. 2007;69:979–985. doi: 10.1212/01.wnl.0000271381.30143.75. [DOI] [PubMed] [Google Scholar]
- 11.Xia W, Wang S, Sun Z, et al. Altered baseline brain activity in type 2 diabetes: A restingstate fMRI study. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Moran C, Phan TG, Chen J, et al. Brain Atrophy in Type 2 Diabetes: Regional distribution and influence on cognition. Diabetes Care. 2013 doi: 10.2337/dc13-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manschot SM, Brands AM, van der Grond J, et al. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- 14.Luchsinger JA, Reitz C, Patel B, et al. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 15.Arvanitakis Z, Wilson RS, Li Y, et al. Diabetes and function in different cognitive systems in older individuals without dementia. Diabetes Care. 2006;29:560–565. doi: 10.2337/diacare.29.03.06.dc05-1901. [DOI] [PubMed] [Google Scholar]
- 16.van den Berg E, Reijmer YD, de Bresser J, et al. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia. 2010;53:58–65. doi: 10.1007/s00125-009-1571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reijmer YD, van den Berg E, de Bresser J, et al. Accelerated cognitive decline in patients with type 2 diabetes: MRI correlates and risk factors. Diabetes Metab Res Rev. 2011;27:195–202. doi: 10.1002/dmrr.1163. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan MD, Katon WJ, Lovato LC, et al. Association of Depression With Accelerated Cognitive Decline Among Patients With Type 2 Diabetes in the ACCORD-MIND Trial. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 20.Arvanitakis Z, Wilson RS, Bienias JL, et al. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 21.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 22.Hassing LB, Johansson B, Nilsson SE, et al. Diabetes mellitus is a risk factor for vascular dementia, but not for Alzheimer's disease: a population-based study of the oldest old. Int Psychogeriatr. 2002;14:239–248. doi: 10.1017/s104161020200844x. [DOI] [PubMed] [Google Scholar]
- 23.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 24.Whitmer RA, Gustafson DR, Barrett-Connor E, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 25.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 26.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer's disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 27.van Harten B, Oosterman JM, Potter van Loon BJ, et al. Brain lesions on MRI in elderly patients with type 2 diabetes mellitus. Eur Neurol. 2007;57:70–74. doi: 10.1159/000098054. [DOI] [PubMed] [Google Scholar]
- 28.Hsu JL, Chen YL, Leu JG, et al. Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. Neuroimage. 59:1098–1105. doi: 10.1016/j.neuroimage.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 29.Tiehuis AM, van der Graaf Y, Visseren FL, et al. Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke. 2008;39:1600–1603. doi: 10.1161/STROKEAHA.107.506089. [DOI] [PubMed] [Google Scholar]
- 30.Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56:1817–1824. doi: 10.2337/db07-0171. [DOI] [PubMed] [Google Scholar]
- 31.Jung HJ, Kim YJ, Eggert S, et al. Age-dependent increases in tau phosphorylation in the brains of type 2 diabetic rats correlate with a reduced expression of p62. Exp Neurol. 2013;248C:441–450. doi: 10.1016/j.expneurol.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Kim B, Backus C, Oh S, et al. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150:5294–52301. doi: 10.1210/en.2009-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planel E, Tatebayashi Y, Miyasaka T, et al. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635–13648. doi: 10.1523/JNEUROSCI.3949-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biessels GJ, Kamal A, Urban IJ, et al. Water maze learning and hippocampal synaptic plasticity in streptozotocin-diabetic rats: effects of insulin treatment. Brain Res. 1998;800:125–135. doi: 10.1016/s0006-8993(98)00510-1. [DOI] [PubMed] [Google Scholar]
- 35.Jolivalt CG, Lee CA, Beiswenger KK, et al. Defective insulin signaling pathway and increased glycogen synthase kinase-3 activity in the brain of diabetic mice: parallels with Alzheimer's disease and correction by insulin. J Neurosci Res. 2008;86:3265–3274. doi: 10.1002/jnr.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L, Teter B, Morihara T, et al. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer's disease intervention. J Neurosci. 2004;24:11120–11126. doi: 10.1523/JNEUROSCI.2860-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chesneau V, Vekrellis K, Rosner MR, Selkoe DJ. Purified recombinant insulindegrading enzyme degrades amyloid beta-protein but does not promote its oligomerization. Biochem J. 2000;351(Pt 2):509–516. [PMC free article] [PubMed] [Google Scholar]
- 38.Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta0amyloid precursor protein intacellular domain in vivo. Proc. Natl. Acad. Sci. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho L, Qin W, Pompl PN, et al. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. Faseb J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 40.Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- 41.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 42.Frolich L, Blum-Degen D, Bernstein HG, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J Neural Transm. 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- 43.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 44.Farris W, Mansourian S, Leissring MA, et al. Partial loss-of-function mutations in insulin-degrading enzyme that induce diabetes also impair degradation of amyloid beta-protein. Am J Pathol. 2004;164:1425–1434. doi: 10.1016/s0002-9440(10)63229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett RG, Hamel FG, Duckworth WC. An insulin-degrading enzyme inhibitor decreases amylin degradation, increases amylin-induced cytotoxicity, and increases amyloid formation in insulinoma cell cultures. Diabetes. 2003;52:2315–2320. doi: 10.2337/diabetes.52.9.2315. [DOI] [PubMed] [Google Scholar]
- 46.Shiiki T, Ohtsuki S, Kurihara A, et al. Brain insulin impairs amyloid-beta(1-40) clearance from the brain. J Neurosci. 2004;24:9632–9637. doi: 10.1523/JNEUROSCI.2236-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu WQ, Walsh DM, Ye Z, et al. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 48.Luchsinger JA, Palmas W, Teresi JA, et al. Improved diabetes control in the elderly delays global cognitive decline. J Nutr Health Aging. 2011;15:445–449. doi: 10.1007/s12603-011-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Zhou K, Wang R, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. This initial report from a clinical trial provides evidence that, even in the absence of diabetes, insulin-based therapy may provide cognitive benefits at early stages of AD
- 52.Searcy JL, Phelps JT, Pancani T, et al. Long-term pioglitazone treatment improves learning and attenuates pathological markers in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2012;30:943–961. doi: 10.3233/JAD-2012-111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long-Smith CM, Manning S, McClean PL, et al. The diabetes drug liraglutide ameliorates aberrant insulin receptor localisation and signalling in parallel with decreasing both amyloid-beta plaque and glial pathology in a mouse model of Alzheimer's disease. Neuromolecular Med. 2013;15:102–114. doi: 10.1007/s12017-012-8199-5. [DOI] [PubMed] [Google Scholar]
- 54.Eriksson A, Attvall S, Bonnier M, et al. Short-term effects of metformin in type 2 diabetes. Diabetes Obes Metab. 2007;9:330–336. doi: 10.1111/j.1463-1326.2006.00611.x. [DOI] [PubMed] [Google Scholar]
- 55.Wang M, Tong JH, Zhu G, et al. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophr Res. 2012;138:54–57. doi: 10.1016/j.schres.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 56.Kalariya NM, Shoeb M, Ansari NH, et al. Antidiabetic drug metformin suppresses endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2012;53:3431–3440. doi: 10.1167/iovs.12-9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barron AM, Rosario ER, Elteriefi R, Pike CJ. Sex-specific effects of high fat diet on indices of metabolic syndrome in 3xTg-AD mice: implications for Alzheimer's disease. PLoS One. 2013;8:e78554. doi: 10.1371/journal.pone.0078554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murakami K, Yokoyama S, Murata N, et al. Insulin receptor mutation results in insulin resistance and hyperinsulinemia but does not exacerbate Alzheimer's-like phenotypes in mice. Biochem Biophys Res Commun. 409:34–39. doi: 10.1016/j.bbrc.2011.04.101. [DOI] [PubMed] [Google Scholar]
- 59.Freude S, Hettich MM, Schumann C, et al. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer's disease. Faseb J. 2009;23:3315–3324. doi: 10.1096/fj.09-132043. [DOI] [PubMed] [Google Scholar]
- 60.Killick R, Scales G, Leroy K, et al. Deletion of Irs2 reduces amyloid deposition and rescues behavioural deficits in APP transgenic mice. Biochem Biophys Res Commun. 2009;386:257–262. doi: 10.1016/j.bbrc.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 63.Deans KA, Sattar N, et al. "Anti-inflammatory" drugs and their effects on type 2 diabetes. Diabetes Technol Ther. 2006;8:18–27. doi: 10.1089/dia.2006.8.18. [DOI] [PubMed] [Google Scholar]
- 64.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 65.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, Dong F, Ren J, et al. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 67. Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. This study demonstrated that high fat diet rapidly induced markers of both neural inflammation and injury in mice, rats, and humans which provides compelling evidence of the damaging effects of obesity on the brain.
- 68.Viscogliosi G, Marigliano V. Alzheimer's disease: how far have we progressed? Lessons learned from diabetes mellitus, metabolic syndrome, and inflammation. J Am Geriatr Soc. 2013;61:845–846. doi: 10.1111/jgs.12242. [DOI] [PubMed] [Google Scholar]
- 69.Griffin WS, Stanley LC, Ling C, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7315. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motta M, Imbesi R, Di Rosa M, et al. Altered plasma cytokine levels in Alzheimer's disease: correlation with the disease progression. Immunol Lett. 2007;114:46–51. doi: 10.1016/j.imlet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 71.Matsuoka Y, Picciano M, Malester B, et al. Inflammatory responses to amyloidosis in a transgenic mouse model of Alzheimer's disease. Am J Pathol. 2001;158:1345–1354. doi: 10.1016/S0002-9440(10)64085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benzing WC, Wujek JR, Ward EK, et al. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 73.Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cote S, Carmichael PH, Verreault R, et al. Nonsteroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2012;8:219–226. doi: 10.1016/j.jalz.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Rodrigue KM. Contribution of cerebrovascular health to the diagnosis of Alzheimer disease. JAMA Neurol. 2013;70:438–439. doi: 10.1001/jamaneurol.2013.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tamaki C, Ohtsuki S, Terasaki T. Insulin facilitates the hepatic clearance of plasma amyloid beta-peptide (1 40) by intracellular translocation of low-density lipoprotein receptor-related protein 1 (LRP-1) to the plasma membrane in hepatocytes. Mol Pharmacol. 2007;72:850–855. doi: 10.1124/mol.107.036913. [DOI] [PubMed] [Google Scholar]
- 78.Deane R, Wu Z, Sagare A, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 79.Terrand J, Bruban V, Zhou L, et al. LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J Biol Chem. 2009;284:381–388. doi: 10.1074/jbc.M806538200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu W, Singh R, Choi CS, et al. Low density lipoprotein (LDL) receptor-related protein 6 (LRP6) regulates body fat and glucose homeostasis by modulating nutrient sensing pathways and mitochondrial energy expenditure. J Biol Chem. 2012;287:7213–7223. doi: 10.1074/jbc.M111.286724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xi YD, Li XY, Ding J, et al. Soy isoflavone alleviates Abeta1-42-induced impairment of learning and memory ability through the regulation of RAGE/LRP-1 in neuronal and vascular tissue. Curr Neurovasc Res. 2013;10:144–156. doi: 10.2174/1567202611310020007. [DOI] [PubMed] [Google Scholar]
- 82.Basta G, Schmidt AM, De Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovasc Res. 2004;63:582–592. doi: 10.1016/j.cardiores.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 84.Yan SD, Zhu H, Zhu A, et al. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat Med. 2000;6:643–651. doi: 10.1038/76216. [DOI] [PubMed] [Google Scholar]
- 85.Yan SD, Chen X, Fu J, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 86.Lue LF, Walker DG, Brachova L, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer's disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- 87.Deane R, Du Yan S, Submamaryan RK, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 88.Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1:1–15. doi: 10.1016/s0047-6374(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt AM, Stern DM. Receptor for age (RAGE) is a gene within the major histocompatibility class III region: implications for host response mechanisms in homeostasis and chronic disease. Front Biosci. 2001;6:D1151–D1160. doi: 10.2741/schmidt. [DOI] [PubMed] [Google Scholar]
- 90.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 91.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 92.Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Irie F, Fitzpatrick AL, Lopez OL, et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the Cardiovascular Health Study Cognition Study. Arch Neurol. 2008;65:89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 95.Hanson AJ, Bayer-Carter JL, Green PS, et al. Effect of apolipoprotein e genotype and diet on apolipoprotein e lipidation and amyloid peptides: randomized clinical trial. JAMA Neurol. 2013;70:972–980. doi: 10.1001/jamaneurol.2013.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cook DG, Leverenz JB, McMillan PJ, et al. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer's disease is associated with the apolipoprotein E-epsilon4 allele. Am J Pathol. 2003;162:313–319. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Craft S, Peskind E, Schwartz MW, et al. Cerebrospinal fluid and plasma insulin levels in Alzheimer's disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 98.Craft S, Asthana S, Schellenberg G, et al. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer's disease differ according to apolipoprotein-E genotype. Ann N Y Acad Sci. 2000;903:222–228. doi: 10.1111/j.1749-6632.2000.tb06371.x. [DOI] [PubMed] [Google Scholar]
- 99.Reger MA, Watson GS, Green PS, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Morley JE. Andropause, testosterone therapy, and quality of life in aging men. Cleve Clin J Med. 2000;67:880–882. doi: 10.3949/ccjm.67.12.880. [DOI] [PubMed] [Google Scholar]
- 101.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hogervorst E, Williams J, Budge M, et al. Serum total testosterone is lower in men with Alzheimer's disease. Neuro Endocrinol Lett. 2001;22:163–168. [PubMed] [Google Scholar]
- 103.Rasmuson S, Nasman B, Carlstrom K, Olsson T. Increased levels of adrenocortical and gonadal hormones in mild to moderate Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;13:74–79. doi: 10.1159/000048637. [DOI] [PubMed] [Google Scholar]
- 104.Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. Jama. 2004;292:1431–1432. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- 105.Rosario ER, Chang L, Head EH, et al. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer's disease. Neurobiol Aging. 2011;32:604–613. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moffat SD, Zonderman AB, Metter EJ, et al. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- 107.Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834–1840. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 108.Kapoor D, Aldred H, Clark S, et al. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30:911–917. doi: 10.2337/dc06-1426. [DOI] [PubMed] [Google Scholar]
- 109.Hougaku H, Fleg JL, Najjar SS, et al. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. Am J Physiol Endocrinol Metab. 2006;290:E234–E242. doi: 10.1152/ajpendo.00059.2005. [DOI] [PubMed] [Google Scholar]
- 110.Goncharov NP, Katsya GV, Chagina NA, Gooren LJ. Three definitions of metabolic syndrome applied to a sample of young obese men and their relation with plasma testosterone. Aging Male. 2008;11:118–122. doi: 10.1080/13685530802204629. [DOI] [PubMed] [Google Scholar]
- 111.Kapoor D, Clarke S, Stanworth R, et al. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156:595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- 112.Bhasin S, Parker RA, Sattler F, et al. Effects of testosterone supplementation on whole body and regional fat mass and distribution in human immunodeficiency virus-infected men with abdominal obesity. J Clin Endocrinol Metab. 2007;92:1049–1057. doi: 10.1210/jc.2006-2060. [DOI] [PubMed] [Google Scholar]
- 113.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154:899–906. doi: 10.1530/eje.1.02166. [DOI] [PubMed] [Google Scholar]
- 114.Lage MJ, Barber BL, Markus RA. Association between androgen-deprivation therapy and incidence of diabetes among males with prostate cancer. Urology. 2007;70:1104–1108. doi: 10.1016/j.urology.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 115.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 116.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 117.Haidar A, Yassin A, Saad F, Shabsigh R. Effects of androgen deprivation on glycaemic control and on cardiovascular biochemical risk factors in men with advanced prostate cancer with diabetes. Aging Male. 2007;10:189–196. doi: 10.1080/13685530701653538. [DOI] [PubMed] [Google Scholar]
- 118.Hogervorst E, Lehmann DJ, Warden DR, et al. Apolipoprotein E epsilon4 and testosterone interact in the risk of Alzheimer's disease in men. Int J Geriatr Psychiatry. 2002;17:938–940. doi: 10.1002/gps.714. [DOI] [PubMed] [Google Scholar]
- 119.Raber J, Bongers G, LeFevour A, et al. Androgens protect against apolipoprotein E4- induced cognitive deficits. J Neurosci. 2002;22:5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pfankuch T, Rizk A, Olsen R, et al. Role of circulating androgen levels in effects of apoE4 on cognitive function. Brain Res. 2005;1053:88–96. doi: 10.1016/j.brainres.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 121.Rizk-Jackson A, Robertson J, Raber J. Tfm-AR modulates the effects of ApoE4 on cognition. J Neurochem. 2008;105:63–67. doi: 10.1111/j.1471-4159.2007.05092.x. [DOI] [PubMed] [Google Scholar]