Abstract
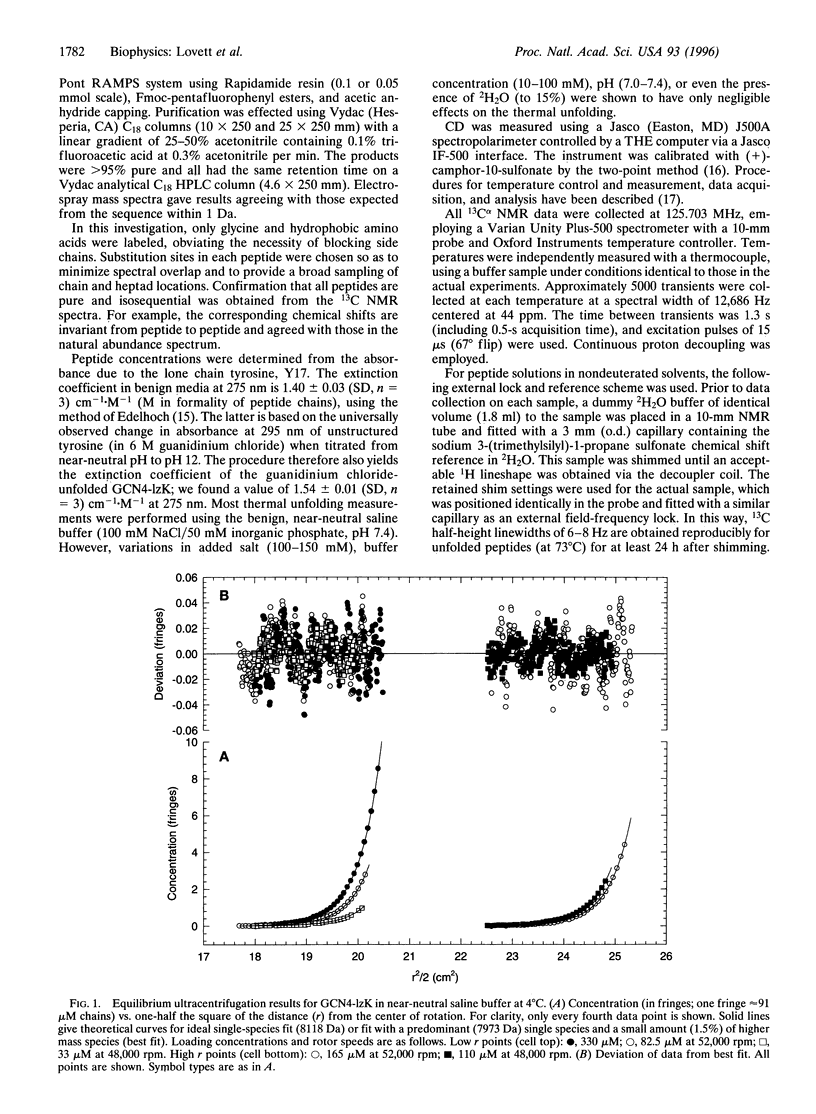
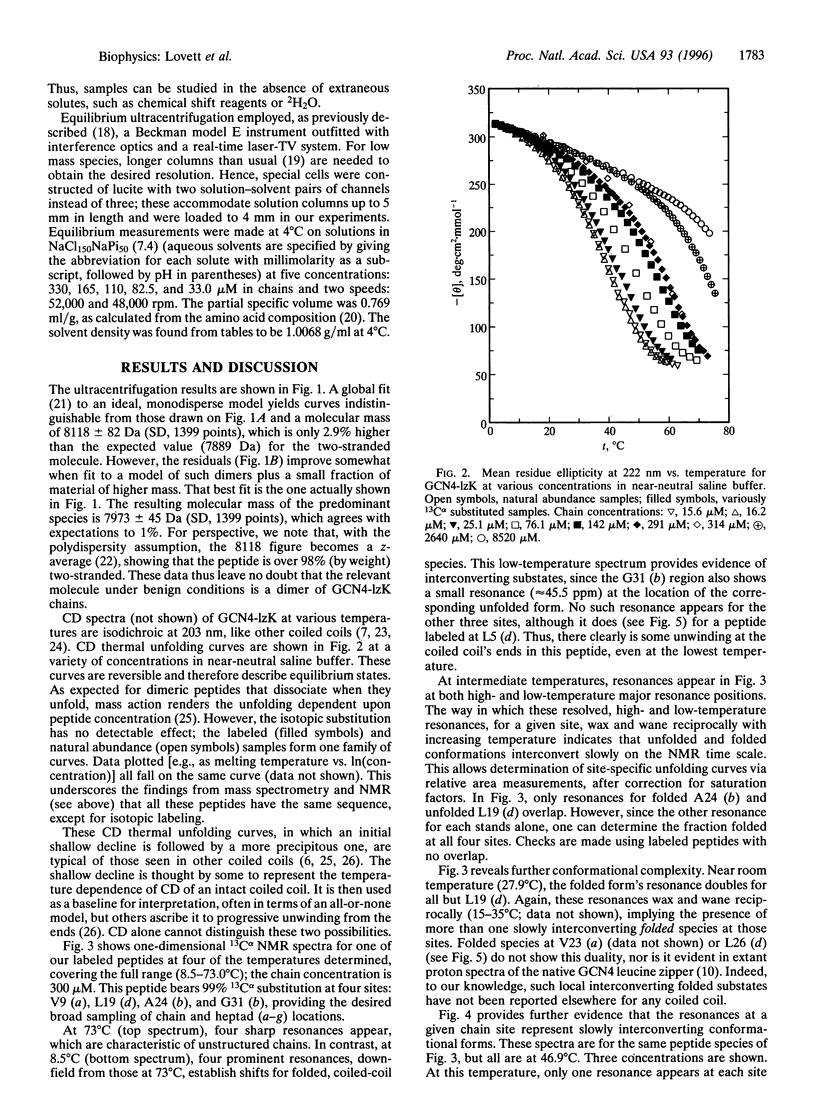
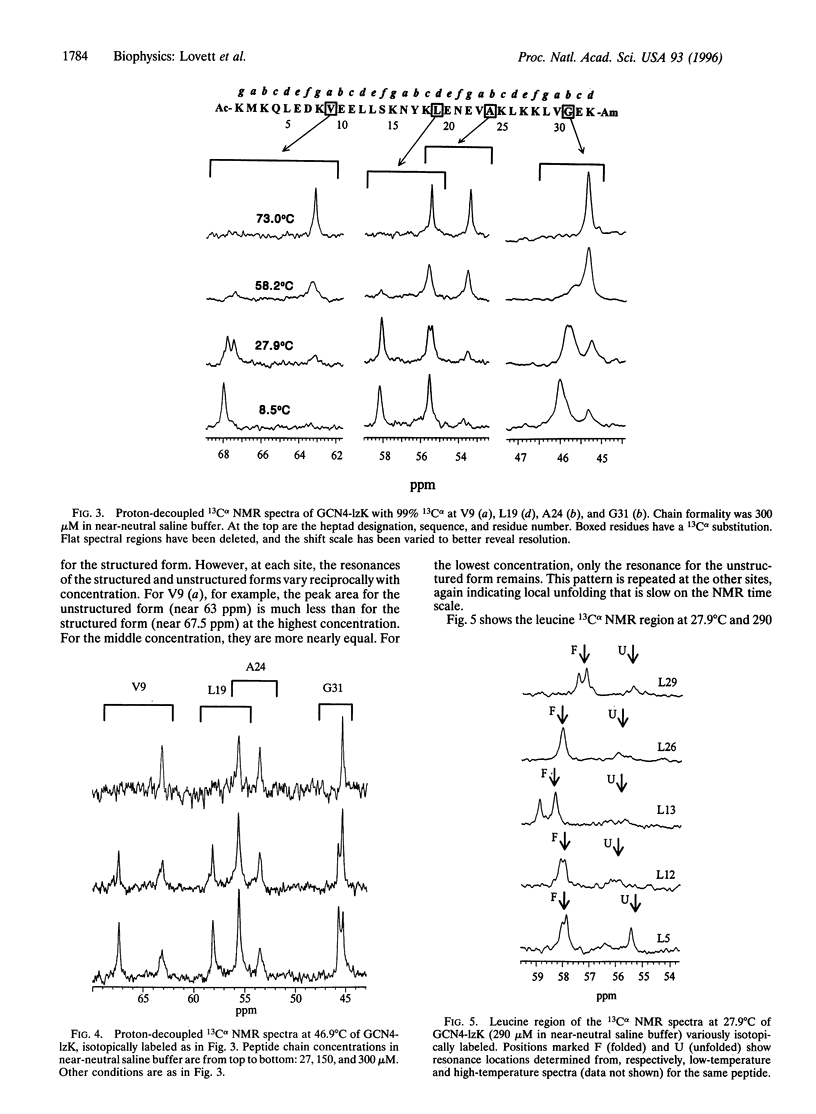
Synthesis of a 33-residue, capped leucine zipper analogous to that in GCN4 is reported. Histidine and arginine residues are mutated to lysine to reduce the unfolding temperature. CD and ultracentrifugation studies indicate that the molecule is a two-stranded coiled coil under benign conditions. Versions of the same peptide are made with 99% 13Calpha at selected sites. One-dimensional 13C NMR spectra are assigned by inspection and used to study thermal unfolding equilibria over the entire transition from 8 to 73 degrees C. Spectra at the temperature extremes establish the approximate chemical shifts for folded and unfolded forms at each labeled site. Resonances for each amino acid appear at both locations at intermediate T, indicating that folded and unfolded forms interconvert slowly (> >2 ms) on the NMR time scale. Moreover, near room temperature, the structured form's resonance is double at several, but not all, sites, indicating at least two slowly interconverting, structured, local conformational substates. Analysis of the dynamics is possible. For example, near room temperature at the Val-9, Ala-24, and Gly-31 positions, the equilibrium constant for interconversion of the two structured forms is near unity and the time scale is > or= 10-20 ms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansevin A. T., Roark D. E., Yphantis D. A. Improved ultracentrifuge cells for high-speed sedimentation equilibrium studies with interference optics. Anal Biochem. 1970 Mar;34:237–261. doi: 10.1016/0003-2697(70)90103-x. [DOI] [PubMed] [Google Scholar]
- Cohen C., Parry D. A. Alpha-helical coiled coils and bundles: how to design an alpha-helical protein. Proteins. 1990;7(1):1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Goodman E. M., Kim P. S. Periodicity of amide proton exchange rates in a coiled-coil leucine zipper peptide. Biochemistry. 1991 Dec 17;30(50):11615–11620. doi: 10.1021/bi00114a002. [DOI] [PubMed] [Google Scholar]
- Holtzer M. E., Crimmins D. L., Holtzer A. Structural stability of short subsequences of the tropomyosin chain. Biopolymers. 1995 Jan;35(1):125–136. doi: 10.1002/bip.360350113. [DOI] [PubMed] [Google Scholar]
- Holtzer M. E., Holtzer A. Alpha-helix to random coil transitions: determination of peptide concentration from the CD at the isodichroic point. Biopolymers. 1992 Dec;32(12):1675–1677. doi: 10.1002/bip.360321209. [DOI] [PubMed] [Google Scholar]
- Holtzer M. E., Holtzer A. Alpha-helix to random coil transitions: interpretation of the CD in the region of linear temperature dependence. Biopolymers. 1992 Nov;32(11):1589–1591. doi: 10.1002/bip.360321116. [DOI] [PubMed] [Google Scholar]
- Johnson M. L., Correia J. J., Yphantis D. A., Halvorson H. R. Analysis of data from the analytical ultracentrifuge by nonlinear least-squares techniques. Biophys J. 1981 Dec;36(3):575–588. doi: 10.1016/S0006-3495(81)84753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S. S., Stafford W. F., 3rd Preferential assembly of the tropomyosin heterodimer: equilibrium studies. Biochemistry. 1991 Jun 11;30(23):5682–5688. doi: 10.1021/bi00237a007. [DOI] [PubMed] [Google Scholar]
- Long C. G., Braswell E., Zhu D., Apigo J., Baum J., Brodsky B. Characterization of collagen-like peptides containing interruptions in the repeating Gly-X-Y sequence. Biochemistry. 1993 Nov 2;32(43):11688–11695. doi: 10.1021/bi00094a027. [DOI] [PubMed] [Google Scholar]
- O'Shea E. K., Klemm J. D., Kim P. S., Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991 Oct 25;254(5031):539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- O'Shea E. K., Rutkowski R., Kim P. S. Evidence that the leucine zipper is a coiled coil. Science. 1989 Jan 27;243(4890):538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- Oas T. G., McIntosh L. P., O'Shea E. K., Dahlquist F. W., Kim P. S. Secondary structure of a leucine zipper determined by nuclear magnetic resonance spectroscopy. Biochemistry. 1990 Mar 27;29(12):2891–2894. doi: 10.1021/bi00464a001. [DOI] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins. Proteins which do not present a single cooperative system. Adv Protein Chem. 1982;35:1–104. [PubMed] [Google Scholar]
- Thompson K. S., Vinson C. R., Freire E. Thermodynamic characterization of the structural stability of the coiled-coil region of the bZIP transcription factor GCN4. Biochemistry. 1993 Jun 1;32(21):5491–5496. doi: 10.1021/bi00072a001. [DOI] [PubMed] [Google Scholar]
- Wendt H., Berger C., Baici A., Thomas R. M., Bosshard H. R. Kinetics of folding of leucine zipper domains. Biochemistry. 1995 Mar 28;34(12):4097–4107. doi: 10.1021/bi00012a028. [DOI] [PubMed] [Google Scholar]
- Wishart D. S., Sykes B. D., Richards F. M. Relationship between nuclear magnetic resonance chemical shift and protein secondary structure. J Mol Biol. 1991 Nov 20;222(2):311–333. doi: 10.1016/0022-2836(91)90214-q. [DOI] [PubMed] [Google Scholar]
- Wrabl J., Holtzer M. E., Holtzer A. Thermal unfolding equilibria in homodimeric chicken gizzard tropomyosin coiled coils. Biopolymers. 1994 Dec;34(12):1659–1667. doi: 10.1002/bip.360341210. [DOI] [PubMed] [Google Scholar]


