Abstract
Although 2 earlier studies reported that aromatic amino acid (AAA) supplementation of children with severe acute malnutrition (SAM) improved whole-body protein anabolism during the early postadmission (maintenance) phase of rehabilitation, it is not known whether this positive effect was maintained during the catch-up growth and recovery phases of treatment. This study aimed to determine whether supplementation with an AAA cocktail (330 mg · kg−1 · d−1) vs. isonitrogenous Ala would improve measures of protein kinetics in 22 children, aged 4–31 mo, during the catch-up growth and recovery phases of treatment for SAM. Protein kinetics were assessed by measuring leucine, phenylalanine, and urea kinetics with the use of standard stable isotope tracer methods in the fed state. Supplementation started at the end of the maintenance period when the children were clinically/metabolically stable and continued up to full nutritional recovery. Three experiments were performed: at the end of maintenance (at ∼13 d postadmission), at mid–catch-up growth (at ∼23 d post- admission when the children had replenished 50% of their weight deficit), and at recovery (at ∼48 d postadmission when they had achieved at least 90% weight for length). Children in the AAA group had significantly faster protein synthesis compared with those in the Ala group at mid–catch-up growth (101 ± 10 vs. 72 ± 7 μmol phenylalanine · kg−1 · h−1; P < 0.05) and better protein balance at mid–catch-up growth (49 ± 5 vs. 30 ± 2 μmol phenylalanine · kg−1 · h−1; P < 0.05) and at recovery (37 ± 8 vs. 11 ± 3 μmol phenylalanine · kg−1 · h−1; P < 0.05). We conclude that dietary supplementation with AAA accelerates net protein synthesis in children during nutritional rehabilitation for SAM.
Introduction
During the early maintenance phase of treatment of a child with severe acute childhood malnutrition (SAM)7 and infection, dietary protein is provided at a rate that is just sufficient to maintain body protein homeostasis, including the increased synthesis of acute phase proteins, without exceeding the child’s capacity to metabolize exogenous protein. After this phase has ended, marked by a return of appetite, the child consumes a high-energy and -protein diet ad libitum to achieve rapid catch-up growth (CUG) (1). However, the need to replenish body proteins and to synthesize aromatic amino acid (AAA)–rich positive and negative acute phase proteins at a high rate during the rapid CUG phase of treatment increases the demand for AAAs (2).
In an earlier study of the effect of AAA supplementation on protein kinetics in children with edematous SAM and infection, Manary et al. (3) reported that urea production was significantly reduced after 24 h of feeding a diet of egg white plus tryptophan, which supplied 142 mg AAA · kg−1 · d−1, compared with milk, which supplied 134 mg · kg−1 · d−1. This finding suggested that the AAA-rich dietary egg white protein was better utilized for protein synthesis than milk protein. However, measurements of whole-body protein turnover failed to show any difference between rates of protein synthesis (and breakdown) between the 2 diets. In a similar study, we tested the effect of AAA supplementation on whole-body protein synthesis in children who were being treated for SAM with concurrent infections by supplementing their early maintenance diet with either 160 mg · kg−1 · d−1 AAAs or an isonitrogenous amount of Ala (4). This amount of AAA was based on our thesis (2) that during acute infections, ∼2 g of body protein must be degraded to provide enough AAA to synthesize 1 g of mixed acute phase proteins and from the observed increase in rates of synthesis of 5 major acute phase proteins in infected malnourished children (5). After 12 d of supplementation during the early maintenance phase of treatment, we reported an increase in both whole-body protein breakdown and synthesis with a significant improvement in net whole-body protein synthesis when protein kinetics were measured with a 13C-leucine tracer model but not with a 2H5-phenylalanine-2H2-tyrosine tracer model (4). Because of the relatively short supplementation period, 12 d postadmission, it was not possible to determine whether the positive effect of AAA supplementation on leucine kinetics and balance was maintained throughout the CUG phase of treatment thereby shortening the time taken to achieve full nutritional recovery, that is when the children had achieved at least 90% weight for length. To answer this question, the present study was conducted in children being treated for SAM. They were randomly assigned to either dietary AAA or isonitrogenous Ala supplementation starting immediately after the early maintenance phase ended and continuing through the rapid CUG phase until they had achieved full nutritional recovery. The primary hypothesis tested is that AAA supplementation will elicit a faster rate of whole-body protein synthesis, thereby accelerating the rate of replenishment of body tissues and shortening the time to achieve nutritional recovery.
Participants and Methods
Participants.
Twenty-two children, 7 girls and 15 boys, who were admitted for treatment of SAM to the metabolic ward of the Tropical Metabolism Research Unit of the University of the West Indies, Kingston, Jamaica, participated in the study. During hospitalization, the children were managed according to a standard treatment protocol as previously described (1). As shown in Table 1, each child had a deficit in body weight for age of >20%, indicating severe undernutrition. The type of SAM, i.e., undernourished, marasmus, kwashiorkor, or marasmic kwashiorkor, was diagnosed on the basis of the Wellcome classification (6) as shown in Table 1. This study was approved by the Medical Ethics Committee of the University Hospital of the West Indies and the Baylor Affiliates Review Board for Human Subject Research of the Baylor College of Medicine. Written informed consent was obtained from at least 1 parent of each child enrolled.
TABLE 1.
Physical and clinical characteristics of the children at admission1
| Clinical characteristics | Ala group (n = 11) | AAA group (n = 11) |
| Age, mo | 14.8 ± 2.3 | 12.6 ± 2.3 |
| Weight, kg | 6.1 ± 0.5 | 6.0 ± 0.5 |
| Length, cm | 67.5 ± 2.5 | 66.8 ± 2.8 |
| Weight-for-age,2 % of expected | 59.1 ± 3.3 | 60.9 ± 2.6 |
| Weight-for-length,2 % of expected | 79.3 ± 2.6 | 80.7 ± 1.9 |
| Length-for-age,2 % of expected | 87.0 ± 2.0 | 88.9 ± 1.4 |
| Diagnosis, n | ||
| Undernourished | 1 | 3 |
| Marasmus | 4 | 2 |
| Kwashiorkor | 4 | 3 |
| Marasmic kwashiorkor | 2 | 3 |
| Gender, n | ||
| M | 8 | 7 |
| F | 3 | 4 |
| Number of infections, n | ||
| 3 | 1 | 2 |
| 2 | 2 | 6 |
| 1 | 7 | 3 |
| 0 | 1 | 0 |
| Hemoglobin, g/dL | 8.6 ± 0.4 | 8.8 ± 0.3 |
| Albumin, g/L | 32.2 ± 2.3 | 29.8 ± 2.0 |
Values are means ± SEMs unless otherwise indicated. Ala is the control group. AAA, aromatic amino acid.
With the use of the WHO growth charts, weight-for-age is calculated by measured weight over expected weight for age × 100; weight-for-length is calculated by measured weight over expected weight for length × 100; length-for-age is calculated by measured length over expected length for age × 100.
Treatment diets.
As previously described by us (1, 7, 8), the children were fed a milk-based maintenance diet that aimed to provide ∼100 kcal · kg−1 · d−1 and ∼1.2 g · kg−1 · d−1 of protein during the early resuscitative and maintenance (clinical/metabolic stabilization) phases of treatment. An energy-dense milk-based diet that provided ∼132 kcal · kg−1 · d−1 and ∼3.1 g · kg−1 · d−1 of protein was fed during the rapid CUG phase (Table 2). The amount offered was gradually increased on the basis of appetite. In addition, both diets were supplemented with vitamins (Tropivite; Federated Pharmaceuticals) and a mineral mix prepared in the Tropical Metabolism Research Unit’s metabolic kitchen. Each child was administered 2 mL/d of the vitamin solution, which contained 6000 IU vitamin A (palmitate), 1600 IU vitamin D (calciferol), 2 mg thiamin HCl, 3.2 mg riboflavin, 120 mg vitamin C (ascorbic acid), 4 mg vitamin B-6, and 28 mg nicotinamide. They also were administered 5 mg folic acid and 2 mL of a mineral mix per kilogram daily. The mineral mix consisted of 37.28 g KCl + 50.84 g MgCl2 · 6H2O + 3.36 g (CH3COO)2Zn · 2H2O/L H2O (BDH Chemicals). During the rapid CUG phase but not in the maintenance phase, the children also were administered 60 mg FeSO4. Over each 24-h period, the children were fed every 3 h during the maintenance phase of treatment followed by 4-h feedings during CUG.
TABLE 2.
Dietary energy, protein, phenylalanine, tyrosine, and leucine intakes in children with severe malnutrition at different stages of nutritional rehabilitation1
|
P |
|||||||||
| EOM |
Mid-CUG |
REC |
Main effect |
||||||
| Ala group | AAA group | Ala group | AAA group | Ala group | AAA group | Clinical phase | Supplementation | Interaction | |
| Energy, kcal · kg−1 · d−1 | 99 ± 3 | 101 ± 1 | 159 ± 7 | 188 ± 6 | 127 ± 8 | 149 ± 14 | <0.0001 | 0.005 | 0.22 |
| Protein, g · kg−1 · d−1 | 1.20 ± 0.04 | 1.23 ± 0.01 | 3.64 ± 0.21 | 4.12 ± 0.13 | 2.88 ± 0.21 | 3.30 ± 0.33 | <0.0001 | 0.035 | 0.37 |
| Total exogenous amino acid intake,2 μmol · kg−1 · h−1 | |||||||||
| Leucine | 48.3 ± 1.4 | 49.3 ± 0.4 | 120 ± 6.6 | 137 ± 4.0 | 98.5 ± 7.0 | 112 ± 9.8 | <0.0001 | 0.037 | 0.39 |
| Phenylalanine | 18.0 ± 0.4 | 18.3 ± 0.7 | 41.9 ± 2.33 | 81.2 ± 1.73,4 | 34.6 ± 2.43 | 74.2 ± 3.13,4 | <0.0001 | <0.0001 | <0.0001 |
| Tyrosine | 14.9 ± 0.5 | 15.3 ± 0.1 | 40.6 ± 2.43 | 76.1 ± 1.53,4 | 30.6 ± 2.43 | 67.2 ± 3.433,4 | <0.0001 | <0.0001 | <0.0001 |
All values are means ± SEMs, n = 11. Means were compared by using repeated-measures 2-factor ANOVA. AAA, aromatic amino acid; EOM, end of maintenance (∼13 d postadmission); mid-CUG, mid–catch-up growth (∼23 d postadmission when the children had replaced 50% of their weight deficit); REC, recovery (∼48 d postadmission when the children had recovered).
Sum of dietary amino acid, AAA supplement, intragastric, and intravenous tracers.
Significantly different from corresponding EOM phase, P < 0.05.
Significantly different from Ala supplementation group in the same clinical phase, P < 0.05.
Study design.
Whole-body and splanchnic phenylalanine, tyrosine, and leucine kinetics were determined by the simultaneous infusion of 2 different stable isotopes of each tracer amino acid at 3 different times during hospitalization, as follows:
1. As a baseline experiment at the end of maintenance (EOM) phase, ∼13 d after admission. At this time the children were still severely malnourished, but they had been treated for infection and were clinically stable as indicated by blood pressure, pulse, and respiration rates; their affect and appetite had improved, and most had lost their edema.
2. At the mid-CUG phase, ∼23 d after admission when the participants were gaining weight rapidly and had replenished 50% of their weight deficit.
3. At nutritional recovery when they had achieved at least 90% weight for length and their rate of weight gain had reached a plateau over 3 consecutive days.
Enrolled participants were randomly assigned to be administered either an AAA cocktail (AAA group), providing 140 mg · kg−1 · d−1 l-phenylalanine, 130 mg · kg−1 · d−1 l-tyrosine (given as 160 mg · kg−1 · d−1 N-acetyl-l-tyrosine), and 60 mg · kg−1 · d−1 l-tryptophan or an isonitrogenous amount, 192 mg · kg−1 · d−1, of l-alanine (Ala group). This AAA supplement was chosen to double the amount consumed at mid-CUG, that is, ∼3.5 g · kg−1 · d−1 of milk protein. The amino acid supplementation started after the EOM experiment and continued until the recovery experiment. During the 2 postsupplementation experiments, the participants were given 50% of total daily AAAs or Ala through a nasogastric tube over a 12-h period.
Isotope infusion protocol.
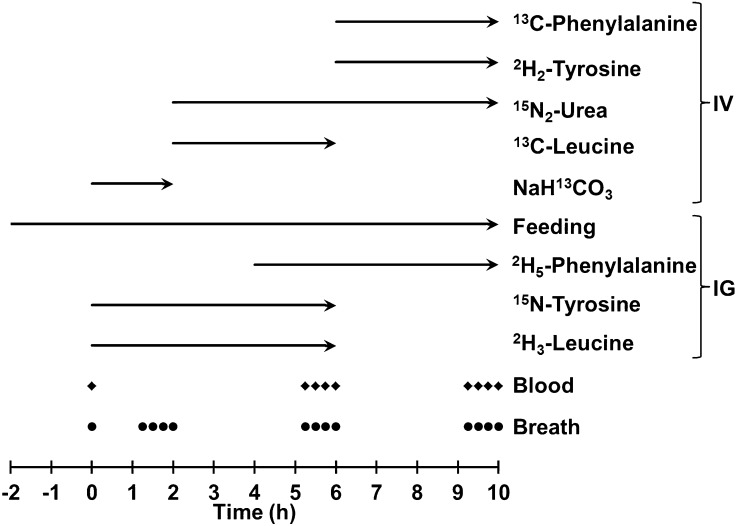
A diagram of the infusion protocol is shown in Figure 1. Sterile solutions of l-13C-phenyalalnine, l-2H5-phenylalanine, l-15N-tyrosine, l-2H2-tyrosine, 15N2-urea, l-13C-leucine, l-2H3-leucine, and 13C-sodium bicarbonate (NaH13CO3) (98–99%; Cambridge Isotope Laboratories) were prepared in 9 g/L NaCl. Approximately 50% of the child’s intake, calculated from the previous day’s intake, was then given over the next 12 h by continuous intragastric infusion.
FIGURE 1.
Schematic diagram of the isotope infusion protocol. IG, intragastric; IV, intravenous.
After 2 h of continuous feeding, a 4-mL blood sample was drawn and baseline breath samples were collected. A priming dose of NaH13CO3 (6 μmol/kg) was administered i.v., followed immediately by continuous infusion of the NaH13CO3 solution at 6 μmol · kg−1 · h−1 for 2 h. Simultaneously, a priming dose of 15N-tyrosine (1 μmol/kg) and 2H3-leucine (6 μmol/kg) were administered i.g. followed immediately by continuous infusion of 15N-tyrosine (1 μmol · kg−1 · h−1) and 2H3-leucine (6 μmol · kg−1 · h−1) for 6 h. After 2 h, the NaH13CO3 infusion was discontinued and primed-continuous i.v. infusions of 15N2-urea (prime = 6 μmol/kg, infusion rate = 6 μmol · kg−1 · h−1) and 13C-leucine (prime = 6 μmol/kg, infusion rate = 6 μmol · kg−1 · h−1) were started and maintained for 8 and 4 h, respectively. After 4 h of starting the infusion protocol, a priming dose of 2H5-phenylalanine (3 μmol/kg) was administered i.g. followed immediately by continuous infusion of 2H5-phenylalanine at a rate of 3 μmol · kg−1 · h−1 for 6 h. At the end of the 13C-leucine infusion, primed-continuous i.v. infusions of 13C-phenylalanine (prime = 3 μmol/kg, infusion rate = 3 μmol · kg−1 · h−1) and 2H2-tyrosine (prime = 1 μmol/kg, infusion rate = 1 μmol · kg−1 · h−1) were started and maintained for 4 h. During the infusions, additional 1-mL blood and breath samples were taken every 15 min as shown in Fig. 1.
Sample analyses.
The blood samples were centrifuged immediately at 1000 × g for 15 min at 4°C, and the plasma was removed and stored immediately at −70°C for later analyses. Plasma amino acids were isolated from 0.2 mL plasma by ion-exchange (Dowex 200×) chromatography and were converted to the n-propyl ester, heptafluorobutyramide derivative as described previously (4). The tracer:tracee ratios of plasma phenylalanine, tyrosine, and leucine were determined by negative chemical ionization GC/MS analysis by selectively monitoring ions at m/z ratios of 383 to 388 (phenylalanine), 595 to 597 (tyrosine), and 349 to 352 (leucine). The plasma α-ketoisocaproic acid tracer:tracee ratio was measured by negative chemical ionization GC/MS of its pentafluorobenzyl derivative monitoring ions at m/z 129–132 (9). The tracer:tracee ratio of plasma urea was determined by electron ionization GC/MS of its 2-pyrimidinol N-tert-butyldimethylsilyl derivative monitoring ions at m/z 153–155 (10). The breath samples were analyzed for 13C abundance in carbon dioxide by gas isotope-ratio MS, monitoring ions at m/z ratios 44 and 45.
Calculations.
Carbon dioxide production (RaCO2) was calculated from the steady state equation
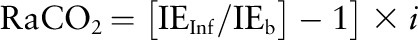 |
where IEInf and IEb are the isotopic enrichments (atom% excess) of bicarbonate in the infusate and carbon dioxide in expired breath at isotopic steady state and i is the rate of infusion of the tracer in μmol · kg−1 · h−1.
The percentage of enteral phenylalanine (or leucine or tyrosine) extracted by the splanchnic tissues was obtained as previously described by us (11):
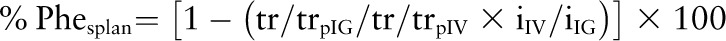 |
where tr/trpIG and tr/trpIV are the plateau tracer:tracee ratios of the i.g. and i.v. tracers and iIG and iIV are the rates of infusion of the i.g. and i.v. tracers. Splanchnic utilization (Phesplan) was obtained as the product of %Phesplan and enteral phenylalanine intake. Total phenylalanine (or leucine or tyrosine or urea) flux was calculated by using its plasma plateau tracer:tracee ratio in the following steady state equation:
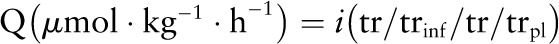 |
where tr/trinf is the tracer:tracee ratio of the infusate and tr/trpl is the plateau tracer:tracee ratio in plasma. Leucine flux was calculated by using the plateau enrichment of α-ketoisocaproic acid derived from 13C-leucine tracer.
Phenylalanine and leucine oxidation (O) was calculated as
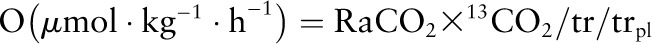 |
Because 2H5-phenylalanine was administered i.g. and 13C-phenylalanine was administered simultaneously with 15N-tyrosine, conversion of phenylalanine (via hydroxylation) to tyrosine, another index of phenylalanine oxidation, was not calculated in the present study. Phenylalanine or leucine used for protein synthesis (nonoxidative disposal) (S) was calculated as flux minus oxidation:
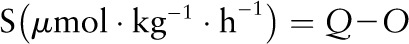 |
Phenylalanine or leucine balance (AABal) was calculated as the difference between intake (I) from the diet and tracer infusions and oxidation:
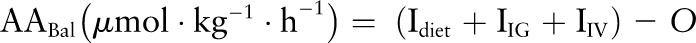 |
To account for the contribution of edema fluid to body weight in the clinical phase 1 experiment, body weights obtained after the loss of edema (i.e., the lowest weight observed from admission to EOM) were used in all calculations.
Statistical analyses.
Data are expressed as means ± SEMs. Differences in the physical and clinical characteristics of the 2 groups at each stage were determined by nonpaired 2-tailed t test. The kinetic data were analyzed by using 2-factor repeated-measures ANOVA with supplementation as the between factor and clinical phase as the repeated factor. If the repeated-measures ANOVA was significant, pairwise comparisons were made by a CONTRAST statement. All statistical analyses were performed by using SAS (version 9.3; SAS Institute); differences were considered significant at P < 0.05.
Results
At admission, age, length, and weight of the participants were not different between the 2 groups (Table 1). All of the children were severely wasted, with a mean weight-for-age of 61% of expected in the AAA group and 59% in the Ala group. At admission, 9 of the 22 participants were hypoalbuminemic, and 20 of the 22 participants were anemic (Table 1). There were no differences between the groups in the time taken for effect and appetite to recover (Ala: 2.8 ± 0.9 d; AAA: 2.5 ± 0.6 d), for loss of edema to occur (Ala: 10.6 ± 3.5 d; AAA: 14.3 ± 3.5 d), and for signs and symptoms of infection(s) to disappear (Ala: 10.4 ± 0.8 d; AAA: 7.8 ± 1.8 d). The time taken to achieve full nutritional recovery from the EOM period was not different between treatment groups (Ala: 29.9 ± 3.9 d; AAA: 25.8 ± 3.1 d). When the children within each group were divided into those with and those without edematous SAM at admission, the time taken to achieve full nutritional recovery was almost identical in the nonedematous children in the Ala and AAA groups (26.2 ± 1.9 d and 25.5 ± 5.1 d, respectively).
Both energy and protein intakes of the participants were significantly higher in mid-CUG and recovery than in the EOM period (Table 2; P < 0.01). Total exogenous leucine, phenylalanine, and tyrosine intakes were higher in both mid-CUG and recovery than during EOM (P < 0.0001). As expected, during mid-CUG and recovery total exogenous phenylalanine and tyrosine intakes were significantly higher in the AAA group (Table 2; P < 0.0001).
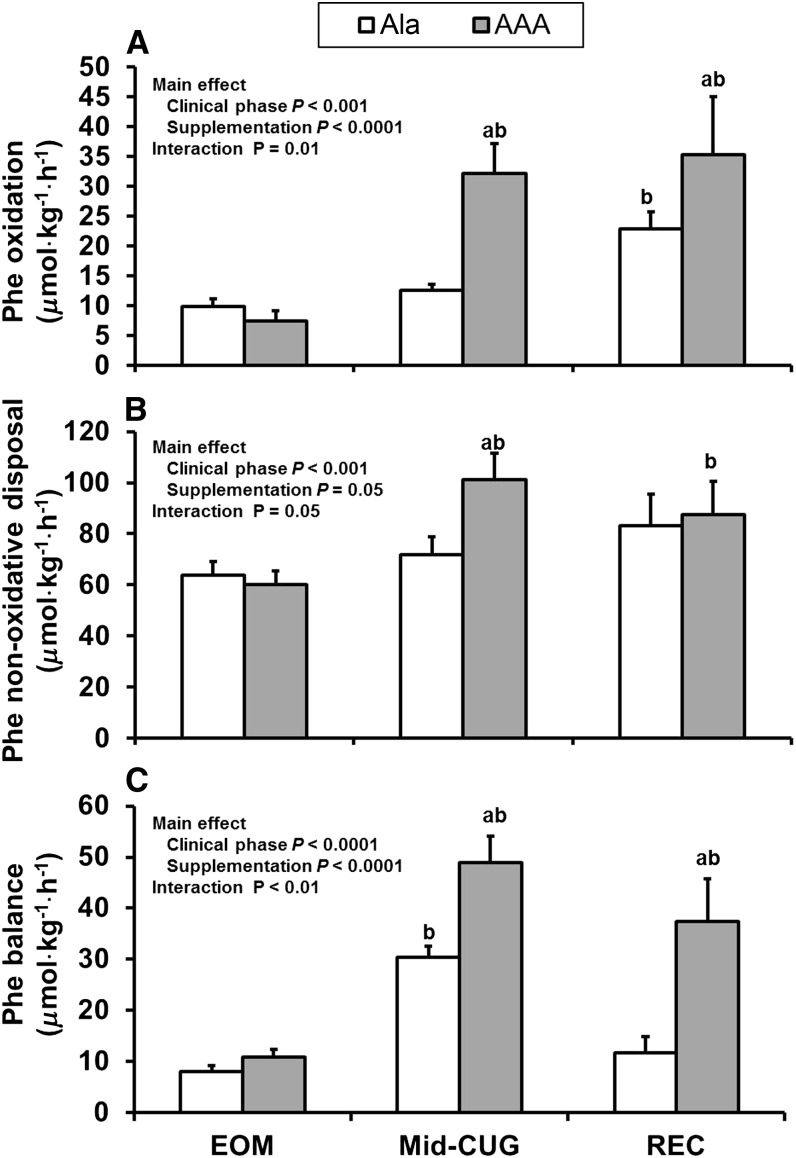
There were significant effects of clinical phase and supplementation and a significant clinical phase by supplementation interaction on total phenylalanine and tyrosine fluxes (P < 0.05; Table 3). In other words, total phenylalanine and tyrosine fluxes at the 3 clinical phases (EOM, mid-CUG, and recovery) were not the same for the Ala and AAA groups. Compared with the Ala group, total phenylalanine and tyrosine fluxes in the AAA group were significantly faster in mid-CUG and recovery phases. There was a significant effect of clinical phase on endogenous phenylalanine and tyrosine fluxes (P = 0.01 and P < 0.001, respectively), but there was no effect of supplementation (Table 3). There was, however, a significant clinical phase by supplementation group interaction in phenylalanine oxidation (P = 0.01), nonoxidative disposal (NOD) (P = 0.05), and balance (P < 0.01) (Fig. 2). In the AAA group, phenylalanine oxidation increased in both mid-CUG and recovery phases (P < 0.01), and in the Ala group phenylalanine oxidation increased only in the recovery phase (P = 0.03). At mid-CUG and recovery, phenylalanine oxidation was significantly higher in the AAA group (P < 0.05). Phenylalanine NOD also increased significantly in the AAA group (P < 0.001) in mid-CUG and recovery phases; and at mid-CUG, the AAA group had higher NOD than the Ala group (P = 0.002). Phenylalanine balance was greater in the AAA group compared with the Ala group at mid-CUG and recovery (P < 0.001).
TABLE 3.
Flux of phenylalanine, tyrosine, leucine, and urea in children with infection and severe malnutrition at the EOM period (baseline) and after supplementation with either alanine or AAAs1
| P | |||||||||
| EOM | Mid-CUG | REC | Main effect | ||||||
| Ala group | AAA group | Ala group | AAA group | Ala group | AAA group | Clinical phase | Supplementation | Interaction | |
| Phenylalanine | μmol · kg−1 · h−1 | μmol · kg−1 · h−1 | μmol · kg−1 · h−1 | ||||||
| Total | 70.9 ± 5.4 | 66.5 ± 4.2 | 83.4 ± 7.0 | 123 ± 12.7*,** | 106 ± 12.2** | 123 ± 19.9** | <0.0001 | 0.006 | 0.01 |
| Endogenous | 52.9 ± 5.4 | 48.1 ± 4.2 | 46.8 ± 6.1 | 59.9 ± 4.8 | 71.5 ± 12.8 | 80.9 ± 15.0 | 0.01 | 0.54 | 0.34 |
| Tyrosine | |||||||||
| Total | 57.3 ± 4.9 | 57.2 ± 10.5 | 69.5 ± 5.4 | 77.2 ± 10.5** | 35.4 ± 4.1** | 84.6 ± 8.3*,** | 0.009 | <0.0001 | <0.0001 |
| Endogenous | 42.4 ± 5.1 | 42.0 ± 10.6 | 28.9 ± 5.5 | 23.6 ± 6.8 | 15.8 ± 5.6 | 25.3 ± 3.7 | 0.0008 | 0.37 | 0.35 |
| Leucine | |||||||||
| Total | 149 ± 8.7 | 151 ± 7.7 | 200 ± 13.7 | 228 ± 15.9 | 232 ± 35.5 | 241 ± 24.4 | 0.0001 | 0.40 | 0.76 |
| Endogenous | 101 ± 8.4 | 101 ± 7.9 | 79.7 ± 15.0 | 91.8 ± 16.8 | 134 ± 38.0 | 129 ± 22.2 | 0.06 | 0.86 | 0.90 |
| Urea | |||||||||
| Total | 174 ± 37.5 | 126 ± 17.6 | 451 ± 50.4 | 498 ± 82.1 | 512 ± 60.1 | 386 ± 29.7 | <0.0001 | 0.18 | 0.12 |
| Endogenous | 168 ± 37.5 | 120 ± 17.6 | 445 ± 50.5 | 492 ± 82.1 | 506 ± 60.1 | 380 ± 29.7 | <0.0001 | 0.18 | 0.12 |
All values are means ± SEMs, n = 11. Means were compared by using repeated-measures 2-factor ANOVA. *Significantly different from Ala supplementation group in the same clinical phase, P < 0.05. **Significantly different from corresponding EOM phase, P < 0.05. AAA, aromatic amino acid; EOM, end of maintenance (∼13 d postadmission); mid-CUG, mid–catch-up growth (∼23 d postadmission when the children had replaced 50% of their weight deficit); REC, recovery (∼48 d postadmission when the children had recovered).
FIGURE 2.
Phenylalanine oxidation (A), nonoxidative disposal (B), and balance (C) in children with SAM at the EOM (∼13 d postadmission), mid-CUG (∼23 d postadmission when the children had replenished 50% of their weight deficit and had been administered either AAA or Ala supplementation for ∼10 d), and recovery (∼48 d postadmission when the children were fully recovered and had been administered either AAA or Ala supplementation for ∼35 d) phases. Values are means ± SEMs (n = 11) determined by using repeated-measures ANOVA. aSignificantly different from the AAA supplementation group in the same clinical phase, P < 0.05. bSignificantly different from the corresponding clinical phase 1, P < 0.05. AAA, aromatic amino acid; EOM, end of maintenance (∼13 d postadmission); mid-CUG, mid–catch-up growth (∼23 d postadmission when the children had replaced 50% of their weight deficit); REC, recovery (∼48 d postadmission when the children had recovered); SAM, severe acute childhood malnutrition.
There was no significant clinical phase by supplementation group interaction in fractional splanchnic uptake of phenylalanine and tyrosine, because the fraction of these amino acids extracted by the splanchnic bed was not different between the groups or between clinical phases (Table 4). There was, however, a significant clinical phase by supplementation group interaction in the amount of phenylalanine and tyrosine extracted by the splanchnic bed (P = 0.03 and 0.004, respectively). Clinical phase and supplementation had significant effects on the amount of phenylalanine and tyrosine extracted by the splanchnic bed (P < 0.001). Compared with the Ala group, the amount of phenylalanine and tyrosine extracted by the splanchnic bed in the AAA group was greater in both mid-CUG and recovery. Phenylalanine uptake by the splanchnic bed was significantly greater in both groups in mid-CUG and recovery phases compared with the EOM phase. Tyrosine uptake by the splanchnic bed was significantly greater in the AAA group in mid-CUG and recovery compared with that in the EOM period.
TABLE 4.
Splanchnic uptake of phenylalanine, tyrosine, and leucine in children with infection and severe malnutrition at the EOM period (baseline) and after supplementation with either alanine or AAAs1
| P | |||||||||
| EOM | Mid-CUG | REC | Main effect | ||||||
| Ala group | AAA group | Ala group | AAA group | Ala group | AAA group | Clinical phase | Supplementation | Interaction | |
| Phenylalanine | |||||||||
| Fraction of SU | 0.33 ± 0.08 | 0.32 ± 0.04 | 0.45 ± 0.07 | 0.36 ± 0.07 | 0.41 ± 0.07 | 0.43 ± 0.06 | 0.17 | 0.51 | 0.44 |
| SU, μmol · kg−1 · h−1 | 4.8 ± 1.1 | 5.0 ± 0.6 | 17.9 ± 3.22 | 28.3 ± 5.52,3 | 14.5 ± 3.02 | 30.2 ± 4.42,3 | <0.0001 | 0.001 | 0.029 |
| Tyrosine | |||||||||
| Fraction of SU | 0.44 ± 0.08 | 0.34 ± 0.08 | 0.41 ± 0.05 | 0.49 ± 0.07 | 0.41 ± 0.07 | 0.46 ± 0.07 | 0.65 | 0.82 | 0.48 |
| SU, μmol · kg−1 · h−1 | 6.2 ± 1.2 | 4.9 ± 1.1 | 15.4 ± 1.9 | 36.9 ± 5.62,3 | 13.7 ± 2.8 | 26.5 ± 3.42,3 | <0.0001 | 0.0005 | 0.004 |
| Leucine | |||||||||
| Fraction of SU | 0.41 ± 0.04 | 046 ± 0.05 | 0.19 ± 0.06 | 0.26 ± 0.08 | 0.27 ± 0.09 | 0.39 ± 0.09 | 0.02 | 0.15 | 0.87 |
| SU, μmol · kg−1 · h−1 | 17.4 ± 1.9 | 19.9 ± 2.4 | 20.3 ± 5.6 | 33.5 ± 10.6 | 23.5 ± 7.2 | 35.3 ± 7.5 | 0.17 | 0.05 | 0.64 |
All values are means ± SEMs, n = 11. Means were compared by using repeated-measures 2-factor ANOVA. AAA, aromatic amino acid; EOM, end of maintenance (∼13 d postadmission); mid-CUG, mid–catch-up growth (∼23 d postadmission when the children had replaced 50% of their weight deficit); REC, recovery (∼48 d postadmission when the children had recovered); SU, splanchnic uptake.
Significantly different from corresponding EOM phase, P < 0.05.
Significantly different from AAA supplementation group in the same clinical phase, P < 0.05.
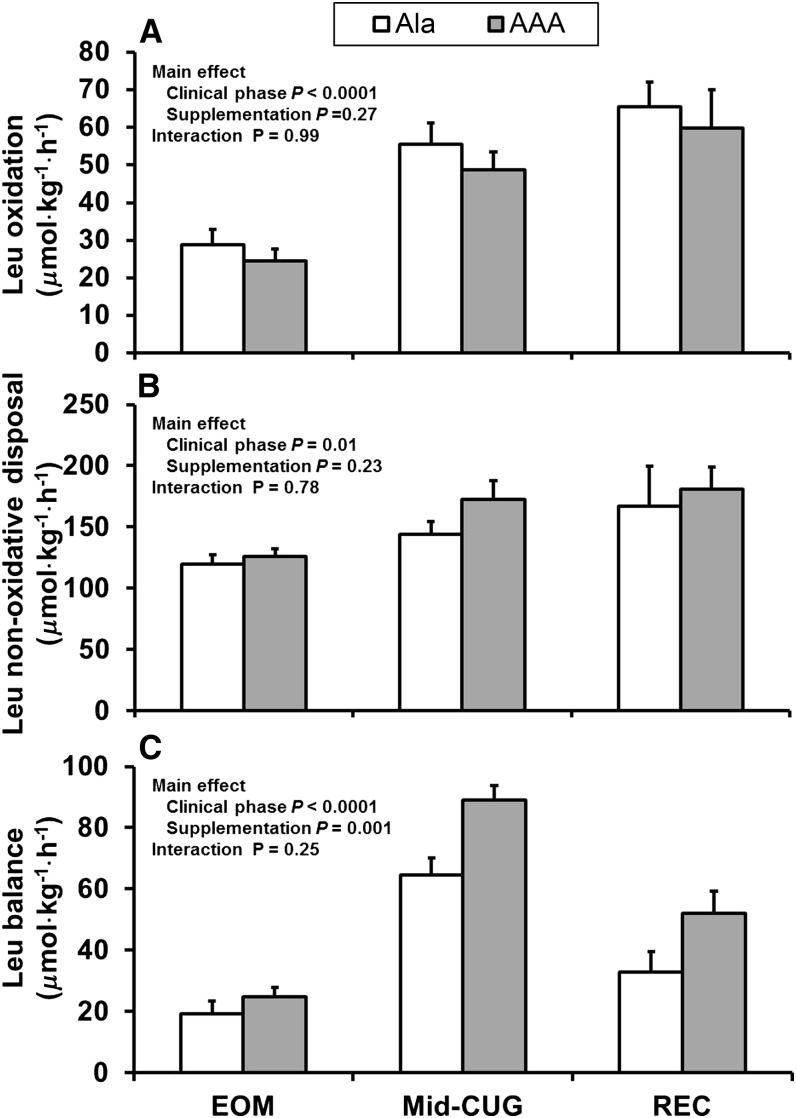
There was a significant effect of clinical phase on both total and endogenous leucine fluxes (Table 3; P < 0.05). Clinical phase had a significant effect on leucine oxidation (P < 0.0001) and leucine NOD (P = 0.01), which were greater in both clinical phases 2 and 3. Leucine balance was significantly affected by both clinical phase (P < 0.0001) and supplementation group (P = 0.001) (Fig. 3). There was no significant clinical phase by supplementation group interaction in fractional splanchnic uptake of leucine, because the fraction extracted by the splanchnic bed was not different between the groups or between clinical phases (Table 4).
FIGURE 3.
Leucine oxidation (A), nonoxidative disposal (B), and balance (C) in children with SAM at the EOM (∼13 d postadmission), mid-CUG (∼23 d postadmission when the children had replenished 50% of their weight deficit and had been administered either AAA or Ala supplementation for ∼10 d), and recovery (∼48 d postadmission when the children were fully recovered and had been administered either AAA or Ala supplementation for ∼35 d) phases. Values are means ± SEMs (n = 11) determined by using repeated-measures ANOVA. AAA, aromatic amino acid; EOM, end of maintenance (∼13 d postadmission); mid-CUG, mid–catch-up growth (∼23 d postadmission when the children had replaced 50% of their weight deficit); REC, recovery (∼48 d postadmission when the children had recovered); SAM, severe acute childhood malnutrition.
When changes in phenylalanine and leucine balances from EOM to mid-CUG were analyzed, there was a significant increase in phenylalanine balance in the AAA group (37.2 ± 5.0 vs. 21.3 ± 2.4 μmol · kg−1 · h−1; P = 0.01) and there was a trend toward a better leucine balance in the AAA group (62.1 ± 6.5 vs. 45.1 ± 6.2 μmol · kg−1 · h−1; P = 0.07).
There was a significant effect of clinical phase on urea production (P < 0.001), but there was no effect of supplementation (Table 3). However, at clinical phase 3, there was a trend in urea production, which was 25% slower in the AAA group (P = 0.06), suggesting a more efficient utilization of dietary protein for protein synthesis.
Discussion
The primary aim of this study was to determine whether AAA supplementation of the rehabilitation diet of children being treated for SAM would lead to an improvement in whole-body protein synthesis during the rapid CUG and recovery phases of rehabilitation. The data demonstrated that supplementation with AAA increased rates of phenylalanine utilization for protein synthesis and improved phenylalanine and leucine balances, indicating an improvement in net protein synthesis in children with SAM at the mid-CUG and recovery phases of treatment.
In an earlier study, we reported that supplementation with AAA in children with edematous SAM increased rates of leucine appearance from protein breakdown and its utilization for protein synthesis with improved leucine balance during the immediate postadmission maintenance phase of treatment. This suggested that AAA supplementation during the early phase of nutritional rehabilitation resulted in an improvement in the efficiency of utilization of dietary protein for protein synthesis (4). However, phenylalanine and tyrosine kinetics did not indicate an improvement in protein synthesis, probably because the phenylalanine-tyrosine tracer model was not sensitive enough in the severely malnourished state. In the current study, however, phenylalanine NOD, an index of whole-body protein synthesis, was greater in the AAA group than in the Ala group during the mid-CUG phase, and phenylalanine balance was improved during both the mid-CUG and recovery phases in a group of children diagnosed with both edematous and nonedematous SAM. Leucine balance was also improved during both the mid-CUG and recovery phases. Compared with the Ala group, leucine balance (P = 0.1) tended to be higher in the AAA group at the mid-CUG and recovery phases, but these differences were not significant, possibly because of our relatively small sample size. Also, a larger leucine pool size relative to turnover rate may have influenced the results in this study compared with those in our earlier study. In the earlier study (4), the children were studied in the acutely malnourished state when the leucine pool would have been markedly smaller compared with the pool sizes in this study at mid-CUG and recovery. Nevertheless, together these data suggest that AAA supplementation of the rehabilitation diet of children with SAM improved their net protein synthesis.
Earlier we proposed that there may be a relative deficiency of AAA during the maintenance phase of treatment of children with SAM plus concurrent infection(s) because their protein intake of 1.2 g · kg−1 · d−1 may not contain enough AAAs to support synthesize of acute phase proteins, which are rich in AAAs (4). We therefore reasoned that during rapid CUG the requirement for AAAs will be even greater to replenish both plasma and body proteins. Because the rate of CUG varies depending on the degree of wasting (12), it is difficult to calculate a fixed amount of AAA supplementation needed to maximize the rate of CUG in children with SAM. It is known, however, that once appetite is restored in children recovering from SAM the rate of weight gain can be 10–20 times the normal rate of weight gain (13). We therefore chose to provide a supplement of 330 mg · kg−1 · d−1 of AAAs, which is the amount contained in ∼3.5 g of milk protein, the average amount of protein consumed during the rapid CUG phase. Hence, the children were consuming ∼14 times the recent WHO-recommended AAA intake of 46.4 mg · kg−1 · d−1 for infants at 1–2 y of age (12). This increased dietary intake of AAAs was associated with a significantly greater splanchnic uptake of phenylalanine. Because phenylalanine hydroxylation and its subsequent oxidation take place primarily in the liver, not surprisingly the increased splanchnic uptake of phenylalanine was associated with a greater rate of oxidation. Despite this greater rate of oxidation, phenylalanine NOD and balance were greater compared with the Ala group, indicating an increase in net protein synthesis in the AAA-supplemented children.
In another earlier study, Manary et al. (3) supplemented the resuscitative diet of children with edematous SAM with 10 mg · kg−1 · d−1 tryptophan, which resulted in the supplemented children being administered ∼142 mg · kg−1 · d−1 of AAAs compared with a control group who were administered ∼134 mg · kg−1 · d−1. They reported that the children in the tryptophan supplementation group had lower rates of urea production after 24 h of supplementation, indicating that the supplement elicited an improvement in the utilization of dietary protein for protein synthesis. In the current study, although there was no significant clinical phase by supplementation group interaction (P = 0.11), mean urea production rate in the AAA group at recovery was 25% less than that in the Ala group (380 vs. 506 μmol · kg−1 · h−1), suggesting that dietary protein was being utilized more efficiently for protein anabolism.
Like our previous study (4), in the current study, despite the positive effect of AAA supplementation on whole-body protein synthesis, this did not translate into a shorter time to achieve nutritional recovery or any other clear improvement in clinical outcome. For example, the time taken to reach full nutritional recovery and the rate of tissue deposition were not different between the 2 groups. It is possible, however, that the composition of tissue deposited by the 2 groups may have been different. That is, that the AAA group laid down more lean tissue than the Ala group because of their consistently better rate of net protein synthesis.
It is noteworthy that in the present study, the AAA and Ala groups included children with both edematous and nonedematous SAM. In the studies by Manary et al. (14) and Reid et al. (4) only children with edematous SAM and infection were studied based on the thesis that they would need more AAAs to make more acute phase proteins because of the higher incidence of systemic infection in children with edematous SAM compared with children with nonedematous SAM. In addition, decreased rates of whole-body protein breakdown in edematous SAM compared with nonedematous SAM (4, 14) suggest that endogenous amino acids released from proteolysis may not provide sufficient AAAs for the synthesis of acute phase proteins. Hence, the expectation that children with edematous SAM might have responded better to AAA supplementation during CUG compared with the response of children with nonedematous SAM. In agreement, there is also evidence that the requirement for cysteine is greater in children diagnosed with edematous SAM than in those with nonedematous SAM during the CUG phase (15).
In summary, children with SAM had significantly faster rates of protein synthesis after supplementation with 330 mg · kg−1 · d−1 AAAs compared with children being administered isonitrogenous Ala supplementation during the CUG growth and recovery phases of treatment. We conclude that dietary supplementation with AAAs improves net protein synthesis in children with SAM during nutritional rehabilitation.
Acknowledgments
The authors thank the laboratory staff—Margaret Frazer, Melanie Del Rosario, and Vy Pham—at the Children's Nutrition Research Center for their excellent work in the analysis of the samples. F.J., A.B., M.R., and T.F. designed the study and supervised various aspects of the study; L.W. and C.T.-B. were responsible for clinical care of the participants and performed the isotope infusions; B.C. prepared all equipment consumables and solutions for the infusions and processed, stored, and shipped samples; J.W.H. supervised laboratory analyses and calculated the final data; and A.B., F.J., and J.W.H. analyzed and interpreted the data and wrote the manuscript. All authors contributed to different aspects of this study, including the design of the study, data collection, sample analysis, data interpretation, and writing of the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AAA, aromatic amino acid; CUG, catch-up growth; EOM, end of maintenance; NOD, nonoxidative disposal; SAM, severe acute childhood malnutrition.
Literature Cited
- 1.Jahoor F, Badaloo A, Reid M, Forrester T. Protein kinetic differences between children with edematous and nonedematous severe childhood undernutrition in the fed and postabsorptive states. Am J Clin Nutr. 2005;82:792–800. [DOI] [PubMed] [Google Scholar]
- 2.Reeds PJ, Fjeld CR, Jahoor F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr. 1994;124:906–10. [DOI] [PubMed] [Google Scholar]
- 3.Manary MJ, Yarasheski KE, Hart CA, Broadhead RL. Plasma urea appearance rate is lower when children with kwashiorkor and infection are fed egg white-tryptophan rather than milk protein. J Nutr. 2000;130:183–8. [DOI] [PubMed] [Google Scholar]
- 4.Reid M, Forrester T, Badaloo A, Heird WC, Jahoor F. Supplementation with aromatic amino acids improves leucine kinetics but not aromatic amino acid kinetics in infants with infection, severe malnutrition, and edema. J Nutr. 2004;134:3004–10. [DOI] [PubMed] [Google Scholar]
- 5.Reid M, Badaloo A, Forrester T, Morlese JF, Heird WC, Jahoor F. The acute-phase protein response to infection in edematous and nonedematous protein-energy malnutrition. Am J Clin Nutr. 2002;76:1409–15. [DOI] [PubMed] [Google Scholar]
- 6.Wellcome Working Party. Classification of infantile malnutrition. Lancet. 1970;2:302–3. [PubMed] [Google Scholar]
- 7.Badaloo A, Reid M, Forrester T, Heird WC, Jahoor F. Cysteine supplementation improves the erythrocyte glutathione synthesis rate in children with severe edematous malnutrition. Am J Clin Nutr. 2002;76:646–52. [DOI] [PubMed] [Google Scholar]
- 8.Reid M, Badaloo A, Forrester T, Morlese JF, Frazer M, Heird WC, Jahoor F. In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. Am J Physiol Endocrinol Metab. 2000;278:E405–12. [DOI] [PubMed] [Google Scholar]
- 9.Hachey DL, Patterson BW, Reeds PJ, Elsas LJ. Isotopic determination of organic keto acid pentafluorobenzyl esters in biological fluids by negative chemical ionization gas chromatography/mass spectrometry. Anal Chem. 1991;63:919–23. [DOI] [PubMed] [Google Scholar]
- 10.Lee B, Dennis JA, Healy PJ, Mull B, Pastore L, Yu H, Aguilar-Cordova E, O'Brien W, Reeds P, Beaudet AL. Hepatocyte gene therapy in a large animal: a neonatal bovine model of citrullinemia. Proc Natl Acad Sci USA. 1999;96:3981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid M, Badaloo A, Forrester T, Heird WC, Jahoor F. Response of splanchnic and whole-body leucine kinetics to treatment of children with edematous protein-energy malnutrition accompanied by infection. Am J Clin Nutr. 2002;76:633–40. [DOI] [PubMed] [Google Scholar]
- 12.FAO/WHO/UNU. Protein and amino acid requirements in human nutrition: report of a joint WHO/FAO/UNU expert consultation. Geneva: FAO/WHO/UNU; 2007. WHO Technical Report Series No.: 935. [Google Scholar]
- 13.Waterlow JC. Treatment of severe PEM. In: Waterlow JC, McGregor S, Tomkins AM, editors. Protein-energy malnutrition. London: Edward Arnold; 2002. p. 164–86. [Google Scholar]
- 14.Manary MJ, Yarasheski KE, Smith S, Abrams ET, Hart CA. Protein quantity, not protein quality, accelerates whole-body leucine kinetics and the acute-phase response during acute infection in marasmic Malawian children. Br J Nutr. 2004;92:589–95. [DOI] [PubMed] [Google Scholar]
- 15.Badaloo A, Hsu JW, Taylor-Bryan C, Green C, Reid M, Forrester T, Jahoor F. Dietary cysteine is used more efficiently by children with severe acute malnutrition with edema compared with those without edema. Am J Clin Nutr. 2012;95:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]