Abstract
Background
Exposure to particulate matter (PM) has been associated with deficits in lung function growth among children in Western countries. However, few studies have explored this association in developing countries, where PM levels are often substantially higher.
Methods
Children (n=3273) aged 6–12 years were recruited from eight schools in four cities. The lung function parameters of forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) were measured using computerized spirometers twice a year for up to three years (1993–1996). Dichotomous samplers placed in each schoolyard were used to measure PM2.5 and PM10 (PM with diameter ≤ 2.5 and ≤ 10, respectively). Multivariable generalized estimating equations were used to examine the association between the quarterly average PM levels and lung function growth over the period of follow-up.
Results
Annual average PM2.5 and PM10 levels in the four cities ranged from 57 to 158 μg/m3 and 95 to 268 μg/m3, respectively. In multivariable models, an increase of 10 μg/m3 of PM2.5 was associated with decreases of 2.7 ml FEV1 (95% confidence interval= −3.5 to −2.0), 3.5 ml FVC (−4.3 to −2.7), 1.4 ml/year FEV1 growth (−1.8 to −0.9), and 1.5 ml/year FVC growth (−2.0 to −1.0). Similar results were seen with PM10 exposure.
Conclusions
Exposure to ambient particulate matter was associated with decreased growth in lung function among Chinese children.
Children are particularly vulnerable to the effects of air pollution because they have a larger lung-to-body-volume ratio, their airway epithelium is more permeable to air pollutants and the lung defense mechanisms against particulate matter (PM) pollution and gaseous pollution are not fully evolved.1 Breathing the same pollutant concentrations, children may have a two- to four-fold higher dose reaching the lung parenchyma compared with adults.2
Numerous epidemiologic studies have reported effects of air pollution on children’s respiratory health.3 Of particular relevance to the present study are recent studies that examined the relationship between long-term exposure to air pollution and decreased lung function growth among children. A Southern California study found that the growth of forced expiratory volume in 1 second (FEV1) was slower in more polluted (higher PM2.5) communities and that movement away from polluted areas resulted in lower deficits in lung function growth.4,5 Similar associations have been found in Poland and Austria.6, 7
However, these studies were carried out in populations exposed to relatively low levels of air pollution (California, ~ 5 to 27 μg/m3 PM2.5; Krakow, Poland, ~33 to 52 μg/m3 suspended particulate matter; Austria: ~ 10 to 30 μg/m3 PM10). People living in many urban areas of developing countries have been exposed to much higher levels of pollution. For example, annual PM2.5 in New Delhi, India, was 102 μg/m3 in 20078; average PM10 levels in urban areas in non-European low income countries range from about 20 to 99 μg/m3, and mega cities in Asia have recorded annual levels to be above 100 μg/m3. 9,10 Whether the lung responses of children living in these highly-polluted cities are similar to those living in Western countries, is unknown. In fact, to the best of our knowledge, no prospective, longitudinal studies have been conducted to examine the effect of ambient PM on lung function growth at higher exposure levels, or have compared the sensitivity of lung function growth in relation to PM2.5 and PM10 exposure. This knowledge gap adds uncertainty to extrapolation of dose-response information from Western countries to developing countries, where PM10 is often the only metric of PM exposure collected. This provided the motivation behind the current study, where we examined the relationship between ambient PM concentrations and lung function growth among children living in four large Chinese cities.
Methods
This prospective semi-ecologic longitudinal study was conducted in the four Chinese cities of Chongqing, Guangzhou, Lanzhou, and Wuhan, and was part of a larger study of respiratory morbidity and air pollution.11–14 During the time of data collection (1993 – 1996), China had not entered its rapid economic development period; people living in the four cities (as well as other cities) had relatively uniform income and lifestyle, and ambient air pollution levels were relatively stable from year to year. 12
Study population
A description of the study population and site selection has been previously published.11–14 In brief, 7621 children residing in eight districts within the four cities were recruited during the years of 1993 through 1996. The study districts were selected with the aims of maximizing the between-city and within-city concentration gradients in the ambient air pollutants of interest. The latter aim was achieved by selecting an urban district and a suburban district in each of the four cities. Within each district, an elementary school was chosen to participate in the study. Subject eligibility was based on the following inclusion criteria: (1) their families had no plans to move within 3 years, and (2) their homes were within 2 km of the schools. Children aged 6–12 years of age were enrolled in the study after informed consent was obtained from the parents.
Of the 7621 children participating in the study on respiratory morbidity (via a questionnaire survey), 3,273 students in grades 2 to 5 were enrolled, from randomly selected classrooms, for lung function tests and were followed for up to 3 years with repeated measurements of lung function twice every year. The study was carried out over the period of 1993–1996. Of the 3273 children, 2480 (76%) had at least three longitudinal measurements of lung function and 1027 (31%) had 5 or more measurements.
Ambient PM measurement
The sampling frequency was stratified by quarter of year. Based on the study design, at least 15 samples were collected in each quarter of 1995 and 1996; and within each city, the samples were collected on the same dates in both the urban and the suburban schools.
PM2.5 and PM10 were collected on a 24-hour integrated basis in the yards of the schools using dichotomous samplers (Sierra -Anderson Model 241). The samples were collected on Teflon filters (2μm pore size) supported by polyolefin rings and the samplers were operated at a total flow rate of 16.7 l/min with 90% of the total flow passing the PM2.5 inlet and 10% of the total flow passing the PM2.5–10 inlet. At the beginning and the end of each 24-hour sampling session bubble flow meters were used to calibrate and ensure constant flow rates of the mass flow controllers used with the dichotomous samplers. Flow rate variations throughout each sampling session were within ± 5%. About 10% field blanks and duplicates were collected for quality control measures. All the air filters were weighed in a climate-controlled room to gravimetrically determine PM2.5 and PM10 concentrations. The method has previously been described in detail.12, 13
Questionnaire survey
Information on residential history, lifestyles, household characteristics, and children’s and parents’ health histories was obtained at baseline through a self-administered questionnaire survey.14 The questionnaire was completed by parents, and included detailed questions on breastfeeding, location and number of years lived in each residence, types and characteristics of dwelling, method of cooking and heating, location of kitchen, types of home ventilation devices (exhaust fan, hood, chimney for cooking or heating), degree of home smokiness and eye irritation during cooking, history and current status of children’s respiratory illnesses and symptoms, parental education levels, parental occupation, parental smoking status, and parental respiratory health histories. The queried information has previously been described in detail.14
Lung function measurement
Lung function was measured using computerized Warren E. Collins Survey II 8 liter water seal volume spirometers situated in each of the eight schools, by six trained technicians. There was at least one technician per city; the same technician oversaw the tests in both urban and rural district schools. Forced vital capacity (FVC) and FEV1 were measured twice a year - once during the warm season and the other during the cold season. Three to 8 measurements per testing session were taken for each student.
The American Thoracic Society’s standard spirometry procedure was used. This required each child to perform at least 3 FVC trials that were acceptable in overall shape, and with at least two acceptable trials that were reproducible (difference < 5%). Of the trials that were deemed acceptable the mean of the two highest FEV1 and FVCs were used to calculate the lung function measures. The spirometers were calibrated, and all the technicians were trained centrally at one location at the beginning of the study; then twice a day, spirometry technicians checked the system at each site for leakage, linearity of analogue to digital responses over the full volume range of the spirometer and volume calibration with a 3-liter syringe. During testing, volume-time curves were recorded on paper charts to provide backup and validation. All values of FVC and FEV1 were adjusted to body temperature and pressure, saturated (BTPS) conditions. We also calculated FEV1/FVC ratios.
Statistical analysis
The outcome data consisted of the results of 6149 pulmonary function tests of 1634 girls and 6056 tests of 1639 boys over the three-year period. Univariate summary statistics and distributional plots were examined for all variables. Ambient concentrations of PM10 and PM2.5 across the cities and districts were explored, and quarterly mean levels were calculated. We used analysis of variance for bivariate analysis to explore associations between quarterly PM exposure, lung function and other potential confounders. We performed preliminary analyses and model-building using multivariate linear regression. Confounders were included in multiple-regression models if they changed the main effect estimate by > 10% and if they were biologically relevant. Because measures of indoor air pollution and home characteristics may not be strictly confounders, but may explain much of the variation in lung function, they were selected using a backwards selection process with cut-off value of P = 0.10.
The study had repeated measures on individual subjects nested within districts in cities. We examined the intraclass correlation of spirometry outcomes at each sampling level (city, district and spirometry technician) using random-effects mixed models. Because we were interested in the marginal effects of PM on lung function, we chose to use generalized estimating equations (GEE).
In order to explore the effect of PM on the growth rate of lung function we carried out a two-stage analysis. In the first stage we estimated the district-specific average growth of lung function after accounting for baseline age, sex, body mass index, asthma status, maternal and paternal educational achievement, fathers’ smoking status, indoor coal use for cooking or heating, and presence of ventilation in the house while accounting for repeated measures and spirometer technician in the GEE model. The second-stage model was an inverse-variance-weighted linear-regression analysis of the district-specific growth in lung function and the average PM2.5 levels and PM10 levels for each district.
Because we had heterogenous lengths of follow-up between spirometry measurements and a wide range of baseline ages among the children in the study, to estimate the population-average lung function growth associated with PM10 or PM2.5 exposure across persons in the districts and also allow for seasonal variation within each district, we created an interaction term between quarterly PM concentration in each district and change in age, accounting for baseline age, confounders, repeated measures, sampling district and spirometry technician in the GEE model. Finally, using the above model, we compared the effects across PM2.5 and PM10, by scaling the effect estimates (sizes) by inter-quartile range (IQR), such that the results reflect changes in lung function growth per year per increase in one IQR of each pollutant.
Differences in the association between PM and lung-function growth by sex were explored using stratified analyses in the GEE models and the two-stage exploratory models. An interaction term (PM x sex) in the two-stage model was also tested.
All analyses were carried out in SAS V9 (SAS Institute, Cary NC). The GEE models were run in the SAS Genmod procedure.
Results
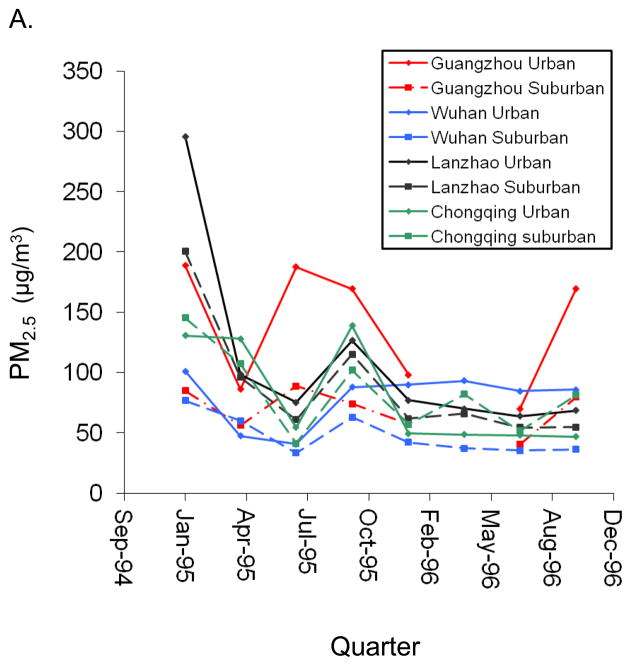
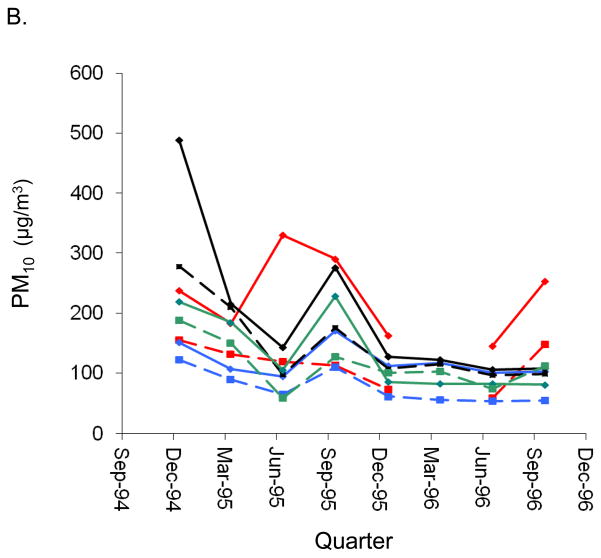
PM2.5 and PM10 levels were highest in the Guangzhou urban district (PM2.5:148 μg/m3 average over 1995–1996) and lowest in the Wuhan suburban district (PM2.5: 52 μg/m3 average over 1995–1996). Annual district-specific mean concentrations of PM10 and PM2.5 are reported in eTable 1 (http://links.lww.com). In addition, there was wide between-city and within-city gradients in long-term ambient PM levels across the years sampled (Figure 1), as reported previously.12, 13
Figure 1.
Quarterly mean concentrations of (A.) PM2.5 and (B.) PM10 (μg/m3) in school yards of the 8 study districts
Mean FEV1 values were 1812 ml (SD= 375) among boys and 1729 ml (416) among girls at the first lung-function measurement (referred to as baseline values). Mean FVC values were 2107 (444) ml and 1955 (460) ml among boys and girls, respectively. Table 1 provides a description of the study population with respect to lung function and the results of preliminary bivariate analyses. Male sex, increasing age, increasing height, and increasing weight were each associated with higher lung function; and the child’s previous diagnosis of asthma was associated with lower FVC and FEV1/FVC ratio. Parental occupation, education and smoking status, as well as number of rooms, measures of indoor air pollution (type of stove, use of coal as cooking/heating fuel, use of ventilation devices) were each associated with lung function.
Table 1.
Description of study population at baselinea
| Variable | No. (%) | FEV Mean (SD) |
FVC Mean (SD) |
FEV/FVC Mean (SD) |
|---|---|---|---|---|
| Sex | ||||
| Boys | 1634 (50) | 1812 (375) | 2107 (444) | 0.8625 (0.0593) |
| Girls | 1639 (50) | 1729 (415) | 1955 (459) | 0.8850 (0.0598) |
| Age | ||||
| <7 | 1440 (44) | 1572 (315) | 1812 (364) | 0.8696 (0.0632) |
| 7 to 9 | 1531 (47) | 1890 (368) | 2163 (430) | 0.8766 (0.0580) |
| >10 | 282 (9) | 2126 (419) | 2429 (488 | 0.8779 (0.0600) |
| Asthma | ||||
| No | 3174 (97) | 1567 (321) | 1789 (362) | 0.8776 (0.0581) |
| Yes | 99 (3) | 1540 (300) | 1836 (396) | 0.8457 (0.0785) |
| Home type | ||||
| Single-story house | 839 (26) | 1493 (326) | 1717 (362) | 0.8699 (0.0619) |
| Apartment building | 2191 (67) | 1590 (313) | 1815 (358) | 0.8779 (0.0589) |
| Other | 230 (7) | 1608 (337) | 1813 (377) | 0.8880 (0.0457) |
| No. of rooms | ||||
| <3 | 1530 (47) | 1540 (320) | 1760 (363) | 0.8768 (0.0584) |
| ≥3 | 1743 (53) | 1589 (320) | 1816 (361) | 0.8764 (0.0596) |
| Father’s education | ||||
| > Primary school | 3088 (94) | 1569 (320) | 1793 (362) | 0.8765 (0.0592) |
| ≤ Primary school | 185 (6) | 1521 (330) | 1735 (372) | 0.8776 (0.0565) |
| Mother’s education | ||||
| > Primary school | 3027 (92) | 1575 (318) | 1799 (361) | 0.8771 (0.0590) |
| ≤ Primary school | 246 (8) | 1461 (333) | 1682 (374) | 0.8698 (0.0592) |
| Father’s Occupation | ||||
| Manual laborer | 1706 (52) | 1543 (322) | 1769 (360) | 0.8730 (0.0613) |
| Non Manual laborer | 1567 (48) | 1592 (317) | 1813 (365) | 0.8805 (0.0563) |
| Mother’s Occupation | ||||
| Manual laborer | 1745 (53) | 1547 (331) | 1776 (368) | 0.8714 (0.0614) |
| Non Manual laborer | 1528 (47) | 1589 (307) | 1806 (357) | 0.8825 (0.0558) |
| Coal use | ||||
| No use | 2242 (69) | 1598 (303) | 1820 (349) | 0.8798 (0.0565) |
| Either heating or cooking | 509 (16) | 1572 (355) | 1794 (398) | 0.8775 (0.0592) |
| Both heating and cooking | 514 (15) | 1427 (325) | 1657 (359) | 0.8612 (0.0666) |
| Presence of ventilation | ||||
| No | 791 (24) | 1614 (310) | 1835 (360) | 0.8823 (0.0552) |
| Yes | 2474 (76) | 1552 (323) | 1776 (363) | 0.8747 (0.0601) |
| Stove type | ||||
| Gas | 2401 (74) | 1590 (311) | 1816 (356) | 0.8777 (0.0582) |
| Other | 844 (26) | 1501 (340) | 1720 (374) | 0.8730 (0.0615) |
| Heating | ||||
| No | 1826 (56) | 1618 (311) | 1837 (360) | 0.8831 (0.0560) |
| Yes | 1435 (44) | 1502 (321) | 1732 (359) | 0.8681 (0.0617) |
| Heating type | ||||
| None/central/gas | 2207 (67) | 1599 (310) | 1819 (357) | 0.8813 (0.0565) |
| Coal stove/fire pit | 1066 (33) | 1499 (332) | 1730 (369) | 0.8667 (0.0630) |
| Duration of heating (months) | ||||
| 0 | 1846 (56) | 1617 (311) | 1836 (359) | 0.8832 (0.0560) |
| 1 | 534 (16) | 1608 (293) | 1825 (338) | 0.8829 (0.0523) |
| 2 | 889 (27) | 1437 (321) | 1674 (361) | 0.8591 (0.0652) |
| Air conditioning | ||||
| No | 2592 (79) | 1571 (332) | 1797 (374) | 0.8752 (0.0604) |
| Yes | 673 (21) | 1551 (272) | 1764 (317) | 0.8815 (0.0529) |
| Father smoker | ||||
| No | 802 (25) | 1560 (324) | 1773 (361) | 0.8805 (0.0565) |
| Yes | 2471 (75) | 1569 (320) | 1795 (364) | 0.8753 (0.0598) |
| Mother smoker | ||||
| No | 3240 (99) | 1568 (321) | 1791 (363) | 0.8768 (0.0591) |
| Yes | 33 (1) | 1446 (266) | 1686 (318) | 0.8596 (0.0520) |
| Area | ||||
| Guangzhou | ||||
| Urban | 415 (13) | 1792 (399) | 2044 (455) | 0.8785 (0.0588) |
| Sub-urban | 411 (14) | 1839 (388) | 2079 (450) | 0.8881 (0.0634) |
| Wuhan | ||||
| Urban | 280 (8) | 1850 (396) | 2078 (454) | 0.8927 (0.0488) |
| Sub-urban | 136 (3) | 1711 (368) | 1923 (425) | 0.8922 (0.0494) |
| Lanzhou | ||||
| Urban | 428 (14) | 1706 (428) | 1997 (502) | 0.8565 (0.0597) |
| Sub-urban | 428 (15) | 1603 (379) | 1876 (434) | 0.8558 (0.0610) |
| Chongqing | ||||
| Urban | 760 (20) | 1801 (347) | 2060 (415) | 0.8776 (0.0566) |
| Sub-urban | 415 (12) | 1862 (406) | 2149 (466) | 0.8688 (0.0648) |
Baseline is defined as the first lung function measurement over the course of the study.
Lung-function level in this population was significantly predicted by age, sex, height, weight, the interaction of weight with sex, and presence of diagnosed asthma. After the backward selection in the linear regression process, the only measures of indoor air pollution remaining in the model were use of coal and presence of a ventilation device. Coal use was associated with a change of −19.0 ml (95% confidence interval= −27.7 to −10.3) FEV1 and a −21.7 ml (−37.8 to −5.6) change in FVC. The use of ventilation devices in the household was associated with a 15.1 (6.0 to 24.2) ml higher FEV1 and an 18.7 (8.0 to 29.3) ml higher FVC, after accounting for age, height, sex, prior diagnosis of asthma, mothers’ education and fathers’ education. Asthma was associated with a decrease (−68.0 ml) in FEV1, but not with FVC. We retained asthma in all models to maintain comparability. We included fathers’ education and mothers’ education as measures of socioeconomic status and fathers’ smoking status as a measure of children’s exposure to environmental tobacco smoke. The prevalence of maternal smoking was very low (~1%) and thus was not included in the analysis.
The final multivariate model accounted for 73% of the variation in FEV1 and 75% of the variation in FVC. Predicted mean FEV1 values for boys and girls were 2002 ml and 1911 ml, respectively; and predicted mean FVC values were 1974 ml and 1838 ml, respectively, all adjusted to the age of 10 years old, after accounting for height, weight, district, spirometry technician, presence of a ventilation device in the house, coal use, fathers’ smoking status, mothers’ education, and fathers’ education.
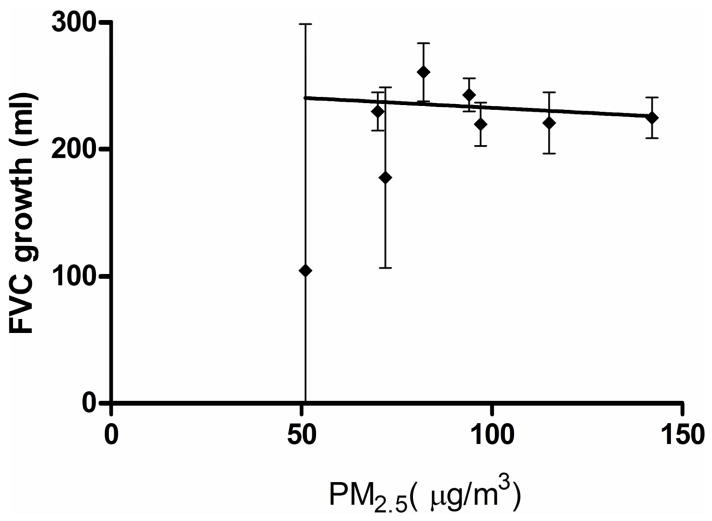
Within the districts, children living in the urban district of Guangzhou (with the highest PM levels) had 63 ml (95% CI = 49 to 78) lower baseline FEV1 and 51 ml (95% CI = 33 to 70) lower baseline FVC than those living in the suburban district of Wuhan (with the lowest PM levels), after adjusting for all the covariates specified above and repeated measures within individual participants. The district-specific lung function growth rates were highest in the Chongqing suburban district for FEV1, (mean = 232 [95%CI: 273 to 190] ml/yr; for FVC, 261 [306 to 216] ml/yr) and lowest in Lanzhou urban and Chongqing urban district for FEV1 (185 ml/yr [226 to 144]) and for FVC (220 ml/yr [253 to 187]). (eTable 1) For every 10 μg/m3 of PM10, average lung function growth across the districts decreased by 1.6 ml/year (95% CI = −4.9 to 1.6) for FVC and 0.3 ml/year (−4.1 to 3.3) for FEV1. Similar results were seen with PM2.5. (Figure 2)
Figure 2.
District-specific estimated average growth in FVC (ml/year) in 1993–1996 plotted against district daily average PM2.5 (μg/m3) over 1995–1996. The line is the association between the PM2.5 and FVC growth estimated from the two stage analysis.
The effect of 10μg/m3 incremental change in quarterly average PM on lung function, from the multivariate GEE models, are reported in Table 2 for all children who were studied during 1995–1996 and had quarterly PM2.5 and PM10 measurements (n = 3158). There were no marked differences in the association between PM and lung function growth by sex (data not shown). The FEV1/FVC ratio declined with exposure to PM but the change was very small. FVC growth decreased by −1.5 ml/year (95% CI= −2.0 to −1.0) per 10μg/m3 increase in PM2.5. When the population was restricted to those who had at least 5 repeated measurements (n=1027), FVC growth per 10 μg/m3 increase in PM2.5 −1.5 ml/year (−2.2 to −0.7). Because variation in growth rates was greatest in Wuhan, which also had the lowest average PM levels, an additional sensitivity analysis was run excluding all children living there. FVC growth per 10μg/m3 increase in PM2.5 was −1.6 ml/year (−2.1 to −1.1). Similar results were seen when sensitivity analyses were run with FEV1 and PM10.
Table 2.
Effect of increase in 10 μg/m3 ambient particulate matter on lung function level (ml) and lung function growth (ml/year) per year
| PM10 | PM2.5 | |||
|---|---|---|---|---|
|
| ||||
| Estimate (95% CI) | Estimate (95% CI) | |||
| FEV1 | −1.6 | (−2.1 to −1.1) | −2.7 | (−3.5 to −2.0) |
| FEV1 growth | −0.7 | (−1.0 to −0.4) | −1.4 | (−1.8 to −0.9) |
| FVC | −1.6 | (−2.2 to −1.2) | −3.5 | (−4.3 to −2.7) |
| FVC growth | −0.7 | (−1.0 to −0.4) | −1.5 | (−2.0 to −1.0) |
| FEV1/FVC | −0.001 | (−0.002 to −0.00) | −0.000 | (−0.000 to −0.002) |
| FEV1/FVC growth | −0.001 | (−0.001 to 0) | −0.001 | (−0.002 to −0.000) |
Controlling for baseline age, sex, height, weight, ventilation, coal use, mother’s education, father’s education, father’s smoking status and time trends. Also, accounting for clustering at district level, spirometry technician and correlation between individual subjects with repeated measures
With every IQR increase in PM10 and PM2.5, deficits in FEV1 growth rate were 5.0 ml/year (95%CI = −7.3 to −2.8) and 8.9 ml/year (−11.9 to −5.9), respectively. Corresponding deficits in FVC growth were 5.1 ml/year (−7.4 to −2.7) and 9.4 ml/year (−12.5 to −6.3).
Discussion
Chronic exposure to high levels of particulate matter were associated with deficits in lung function and decreased development of lung function of elementary-school children living in urban China. The effect sizes per IQR were larger for PM2.5 than for PM10, indicating that PM2.5 had a stronger effect than did PM10 on lung function growth in this population. This may reflect deeper penetration of PM2.5 into the lower respiratory tract, or higher measurement error in allocating personal exposure to PM10 from the ambient monitors, given that spatial variation in PM10 is higher than that of PM2.5.
FEV1 and FVC growth were both affected by PM2.5 and PM10, with very small changes in FEV1/FVC ratio growth. FEV1/FVC ratio is considered to be a marker of obstructive diseases.15 Even though the effects on the ratio were small, because of the inherently higher measurement error (the ratio combines the measurement error in both FEV and FVC measurements), the effect seen is important and suggests early changes that could lead to diseases such as asthma and chronic obstructive pulmonary disease (COPD). However, we also observed a decrease in FVC growth, indicating that the effects of PM exposure had a restrictive component. Though we cannot be certain of the underlying etiopathology, animal studies suggest that postnatal PM exposure is associated with decreased proliferation of lung epithelium and development of lung architecture, which has implications for gas exchange and overall growth of the lungs.16, 17
Our results are similar to results of other studies. In a study carried out in 3170 Mexico City children, aged 8 to 11 years, from 1996 to 1999, there were decreases in FVC and FEV1 growth associated with air pollution, as well as an increase in the FEV1/FVC ratio, which the authors suggested could arise from restrictive changes.18 The Children’s Health Study (CHS) of 1759 children aged 10 years followed until the age of 18, in 12 communities in Southern California, also found lower FEV1 growth, but not FVC growth, in association with ambient PM exposure.4 A similar pattern, of lower FEV1 growth but not FVC growth in association with exposure to winter PM, was seen in the study carried out by Horak et al among 975 Austrian children aged approximately 8 at baseline and followed for three years (1994–1997).7 In another study among 1983 children in Guangzhou, China (2006–2007), FEV1 growth but not FVC growth over six months, was lower among the children living in the more highly polluted district as compared with those living in the less polluted district.22
We summarize the estimated effects of PM10 and FEV1 growth across these studies in Table 3. The CHS,4 Mexico City18 and Austrian7 studies were included because these study designs were similar to the current study and children were followed for at least two years. The Guangzhou study22 was excluded because it followed the children for only six months and did not specifically estimate the effect of PM10 on FEV1 growth.
Table 3.
Comparison of the association of PM10 and FEV1 growth in 4 study sites
| Author | Country (years) | No. | Baseline age | Exposure range (μg/m3) | FEV1 growth βa (95% CI) | Covariates |
|---|---|---|---|---|---|---|
| Horak 7 | Austria (1994–1997) | 975 | Grade 2–3 | 11–30 | −83.9 (NR) | sex, baseline height, change in height, atopy, ETS, baseline lung function. |
| Gauderman4 | USA (1993–2001) | 1759 | 10 years | 15–75 | −1.99 (0.31, −4.29) | height, BMI, BMI2, race, asthma, smoking, ETS, exercise (stratified by sex) |
| Rojas-Martinez15 | Mexico (1996–1999) | 3170 | 8–11 years | 53–97 | −7.9(−6.04, −9.88) | age, BMI, height, height by age, time outdoors, ETS, study phase (stratified by sex) |
| Roy et al (current study) | China (1993–1996) | 3273 | 6–12 years | 95–232 | −0.7(−0.4, −1.0) | baseline age, height, weight, sex, ETS, home ventilation, coal use, parental education, test date |
change in growth rate (ml/year) per 10 μg/m3 of PM10
NR indicates not reported; ETS, environmental tobacco smoke.
In comparison with these earlier studies, our study found a more modest effect of 1.3 ml/year decrease in FEV1 growth for every 10 μg/m3 increase in PM2.5 and 0.7 ml/year deficit in FEV1 growth with every 10 μg/m3 increase in PM10. There could be various explanations for this observation. Each study utilized different analytic approaches and there is considerable variation in effects within the previous studies. The PM concentrations in our study were about 2–10 times higher than those reported in the previous studies,4, 12, 18,21, 22 and the Chinese children under study might have had higher prenatal and lifetime exposures to air pollution. Age-adjusted mean FEV1 for the Chinese children at age 10 was lower by almost 70 to 80 ml than that of the same-aged California children participating in the CHS.4 It is possible that the deficits in lung development had already taken place due to lifetime exposures to high levels of air pollution and that the smaller effect seen here reflect a saturation of the response or depletion of susceptible individuals in this population. Lifetime exposure to high ambient air pollution may cause biologic adaptation and make lung-function growth less sensitive due to per-unit increases in PM, as compared with those living in Western cities with lower ambient PM concentrations.
A recent study suggests that exposure- response relationships of PM exposure with cardiovascular mortality could also have steeper effects at lower exposure ranges as compared with higher exposure ranges.23 An insightful discussion of the possible nonlinear effects of PM exposure from different sources is presented in a recent paper by Smith and Peels.24 Nonlinear associations have been documented with other environmental pollutant health effects such as lead exposure and IQ deficits in children,25 cigarette smoking and bladder cancer,26 and polyaromatic hydrocarbon exposure and DNA adducts. 27
Our results, taken with findings of previously published studies, suggest a nonlinear dose-response curve, with larger deficits per unit PM seen at lower exposures. This would need to be confirmed by pooled analyses of all the studies exploring this association.
Other potential reasons for the difference in results from our study compared with other studies include: (1) Possible lower effect estimates due to differences in growth rates and susceptibility across age groups. Our study had the youngest age group (5–12 years vs. 8 and above in the other studies). (2) Accurate and consistent spirometric measurements of lung function for young children are known to be challenging. Though we took considerable precautions to maintain quality, measurement error could bias the association toward the null, causing us to detect a smaller effect than that seen in other older children. (3) Differences in exposure assessment methods as well as exposure ranges in the various studies.(4) Lung function differences due to genetic, lifestyle, nutritional, or environmental differences among the Chinese four-cities study children and the other populations studied.(5) Lower effect estimates due to the absence of a reference group with very low exposure in this study population.
This study had strengths and limitations. The study provides data on a range of exposures higher than that seen in previous studies. The longitudinal nature of the study allows us to explore the temporal nature of the deficits in lung function growth affected by PM pollution. We also carried out various sensitivity analyses in order to ensure that the results of the study did not suffer from selection bias due to loss to follow-up. We explored and controlled for common confounders and important predictors; socioeconomic status was ascertained by maternal and paternal education, occupation, and the type of house and number of rooms in the house. Indoor air pollution measures were adjusted for, including environmental tobacco smoke, presence of ventilation in the house, and indoor coal use for cooking/heating. We did not find important associations between exposure to environmental tobacco smoke and lung function. This could be due to the low variation in smoking rates seen in this population (75% of all fathers were smokers and 99% of all mothers did not smoke). Although we explored a large number of questionnaire-based factors associated with indoor air pollution, only the presence of ventilation devices and coal use were associated with lung function. This is probably due to potential measurement error inherent in questionnaire information compared with actual measurements of indoor air pollution.
We also explored effect modification by sex, but did not find any difference in air-pollution-associated lung function growth rates. We had expected such a difference, given that this was noted in the Guangzhou study22 and the CHS.7 However, the children in both of those studies were older than our study population and, in the case of the CHS, were followed over the period of adolescence where sex differences are more overt. It is possible that sex does not modify the effect of air pollution on lung function growth prior to puberty.
We were unable to ascertain the role of other ambient air pollutants (such as sulfur dioxide and nitrogen dioxide) because we did not measure them directly, and because the government central-site monitoring data provided too few days within a quarter to be useful for estimating quarterly means.12 However, sensitivity analyses in previous publications using two-pollutant or multi-pollutant models did not alter the direction of the associations or provide a better model fit when gaseous co-pollutants were adjusted for.4, 18 Another strength of this study is that PM10 and PM2.5 measurements were carried out in the yards of the schools and all study children also lived within 2 km of the schools; thus the assessment of exposure from this measurement routine is better than information from central monitoring sites, as in most studies. Although we were unable to carry out air sampling every day, the random selection of sampling dates within a quarter should accurately capture the local quarterly mean concentrations.
In conclusion, exposure to ambient PM is associated with deficits in lung function and decreased lung function growth among Chinese children in cities where ambient PM levels were nearly 10 times higher than PM levels measured in Western cities.
Supplementary Material
Acknowledgments
Financial support: The study was funded by the U.S. EPA and the Chinese government. The current work of Drs. Zhang and Roy is partly supported by NIEHS Center grants (P30 ES05022, and 5P30ES007048).
We are grateful to the study participants.
Footnotes
Financial interest declaration: The authors declare they have no competing financial interests.
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com). This content is not peer-reviewed or copy-edited; it is the sole responsibility of the author.
References
- 1.Schwartz J. Air pollution and children’s health. Pediatrics. 2004 Apr;113(4 Suppl):1037–43. [PubMed] [Google Scholar]
- 2.Ginsberg GL, Foos BP, Firestone MP. Review and analysis of inhalation dosimetry methods for application to children’s risk assessment. J Toxicol Environ Health A. 2005 Apr 23;68(8):573–615. doi: 10.1080/15287390590921793. [DOI] [PubMed] [Google Scholar]
- 3.Gotschi T, Heinrich J, Sunyer J, Kunzli N. Long-term effects of ambient air pollution on lung function: A review. Epidemiology. 2008 Sep;19(5):690–701. doi: 10.1097/EDE.0b013e318181650f. [DOI] [PubMed] [Google Scholar]
- 4.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E, Margolis H, Bates D, Peters J. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004 Sep 9;351(11):1057–67. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 5.Avol EL, Gauderman WJ, Tan SM, London SJ, Peters JM. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med. 2001 Dec 1;164(11):2067–72. doi: 10.1164/ajrccm.164.11.2102005. [DOI] [PubMed] [Google Scholar]
- 6.Jedrychowski W, Flak E, Mroz E. The adverse effect of low levels of ambient air pollutants on lung function growth in preadolescent children. Environ Health Perspect. 1999 Aug;107(8):669–74. doi: 10.1289/ehp.99107669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horak F, Jr, Studnicka M, Gartner C, Spengler JD, Tauber E, Urbanek R, Veiter A, Frischer T. Particulate matter and lung function growth in children: A 3-yr follow-up study in austrian schoolchildren. Eur Respir J. 2002 May;19(5):838–45. doi: 10.1183/09031936.02.00512001. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Environment & Forests. State of Environment Report: India-2009. MOEF, Govt. of India; 2009. [Google Scholar]
- 9.Anderson HR, Ruggles R, Pandey KD, Kapetanakis V, Brunekreef B, Lai CK, Strachan DP, Weiland SK ISAAC Phase One Study Group. Ambient particulate pollution and the world-wide prevalence of asthma, rhinoconjunctivitis and eczema in children: Phase one of the international study of asthma and allergies in childhood (ISAAC) Occup Environ Med. 2010 May;67(5):293–300. doi: 10.1136/oem.2009.048785. [DOI] [PubMed] [Google Scholar]
- 10.HEI International Scientific Oversight Committee. Health Effects of Outdoor Air Pollution in Developing Countries of Asia: A Literature Review. Boston, MA: HEI Publications; 2004. Report No.: Special report 15. [Google Scholar]
- 11.Qian Z, He Q, Kong L, Xu F, Wei F, Chapman RS, Chen W, Edwards RD, Bascom R. Respiratory responses to diverse indoor combustion air pollution sources. Indoor Air. 2007 Apr;17(2):135–42. doi: 10.1111/j.1600-0668.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- 12.Qian Z, Zhang J, Wei F, Wilson WE, Chapman RS. Long-term ambient air pollution levels in four chinese cities: Inter-city and intra-city concentration gradients for epidemiological studies. J Expo Anal Environ Epidemiol. 2001 Sep-Oct;11(5):341–51. doi: 10.1038/sj.jea.7500170. [DOI] [PubMed] [Google Scholar]
- 13.Wei F, Teng E, Wu G, Hu W, Wilson WE, Chapman RS, Pau JC, Zhang J. * Ambient concentrations and elemental compositions of PM10 and PM2.5 in four Chinese cities. Environmental Science & Technology. 1999;33(23):4188–4193. [Google Scholar]
- 14.Zhang JJ, Hu W, Wei F, Wu G, Korn LR, Chapman RS. Children’s respiratory morbidity prevalence in relation to air pollution in four chinese cities. Environ Health Perspect. 2002 Sep;110(9):961–7. doi: 10.1289/ehp.02110961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyatt RE, Scanlon PD, Nakamura M. Interpretation of Pulmonary Function Tests. 3. New York: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 16.Frischer T, Studnicka M, Gartner C, Tauber E, Horak F, Veiter A, Spengler J, Kuhr J, Urbanek R. Lung function growth and ambient ozone: A three-year population study in school children. Am J Respir Crit Care Med. 1999 Aug;160(2):390–6. doi: 10.1164/ajrccm.160.2.9809075. [DOI] [PubMed] [Google Scholar]
- 17.Lee D, Wallis C, Wexler AS, Schelegle ES, Van Winkle LS, Plopper CG, Fanucchi MV, Kumfer B, Kennedy IM, Chan JK. Small particles disrupt postnatal airway development. J Appl Physiol. 2010 Jul 15; doi: 10.1152/japplphysiol.00295.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rojas-Martinez R, Perez-Padilla R, Olaiz-Fernandez G, Mendoza-Alvarado L, Moreno-Macias H, Fortoul T, McDonnell W, Loomis D, Romieu I. Lung function growth in children with long-term exposure to air pollutants in mexico city. Am J Respir Crit Care Med. 2007 Aug 15;176(4):377–84. doi: 10.1164/rccm.200510-1678OC. [DOI] [PubMed] [Google Scholar]
- 19.Pinkerton KE, Zhou YM, Teague SV, Peake JL, Walther RC, Kennedy IM, Leppert VJ, Aust AE. Reduced lung cell proliferation following short-term exposure to ultrafine soot and iron particles in neonatal rats: Key to impaired lung growth? Inhal Toxicol. 2004;16(Suppl 1):73–81. doi: 10.1080/08958370490443123. [DOI] [PubMed] [Google Scholar]
- 20.Fanucchi MV, Wong VJ, Hinds D, Tarkington BK, Van Winkle LS, Evans MJ, Plopper CG. Repeated episodes of exposure to ozone alters postnatal development of distal conducting airways in infant rhesus monkeys. Am J Respir Crit Care Med. 2000;161:A615. [Google Scholar]
- 21.Castro HA, Cunha MF, Mendonca GA, Junger WL, Cunha-Cruz J, Leon AP. Effect of air pollution on lung function in schoolchildren in rio de janeiro, brazil. Rev Saude Publica. 2009 Feb;43(1):26–34. doi: 10.1590/s0034-89102009000100004. [DOI] [PubMed] [Google Scholar]
- 22.He QQ, Wong TW, Du L, Jiang ZQ, Gao Y, Qiu H, Liu WJ, Wu JG, Wong A, Yu TS. Effects of ambient air pollution on lung function growth in chinese schoolchildren. Respir Med. 2010 May 12; doi: 10.1016/j.rmed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Pope CA, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke. Circulation. 2009;120:941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 24.Smith KR, Peel JL. Mind the Gap. Environ Health Perspect. 2010;118(12):1643–1645. doi: 10.1289/ehp.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environ Health Perspect. 2005 Jul;113(7):894–9. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vineis P, Kogevinas M, Simonato L, Brennan P, Boffetta P. Levelling-off of the risk of lung and bladder cancer in heavy smokers: an analysis based on multicentric case-control studies and a metabolic interpretation. Mutat Res. 2000;463:103–110. [PubMed] [Google Scholar]
- 27.Lewtas J, Walsh D, Williams R, Dobias L. Air pollution exposure-DNA dosimetry in humans and rodents: evidence for non-linearity at high doses. Mutat Res. 1997;378:51–63. doi: 10.1016/s0027-5107(97)00097-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.