Abstract
Hearing thresholds have been shown to exhibit periodic minima and maxima, a pattern known as threshold microstructure. Microstructure has previously been linked to spontaneous otoacoustic emissions (SOAEs) and normal cochlear function. However, SOAEs at high frequencies (>4 kHz) have been associated with hearing loss or cochlear pathology in some reports. Microstructure would not be expected near these high-frequency SOAEs. Psychophysical tuning curves (PTCs), the expression of frequency selectivity, may also be altered by SOAEs. Prior comparisons of tuning between ears with and without SOAEs demonstrated sharper tuning in ears with emissions. Here, threshold microstructure and PTCs were compared at SOAE frequencies ranging between 1.2 and 13.9 kHz using subjects without SOAEs as controls. Results indicate: (1) Threshold microstructure is observable in the vicinity of SOAEs of all frequencies; (2) PTCs are influenced by SOAEs, resulting in shifted tuning curve tips, multiple tips, or inversion. High frequency SOAEs show a greater effect on PTC morphology. The influence of most SOAEs at high frequencies on threshold microstructure and PTCs is consistent with those at lower frequencies, suggesting that high-frequency SOAEs reflect the same cochlear processes that lead to SOAEs at lower frequencies.
INTRODUCTION
The cochlear amplifier allows detection of low-level sounds (Dallos, 2008). Otoacoustic emissions, signals generated in the cochlea and recordable in the ear canal are a product of this amplification process (Kemp, 1978). Spontaneous otoacoustic emissions (SOAEs) are recordable in the absence of deliberate external acoustic stimulation (see Shera, 2003 for a review and theory). The relationship between SOAEs and various aspects of auditory function has been explored in many species including human, pony, chinchilla, guinea pig, mosquito, frog, lizard, rhesus monkey, and barn owl (Clark et al., 1984; Ruggero et al., 1984; Lonsbury-Martin et al., 1988; Martin et al., 1988; Mayhew et al., 1995; Taschenberger and Manley, 1997; Robert and Gopfert, 2002; Manley, 2004; Nuttall et al., 2004). Following this line of work, our goal is to examine the relationship between SOAEs, hearing thresholds, and psychophysical tuning curves (PTCs) over a wide range of frequencies in human subjects.
Two mechanistic biophysical models involving either local oscillators or global standing wave patterns in the cochlea have been proposed as the source of SOAEs (Gold, 1948; Kemp, 1979; Talmadge et al., 1998; Shera and Guinan, 1999; Shera, 2003). The global models involve multiple internal reflections between certain locations in the cochlea and the basal cochlear junction with the middle ear. SOAEs are found at frequencies corresponding to the locations in the cochlea where these reflections meet phase criteria conducive to constructive summing (Kemp, 1979; Shera, 2003). This general class of models can readily explain the characteristic frequency spacing observed in SOAEs and other otoacoustic and psychoacoustic structure—broadly defined as the pseudo periodic pattern of peaks and valleys as a function of frequency or distance from the base of the cochlea. In contrast, local models attribute SOAEs to isolated generators (e.g., outer hair cells) that oscillate beyond the normal limits presumably due to malfunction of feedback control (Martin and Hudspeth, 1999, 2001). The reader is directed to Shera (2003) for a comparative evaluation of these model classes.
In parallel to the development of these mechanistic models, a debate about whether SOAEs can be generated by local cochlear pathologies has also emerged. Some SOAEs in non-human animals (e.g., chinchilla, primates, etc.) have been linked to cochlear pathology. For example, Clark et al. (1984) were able to induce relatively high-frequency SOAEs (approximately 4 to 6 kHz) in two chinchillas after long-term exposure to octave-band noise. Histopathologic analysis revealed cochlear regions nearly void of normal outer hair cells associated with the characteristic-frequency region of the SOAEs. In a young American Eskimo dog, an SOAE of approximately 9 kHz and 55 dB sound pressure level (SPL) was found at a transition frequency between good and bad sensitivity as measured by auditory brainstem responses (Ruggero et al., 1984). In a guinea pig, an SOAE was measured at 15 kHz along with concomitant spontaneous basilar membrane motion at the 15 kHz tonotopic place (Nuttall et al., 2004).
Comparable case reports in humans are also available. Mathis et al. (1991) described a child with a 55 dB SPL SOAE at 5.6 kHz with a hearing loss between 4 and 8 kHz. A similar connection between a 6.1 kHz SOAE and elevated (i.e., poorer) thresholds was also reported in a 25 yr-old man (Yamamoto et al., 1987). An earlier report connected a 7.5 kHz SOAE to poorer thresholds slightly below the emission frequency in an adult (Ruggero et al., 1983). Taken together, these data indicate that SOAEs at high frequencies (e.g., >4 kHz) can be associated with anatomical anomalies in the cochleae of animals or behavioral threshold elevation in human studies.
SOAEs linkable to cochlear pathology or hearing loss have almost exclusively been observed at high frequencies in both humans (Ruggero et al., 1983; Yamamoto et al., 1987; Mathis et al., 1991) and other species such as chinchilla (Clark et al., 1984) and dog (Ruggero et al., 1984). Furthermore, damage-related SOAEs are often, but not exclusively, observed in cases of notched or abruptly sloping hearing losses (e.g., Ruggero et al., 1983; Mathis et al., 1991), although there have been reports in ears with losses of flat configuration (Yamamoto et al., 1987) as well. Although there are a number of reports linking high-frequency SOAEs with hearing impairment or cochlear damage, the vast majority of SOAEs seem to be by-products of normal cochlear function. In fact, most SOAEs are recorded from normal-hearing human ears, with reported prevalence ranging from 30% to 80% (Strickland et al., 1985; Lonsbury-Martin et al., 1991; Probst et al., 1991; Penner et al., 1993; Talmadge et al., 1993; Kuroda, 2007). The majority of these SOAEs are found below 4 kHz.
The case reports of SOAEs at high frequencies associated with cochlear pathology lead to the question: Are there two classes of SOAEs, one due to normal cochlear function, commonly evident in the lower frequencies, and the other connected to cochlear pathology and shown in several studies to occur at higher frequencies?
The influence of SOAEs on other measures of auditory function has been examined previously. Individuals with multiple SOAEs appear to have better behavioral thresholds in the low and mid frequencies (i.e., from approximately 1 to 6 kHz) than those without SOAEs (Moulin et al., 1991; McFadden and Mishra, 1993). In a comparison of 11 subjects with 4 or more SOAEs to 14 subjects with none, subjects with SOAEs demonstrated better behavioral hearing thresholds in both ears (McFadden and Mishra, 1993). Additionally, Moulin et al. (1991) demonstrated better thresholds in individuals with diagnosed sensorineural hearing loss and SOAEs than those with hearing loss but no SOAEs. Behavioral thresholds often exhibit microstructure (Elliott, 1958), sometimes with depths as large as 15 dB. Threshold minima (best sensitivity) generally coincide with the frequencies of SOAEs (Kemp, 1979; Burns, 2009; Lee and Long, 2012). This linkage between SOAEs and threshold microstructure has been widely recognized at low frequencies. What remains unknown is whether or not the enhancement in behavioral hearing thresholds associated with SOAEs manifests at high frequencies as well. The reports linking high-frequency SOAEs to cochlear pathology suggest that they may have different physiological underpinnings than low-frequency SOAEs.
The presence of SOAEs has also been shown to influence measures of frequency selectivity via PTCs. PTCs represent masker levels needed to just mask a tonal probe fixed in frequency and level as a function of different masker frequencies. Micheyl and Collet (1994) investigated the relationship between frequency selectivity and SOAEs for probe frequencies of 1, 2, and 4 kHz in a tone-on-tone masking paradigm. Subjects with SOAEs exhibited sharper PTCs than subjects without SOAEs at 2 kHz, but not at lower (1 kHz) or higher (4 kHz) frequencies. Micheyl and Collet attributed their finding to extraordinary active processes in the cochlea in the subjects with SOAEs. However, since PTCs were not assessed specifically near or at SOAE frequencies, it is difficult to make a direct connection. Others have examined the influence of SOAEs on PTCs within a single subject, comparing tuning in the ear with SOAEs to the ear without. The results indicate that emitting ears may have better frequency selectivity at frequencies where SOAEs are present as compared to either another frequency in the same ear or an equivalent frequency in non-emitting ears (Bright, 1985, 2007). It is also possible that differences in the perception of maskers or the probe tone in the vicinity of SOAEs is at the root of the observed differences—a notion explored further below.
The measurement of PTCs in the proximity of SOAEs is complicated by the interaction between the SOAE and the external (probe) tone. External tones are known to cause beating with, as well as entrain, an SOAE (Long, 1998). The transition between beating and the absence thereof (i.e., entrainment) depends in part on the amplitudes of the SOAE and the external tone (Long, 1998). Introducing external tones in the presence of SOAEs can cause deviations in SOAE characteristics. For example, presenting tones lower in frequency than the SOAE has been shown to decrease (and occasionally, increase) SOAE frequency and amplitude with the amount of shift dependent on the external tone level (Long, 1998). SOAE behavior in response to higher frequency tones may be more complex; both pushing and pulling of the SOAE in frequency have been observed. In addition, SOAEs themselves are known to fluctuate in both level and frequency over time These interactions between SOAEs and external signals complicate the measurement of PTCs. Long (1984) measured PTCs in subjects with and without microstructure (and presumably, with and without SOAEs although they were not explicitly measured) and found irregularities in PTCs in subjects with threshold microstructure when PTCs were obtained at threshold minima. PTCs in subjects with SOAEs are thus complicated in an unpredictable manner.
While there has been some work exploring the relationship between hearing sensitivity, frequency selectivity, and SOAEs, it has been largely limited to frequencies below 4 kHz. Moreover, PTCs have not always been reported at the SOAE frequency. Further, these relationships have not been explored in the context of a possible dichotomy of sources or mechanisms for low- versus high-frequency SOAEs. Differences in threshold patterns and PTCs between subjects with normal and impaired cochlear function can be expected if an SOAE is associated with pathology of the cochlea. For example, broadened PTCs with tips shifted away from frequencies corresponding to behavioral hearing loss have been demonstrated empirically and modeled for subjects with sensory/neural hearing losses (Warnaar et al., 2013). Inversions have also been observed (Carney and Nelson, 1983). Elevation in threshold and disappearance of pronounced threshold minima have been observed in subjects with induced acoustic trauma (Furst et al., 1992). The vulnerability of PTCs and threshold microstructure to cochlear damage has thus been demonstrated. We were motivated to explore differences in thresholds and PTCs between subjects with low- and high-frequency SOAEs over an extensive range of frequencies using a comparison group of subjects with no measurable emissions. We postulated that subjects with damage-related SOAEs would lack typical threshold microstructure and demonstrate broadened PTCs. Further, we speculated that this was more likely to occur at high frequencies, consistent with previous reports of damage-related SOAEs. Specifically, we sought to answer the following three research questions: (1) Do threshold patterns at SOAE frequencies differ between subjects with SOAEs and those with no measurable SOAEs; (2) do PTC patterns at SOAE frequencies differ between subjects with and without SOAEs; and (3) do anomalous patterns of threshold microstructure and PTCs predominantly appear around SOAEs in a particular frequency range? A priori, it is difficult to determine a precise cutoff frequency between low- and high-frequency SOAEs. Case reports in the literature seem to suggest that an apt cutoff frequency in humans might be approximately 4 kHz. We use 4.3 kHz as an arbitrary boundary to distinguish low- and high-frequency SOAEs as this cut off allowed equal numbers of subjects with low- and high-frequency SOAEs.
METHODS
Subjects
Thirty-four human subjects (7 males, 27 females), between 19 and 35 yrs old [mean = 23 yr, standard deviation (SD) = 4.4] participated in this study. Tympanometry was performed in test ears (Interacoustics Audio Traveler AA220, Interacoustics, Assens, Denmark). All subjects had normal equivalent ear canal volume, peak pressure, and compliance (Roup et al., 1998). Subjects were chosen to form pairs based on similarity in hearing thresholds near the SOAE frequency of the SOAE+ subject. Generally, all subjects were healthy young adults without complaints of hearing loss.
Subjects with SOAEs (SOAE+) were individually matched to subjects with no measurable SOAEs at any frequency (SOAE−) by age, gender, ear, and threshold. An SOAE− subject was paired with an SOAE+ subject as closely as possible using these four parameters. For example, pair 1 contained 2 females, 25 and 27 yrs of age. Thresholds at 10 of the closest test frequencies surrounding the SOAE were matched within 5 dB. Table TABLE I. provides detailed information about the age, gender, ear, and threshold matching between the SOAE+ and SOAE− groups. The presence or absence of threshold microstructure was not used to classify a subject as SOAE+ or SOAE−. Matching on the 4 parameters was carried out as follows: Age (±4 yrs in 16/18 pairs and ±9 yrs in the remaining 2 pairs), gender (17/18 pairs), ear (18/18 pairs), and threshold at 10 frequencies surrounding the SOAE (±4 dB SPL; in 17/18 pairs). Typically, subject matching was carried out such that one SOAE+ subject was matched to one SOAE− subject. However, in two instances, SOAE+ subjects with multiple SOAEs were matched to two SOAE− subjects (thus n = 16 SOAE + subjects and n = 18 SOAE− subjects).
TABLE I.
Pair number, gender, test ear, SOAE frequency (Hz), age (years), and average threshold (dB SPL) for SOAE+ and SOAE− subjects, respectively. Threshold values are an average for each subject at 11 frequencies centered on the SOAE frequency. Note that pairs 3 and 10 used the same female SOAE+ subject (the two ears were tested during separate experimental sessions). Pairs 5 and 8 also used the same female SOAE+ subject (both right ear, tested on separate days for each frequency). There was one male-female match (pair 17). Aside from gender, this pair was matched on all other variables. Age and thresholds of the SOAE+ subject in the pair are presented first in the last two columns.
| Pair number | Gender | Ear | Frequency (Hz) | Age in yrs | Average threshold (dB SPL) around test frequency |
|---|---|---|---|---|---|
| 1 | F | L | 1275 | 25, 27 | 11.88, 11.43 |
| 2 | M | R | 1358 | 20, 19 | 21.80, 18.44 |
| 3 | F | R | 1372 | 22, 22 | 30.20, 30.25 |
| 4 | M | R | 1455 | 26, 24 | 23.65, 18.95 |
| 5 | F | R | 1708 | 19, 21 | 17.65, 18.71 |
| 6 | F | R | 2270 | 21, 22 | 17.37, 20.16 |
| 7 | F | R | 2647 | 19, 20 | 16.66, 19.86 |
| 8 | F | R | 3007 | 19, 26 | 23.48, 22.44 |
| 9 | F | L | 4330 | 35, 26 | 17.50, 19.92 |
| 10 | F | L | 4575 | 22, 24 | 19.38, 11.83 |
| 11 | F | R | 5717 | 23, 27 | 22.64, 19.14 |
| 12 | F | L | 6908 | 21, 18 | 26.56, 26.15 |
| 13 | M | L | 7808 | 20, 21 | 20.17, 21.79 |
| 14 | F | R | 8727 | 22, 21 | 32.56, 30.16 |
| 15 | F | R | 9977 | 29, 21 | 39.03, 37.10 |
| 16 | F | R | 10 585 | 19, 21 | 29.25, 31.75 |
| 17 | F, M | R | 13 245 | 24, 27 | 39.40, 42.40 |
| 18 | F | R | 13 952 | 25, 22 | 55.96, 55.50 |
Instrumentation
Custom software running on a personal computer was used to calibrate sound sources, record SOAEs, and generate signals for threshold measurements. For PTC measurements, Psycon software, version 2.06 (Kwon, 2012) was used for signal generation. Analog-to-digital and digital-to-analog conversion was performed using a sampling rate of 44 100 Hz (24 bit; MOTU828 MKII input/output Firewire device, MOTU, Cambridge, MA) for SOAE and threshold measurements. Digital signals were generated by custom software, converted to analog signals by the soundcard, amplified by an Etymotic Research H4C low distortion power amplifier (Etymotic Research, Elk Grove Village, IL), and delivered to MB Quart 13.01HX transducers (Maxxsonics, Lake Zurich, IL). The drivers were coupled to an ER-10B+ probe assembly. Ear canal acoustic signals (i.e., SOAEs) were recorded using an Etymotic Research ER-10B+ microphone and preamplifier (+20 dB gain), digitized (by the soundcard), and stored on the hard disk for offline analysis. The ER-10B+ probe assembly was also used to deliver signals for threshold measurements. For PTC measurements, signals were routed through an Echo Audiofire2 Firewire audio interface (24 bit) (Echo, Santa Barbara, CA).1
In order to deliver stimuli to the tympanic membrane with accurate SPL across a wide frequency range, a multi-step calibration procedure was utilized. This was achieved by accurately measuring the frequency response of the transducers, estimating the SPL delivered at each frequency to the microphone of an ear simulator for different depths of insertion, estimating the depth of insertion in each subject's ear canal, and then applying the appropriate correction for that depth of insertion to obtain an approximately constant SPL at the ear drum (see Lee et al., 2012 for details). Subject and insertion-specific calibration was carried out during each experimental session for threshold measurements between 0.125 and 20 kHz. Subjects were encouraged to remain as still and quiet as possible during measurements and to alert the examiner if any noticeable probe slippage occurred. All measurements were made in a sound-treated audiometric booth with subjects seated comfortably in a reclining chair. The study procedures were approved by the Institutional Review Board of Northwestern University (Evanston, IL).
Measurements
SOAEs
SOAE measurements were made approximately 10 min after the subject's arrival at the test booth. Consent paperwork, otoscopy, and tympanometry were performed in this initial period before the measurement of SOAEs. Three-minute recordings from the ear canal were obtained without external stimulation and filtered using a 300-Hz high-pass filter. The peak frequency and amplitude of the SOAE were determined after spectral averaging 1-s long contiguous windows of the recorded waveform yielding a final frequency resolution of 1 Hz. Typically, the SOAE with the greatest amplitude was selected for assessment following visual examination of the spectrum, but in some cases, lower-amplitude SOAEs were chosen in order to maximize the frequency range of study across subjects. All selected SOAEs were at least 3 dB above the noise floor. Before each test session, SOAEs were re-recorded. SOAEs often fluctuated in frequency and amplitude over time, thus threshold microstructure frequencies varied slightly between sessions for most subjects. The SOAEs of interest were recorded with a level of at least 3 dB above the noise floor in all test sessions.
Thresholds
The SOAE frequency (and hence, the center frequency for microstructure measurements) was explicitly obtained at the initiation of each test session based on the SOAE recording from that session. Note that the SOAE frequency itself often fluctuated across test sessions and the microstructure evaluation was adjusted accordingly. Threshold was measured in 0.003 octave-steps over a 1/16-octave range around the test frequency to obtain the microstructure pattern.
Behavioral audiometry was performed with custom software using the signal delivery system described above. Pure tone thresholds were obtained in the test ear at octave and inter-octave frequencies between 0.125 and 8 kHz, as well as at 10, 11.2, 12.5, 14, 15, 16, 17, 18, 19, and 20 kHz in accordance with ISO recommendations (ISO, 1998).
A modified Békésy tracking procedure was employed for all threshold measurements using 250 ms pulsed tones (25 ms rise and fall times) presented two times per second (for additional details, see Lee et al., 2012). Signal attenuation was under control of the subject via a mouse. The subject was instructed to depress the mouse button as long as the signal was audible and release it when the signal became inaudible. Thus, the depressed state of the mouse button caused the signal to be attenuated and the released state caused the signal level to increase. Signals were attenuated in 6 dB steps through the first two reversals and in 2 dB steps thereafter. The midpoints of six upward tracks between reversals were averaged to ascertain a threshold at each frequency. Threshold convergence occurred when the standard error of the mean of the midpoints was less than 1. The thresholds of some SOAE+ subjects were gathered on three separate days and averaged. However, as the within-subjects variability was minimal between sessions, thresholds from 0.125 to 20 kHz were tested only once for the remainder of the SOAE+ subjects and all SOAE− subjects. SOAE− subjects were tested at the same frequencies as their SOAE+ counterpart. Threshold microstructure measurements were repeated on 3 days separated by at least 24 h and averaged. Four subjects (including SOAE− subjects 3, 14, and 15 and SOAE+ subject 17) were unavailable for a third day of threshold microstructure assessment. For these subjects, threshold averages were based on two days of measurement.
Psychophysical tuning curves
PTCs were measured at SOAE frequencies using a simultaneous masking paradigm, where the masker and probe were presented at the same time. Prior to obtaining PTCs, an SOAE recording was done to determine the probe frequency. The probe frequency was set to the SOAE frequency. A 500-ms probe (40-ms raised-cosine ramps) with an inter-stimulus interval of 400 ms was used. Narrowband noise with 0.32-kHz bandwidth (duration of 500 ms with 40-ms raised-cosine ramps) was used as the masker. This masker bandwidth has resulted in the smoothest PTCs in other experiments and has been suggested for probe frequencies above 1.5 kHz (Kluk and Moore, 2004; Sęk et al., 2005). Masker frequencies spanning ±30% of the probe frequency were typically used. In some instances, it was necessary to extend the range to ±50% of the probe to capture a tuning curve with distinct low- and high-frequency “tails.” Between 5 and 12 masker frequencies were used for each PTC measurement. The variation was based on both the number of masker frequencies needed to capture a complete tuning curve, as described above, and time limitations. Typically, PTCs were obtained during one experimental session. In some cases, time constraints resulted in measurements made on two separate days. For these subjects, measurement was repeated at random masker frequencies to ensure between-session reliability.
Detection threshold in quiet (absolute threshold) was measured for the probe. This initial measurement also served to acquaint the subject with the measurement procedure. The absolute threshold value was used to determine the appropriate probe level. The minimum masker levels needed to mask the probe at a fixed level of 10 dB SL (i.e., 10 dB higher than absolute threshold in SPL) were then measured using a two-alternative forced-choice paradigm with a three-down one-up adaptive procedure which estimates the 79.4% point on the psychometric functions (Levitt, 1971). The step size for the masker level was 6 dB for the first 3 reversals, and reduced to 2 dB for the last 4. The final four reversals were averaged to estimate threshold for a run. Final threshold values were based on a minimum of two runs at each masker frequency, or until the SD between trials was below 3 dB. The SD was 3 dB or less for 66% of the final thresholds and it was 5 dB or less for 88%. Typically, 2 to 4 trials per masker frequency were needed to reach a SD of <5 dB.
Analyses
Threshold microstructure was assessed both qualitatively (visually) and quantitatively. In the past, most reports of threshold microstructure have been largely qualitative. Here, we employed a variation of the algorithm developed by Heise et al. (2008) to quantify threshold microstructure characteristics. Briefly, each averaged threshold curve was linearly interpolated in 1 Hz steps using matlab. Smoothing was then performed using a moving average using a window span of 10% of the data length and 1-point step between windows. Minima and maxima were identified as points where the derivative changed sign. The average difference between each minimum and two adjacent maxima was calculated and deemed “microstructure depth.” Depths of ≥5 dB were considered “true” threshold microstructure, a conservative criterion consistent with previous reports (e.g., Horst and de Kleine, 1999). The limited range of threshold measurement (1/16-octave) prohibited a complete calculation of spacing or periodicity, but the octave spacing between the two extrema used for calculations are reported here for reference. Threshold curves from SOAE+ and SOAE− subjects were evaluated using these criteria. Each subject was assigned a microstructure status (present or absent) based on the 5 dB criterion.
PTC data were analyzed offline using matlab. Qualitative and quantitative comparisons of PTCs were extracted following linear interpolation (0.01 Hz steps) between data points. Quantitative analysis of PTCs included identification of tip frequency and sharpness of tuning. The tip frequency was identified as that corresponding to the lowest masker threshold (in dB SPL) on the curve. When tuning curves were inverted, the tip frequency was identified as the highest masker threshold as opposed to the lowest. Sharpness of tuning was assessed via calculation of quality factors (either Q10 or Q30) following interpolation, as described above. The quality factors, Q10 and Q30 were calculated by dividing the center frequency (PTC tip) by the bandwidth 10 or 30 dB from the tip, respectively. High values of Q10 or Q30 correspond to sharper tuning and hence narrower PTCs.
For subjects with inverted PTCs (i.e., threshold maximum aligned with the probe frequency), an inverted Q10 was calculated by using the peak (as opposed to the tip) as the center frequency. Because this metric does not capture frequency selectivity in the same way as the standard Q10, data from these subjects were removed from statistical analysis.
Behavioral thresholds and PTC characteristics were compared between the SOAE+ and SOAE− groups using Wilcoxon Signed Rank Tests in SPSS (version 19.0). A significance level of 5% was established for all statistical tests. Comparisons of mean threshold at standard and extended high frequencies were done via paired-samples t-tests. Bonferroni corrections were applied to account for repeated measures. Because the assumption of normal distribution underlying traditional statistical techniques (e.g., t-tests) could not be assumed for microstructure and PTC data, the non-parametric Wilcoxon Signed Rank Test was used to make between-groups (SOAE+ vs SOAE−) comparisons for PTC parameters including tip frequency, and tuning (Q10 and Q30). In addition, PTC outcomes were compared between SOAE+ subjects with low- and high-frequency emissions using Mann-Whitney U Tests. The 18 subjects were divided into 2 groups of 9 using 4.3 kHz as the cutoff between low and high frequencies. Changing the frequency demarcation between groups to slightly higher or lower frequencies did not alter the statistical findings reported here.
RESULTS
Hearing thresholds at standard and extended high frequencies
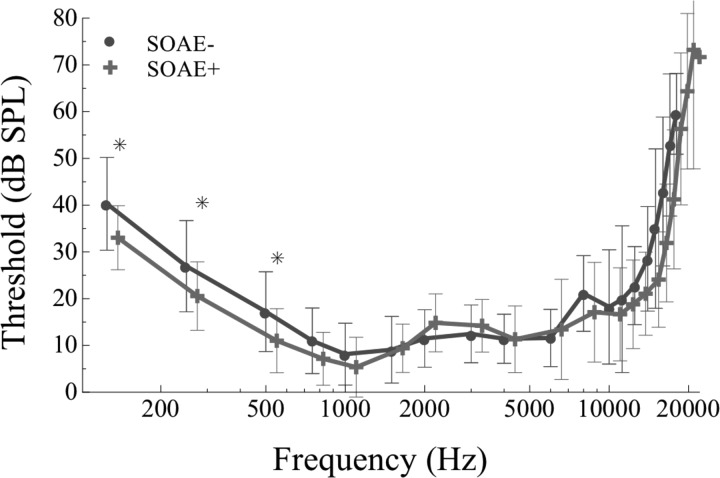
Thresholds from 0.125 to 20 kHz were compared between SOAE+ and SOAE− subjects, revealing generally comparable thresholds across frequency (Fig. 1). Thresholds above 17 kHz exceeded the maximum output of the system in some subjects limiting statistical comparison to 16 pairs at and below 6 kHz, 15 pairs between 6 and 11.2 kHz, 14 pairs between 12.5 and 15 kHz, 13 pairs at 16 kHz, and 12 pairs at 17 kHz. Throughout the entire frequency range, there was a non-significant trend for better thresholds in SOAE+ subjects, with the greatest difference evident at frequencies below 0.750 kHz. Significantly better thresholds were identified at 0.125, 0.25, and 0.5 kHz in SOAE+ subjects via paired samples t-test after Bonferroni adjustment for multiple comparisons (p < 0.05, n = 17 pairs; Fig. 1). At all other frequencies, thresholds were not statistically different between the SOAE+ and SOAE− groups.
Figure 1.
(Color online) Behavioral hearing thresholds for SOAE+ (crosses, n = 16) and SOAE− (circles, n = 18) subjects. Symbols represent mean data and error bars are ±SD of the mean. Global thresholds were assessed once for the majority of subjects. Significantly better thresholds are observable inthe SOAE+ group at 0.125, 0.250, and 0.5 kHz (marked by asterisks, p< 0.05).
Threshold microstructure
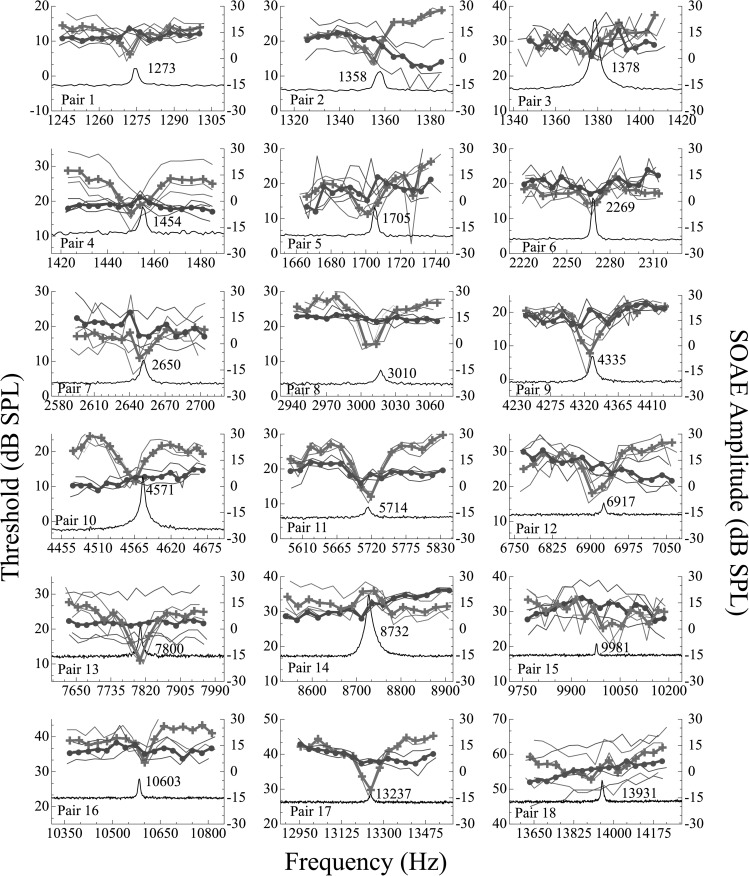
SOAEs were identified between approximately 1.2 and 13.9 kHz. SOAE+ subjects had between 1 and 20 SOAEs per ear with an average of 5.82. Microstructure data are presented for each subject pair in Fig. 2. Together these 18 threshold plots demonstrate that consistent threshold microstructure is measurable in the majority of SOAE+ subjects regardless of SOAE frequency. Moreover, microstructure at high frequencies was often just as prominent as that at low frequencies (pairs 10, 11, 13, and 17 in Fig. 2). In some instances, SOAE+ subjects had only a subtle threshold microstructure (e.g., subjects 15 and 18) upon visual inspection. SOAE− subjects occasionally demonstrated slight threshold rippling, but none with depths satisfying our criterion of ≥5 dB to be categorized as microstructure. Nonetheless, the presence of SOAE-related mechanical activity inside the cochlea of SOAE− subjects that fails to manifest as ear canal SOAEs due to conditions related to reverse transmission cannot be ruled out as a potential factor in this rippling.
Figure 2.
(Color online) Behavioral thresholds in dB SPL as a function of frequency for all subject pairs (n = 18 pairs). Microstructure data for SOAE+ subjects are indicated by the crosses and for SOAE− subjects, the circles. The right ordinate represents SOAE amplitude in dB SPL. The black traces are SOAE spectra in SOAE+ subjects with the frequency indicated next to the peak. Thresholds from each of three sessions are presented as thin lines. Mean data (thick lines) are based on the average of all three trials. The SOAEs under study encompass a wide array of levels and frequencies. Subjects with SOAEs demonstrate microstructure regardless of emission frequency or amplitude with the exception of three cases. Note that SOAE+ subject 14 demonstrates an inverted microstructure pattern, while SOAE+ subjects 15 and 18 do not exhibit microstructure that satisfies the 5 dB criterion.
Quantitative analyses were performed on the average threshold curve for each subject. Results of this analysis can be viewed in Table TABLE II.. The microstructure depth, spacing between adjacent extrema, and microstructure status (present or absent) between the interpolated and smoothed data is indicated for each of the 36 subjects. The latter metric reflects the faithfulness of the analysis at representing the original data. All of the SDs are <0.30, suggesting that smoothed data accurately represented the raw data. Microstructure status was categorized dichotomously as present or absent using ≥5 dB as the criterion for “present.” This quantification corroborates the qualitative findings reported above; namely, 16 of the 18 SOAE+ subjects demonstrated microstructure when analyzed in this way. The two who did not were subjects 15 and 18 who, upon visual analysis, were also noted to lack threshold microstructure. In addition, subject 14 was unique in that the thresholds exhibited microstructure, but the pattern was inverted. SOAE− subjects, although not expected to have microstructure at a frequency where they had no measurable emissions, sometimes demonstrated slight threshold rippling. After quantifying the observed threshold traces, none of the 18 had threshold microstructures with depths ≥5 dB, although some cases were close (e.g., SOAE− subject 3 with a depth of 4.5 dB and subject 7 with a depth of 3.8 dB; see Fig. 2, Table TABLE II.).
TABLE II.
Results of quantitative microstructure analysis. Columns indicate the pair number, SOAE status (present+ or absent−), microstructure depth between adjacent extrema (in dB), spacing between adjacent extrema (in octaves), the average SD between the interpolated and smoothed data, and the microstructure status (present or absent) of each subject using a 5 dB depth criterion. Bolded values in the depth column highlight depths ≥5 dB.
| Pair number | Subject SOAE status (+ or −) | Depth (dB) | Spacing between maxima (octaves) | SD (interpolated and smoothed data) | Microstructure status (present or absent) |
|---|---|---|---|---|---|
| 1 | + | 5.608 | 0.030 | 0.095 | Present |
| 1 | − | 2.342 | 0.017 | 0.074 | Absent |
| 2 | + | 8.182 | 0.266 | 0.089 | Present |
| 2 | − | N/A | N/A | 0.055 | Absent |
| 3 | + | 6.294 | 0.035 | 0.111 | Present |
| 3 | − | 4.524 | 0.025 | 0.174 | Absent |
| 4 | + | 11.055 | 0.044 | 0.134 | Present |
| 4 | − | 2.21 | 0.023 | 0.070 | Absent |
| 5 | + | 11.875 | 0.052 | 0.082 | Present |
| 5 | − | 3.681 | 0.017 | 0.178 | Absent |
| 6 | + | 7.182 | 0.017 | 0.156 | Present |
| 6 | − | 3.30 | 0.019 | 0.218 | Absent |
| 7 | + | 6.677 | 0.021 | 0.210 | Present |
| 7 | − | 3.83 | 0.016 | 0.169 | Absent |
| 8 | + | 12.887 | 0.038 | 0.254 | Present |
| 8 | − | 1.181 | 0.020 | 0.056 | Absent |
| 9 | + | 13.77 | 0.029 | 0.247 | Present |
| 9 | − | 3.491 | 0.021 | 0.151 | Absent |
| 10 | + | 11.654 | 0.037 | 0.154 | Present |
| 10 | − | N/A | N/A | 0.094 | Absent |
| 11 | + | 14.772 | 0.029 | 0.286 | Present |
| 11 | − | 3.328 | 0.026 | 0.117 | Absent |
| 12 | + | 12.3 | 0.029 | 0.222 | Present |
| 12 | − | N/A | N/A | 0.162 | Absent |
| 13 | + | 13.649 | 0.024 | 0.227 | Present |
| 13 | − | 1.02 | 0.020 | 0.042 | Absent |
| 14 | + | 5.721 | 0.020 | 0.202 | Present |
| 14 | − | N/A | N/A | 0.153 | Absent |
| 15 | + | N/A | N/A | 0.270 | Absent |
| 15 | − | 1.553 | 0.015 | 0.127 | Absent |
| 16 | + | 6.757 | 0.020 | 0.146 | Present |
| 16 | − | N/A | N/A | 0.117 | Absent |
| 17 | + | 14.818 | 0.040 | 0.204 | Present |
| 17 | − | N/A | N/A | 0.067 | Absent |
| 18 | + | N/A | N/A | 0.167 | Absent |
| 18 | − | N/A | N/A | 0.055 | Absent |
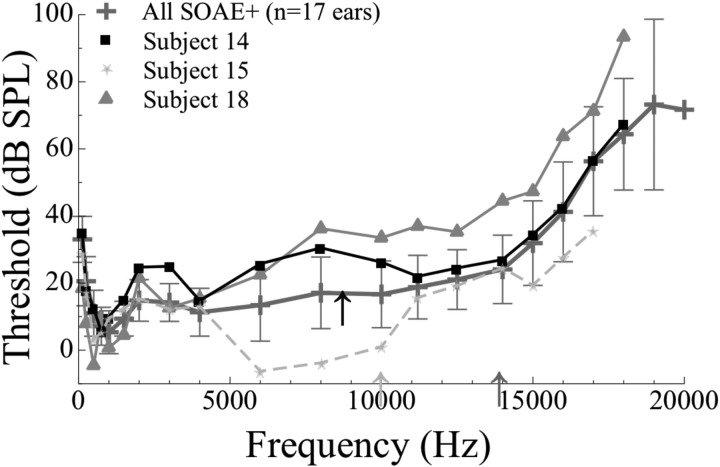
Subjective (visual) and objective analyses revealed three anomalous cases from the SOAE+ group, all at high frequencies in Fig. 2. Subject 14 demonstrated an inverted microstructure pattern with a threshold maximum corresponding to a high-level SOAE (three-day average amplitude of approximately 15 dB SPL) at 8.7 kHz. Though inverted, the depth of the microstructure exceeded 5 dB. Subject 15 exhibited a slightly variable threshold pattern, but no microstructure identifiable using our algorithm, a modification of that of Heise et al. (2008). This subject exhibited a high-frequency, low level SOAE (9.99 kHz, −8.55 dB SPL). Similar to subject 15, the third case (subject 18) had an SOAE at 13.9 kHz (−8.67 dB SPL) and exhibited a threshold pattern drifting upwards with frequency, but no discernable threshold minimum. These three cases provided the only instances where auditory hypersensitivity was not visually (i.e., subjectively) detected at an SOAE frequency, nor was it discovered using our objective analytical tool. To understand the hearing statuses of subjects 14, 15, and 18 better, average thresholds from 0.125 to 20 kHz in the complete SOAE+ group (n = 16 subjects, 17 ears) were examined along with thresholds at the same frequencies from these three individuals (Fig. 3). Subjects 14, 15, and 18 are indicated by black squares, light gray stars, and dark gray triangles, respectively. Note that the thresholds are somewhat elevated in the frequency region of these high-frequency SOAEs for subjects 14 and 18 (i.e., at approximately 8.7 kHz for subject 14 and at 13.9 kHz for subject 18). For subject 15, thresholds were elevated at frequencies directly above the SOAE. Subjects 15 and 18 were similar in this way, whereas subject 14 had a more confined elevation of thresholds at frequencies surrounding the SOAE. The elevated thresholds and the lack of or altered microstructure patterns in these three subjects may be suggestive of the corresponding SOAEs being associated with cochlear pathology.
Figure 3.
(Color online) Global hearing thresholds for subjects with anomalous threshold microstructure patterns (subject 14, black squares; subject 15, light gray stars; and subject 18, dark gray triangles). The SOAE+ mean threshold data are also supplied (crosses, n = 17 ears). Three-day average SOAE amplitude and frequency are indicated for each subject by corresponding arrows. Compared to the mean thresholds of the SOAE+ group, the thresholds at 8 kHz in subject 14 and thresholds between 8 and 18 kHz in subject 18 are elevated. Subject 15 possesses thresholds within or below the rest of the SOAE+ group mean, but the corner frequency of the audiogram appears to be directly above the SOAE. A high level SOAE was recorded at 8.7 kHz in subject 14. Subject 15 and 18 had lower amplitude, but high-frequency SOAEs as shown by the arrows. In these three exceptional cases, the expected patterns of threshold microstructure were not observed.
Psychophysical tuning curves
Individual data
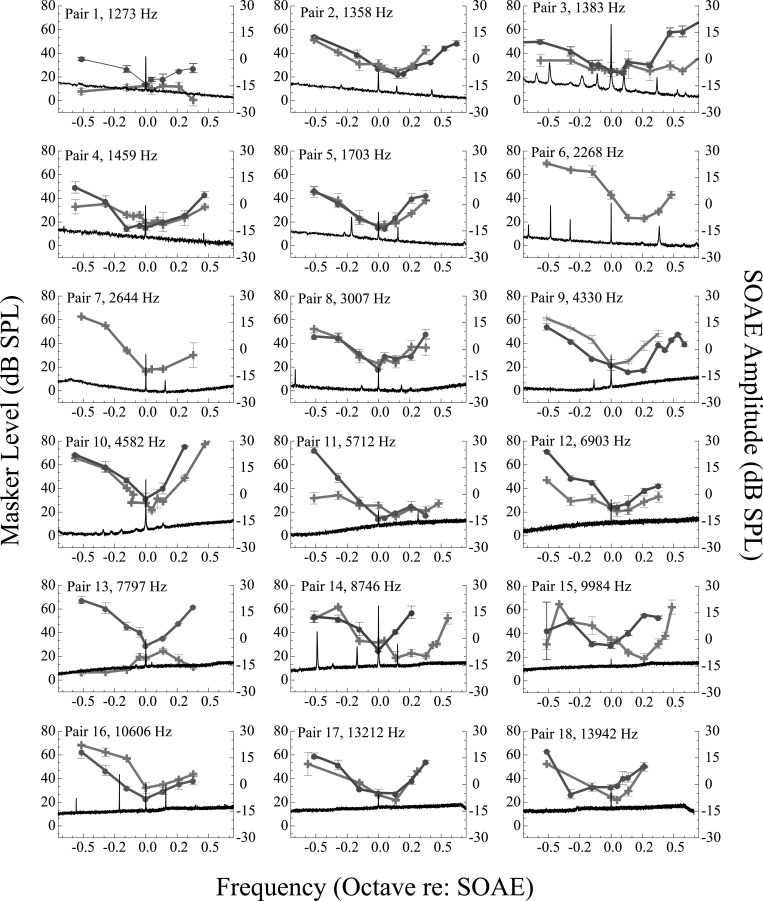
Individual PTCs for all subject pairs are shown in Fig. 4 along with one representative SOAE spectrum. Multiple SOAEs are often visible in the displayed frequency range, but the SOAE of interest aligns with 0 octaves re: SOAE. Tuning curves obtained from SOAE− subjects were mostly typical in their morphology, i.e., they demonstrated characteristic “V” shapes and tips (i.e., minimum masker threshold) at the probe frequency as shown in previous reports (e.g., Kluk and Moore, 2004). In contrast, the morphology of PTCs from the SOAE+ group was distinctly complex and heterogeneous. Commonly, PTCs from SOAE+ subjects exhibited the following characteristics: Typical V-shape (i.e., PTC appeared similar to those of SOAE− subjects, see SOAE+ subject 5); inverted (i.e., the tip of the PTC occurred at the highest masker level instead of the lowest, see SOAE+ subject 1); double-tipped (i.e., PTC demonstrated a non-monotonicity such that two or more minima were ≥4 dB below the nearest adjacent maxima, see SOAE+ subject 14); or shifted tip (tuning curve tip ≥0.10 octave above the SOAE frequency, see SOAE+ subject 15). Some of the PTCs demonstrated more than one of these irregularities. For instance, SOAE+ subject 3 produced a jagged, multi-lobed tuning curve with a shallow tail.
Figure 4.
(Color online) PTCs as a function of frequency for all subject pairs. The PTCs of SOAE+ and SOAE− subjects are indicated by the crosses and circles, respectively. SOAE+ subjects 6 and 7 have no match, thus data are presented unpaired. Error bars are ±1 SD of the mean. Spectra of the SOAEs under study are visible in the lower portion of each panel (black trace) and align with 0 octaves re: SOAE. Subjects with multiple SOAEs are more likely to demonstrate PTCs with shifted tips, as well as PTCs with multiple minima. Subjects with high-frequency SOAEs tend to have PTCs with shifted tips compared to subjects with low-frequency SOAEs. Emission amplitude appears unrelated to these anomalies.
The probability of observing a PTC with atypical morphology was greater in the SOAE+ group. Many subjects from the SOAE+ group (8 out of 18) demonstrated PTCs lacking pronounced tips or the characteristic V-shape. In contrast, expected tuning patterns (i.e., V shape with a tip at the probe frequency) were observed in most of the SOAE− subjects, although some demonstrated nuanced oddities in their PTCs as well (e.g., shifted tip and high-frequency irregularity such as SOAE− subject 9). In another example, SOAE− subject 11 showed a pattern of tuning with a markedly shallow high-frequency side. In general, PTCs with multiple or shifted tips occurred more often at higher frequencies (>4 kHz) than lower frequencies. Two SOAE+ subjects (subjects 1 and 13), exhibited inverted tuning patterns wherein the highest masker levels, not the lowest, occurred at the probe frequency. These inverted PTCs occurred for a low-frequency and a high-frequency SOAE (1.2 kHz in pair 1, 7.8 kHz in pair 13).
Two PTCs (SOAE+ subjects from pairs 3 and 14) were particularly atypical in their expanded tip regions, with indications of more than one minimum. These tuning curves demonstrated two minima ≥4 dB below the nearest maxima. One emerged at a low-frequency (1.4 kHz, pair 3) and the other at a higher frequency (8.7 kHz, pair 14). This feature was subtle and this degree of jaggedness only occurred in two subjects. Hence, it is difficult to make any conclusions concerning the potential cause for the observed behavior.
By far, the most commonly observed morphological feature of the PTCs from SOAE+ subjects was tip shifting. Only four PTCs in the SOAE+ group (see pairs 6, 11, 14, and 17 in Fig. 4) had tips ≥0.10 octaves above the SOAE, but 11 additional PTCs demonstrated tips shifted above the probe to a lesser extent (see pairs 1–4, 11, and 13–18 in Fig. 4).
Group data
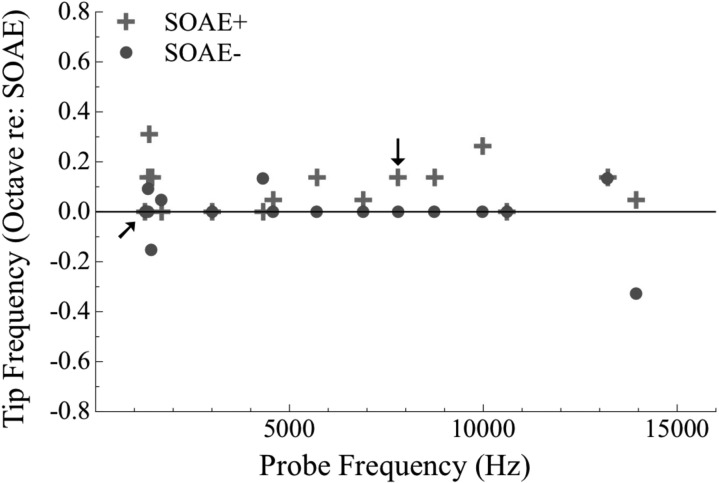
Figure 5 depicts the relationship between tip frequency and probe frequency in both groups. The majority of SOAE− subjects demonstrated PTC tips at the probe frequency while SOAE+ subjects often had tuning curves with tips displaced upwards in frequency regardless of probe frequency. The deviation in tip frequency from the probe frequency was significant between the SOAE+ and SOAE− groups according to a Wilcoxon Signed Rank Test (Z = −2.312, p = 0.021, n = 14). However, the deviation in tip frequency did not differ between subjects with low- and high-frequency SOAEs according to a Mann-Whitney U Test (p > 0.05, n = 14) using subjects 1–9 in the low-frequency group and 10–18 in the high-frequency group. Data from subjects with inverted PTCs are indicated by arrows, but not included in statistical analysis. In two SOAE− subjects, the tip occurred below the probe frequency (see Fig. 4, SOAE− subjects 4 and 18).
Figure 5.
(Color online) Average tip frequency (octave re: SOAE) as a function of probe frequency (Hz) for SOAE+ subjects (crosses) and SOAE− subjects (circles; n = 16 per group). Tip frequency for unpaired data (subjects 6 and 7) are not presented. PTC tip frequencies tend to be higher than probe frequencies in SOAE+ subjects. The majority of SOAE− subjects demonstrate PTC tips aligned with probe frequencies. Arrows indicate values from inverted tuning curves. There is a statistically significant difference in tip frequency between SOAE+ and SOAE− subjects according to a Wilcoxon Signed Rank Test (p < 0.05, n = 14). Tip frequencies between SOAE+ subjects with low- and high-frequency emissions (using 4.3 kHz has the cutoff) did not differ significantly. Tip frequencies from inverted PTCs were not included in these analyses.
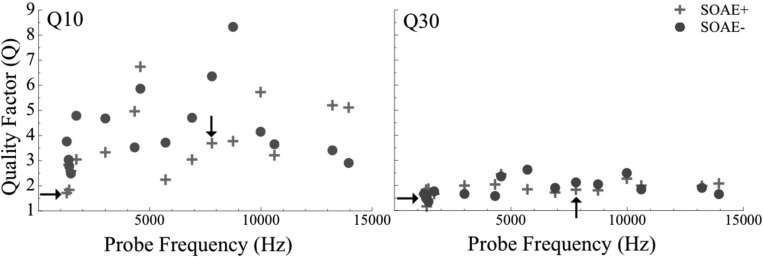
Sharpness of tuning was evaluated via two traditionally used quality factors, Q10 and Q30. Figure 6 shows individual Q10 (left) and Q30 data (right) for SOAE+ (crosses) and SOAE− (circles) subjects. The average tuning values in SOAE+ subjects were 3.84 (SD = 1.37) for Q10 and 1.98 (SD = 0.34) for Q30 using data only from subjects with non-inverted PTCs (N = 16). For SOAE− subjects, the values were similar: Average of 4.26 (SD = 1.53) for Q10 and 1.88 (SD = 0.37). There was not a significant difference in sharpness of tuning between the SOAE+ and SOAE− subjects according to a Wilcoxon Signed Rank Test (p > 0.05, n = 14 pairs [unmatched subjects 6 and 7 also excluded from analysis]). In addition, within the SOAE+ group, Q10 and Q30 were not significantly different between subjects with low- and high-frequency SOAEs according to a Mann-Whitney U Test (p > 0.05, n = 14). Data from subjects with inverted tuning curves were included in Fig. 6 (marked by arrows) but were not used for statistical analysis.
Figure 6.
(Color online) Sharpness of PTCs measured by Q10 (left) and Q30 (right) as a function of probe frequency (Hz). SOAE+ data are presented as crosses and SOAE− data as circles (n = 16 per group). Tuning data for subjects 6 and 7 were removed because control subjects were unavailable for PTC assessment. No frequency-dependent relation between tuning and frequency is observed. Arrows indicate tuning values for subjects with inverted PTCs. The difference in Q10 and Q30 is not statistically significant between SOAE+ and SOAE− subjects according to a Wilcoxon Signed Rank Test (p > 0.05, n = 14 pairs). Similarly, no difference in tuning values was observed between SOAE+ subjects with low-frequency emissions compared to those in the high-frequency group (>4.3 kHz) according to a Mann-Whitney U Test. Tuning data from inverted PTCs were not included in these analyses.
In summary, there were no statistically reliable differences in Q10 and Q30 between the SOAE+ and SOAE− groups, although PTC tip alignment with the probe was significantly different between subjects with and without SOAEs. Tuning curve inversion, multiple tips, and tip shifting were observed in many SOAE+ subjects, but tuning curves measured in the proximity of SOAEs above 4 kHz exhibited these behaviors more commonly.
DISCUSSION
Many previous reports of threshold microstructure have demonstrated a connection between SOAEs and threshold minima in normal hearing subjects (e.g., Zwicker and Schloth, 1984; Long and Tubis, 1988; Lee and Long, 2012). In contrast, some high-frequency SOAEs have been associated with hearing loss and cochlear pathology in the literature (Ruggero et al., 1983; Yamamoto et al., 1987; Mathis et al., 1991). Hence, an apparent dichotomy between low- and high-frequency SOAEs has emerged. Aside from the occasional case report, though, evidence has been lacking to unequivocally link high-frequency emissions and cochlear pathology. Before evaluating differences in psychoacoustical behavior between subjects with low- and high-frequency SOAEs, we endeavored to determine if the presence of SOAEs themselves would influence tuning and thresholds. Hence, we first compared the two behaviors between subjects with and without SOAEs and then delved further into the SOAE+ group to determine whether or not abnormal behavioral patterns were associated with SOAEs in a particular frequency range. The objective of these experiments was to confirm or refute frequency-specific influences of SOAEs on threshold microstructure and psychophysical tuning. To this end, a control group of individuals with no measurable SOAEs was used for reference (SOAE−). Subjects with SOAEs (SOAE+ group) demonstrated microstructure depth at SOAE frequencies in the majority of cases of at least 5 dB. In contrast, though predictably, SOAE− subjects did not exhibit microstructure in the isolated frequency range investigated. Note that the range of threshold exploration was quite limited; therefore we cannot exclude the possibility that SOAE− subjects may indeed have threshold microstructure at frequencies not examined here. For PTCs, SOAE+ subjects had significantly shifted tuning curve tips (toward higher frequencies) compared to SOAE− subjects. No other statistically significant differences between SOAE+ and SOAE− subjects were identified. In general the effects of SOAEs on the behavioral measures did not appear to be systematically dependent on SOAE amplitude.
Among individuals with measurable SOAEs, no substantial differences were found in the microstructures between those with low- and high-frequency SOAEs. While we arbitrarily chose an approximate boundary at 4.3 kHz to segregate low- and high-frequency emissions, moving this fence to any other frequency would yield the same result: No statistically significant differences in threshold microstructure and PTCs recorded in the vicinity of high- and low-frequency SOAEs. Although traditional quantifications of tuning (e.g., Q10) did not differ markedly between the SOAE+ and SOAE− groups, SOAE+ subjects often exhibited atypical patterns of tuning such as inversion, tip shifting, or multiple tips. Such irregularities were more likely to occur for subjects with high-frequency SOAEs than low-frequency SOAEs. Taken together, the observations presented herein support the notion that the vast majority of human SOAEs are not associated with cochlear damage, and when high-frequency emissions are linked to pathology, it is the exception, not the rule. Here, we discuss these results against the background of current theories of SOAE generation, previous literature comparing hearing sensitivity between ears with and without SOAEs, and the influence of SOAEs on behavioral threshold and tuning across frequency.
SOAE generation
In some sense, the experiments reported here were performed as a means of exploring contrasting models of SOAE generation, i.e., the model proposed by Gold (1948) and others (Martin and Hudspeth, 1999, 2001) versus a model of stabilized cochlear standing waves (e.g., Shera, 2003). We made the simple assumption based on reports in the literature that such pathology-related SOAEs would likely be observable at higher frequencies and that such SOAEs would not be associated with threshold microstructure and sharp, V-shaped PTCs in their vicinity.
Our results suggest a common source for SOAEs at all frequencies as we observed characteristic threshold microstructure and normal tuning behavior for the great majority of the SOAEs studied at frequencies between approximately 1 and 14 kHz. Isolated instances of inverted threshold microstructure and PTCs were observed, but were equally likely to be seen near low- or high-frequency SOAEs. However, the propensity for shifted tips and other anomalous tuning curve features was greater near SOAEs at higher frequencies.
Comparing hearing sensitivity in ears with low- and high-frequency SOAEs
Overall hearing sensitivity has been shown to be better in ears with SOAEs than those without (Moulin et al., 1991; McFadden and Mishra, 1993; Kuroda, 2007). Such a comparison cannot be readily made from our data as hearing thresholds around SOAE frequencies were matched within subject pairs. The SOAE+ group had statistically significantly better thresholds at 0.125, 0.25, and 0.5 kHz (see Fig. 1). It should be noted that none of the SOAEs studied were lower in frequency than 1 kHz. Therefore thresholds were not matched within pairs by design at these frequencies.
Given the reports linking high-frequency (and high level) SOAEs to hearing loss in humans (Huizing and Spoor, 1973; Ruggero et al., 1983; Yamamoto et al., 1987; Mathis et al., 1991) and animals (Clark et al., 1984; Ruggero et al., 1984; Nuttall et al., 2004), we expected threshold microstructure to be either absent or altered near SOAEs at high frequencies. Contrary to this expectation, SOAE+ ears produced normal-like threshold microstructures across the wide frequency range examined. Threshold microstructure was repeatable for almost all subjects, including those with the highest frequency SOAEs. Moreover, high-frequency threshold microstructure was often as sizeable as that observed at low frequencies (cf. subjects 4 and 17, with depths of 11.06 and 14.82 dB, respectively; Table TABLE II.). None of the control subjects demonstrated threshold variation that could be identified as microstructure, according to our criterion depth of ≥5 dB, consistent with previous findings of Lee and Long (2012). However, it should be noted that this finding does not unequivocally indicate there is no threshold microstructure in SOAE− subjects at any frequency and in fact, some SOAE− subjects exhibited depths >3 dB (including subjects 3, 5, 6, 7, 9, and 11). The frequency range under study was very limited in the experiments reported here (1/16-octave). SOAE− subjects may have repeatable microstructure in other, unobserved, frequencies, corresponding to cochlear activity with insufficient energy to be measurable in the ear canal (i.e., low-level SOAEs).
The microstructures obtained from the SOAE+ subjects 14, 15, and 18 were exceptional (see Fig. 3). The SOAE+ subject in pair 14 exhibited inverted microstructure associated with a high-frequency emission (8.7 kHz). SOAE+ subjects 15 and 18 lacked microstructure associated with SOAEs around 9 and 14 kHz, respectively. It should also be noted that subject 18 presented with multiple SOAEs, though the SOAE studied here was the highest in frequency. The inverted microstructure pattern observed in the SOAE+ subject in pair 14 may be related to the exceptionally high level of the emission (approximately 15 dB SPL). However, it is interesting that both subjects 15 and 18 had markedly poorer thresholds at frequencies just higher than the SOAEs. The thresholds of these two subjects from 0.125 to 20 kHz differed with respect to how they compared to the SOAE+ group average (Fig. 3). Subject 18 had poorer thresholds than the group mean at frequencies ≥8 kHz whereas subject 15's thresholds were as good or better than the group average up to 17 kHz. Subject 14 presented with a notched audiogram, while 18 had a more widespread elevation of threshold, beginning around 6 kHz. The microstructure patterns from these three anomalous cases are consistent with the notion of cochlear damage in the vicinity of the SOAEs. Although the thresholds of subject 15 were not poorer than the SOAE+ group average, subject 15's thresholds directly above the SOAE frequency were elevated compared to the subject's own thresholds at lower frequencies (i.e., the subject had a high-frequency sloping audiogram, with the corner frequency corresponding to the SOAE frequency). The effects of intermodulation distortion (between the probe and the SOAE or between multiple SOAEs within the ear) as an explanation for these unusual microstructure patterns can be largely ruled out, as such an interference would be highly frequency-specific and the threshold elevation observed in these cases was wide, as can be observed in Fig. 3. However, the scarcity of these observations prevents us from making a more definitive connection between cochlear damage and these particular high-frequency SOAEs. After screening nearly 100 subjects, only one high level, high-frequency SOAE was found—the 8.7 kHz SOAE in pair 14. Overall, our results provide evidence that most high-frequency, low amplitude SOAEs influence behavioral hearing sensitivity in a way comparable to low-frequency SOAEs. Higher amplitude emissions may represent a unique class of SOAEs, with differential effects on threshold. In summary, although caution should be exercised in correlating such SOAEs with cochlear damage, the widespread threshold elevation visible in Fig. 3 does support the postulation that the lack of microstructure in these SOAE+ ears might be related to pathology. That these SOAEs happened to occur at relatively high frequencies (i.e., >8 kHz) may be coincidental, but lends further credence to the argument that if damage-related SOAEs exist, they are more likely to occur at higher frequencies.
One limitation of the present work is that thresholds were assessed over a narrow frequency range around SOAEs. The span covered for each subject was 1/16-octave, which resulted in our ability to capture only very narrow spacing between adjacent extrema. If anything, that means the reported depths here are underestimates of the potential depth of the microstructure in SOAE+ subjects. Measuring threshold microstructure over multiple periods spanning a broader frequency range would lead to more comprehensive estimates of threshold microstructure.
Nearly all SOAE+ subjects (n = 16) demonstrated pronounced threshold minima at SOAE frequencies (crosses, Fig. 2). The frequencies of the microstructure minima were closely aligned with those of the SOAEs. At times, there was a slight discrepancy between average threshold minima and SOAE frequencies (e.g., Fig. 2, SOAE+ subject 16). Small mismatches between microstructure minima and SOAE frequency have been observed before (Long, 1998; Smurzynski and Probst, 1998; Lee and Long, 2012) and attributed to inherent fluctuations in SOAE level and frequency (Whitehead, 1991; Smurzynski and Probst, 1998). In these reports SOAEs have been limited to frequency regions we are labeling as low frequencies with shifts in frequency observed in either direction. Specifically, the majority of SOAEs examined by Whitehead (1991) exhibited downward shifts in frequency. Some emissions explored by Long (1998) also demonstrated pulling to lower frequencies in the presence of lower-frequency external tones. In contrast, the SOAE examined by Smurzynski and Probst (1998) shifted upwards, drawing the microstructure minimum with it. If shifts in microstructure minima are driven solely by shifts in SOAE frequency over time, it is hard to make a definitive conclusion about SOAE drift and threshold minima from our dataset, as the SOAEs were not monitored over the course of threshold recording. Further investigation of the relationship between SOAE frequency, presentation of external tones, and behavioral responses is thus warranted.
The influence of SOAEs on PTCs
Previous studies have shown that both SOAEs and cochlear damage can alter the morphology of PTCs. For example, Long (1984) found that PTC tips sometimes align with nearby threshold minima (and presumably, with SOAEs) and Carney and Nelson (1983) identified inverted PTCs in subjects with hearing impairment. However, PTCs have not been examined in ears with high-frequency, possibly damage-related, SOAEs. Based on previous findings, we hypothesized anomalous tuning patterns in subjects with damage-related SOAEs at any frequency. We also expected some degree of tip shifting due to entrainment (Long, 1988).
SOAE− subjects consistently demonstrated PTCs with predictable V-shaped morphology (see Fig. 4). In two cases (subjects 4 and 18), the PTC tips occurred below the probe frequency. It is possible that this occurred because low level SOAEs were present but not identified during the initial SOAE assessment. In contrast, SOAE+ subjects frequently exhibited atypical PTC morphology irrespective of frequency. Irregularities in PTCs in the vicinity of SOAEs can be explained by the psychoacoustical consequences of the interaction between external stimuli and any SOAEs. In particular, external tones in the vicinity of SOAEs have been shown to induce a sensation of beating prior to the SOAE being entrained by the external tone (Long, 1988). Fluctuations in the frequency of the SOAE, when large in magnitude, would cause beating, aiding detection of the probe, and requiring a higher masker level. In contrast, fluctuations in SOAE amplitude would result in fluctuations in the perceived probe level, thereby necessitating a change in the level of the masker required to just mask the probe tone. Therefore it is not surprising that PTCs measured near SOAEs were more variable in their morphology.
An example of the influence of SOAEs on PTCs is the frequent occurrence of multiple tips. PTCs with this type of morphology were found at low (1.4 kHz) and high (8.7 kHz) SOAE frequencies (see Fig. 5, pairs 3 and 14). Both PTCs were measured in subjects with emissions >15 dB SPL in amplitude. Interestingly, these PTCs were most likely to occur in subjects with several closely spaced SOAEs with the secondary tips often aligned in frequency with the adjacent SOAEs. This would suggest that the adjacent SOAEs, when in the noise band, provided additional masking, thereby reducing the level of the masking noise required to just mask the probe tone.
PTC tips were commonly shifted above the probe frequency in SOAE+ subjects (see Fig. 5) with shifts ≥0.10 octaves in approximately half the cases. PTC tip shifting toward higher frequencies is not unlike previous reports in which SOAE suppression curves are displaced toward higher frequencies (Ruggero et al., 1983; Wilson, 1980). There are complex interactions between the probe tone (which was set at the initial SOAE frequency in these experiments), the SOAE itself (which may drift in frequency and amplitude over the course of an experimental session), the masker, and any adjacent SOAEs. Introduction of a low-level probe tone may itself produce alterations in SOAE amplitude or frequency, may cause probe-SOAE entrainment, or release an SOAE from the suppression caused by adjacent SOAEs. SOAEs are known to drift in frequency by approximately 10 to 20 Hz, most commonly toward lower frequencies. The speed of fluctuations in SOAE amplitude or frequency is not fully specified. SOAEs have been shown to fluctuate with heartbeat and blood flow with indications that these fluctuations are primarily in the form of frequency modulation (Long and Talmadge, 1997), and weaker indications of amplitude modulation (Ren et al., 1995). The temporal dynamics of suppression of SOAEs either by external tones or by adjacent SOAEs have also been investigated (Murphy et al., 1995a,b, 1996). Our data seem to indicate that suppression of the target SOAE by the probe releases an adjacent SOAE (from suppression) at a higher frequency. The interaction of this just released SOAE with the probe makes detection of the probe easier, thereby demanding a higher masker level. When the masker effectively suppresses the adjacent SOAE, this perceptual enhancement is eliminated, yielding the tip of the PTC, shifted from the original SOAE frequency. Consistent with this reasoning, PTC tip shifting was more likely to occur in subjects with multiple SOAEs.
In two exceptional cases, PTCs were inverted (Fig. 4, pairs 1 and 13). The initial expectation was that inverted tuning curves would be found in damaged ears emitting high-frequency SOAEs, but these PTCs were measured at a low- and a high-frequency (approximately 1.3 and 7.8 kHz, respectively). Both observations of inverted PTCs were found in subjects with relatively high-level SOAEs (approximately 0 dB SPL). However, PTCs around other SOAEs of similar levels were not inverted. Although the SOAE levels in these subjects were relatively high (approximately 0 dB SPL), other SOAEs of similar amplitude did not result in inversion of PTCs (e.g., SOAE+ subject 3 in Fig. 4). Therefore, inversion of the PTCs in these cases cannot be linked solely to their relatively high levels. Caution should be exercised in any interpretation of these data due to the paucity of inverted PTC observations. Other investigators have identified inverted PTCs, but only for subjects with confirmed cochlear losses (Carney and Nelson, 1983). In our study, both SOAE+ subjects with inverted PTCs exhibited hearing thresholds from 0.125 to 20 kHz, in good agreement with the group average and prominent threshold microstructure (Fig. 2, SOAE+ subjects 1 and 13). Thus, it seems unlikely that the inverted PTCs in these cases were due to cochlear damage. It appears much more likely that the inversion of these tuning curves was caused by an increase in SOAE level during measurement.
Sharpness of tuning, as indicated by Q10 and Q30, neither varied systematically by SOAE frequency, nor did it differ greatly between SOAE+ and SOAE− subjects (Fig. 6). Micheyl and Collett (1994) compared PTCs in subjects with and without SOAEs and noted sharper tuning at 2 kHz in persons with SOAEs. More recently, Bright (2007) showed that ears with SOAEs have sharper tuning than those without. The present work does not corroborate these earlier findings, although it is difficult to make direct comparisons due to procedural differences. Most prior investigations of tuning in SOAE+ subjects did not examine PTCs at precise SOAE frequencies, as we did here. Bright (1985, 2007) did evaluate tuning at SOAE frequencies and observed some PTCs with irregularities such as “W” shapes, but even so, the Q10 values reported in that study were higher at emission frequencies than non-emission frequencies within or between ears. About half of the SOAEs examined in the present investigation were recorded at frequencies above 4 kHz. In contrast, most previous assessments of SOAEs and tuning investigated frequencies below 4 kHz. Here, we found average Q10 values of 3.84 (SOAE+) and 4.26 (SOAE−). Q30 values were lower than Q10, with a mean of 1.98 for the SOAE+ group and 1.88 for the SOAE− subjects. Note these averages reflect only non-inverted PTCs (i.e., n = 16). Our tuning values are consistent with previous reports, such as the Q10 of 4.9 for a probe frequency of 4 kHz and 320-Hz bandwidth masker (Kluk and Moore, 2004). To the best of our knowledge, the only published assessment of tuning at precise SOAE frequencies in multiple subjects is that by Bright (1985). A wide range of Q10 values were reported in that study, from 2 to 18.6, using a continuously presented sinusoidal masker. The range of Q10's reported in the present study is thus consistent with previous reports and more importantly, not strikingly different between subjects with and without SOAEs. Furthermore, no consistent frequency-dependent trend was observed for tuning. The slight differences in Q10 between this study and others may be related to the variable shapes of the PTCs recorded in our SOAE+ group. Objective quantification of tuning using Q10 or Q30 is questionable for tuning curves with exaggerated shapes (e.g., SOAE+ subject 3). Whether or not this metric accurately reflects frequency selectivity in such cases is debatable. Nonetheless, the agreement in Q10 between previous reports (e.g., Kluk and Moore, 2004) and our data, as well as the characteristic V-shape of the curves in SOAE− subjects, confirms that tuning was measured and assessed appropriately in this study. We are not aware of any other report of tuning at high frequencies near SOAEs. To summarize, in the present study, tuning was approximately equivalent, and if anything, the tuning of SOAE+ ears was less sharp than SOAE− ears (Fig. 6). However, these results should be interpreted with caution as atypical PTC morphology in SOAE+ ears posed a challenge in quantifying Q10 and Q30.
CONCLUDING REMARKS
The question of whether some SOAEs observed in human ears are due to cochlear pathology remains unresolved. These data demonstrate that most SOAEs at high frequencies are attributable to normal cochlear function much like that observed at lower frequencies before. These SOAEs, at frequencies as high as approximately 14 kHz, were associated with prominent threshold microstructure. Three SOAE+ subjects among the 18 examined here exhibited an absence or inversion of their threshold microstructure patterns allowing them to be speculatively associated with cochlear damage. The PTCs measured in one of these two subjects (subject 14) was also broadened compared to the SOAE− subject in that subject pair. PTCs proved to be much complicated in the vicinity of SOAEs to be useful in discerning the mechanisms responsible for SOAE generation. Damage-related SOAEs may certainly exist, but they seem to be quite rare. There certainly appears to be no reason to consider any general association between high-frequency SOAEs and cochlear damage.
ACKNOWLEDGMENTS
The authors wish to thank James Dewey, Gayla Poling, Mario Ruggero, Sumaya Sidique, Jonathan Siegel, Beverly Wright, and Wei Zhao for helpful comments of earlier drafts of this manuscript and stimulating discussion. Additional thanks are due to Julia Lee for help with the statistical analyses, Efoe (Femi) Nyatepe-Coo for assistance with data collection, and Samir Datta and Stephen Stachowiak for assistance with data analysis. This work was supported by a grant from the NIH (Grant No. DC008420) and Northwestern University.
Portions of this work were presented in “The influence of spontaneous otoacoustic emissions on threshold microstructure and psychophysical tuning,” in Proceedings of Association for Research in Otolaryngology Conference, San Diego, CA, February 2012.
Footnotes
Two different audio interface devices were used for the SOAE and PTC measurements, as the custom software used to measure PTCs was not compatible with the MOTU828.
References
- Bright, K. E. (1985). “Microstructure audiograms and psychophysical tuning curves from ears with spontaneous otoacoustic emissions,” Doctoral dissertation, University of Arizona, Tucson. [Google Scholar]
- Bright, K. E. (2007). “Spontaneous otoacoustic emissions in populations with normal hearing sensitivity,” in Otoacoustic Emissions, edited by Robinette M. S. and Glattke T. J. (Thieme Medical Publishers, Inc., New York: ), pp. 69–86. [Google Scholar]
- Burns, E. M. (2009). “Long-term stability of spontaneous otoacoustic emissions,” J. Acoust. Soc. Am. 125, 3166–3176. 10.1121/1.3097768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney, A. E., and Nelson, D. A. (1983). “An analysis of psychophysical tuning curves in normal and pathological ears,” J. Acoust. Soc. Am. 73, 268–278. 10.1121/1.388860 [DOI] [PubMed] [Google Scholar]
- Clark, W. W., Kim, D. O., Zurek, P. M., and Bohne, B. A. (1984). “Spontaneous otoacoustic emissions in chinchilla ear canals: Correlation with histopathology and suppression by external tones,” Hear. Res. 16, 299–314. 10.1016/0378-5955(84)90119-9 [DOI] [PubMed] [Google Scholar]
- Dallos, P. (2008). “Cochlear amplification, outer hair cells and prestin,” Curr. Opin. Neurobiol. 18, 370–376. 10.1016/j.conb.2008.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, E. (1958). “A ripple effect in the audiogram,” Nature 181, 1076. 10.1038/1811076a0 [DOI] [PubMed] [Google Scholar]
- Furst, M., Reshef, I., and Attias, J. (1992). “Manifestations of intense noise stimulation on spontaneous otoacoustic emission and threshold microstructure: Experiment and model,” J. Acoust. Soc. Am. 91, 1003–1014. 10.1121/1.402626 [DOI] [PubMed] [Google Scholar]
- Gold, T. (1948). “Hearing. II. The physical basis of the action of the cochlea,” Proc. R. Soc. Med. 135, 429–498. 10.1098/rspb.1948.0025 [DOI] [Google Scholar]
- Heise, S. J., Verhey, J. L., and Mauermann, M. (2008). “Automatic screening and detection of threshold fine structure,” Int. J. Audiol. 47, 520–532. 10.1080/14992020802089473 [DOI] [PubMed] [Google Scholar]
- Horst, J. W., and de Kleine, E. (1999). “Audiogram fine structure and spontaneous otoacoustic emissions in patients with Meniere's disease,” Audiology 38, 267–270. 10.3109/00206099909073033 [DOI] [PubMed] [Google Scholar]
- Huizing, E. H., and Spoor, A. (1973). “An unusual type of tinnitus. Production of a high tone by the ear,” Arch. Otolaryngol. 98, 134–136. 10.1001/archotol.1973.00780020140017 [DOI] [PubMed] [Google Scholar]
- International Standards Organization (1998). (ISO 389-5–1998). Acoustics: Reference zero for the calibration of audiometric equipment. Part 5: Reference equivalent threshold sound pressure levels for pure tones in the frequency range 8 kHz to 16 kHz (International Standards Organization, Geneva, Switzerland: ). [Google Scholar]
- Kemp, D. T. (1978). “Stimulated acoustic emissions from within the human auditory system,” J. Acoust. Soc. Am. 64, 1386–1391. 10.1121/1.382104 [DOI] [PubMed] [Google Scholar]
- Kemp, D. T. (1979). “The evoked cochlear mechanical response and the auditory microstructure–Evidence for a new element in cochlear mechanics,” Scand. Audiol. Suppl. 9, 35–47. [PubMed] [Google Scholar]
- Kluk, K., and Moore, B. C. (2004). “Factors affecting psychophysical tuning curves for normally hearing subjects,” Hear. Res. 194, 118–134. 10.1016/j.heares.2004.04.012 [DOI] [PubMed] [Google Scholar]
- Kuroda, T. (2007). “Clinical investigation on spontaneous otoacoustic emission (SOAE) in 447 ears,” Auris. Nasus. Larynx 34, 29–38. 10.1016/j.anl.2006.09.023 [DOI] [PubMed] [Google Scholar]
- Kwon, B. J. (2012). “AUX: A scripting language for auditory signal processing and software packages for psychoacoustic experiments and education,” Behav. Res. Methods 44, 361–373. 10.3758/s13428-011-0161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J., Dhar, S., Abel, R., Banakis, R., Grolley, E., Zecker, S., and Siegel, J. (2012). “Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration,” Ear Hear. 33, 315–329. 10.1097/AUD.0b013e31823d7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J., and Long, G. (2012). “Stimulus characteristics which lessen the impact of threshold fine structure on estimates of hearing status,” Hear. Res. 283, 24–32. 10.1016/j.heares.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, Suppl. 2, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Long, G. R. (1984). “The microstructure of quiet and masked thresholds,” Hear. Res. 15, 73–87. 10.1016/0378-5955(84)90227-2 [DOI] [PubMed] [Google Scholar]
- Long, G. (1998). “Perceptual consequences of the interactions between spontaneous otoacoustic emissions and external tones. I. Monaural diplacusis and after tones,” Hear. Res. 119, 49–60. 10.1016/S0378-5955(98)00032-X [DOI] [PubMed] [Google Scholar]
- Long, G. R., and Talmadge, C. L. (1997). “Spontaneous otoacoustic emission frequency is modulated by heartbeat,” J. Acoust. Soc. Am. 102, 2831–2848. 10.1121/1.420339 [DOI] [PubMed] [Google Scholar]
- Long, G. R., and Tubis, A. (1988). “Investigations into the nature of the association between threshold microstructure and otoacoustic emissions,” Hear. Res. 36, 125–138. 10.1016/0378-5955(88)90055-X [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin, B. L., Martin, G. K., Probst, R., and Coats, A. C. (1988). “Spontaneous otoacoustic emissions in a nonhuman primate. II. Cochlear anatomy,” Hear. Res. 33, 69–93. 10.1016/0378-5955(88)90021-4 [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin, B. L., Whitehead, M. L., and Martin, G. K. (1991). “Clinical applications of otoacoustic emissions,” J. Speech Hear. Res. 34, 964–981. [DOI] [PubMed] [Google Scholar]
- Manley, G. A. (2004). “Spontaneous otoacoustic emissions in monitor lizards,” Hear. Res. 189, 41–57. 10.1016/S0378-5955(03)00367-8 [DOI] [PubMed] [Google Scholar]
- Martin, G. K., Lonsbury-Martin, B. L., Probst, R., and Coats, A. C. (1988). “Spontaneous otoacoustic emissions in a nonhuman primate. I. Basic features and relations to other emissions,” Hear. Res. 33, 49–68. 10.1016/0378-5955(88)90020-2 [DOI] [PubMed] [Google Scholar]
- Martin, P., and Hudspeth, A. J. (1999). “Active hair-bundle movements can amplify a hair cell's response to oscillatory mechanical stimuli,” Proc. Natl. Acad. Sci. U.S.A. 96, 14306–14311. 10.1073/pnas.96.25.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, P., and Hudspeth, A. J. (2001). “Compressive nonlinearity in the hair bundle's active response to mechanical stimulation,” Proc. Natl. Acad. Sci. U.S.A. 98, 14386–14391. 10.1073/pnas.251530498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis, A., Probst, R., De Min, N., and Hauser, R. (1991). “A child with an unusually high-level spontaneous otoacoustic emission,” Arch. Otolaryngol. Head Neck Surg. 117, 674–676. 10.1001/archotol.1991.01870180110021 [DOI] [PubMed] [Google Scholar]
- Mayhew, I. G., Preston, S. E., Hannant, D., Washbourne, J. R., Johnson, C. B., and Phillips, T. J. (1995). “Spontaneous otoacoustic emission in a pony,” Vet. Rec. 136, 419. 10.1136/vr.136.16.419 [DOI] [PubMed] [Google Scholar]
- McFadden, D., and Mishra, R. (1993). “On the relation between hearing sensitivity and otoacoustic emissions,” Hear. Res. 71, 208–213. 10.1016/0378-5955(93)90036-Z [DOI] [PubMed] [Google Scholar]
- Micheyl, C., and Collet, L. (1994). “Interrelations between psychoacoustical tuning curves and spontaneous and evoked otoacoustic emissions,” Scand. Audiol. 23, 171–178. 10.3109/01050399409047504 [DOI] [PubMed] [Google Scholar]
- Moulin, A., Collet, L., Delli, D., and Morgon, A. (1991). “Spontaneous otoacoustic emissions and sensori-neural hearing loss,” Acta. Otolaryngol. 111, 835–841. 10.3109/00016489109138419 [DOI] [PubMed] [Google Scholar]
- Murphy, W. J., Talmadge, C. L., Tubis, A., and Long, G. R. (1995a). “Relaxation dynamics of spontaneous otoacoustic emissions perturbed by external tones. I. Response to pulsed single-tone suppressors,” J. Acoust. Soc. Am. 97, 3702–3710. 10.1121/1.412387 [DOI] [PubMed] [Google Scholar]
- Murphy, W. J., Tubis, A., Talmadge, C. L., and Long, G. R. (1995b). “Relaxation dynamics of spontaneous otoacoustic emissions perturbed by external tones. II. Suppression of interacting emissions,” J. Acoust. Soc. Am. 97, 3711–3720. 10.1121/1.412388 [DOI] [PubMed] [Google Scholar]
- Murphy, W. J., Tubis, A., Talmadge, C. L., Long, G. R., and Krieg, E. F. (1996). “Relaxation dynamics of spontaneous otoacoustic emissions perturbed by external tones. III. Response to a single tone at multiple suppression levels,” J. Acoust. Soc. Am. 100, 3979–3982. 10.1121/1.417217 [DOI] [PubMed] [Google Scholar]
- Nuttall, A. L., Grosh, K., Zheng, J., de Boer, E., Zou, Y., and Ren, T. (2004). “Spontaneous basilar membrane oscillation and otoacoustic emission at 15 kHz in a Guinea pig,” J. Assoc. Res. Otolaryngol. 5, 337–348. 10.1007/s10162-004-4045-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner, M. J., Glotzbach, L., and Huang, T. (1993). “Spontaneous otoacoustic emissions: Measurement and data,” Hear. Res. 68, 229–237. 10.1016/0378-5955(93)90126-L [DOI] [PubMed] [Google Scholar]
- Probst, R., Lonsbury-Martin, B. L., and Martin, G. K. (1991). “A review of otoacoustic emissions,” J. Acoust. Soc. Am. 89, 2027–2067. 10.1121/1.400897 [DOI] [PubMed] [Google Scholar]
- Ren, T., Zhang, M., Nuttall, A. L., and Miller, J. M. (1995). “Heart beat modulation of spontaneous otoacoustic emissions in Guinea pig,” Acta Oto-Laryngol. 115, 725–731. 10.3109/00016489509139393 [DOI] [PubMed] [Google Scholar]
- Robert, D., and Gopfert, M. C. (2002). “Novel schemes for hearing and orientation in insects,” Curr. Opin. Neurobiol. 12, 715–720. 10.1016/S0959-4388(02)00378-1 [DOI] [PubMed] [Google Scholar]
- Roup, C. M., Wiley, T. L., Safady, S. H., and Stoppenbach, D. T. (1998). “Tympanometric screening norms for adults,” Am. J. Audiol. 7, 55–60. 10.1044/1059-0889(1998/014) [DOI] [PubMed] [Google Scholar]
- Ruggero, M. A., Kramek, B., and Rich, N. C. (1984). “Spontaneous otoacoustic emissions in a dog,” Hear. Res. 13, 293–296. 10.1016/0378-5955(84)90083-2 [DOI] [PubMed] [Google Scholar]
- Ruggero, M. A., Rich, N. C., and Freyman, R. (1983). “Spontaneous and impulsively evoked otoacoustic emissions: Indicators of cochlear pathology?,” Hear. Res. 10, 283–300. 10.1016/0378-5955(83)90094-1 [DOI] [PubMed] [Google Scholar]
- Sęk, A., Alcántara, J., Moore, B. C., Kluk, K., and Wicher, A. (2005). “Development of a fast method for determining psychophysical tuning curves,” Int. J. Audiol. 44, 408–420. 10.1080/14992020500060800 [DOI] [PubMed] [Google Scholar]
- Shera, C. A. (2003). “Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves,” J. Acoust. Soc. Am. 114, 244–262. 10.1121/1.1575750 [DOI] [PubMed] [Google Scholar]
- Shera, C. A., and Guinan, J. J., Jr. (1999). “Evoked otoacoustic emissions arise by two fundamentally different mechanisms: A taxonomy for mammalian OAEs,” J. Acoust. Soc. Am. 105, 782–798. 10.1121/1.426948 [DOI] [PubMed] [Google Scholar]
- Smurzynski, J., and Probst, R. (1998). “The influence of disappearing and reappearing spontaneous otoacoustic emissions on one subject's threshold microstructure,” Hear. Res. 115, 197–205. 10.1016/S0378-5955(97)00193-7 [DOI] [PubMed] [Google Scholar]
- Strickland, E. A., Burns, E. M., and Tubis, A. (1985). “Incidence of spontaneous otoacoustic emissions in children and infants,” J. Acoust. Soc. Am. 78, 931–935. 10.1121/1.392924 [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Long, G. R., Murphy, W. J., and Tubis, A. (1993). “New off-line method for detecting spontaneous otoacoustic emissions in human subjects,” Hear. Res. 71, 170–182. 10.1016/0378-5955(93)90032-V [DOI] [PubMed] [Google Scholar]
- Talmadge, C. L., Tubis, A., Long, G. R., and Piskorski, P. (1998). “Modeling otoacoustic emission and hearing threshold fine structures,” J. Acoust. Soc. Am. 104, 1517–1543. 10.1121/1.424364 [DOI] [PubMed] [Google Scholar]
- Taschenberger, G., and Manley, G. A. (1997). “Spontaneous otoacoustic emissions in the barn owl,” Hear. Res. 110, 61–76. 10.1016/S0378-5955(97)00070-1 [DOI] [PubMed] [Google Scholar]
- Warnaar, B., Jepsen, M. L., and Dreschler, W. A. (2013). “Simulating psychophysical tuning curves in listeners with dead regions,” Int. J. Audiol. 52, 533–544. 10.3109/14992027.2013.795247 [DOI] [PubMed] [Google Scholar]
- Whitehead, M. L. (1991). “Slow variations of the amplitude and frequency of spontaneous otoacoustic emissions,” Hear. Res. 53, 269–280. 10.1016/0378-5955(91)90060-M [DOI] [PubMed] [Google Scholar]
- Wilson, J. P. (1980). “Evidence for a cochlear origin for acoustic re-emissions, threshold fine-structure and tonal tinnitus,” Hear. Res. 2, 233–252. 10.1016/0378-5955(80)90060-X [DOI] [PubMed] [Google Scholar]
- Yamamoto, E., Takagi, A., Hirono, Y., and Yagi, N. (1987). “A case of ‘spontaneous otoacoustic emission,’ ” Arch. Otolaryngol. Head Neck Surg. 113, 1316–1318. 10.1001/archotol.1987.01860120062009 [DOI] [PubMed] [Google Scholar]
- Zwicker, E., and Schloth, E. (1984). “Interrelation of different oto-acoustic emissions,” J. Acoust. Soc. Am. 75, 1148–1154. 10.1121/1.390763 [DOI] [PubMed] [Google Scholar]