Abstract
Background and Purpose
Diffusion-weighted imaging (DWI) is recommended for the evaluation of transient ischemic attack. Perfusion imaging can increase the yield of MRI in transient ischemic attack. We evaluated automated bolus perfusion (the time when the residue function reaches its maximum [TMax] and mean transit time [MTT]) and arterial spin labeling (ASL) sequences for the detection of ischemic lesions in patients with transient ischemic attack.
Methods
We enrolled consecutive patients evaluated for suspicion of acute transient ischemic attack by multimodal MRI within 36 hours of symptom onset. Two independent raters assessed the presence and location of ischemic lesions blinded to the clinical presentation. The prevalence of ischemic lesions and the interrater agreement were1410 assessed.
Results
From January 2010 to 2011, 93 patients were enrolled and 90 underwent perfusion imaging (69 bolus perfusion and 76 ASL). Overall, 25 of 93 patients (27%) were DWI-positive and 14 (15%) were perfusion-positive but DWI-negative (ASL n=9; TMax n=9; MTT n=2). MTT revealed an ischemic lesion in fewer patients than TMax (7 versus 20, P=0.004). Raters agreed on 89% of diffusion-weighted imaging cases, 89% of TMax, 87% o10f010 MTT, and 90% of ASL cases. The interrater agreement was good for DWI, TMax, and ASL (κ =0.73, 0.72, and 0.74, respectively) and fair for MTT (κ=0.43). Diffusion and/or perfusion were positive in 39 of 69 (57%) patients with a discharge diagnosis of possible ischemic event.
Conclusions
Our results suggest that in patients referred for suspicion of transient ischemic attack, automated TMax is more sensitive than MTT, and both ASL and TMax increase the yield of MRI for the detection of ischemic lesions.
Keywords: cerebrovascular disease/stroke, MRI, perfusion imaging, transient ischemic attack
Transient ischemic attacks (TIAs) affect 250 000 patients per year, and >10 000 will experience a stroke within a few days.1–4 Urgent specialized management of TIA appears to reduce the risk of further stroke by up to 80%.5,6 An accurate diagnosis of TIA is essential to initiate adequate acute management but is often difficult. Cohort studies report that 25% to 50% of patients referred for suspicion of TIA have a final diagnosis of a nonischemic event.6,7 In addition, there is a substantial lack of agreement regarding TIA diagnosis among emergency physicians, neurologists, and even stroke specialists, whose judgment is often considered the gold standard.8–11 Brain imaging can detect ischemic “footprints” that confirm the ischemic nature of transient neurological symptoms.12 For approximately a decade, multimodal brain MRI has been used for the management of patients with acute TIA although never evaluated prospectively.13–15 In recent meta-analyses, diffusion-weighted imaging (DWI) demonstrates an acute ischemic lesion among 30% of the patients referred for the evaluation of TIAs, although these lesions are very subtle.16
Mean transit time (MTT) and time to peak maps can reveal ischemic lesions in patients with TIA with a negative DWI.17,18 Our group has reported that manually processed MTT maps can detect ischemic lesions in approximately one third of the patients with TIA who exhibit an ischemic lesion and that half of them have a negative DWI.19 Since then, we have developed the RApid processing of PerfusIon and Diffusion (RAPID) software, which automatically produces deconvolved MTT and the time when the residue function reaches its maximum (TMax) sequences.20 Additionally, we have implemented the use of arterial spin labeling (ASL) automatically processed by commercially available software.20,21 Both sequences have been incorporated in the Stanford MRI protocol used to evaluate patients with acute TIA. Neither ASL nor automated deconvolved perfusion measures have previously been assessed in patients with TIA. Therefore, we sought to evaluate the sensitivity and interrater agreement of automated TMax, MTT, and ASL maps for the detection of ischemic lesions in a cohort of consecutive patients referred for suspicion of TIA.
Methods
Patient Population
Between January 2010 and January 2011, consecutive patients evaluated in the emergency department by our stroke team at the request of the emergency physician for transient neurological symptoms of suspected cerebrovascular etiology that lasted <24 hours, in the absence of any revascularization therapy, were prospectively collected into a TIA registry. Per protocol, these patients were monitored in the emergency department observation unit, and if they had no contraindications, they underwent a multimodal MRI within 12 hours of arrival. Clinical information was prospectively collected during TIA evaluation. Patients scanned within 36 hours after symptom onset were enrolled. The final diagnosis was assigned at discharge by the treating stroke neurologist using clinical information, imaging, and laboratory results. Events were classified as: (1) definite; (2) possible; or (3) unlikely ischemic. The clinically symptomatic location of symptoms (hemisphere or posterior fossa/posterior cerebral artery) was determined based on the history obtained by the attending stroke neurologist before reviewing MRI scans. This study has been approved by the Stanford Administrative Panel on Human Subjects in Medical Research. A waiver of consent was obtained from the Stanford Institutional Review Board that approved this study.
Imaging
Multimodal MRI was performed on a 1.5-T scanner (GE Signa, Waukesha, WI). Sequences obtained were DWI, MR angiography, bolus perfusion and ASL, T1 with gadolinium, gradient echo, T2, brain and neck MR angiography, and fluid-attenuated inversion recovery. DWI was performed with a b-value of 1000 s/mm2; TR/TE 6000/70 ms; field of view 24 cm; matrix 128×128; slice thickness 5 mm skip 1.5 mm; gradients in 3 tetrahedrally encoded directions to create isotropic DWI, and apparent diffusion coefficient maps. ASL maps have been processed as previously described.21 Pseudocontinuous background-suppressed 3-dimensional fast-spin-echo ASL was acquired (TR/TE/label time/postlabel delay 5500/2.5/1500/2000 ms). Cerebral blood flow maps were created using an automated script on the scanner console. Bolus perfusion was performed using gradient-echo echo planar image during passage of 0.1 mmol/kg of either gadopentetate dimeglumine or gadodiamide. Image readout was performed using standard single-shot echo planar image (TR/TE 2000/60 ms). Hemodynamic maps (TMax and MTT) were created using in-house-developed automated arterial input function detection and delay-invariant deconvolution software called RAPID.20
Image Evaluation
MRIs were reviewed by 1 stroke neurologist (J.-M.O.) and 1 neuroradiologist (G.Z.) blinded to all clinical information including symptom side and final diagnosis. Before the evaluation, the raters separately reviewed the MRI of 20 TIA cases from another clinical data set and defined the criteria of a focal ischemic lesion on DWI and perfusion. An acute focal DWI lesion was defined by hyperintensity on b=1000 images. Fluid-attenuated inversion recovery and T2 were reviewed to rule out “shine through.” A corresponding decrease in apparent diffusion coefficient values was confirmatory but was not required. An acute bolus perfusion lesion was defined by the presence of a focal increase of TMax or MTT in a vascular distribution. An ASL lesion was defined by the presence of focal slow flow (arterial transit artifact) in a vascular distribution. Patients with ischemic lesion corresponding to chronic infarction on fluid-attenuated inversion recovery were excluded. Perfusion lesions “agreed” with the suspected hemisphere if they agreed with the reported symptoms and/or there was a concurrent DWI lesion in that location (regardless of reported symptoms). If the posterior circulation was suspected, cases were counted as “agreement” with symptoms if they had a diffusion and/or perfusion lesion in the posterior fossa or posterior cerebral artery territory.
Statistics
Statistical analyses were performed using SPSS (Version 19; SPSS Inc, Chicago, IL). We used χ2, McNemar, and t test to compare continuous variables. A 2-sided probability value of <0.05 was considered statistically significant. Interrater reliability was assessed using a κ statistic. A κ >0.6 was considered good agreement, and a κ >0.8 was considered excellent.22
Results
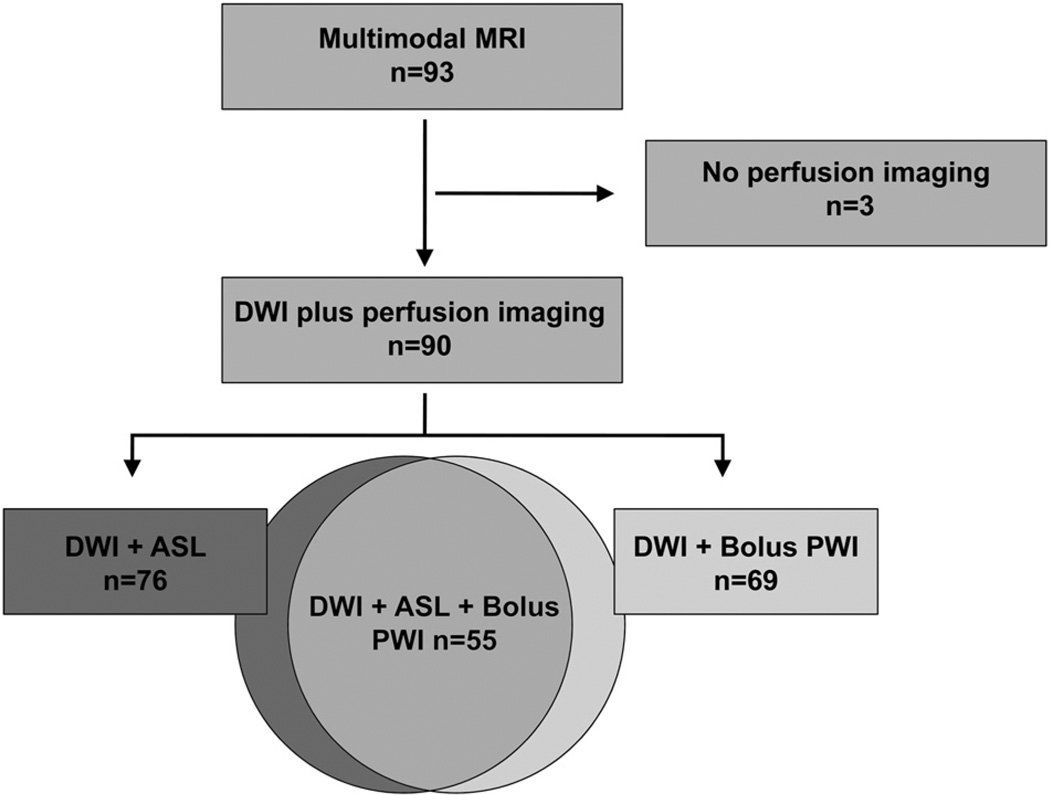
From January 2010 to 2011, 104 patients were evaluated in the emergency department by our stroke team for transient neurological symptoms of presumed vascular mechanism. Eleven patients did not undergo MRI due to: a pacemaker (5), claustrophobia (2), an old metallic clip (1), and other (3). Ninety-three underwent an acute MRI after a median delay of 16.7 hours (interquartile range, 11–26) after symptom onset. Every patient underwent DWI and 90 (97%) received at least 1 perfusion modality (69 bolus perfusion-weighted image, 76 ASL; Figure 1). Baseline characteristics are summarized in Table 1. None of the patients were symptomatic at the time of MRI. After the review of the MRI by the 2 raters, all disagreement was adjudicated by consensus of the 2 readers, and in all cases, agreement was reached.
Figure 1.
Flow chart. DWI indicates Diffusion-weighted imaging; PWI, perfusion-weighted imaging; ASL, arterial spin labeling.
Table 1.
Patient Population
| Demographics | Full Cohort (n=93) |
Definite or Possible TIA (n=69; 74%) |
Unlikely TIA (n=24; 26%) |
|---|---|---|---|
| Age, mean±SD | 69.2±16.3 y | 70±16.5 y | 66.8±15.8 y |
| Sex, female no. (%) | 57 (61%) | 42 (61%) | 15 (62%) |
| ABCD2 median (IQR) | 3 (3–4) | 3 (3–4) | 3 (3–4) |
| Hypertension, no. (%) | 53 (57%) | 40 (58%) | 13 (54%) |
| Diabetes, no. (%) | 20 (22%) | 15 (22%) | 5 (21%) |
| Hyperlipidemia, no. (%) | 48 (52%) | 36 (52%) | 12 (50%) |
| Current smoker, no. (%) | 5 (5%) | 2 (3%) | 3 (13%) |
| Atrial fibrillation, no. (%) | 14 (15%) | 12 (17%) | 2 (8%) |
| Coronary artery disease, no. (%) | 9 (10%) | 6 (9%) | 3 (13%) |
| Prior stroke, no. (%) | 20 (22%) | 17 (25%) | 3 (13%) |
| MRI delay median (IQR) | 16.8 (11–26 h) | 16.4 (10–26 h) | 19.3 (14–29 h) |
TIA indicates transient ischemic attack; IQR, interquartile range.
Full Cohort (n=93)
An ischemic lesion was identified on either diffusion or perfusion imaging (TMax, MTT, or ASL) in 39 of 93 (42%) patients who underwent an acute MRI (25 on DWI and 29 on perfusion; Table 2). Perfusion imaging revealed an ischemic lesion in 14 patients who had a negative DWI. Six of 93 patients (6%) had a symptomatic intra- or extracranial stenosis. They all had either a corresponding acute diffusion (n=4) or perfusion lesion (n=5). Two patients had nonischemic perfusion lesions (both on TMax and ASL, 1 on MTT). The first case had a venous angioma as revealed by gradient echo. The second case had a fetal posterior cerebral artery revealed by MR angiography, which can cause perfusion asymmetry.23 These cases were recognized as nonischemic and were counted as perfusion imaging negative in further analyses.
Table 2.
Yield of Multimodal MRI for the Detection of Ischemic Lesions in the Full Cohort
| Full Cohort | All Patients (n=93) |
DWI+MTT (n=69) |
DWI+TMax (n=69) |
DWI+ASL (n=76) |
DWI+TMax+MTT+ASL (n=55) |
|---|---|---|---|---|---|
| DWI+, no. (%) | 25 (27%) | 18 (26%) | 18 (26%) | 23 (30%) | 16 (29%) |
| Perfusion+, no. (%) | 29 (31%) | 7 (10%) | 20 (29%) | 20 (26%) | 21 (38%) |
| DWI+ and perfusion+, no. (%) | 15 (16%) | 5 (7%) | 11 (16%) | 13 (17%) | 12 (22%) |
| DWI− and perfusion+, no. (%) | 14 (15%) | 2 (3%) | 9 (13%) | 7 (9%) | 9 (16%) |
| DWI+ and perfusion−, no. (%) | 10 (11%) | 13 (19%) | 7 (10%) | 10 (13%) | 4 (7%) |
| DWI+ and/or perfusion+ | 39 (42%) | 20 (29%) | 27 (39%) | 30 (39%) | 25 (45%) |
Results are presented per subgroup defined by the MRI sequence performed: all patients, patients who underwent bolus perfusion (TMax or MTT), ASL, and both TMax and ASL.
DWI indicates diffusion-weighted imaging; MTT, mean transit time; TMax, time of maximum drug concentration; ASL, arterial spin labeling.
Diffusion Imaging (n=93)
An acute ischemic lesion on DWI was found among 25 of 93 (27%) of the patients (Table 2). The 2 readers agreed on the presence of a DWI lesion in 89% (95% CI, 81–94) of the cases and the interrater agreement was good (κ=0.73; 95% CI, 0.57–0.89).
Bolus Perfusion (n=69)
Bolus perfusion was attempted in 69 of 93 patients. A poor bolus occurred in 2 of 69 (3%) patients, although images were of adequate quality for RAPID processing and subsequent interpretation. The most frequent contraindication to bolus perfusion was severe renal insufficiency (n=11). TMax demonstrated an ischemic lesion in 20 of 69 (29%) patients and 9 of 20 (45%) had a negative DWI (Figure 2). The overall agreement between raters was 89% (95% CI, 81–94) and the interrater agreement was good (κ=0.72; 95% CI, 0.56–0.88).
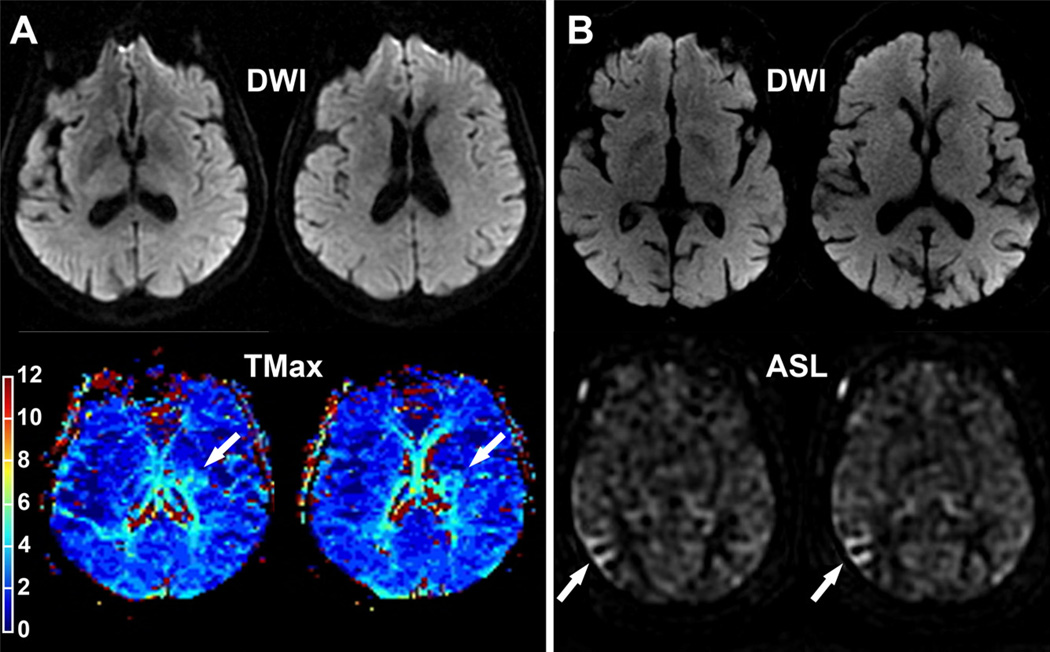
Figure 2.
A, A 75-year-old patient referred for a transient episode of right-sided weakness (ABCD2=4). MRI was performed 5 hours after symptom onset. Diffusion-weighted sequence was negative. TMax revealed a perfusion lesion in the deep left MCA territory (arrow). This lesion progressed into infarction 4 days later when the patient developed a persistent right-sided weakness. B, An 82-year-old right-handed patient with atrial fibrillation referred for a transient episode of left-sided weakness. ABCD2 score=6. MRI was performed 22 hours after symptom onset. Acute DWI was negative. ASL identified hypoperfusion in the right MCA territory (arrow). TMax the time when the residue function reaches its maximum; MCA, middle cerebral artery; DWI, diffusion-weighted imaging; ASL, arterial spin labeling.
MTT revealed an ischemic lesion in fewer patients than with TMax (n=7; P=0.004), and 2 of 7 (29%) had a negative DWI versus 9 of 20 (45%) using TMax. The overall agreement was 87% (95% CI, 78–93) and the interrater agreement was fair (κ=0.43; 95% CI, 0.16–0.70; Table 2).
Arterial Spin Labeling (n=76)
ASL was attempted in 78 of 93 patients and failed in 2 cases (3%) due to patient motion. We detected an ischemic lesion on ASL in 20 of 76 patients (26%) who underwent ASL, and 7 of 20 (35%) had a negative DWI (Figure 2). The overall agreement was 90% and the interrater agreement was good (κ=0.74; 95% CI, 0.57– 0.90).
Bolus Perfusion and ASL (n=55)
Fifty-five of 93 patients (59%) underwent DWI, bolus perfusion, and ASL. Imaging demonstrated an acute DWI lesion in 16 of 55 (29%) patients. ASL revealed an ischemic lesion in 19 of 55 (35%), TMax in 17 of 55 (31%), and MTT in 5 of 55 (9%). In this subgroup, MTT identified fewer ischemic lesion than TMax (P=0.042) or ASL (P=0.001). Every ischemic lesion identified by MTT was captured either by TMax or ASL. TMax and ASL were equivalent (P=0.7).
ASL and/or TMax were positive among 23 of 55 patients (42%; ASL+TMax n=13; ASL alone n=6; and TMax alone n=4). Combined ASL and TMax identified an ischemic lesion among 14 of 16 patients (88%) with a positive DWI (TMax+ASL n=9; ASL alone n=4; TMax alone n=1). Combined ASL and TMax demonstrated an ischemic lesion among 9 DWI-negative patients (ASL+TMax n=4; ASL alone n=2; and TMax alone n=3). In this subgroup, DWI and/or TMax and/or ASL were positive among 25 of 55 (45%) patients.
Definite or Possible Cerebrovascular Events (n=69)
Sixty-nine patients (74%) had a discharge diagnosis of “possible or definite” ischemic event. Diffusion and/or perfusion imaging were positive among 39 of 69 (57%) of these patients. The yield of perfusion imaging in this subgroup is presented in Table 3. Nine patients (13%) had presumed posterior fossa or posterior cerebral artery territory symptoms and 60 had hemispheric symptoms. In 4 DWI-negative patients, the perfusion measures did not agree with the presumed symptom location. Three of these 4 patients were in atrial fibrillation versus 12% of the rest of the cohort (Fisher exact, P=0.01). In 3 of these 4 discordant cases, both ASL and TMax were positive in an identical area. All 4 patients were either in atrial fibrillation and/or had a concordant perfusion lesion on TMax and ASL.
Table 3.
Yield of Multimodal MRI for the Detection of Ischemic Lesions in the Subgroup of Patients With a Discharge Diagnosis of Definite or Possible TIA
| Definite and Possible TIA | All Patients (n=69) |
DWI+MTT (n=51) |
DWI+TMax (n=51) |
DWI+ASL (n=57) |
DWI+TMax+MTT+ASL (n=40) |
|---|---|---|---|---|---|
| DWI+, no. (%) | 25 (36%) | 18 (35%) | 18 (35%) | 23 (40%) | 16 (40%) |
| Perfusion+, no. (%) | 29 (42%) | 7 (14%) | 20 (39%) | 20 (35%) | 21 (53%) |
| DWI+ and perfusion+, no. (%) | 15 (22%) | 5 (10%) | 11 (22%) | 13 (23%) | 12 (30%) |
| DWI− and perfusion+, no. (%) | 14 (20%) | 2 (4%) | 9 (18%) | 7 (12%) | 9 (23%) |
| DWI+ and perfusion−, no. (%) | 10 (14%) | 13 (25%) | 7 (14%) | 10 (18%) | 4 (10%) |
| DWI+ and/or perfusion+ | 39 (57%) | 20 (39%) | 27 (53%) | 30 (53%) | 25 (63%) |
TIA indicates transient ischemic attack; DWI, diffusion-weighted imaging; MTT, mean transit time; TMax, time of maximum drug concentration; ASL, arterial spin labeling.
Discussion
Our results demonstrate that automated sequences TMax, MTT, and ASL increased the yield of MRI for the detection of ischemic lesions in patients referred for suspicion of TIA. TMax and ASL were more sensitive than MTT. The combination of TMax and ASL appears to provide the highest yield and certainty for detecting perfusion abnormalities.
As opposed to TMax, ASL does not require any contrast injection and can be performed in every patient undergoing an MRI for TIA workup. ASL has not previously been assessed in the evaluation of patients with TIA. The ASL maps were interpretable in 97% of the patients. The sensitivity of ASL to detect an ischemic lesion among DWI-negative patients was comparable to TMax. The combination of TMax and ASL detected a higher number of ischemic lesions than either sequence alone and captured every lesion found by MTT. The overall agreement observed with TMax (89%, κ=0.72) and ASL (90%, κ=0.74) was comparable to that found with DWI (89%, κ=0.73).
Two previous studies showed that MTT could identify ischemic lesions in patients with TIA.18,19 We are comparing, for the first time, 2 automated perfusion sequences that can be used in clinical practice. Our results suggest that, as processed by RAPID, the yield of TMax was higher than MTT for the detection of ischemic lesions in patients with acute TIA. One study suggests that time to peak, the nondeconvolved commercially available version of TMax, could also increase the yield of MRI for the detection of ischemic lesions among patients with TIA.17 RAPID is the only bolus perfusion software available on our scanners and does not produce time to peak maps; therefore, we were unable to include time to peak maps in this study.
Previous studies selected patients with final diagnoses of definite ischemic events.17–19 This may have resulted in a higher yield of MRI than would be expected in consecutive cohort sample. In our study, patient characteristics were in line with large cohort studies6,16 (75% with a final diagnosis of possible/definite ischemic event and 27% of positive DWI), suggesting that our cohort was a representative sample of patients typically evaluated for TIA in clinical practice.
The results of our study also underline several limitations of acute MRI in the evaluation of acute TIA. In contrast to acute stroke, in which lesions are obvious and easily outlined on MRI studies, TIA ischemic lesions can be subtle and the possibility of false-positive exists. In the present study, the location of the perfusion lesion was not always consistent with the presumed location of the symptoms. Three patients were in atrial fibrillation, which may account for perfusion abnormalities in an asymptomatic territory. Additionally both ASL and TMax sequences agreed in 3 of the 4 discordant cases. All of these 4 patients were considered as having experienced an ischemic event by the stroke neurologist. Therefore, we believe the perfusion lesions in these 4 cases are unlikely to be false-positive. A limitation of the study is that the results of the MRI scans were evaluated retrospectively, not at a time when their interpretation could have an impact on patient management. Finally, the study was conducted using a single type of MRI (1.5 T); the type of scanner (make and magnet strength) may modify the yield of each sequence.
The results and the limitations of this study suggest that multimodal MRI including DWI, TMax, and ASL may be a powerful tool for the evaluation of acute TIA. These results should be validated in a prospective multicenter study evaluating real-time perfusion interpretation and its impact on patient management and prognosis.
Acknowledgments
Sources of Funding
Dr Kleinman is supported by the Stanford School of Medicine Medical Scholars Program.
Footnotes
Disclosures
None.
References
- 1.Johnston S, Gress D, Browner W, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–2906. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 2.Johnston S, Rothwell P, Nguyen-Huynh M, Giles M, Elkins J, Berstein A, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. 2007;369:283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 3.Chandratheva A, Mehta Z, Geraghty O, Marquardt L, Rothwell P. Population-based study of risk and predictors of stroke in the first few hours after a TIA. Neurology. 2009;72:1941–1947. doi: 10.1212/WNL.0b013e3181a826ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giles M, Rothwell P. Risk of stroke early after transient iscaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2007;6:1063–1072. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- 5.Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432–1442. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
- 6.Lavallee PC, Meseguer E, Abboud H, Cabrejo L, Olivot JM, Simon O, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6:953–960. doi: 10.1016/S1474-4422(07)70248-X. [DOI] [PubMed] [Google Scholar]
- 7.Olivot JM, Wolford C, Castle J, Mlynash M, Schwartz NE, Lansberg MG, et al. Two aces: transient ischemic attack work-up as outpatient assessment of clinical evaluation and safety. Stroke. 2011;42:1839–1843. doi: 10.1161/STROKEAHA.110.608380. [DOI] [PubMed] [Google Scholar]
- 8.Koudstaal PJ, van Gijn J, Staal A, Duivenvoorden HJ, Gerritsma JG, Kraaijeveld CL. Diagnosis of transient ischemic attacks: improvement of interobserver agreement by a check-list in ordinary language. Stroke. 1986;17:723–728. doi: 10.1161/01.str.17.4.723. [DOI] [PubMed] [Google Scholar]
- 9.Kraaijeveld CL, van Gijn J, Schouten HJ, Staal A. Interobserver agreement for the diagnosis of transient ischemic attacks. Stroke. 1984;15:723–725. doi: 10.1161/01.str.15.4.723. [DOI] [PubMed] [Google Scholar]
- 10.Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis. 2008;26:630–635. doi: 10.1159/000166839. [DOI] [PubMed] [Google Scholar]
- 11.Castle J, Mlynash M, Lee K, Caulfield AF, Wolford C, Kemp S, et al. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke-trained neurologists. Stroke. 2010;41:1367–1370. doi: 10.1161/STROKEAHA.109.577650. [DOI] [PubMed] [Google Scholar]
- 12.Ay H, Oliveira-Filho J, Buonanno FS, Schaefer PW, Furie KL, Chang YC, et al. ‘Footprints’ of transient ischemic attacks: a diffusion-weighted MRI study. Cerebrovasc Dis. 2002;14:177–186. doi: 10.1159/000065682. [DOI] [PubMed] [Google Scholar]
- 13.Redgrave JN, Coutts SB, Schulz UG, Briley D, Rothwell PM. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke. 2007;38:1482–1488. doi: 10.1161/STROKEAHA.106.477380. [DOI] [PubMed] [Google Scholar]
- 14.Coutts SB, Simon JE, Eliasziw M, Sohn CH, Hill MD, Barber PA, et al. Triaging transient ischemic attack and minor stroke patients using acute magnetic resonance imaging. Ann Neurol. 2005;57:848–854. doi: 10.1002/ana.20497. [DOI] [PubMed] [Google Scholar]
- 15.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 16.Giles MF, Albers GW, Amarenco P, Arsava EM, Asimos AW, Ay H, et al. Early stroke risk and ABCD2 score performance in tissue- vs time-defined TIA: a multicenter study. Neurology. 2011;77:1222–1228. doi: 10.1212/WNL.0b013e3182309f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Restrepo L, Jacobs MA, Barker PB, Wityk RJ. Assessment of transient ischemic attack with diffusion- and perfusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25:1645–1652. [PMC free article] [PubMed] [Google Scholar]
- 18.Krol AL, Coutts SB, Simon JE, Hill MD, Sohn CH, Demchuk AM. Perfusion MRI abnormalities in speech or motor transient ischemic attack patients. Stroke. 2005;36:2487–2489. doi: 10.1161/01.STR.0000185936.05516.fc. [DOI] [PubMed] [Google Scholar]
- 19.Mlynash M, Olivot JM, Tong DC, Lansberg MG, Eyngorn I, Kemp S, et al. Yield of combined perfusion and diffusion MR imaging in hemispheric TIA. Neurology. 2009;72:1127–1133. doi: 10.1212/01.wnl.0000340983.00152.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straka M, Albers GW, Bammer R. Real-time diffusion–perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–1037. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaharchuk G, Bammer R, Straka M, Shankaranarayan A, Alsop DC, Fischbein NJ, et al. Arterial spin-label imaging in patients with normal bolus perfusion-weighted MR imaging findings: pilot identification of the borderzone sign. Radiology. 2009;252:797–807. doi: 10.1148/radiol.2523082018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landis R, Koch G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 23.Wentland A, Rowley H, Vigen K, Field A. Fetal origin of the posterior cerebral artery produces left–right asymmetry on perfusion imaging. AJNR Am J Neuroradiol. 2010;31:448–453. doi: 10.3174/ajnr.A1858. [DOI] [PMC free article] [PubMed] [Google Scholar]