Significance
Bones adapt to mechanical forces in youth to increase their size and strength but are more at risk for breaking later in life. Do the skeletal benefits of physical activity in youth persist with aging? Here we show at an upper extremity site that half of the benefit in bone size and one-third of the benefit in bone strength obtained from physical activity during youth are maintained throughout life, even though the bone mass benefits are lost. When physical activity was continued during aging, some mass and more strength benefits were preserved. These data suggest that physical activity during youth should be encouraged for lifelong bone health, with the focus being optimization of bone size rather than increasing mass.
Keywords: exercise, intracortical remodeling, osteoporosis, peak bone mass
Abstract
The skeleton shows greatest plasticity to physical activity-related mechanical loads during youth but is more at risk for failure during aging. Do the skeletal benefits of physical activity during youth persist with aging? To address this question, we used a uniquely controlled cross-sectional study design in which we compared the throwing-to-nonthrowing arm differences in humeral diaphysis bone properties in professional baseball players at different stages of their careers (n = 103) with dominant-to-nondominant arm differences in controls (n = 94). Throwing-related physical activity introduced extreme loading to the humeral diaphysis and nearly doubled its strength. Once throwing activities ceased, the cortical bone mass, area, and thickness benefits of physical activity during youth were gradually lost because of greater medullary expansion and cortical trabecularization. However, half of the bone size (total cross-sectional area) and one-third of the bone strength (polar moment of inertia) benefits of throwing-related physical activity during youth were maintained lifelong. In players who continued throwing during aging, some cortical bone mass and more strength benefits of the physical activity during youth were maintained as a result of less medullary expansion and cortical trabecularization. These data indicate that the old adage of “use it or lose it” is not entirely applicable to the skeleton and that physical activity during youth should be encouraged for lifelong bone health, with the focus being optimization of bone size and strength rather than the current paradigm of increasing mass. The data also indicate that physical activity should be encouraged during aging to reduce skeletal structural decay.
Physical activity is recommended for skeletal health because bones adapt to elevated mechanical loading. However, a disparity exists between the time when the skeleton shows greatest plasticity to mechanical loads (during youth) and when it is most at risk for failure (during aging) (1, 2). Do the skeletal benefits derived from physical activity-related loading during youth persist with aging? A popular hypothesis is that physical activity increases peak bone mass to prime the skeleton against the bone loss occurring during aging (3). Prospective observational studies suggest some of the benefits in bone mass generated through physical activity during youth persist into early adulthood (4–9); however, the prospective assessment of lifelong benefits is not practically feasible. Instead, the lifelong skeletal benefits of physical activity during youth can be explored using cross-sectional studies comparing former athletes with controls. Although cross-sectional studies typically do not control for selection bias and secular variations in activity levels, current data suggest that cessation of physical activity after youth is associated with the eventual return of bone mass to control levels (10).
Although the benefits in bone mass acquired through physical activity during youth may be lost, some of the benefits in bone size and strength may persist throughout life. For the purposes of the current work, “bone size” refers to total cross-sectional area, and “bone strength” refers to torsional rigidity. The torsional rigidity of a tubular bone is dependent on its polar moment of inertia, which is calculated from the radii of its outer periosteal (rp) and inner endocortical (re) surfaces as π(rp4 − re4)/2. This relationship demonstrates that a bone is stronger if its material is distributed further from its central axis and that periosteal surface changes have a greater influence on strength than changes on the endocortical surface. For example, assuming constant bone material properties and a typical rp: re ratio of 1.8, a 5% increase in rp (equating to 10% and 15% increases in bone size and mass, respectively) results in a 24% increase in strength. If the same mass of bone added to the periosteal surface was simultaneously removed from the endocortical surface, re would increase by 15%, but the bone would still be 16% stronger because of its 5% greater rp (i.e., size). Because physical activity during youth preferentially deposits new bone on the outer periosteal surface to increase bone size (11–13), and bone loss during aging occurs primarily on the endocortical surface to decrease mass (14, 15), the benefits in bone size and strength acquired through physical activity during youth have the potential to remain independent of the maintenance of benefits in bone mass.
Cross-sectional and prospective observational studies have suggested that some of the benefits in bone size and strength acquired through physical activity during youth persist into early adulthood (5, 8, 16, 17), but whether these benefits persist throughout life remains unanswered. We demonstrated that mechanical loading during a period of rapid skeletal growth conferred lifelong benefits in bone size and strength in rodent models (18, 19). To explore whether the same phenomenon occurs in humans, the current study used a uniquely controlled cross-sectional study design that compared differences in humeral diaphysis properties between the throwing and nonthrowing arms of professional Major (MLB) and Minor (MiLB) League Baseball players at different stages of their careers with the differences between the dominant and nondominant arms in age-matched controls. The use of baseball players was inspired by the bilateral asymmetry initially observed in tennis players by Jones et al. (20) and minimizes the influence of selection bias because the unilateral upper extremity loading and humeral adaptation associated with overhand throwing (21–23) enables the nonthrowing arm to serve as an internal control site for inherited and other systemic traits. Similarly, use of MLB/MiLB players reduces secular variations in physical activity levels because individuals who reached professional level baseball typically threw with high volume from a young age, with throwing being the primary unilateral dominant training modality. Also, the game of baseball has not changed in more than 100 y, and the ball weight and the distance and speed at which the ball is thrown have remained relatively constant across generations. Another distinct advantage of studying former MLB/MiLB players (compared with even tennis players) is that they often retire completely from throwing activities once they stop professional play, allowing us to explore the skeletal benefits of physical activity long after the subjects’ return to habitual loading. By comparing the differences between the throwing and nonthrowing arms in throwing athletes with the differences between the dominant and nondominant arms in age-matched controls, we isolated the skeletal benefits of throwing from the differences caused by the elevated habitual unilateral loading associated with simple arm dominance.
Using professional baseball players as a model, we addressed: (i) the magnitude and location of adaptations associated with throwing-related physical activity; (ii) whether the skeletal benefits of throwing-related physical activity persist lifelong once throwing is ceased; and (iii) whether there are skeletal benefits of continued throwing-related physical activity in later adulthood.
Results
Overhand Throwing Loads the Humerus and Induces Substantial Humeral Adaptation.
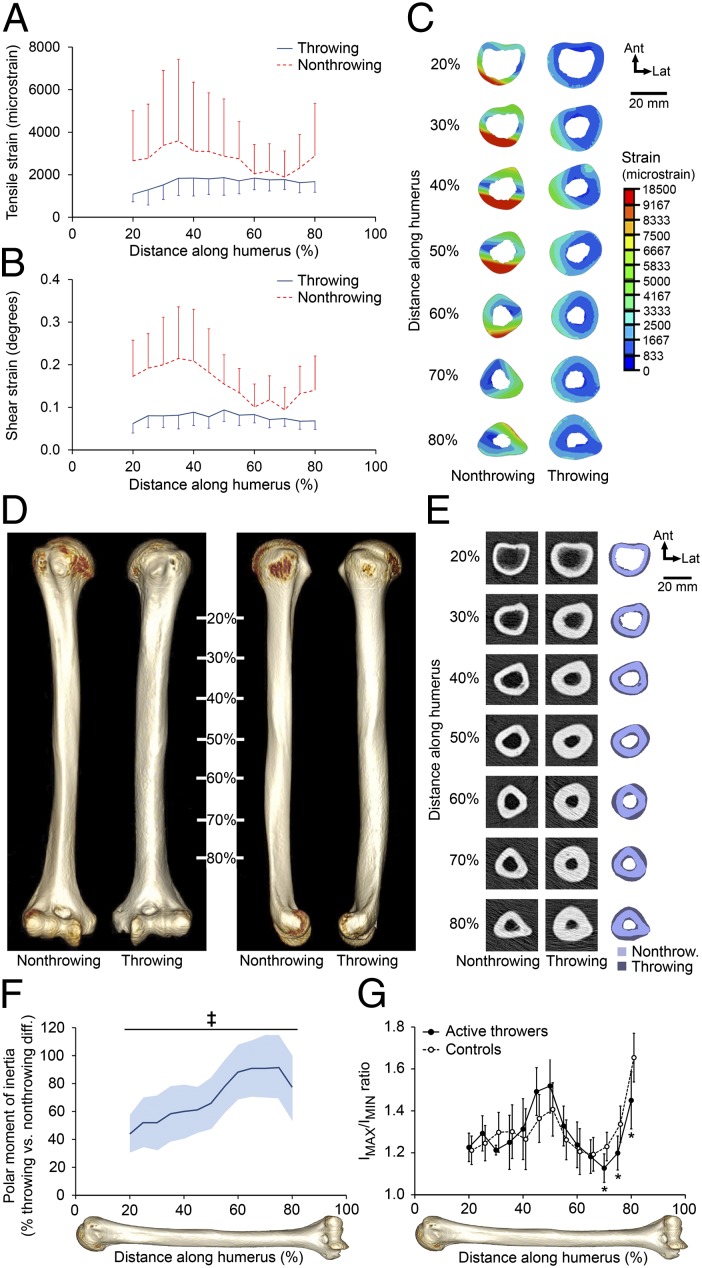
Using a subject-specific musculoskeletal model of the upper limb and a CT-based finite-element model of the humerus (24), we calculated strains within the humeral diaphysis of a MLB player at the time of maximal joint torques during a fastball pitch (Fig. S1). In the throwing arm, median and peak tensile strains (change in element length) within each humeral cross-section ranged from 1,100–1,900 microstrain (Fig. 1A) and 2,588–4,885 microstrain (Fig. 1C), respectively. Median cross-sectional shear strains (change in element angle) ranged from 0.06–0.09° (Fig. 1B). Both the tensile and shear strains were increased and showed greater variability when the same throwing forces were applied to the collateral (nonthrowing) humerus, suggesting that adaptation of the humerus in the throwing arm reduced tissue-level deformation and its spatial variability (Fig. 1 A–C).
Fig. 1.
Overhand throwing loads the humeral diaphysis, inducing skeletal adaptation. (A and B) Median (and median absolute deviation) cross-sectional tensile (A) and shear (B) strains in the humerus of the throwing arm of an MLB player toward the end of the cocking stage of a fastball pitch showed strains throughout the diaphysis. Both tensile and shear strains were increased when the same forces were applied to the collateral, nonthrowing humerus. (C) Cross-sectional distribution of peak tensile strain in the bilateral humerus demonstrated reduced strains in the throwing arm. (D) Reconstructed CT images of the bilateral humerii in a representative MLB/MiLB player demonstrated a more robust diaphysis with visibly broader diameter on the throwing side. (E) Cross-sectional images of the humerii in D revealed substantially greater total and cortical bone areas and cortical thickness and smaller medullary area in the throwing arm. (F) Throwing substantially increased torsional bone strength (indicated by density-weighted polar moment of inertia) along the entire diaphysis, with strength nearly doubled toward the distal diaphysis. Data show the mean percent difference and 95% CI (shaded area) between the throwing arm and the nonthrowing arm in throwers normalized to the differences between the dominant arm and the nondominant arm in controls (‡P < 0.001, unpaired t test). (G) The distal diaphysis in throwers had a more circular cross-section than seen in controls, as indicated by a maximum:minimum (IMAX:IMIN) second moment of area ratio closer to 1 (*P < 0.05, ANCOVA with the contralateral arm as the covariate).
Adaptation to throwing-related strain was assessed via quantitative CT (QCT) of the humeral diaphysis in active throwers (MLB/MiLB pitchers) (n = 9; age = 27.9 ± 2.2 y) and age-matched controls (n = 8; age = 27.1 ± 3.8 y) (Table S1). Active throwers had a more robust diaphysis within their throwing arm than in their nonthrowing arm (Fig. 1 D and E). The differences in torsional strength (indicated by density-weighted polar moment of inertia) between the throwing arm and the nonthrowing arm were substantially greater (≥44%) along the entire diaphysis in throwers as compared with the differences between the dominant arm and the nondominant arm in controls (all P < 0.001) (Fig. 1F). The greatest adaptation was observed distally, with throwers having 91% [95% confidence interval (CI) = 65–118%, P < 0.001] greater side-to-side differences in torsional strength (measured at 75% of the humeral length from its proximal end) compared with controls. The largest difference in torsional strength between the throwing arm and the nonthrowing arm observed within an individual thrower was 158% (Fig. S2). The ratio between the maximum and minimum second moments of area in the distal humeral diaphysis of throwers was closer to 1 than in controls (Fig. 1G), suggesting the development of a more circular bone designed to resist torsion.
The greater torsional strength of the humeral diaphysis in the throwing arm of active throwers resulted from greater total cross-sectional area/size and smaller medullary area (Fig. 1E and Fig. S3 E and G). The pattern of adaptation for total area approximated that of torsional strength, with greatest adaptation observed distally. At 75% of humeral length from its proximal end, the side-to-side differences in total area/size were 41% greater in throwers than in controls. The greater size of the diaphysis in the throwing arm suggests throwing increased periosteal bone formation, whereas the smaller medullary area suggests that throwing increased bone formation and/or decreased bone resorption on the endocortical surface. These surface-specific modeling changes contributed to ∼50% greater mineral content, area, and thickness of cortical bone throughout the diaphysis in the throwing arm (Fig. 1E and Fig. S3 C, F, and H). Throwing had a small negative effect on cortical volumetric bone mineral density, depending on the location of the tomographic slice (Fig. S3A).
Throwing-Related Physical Activity During Youth Confers a Lifelong Benefit in Bone Size and Strength.
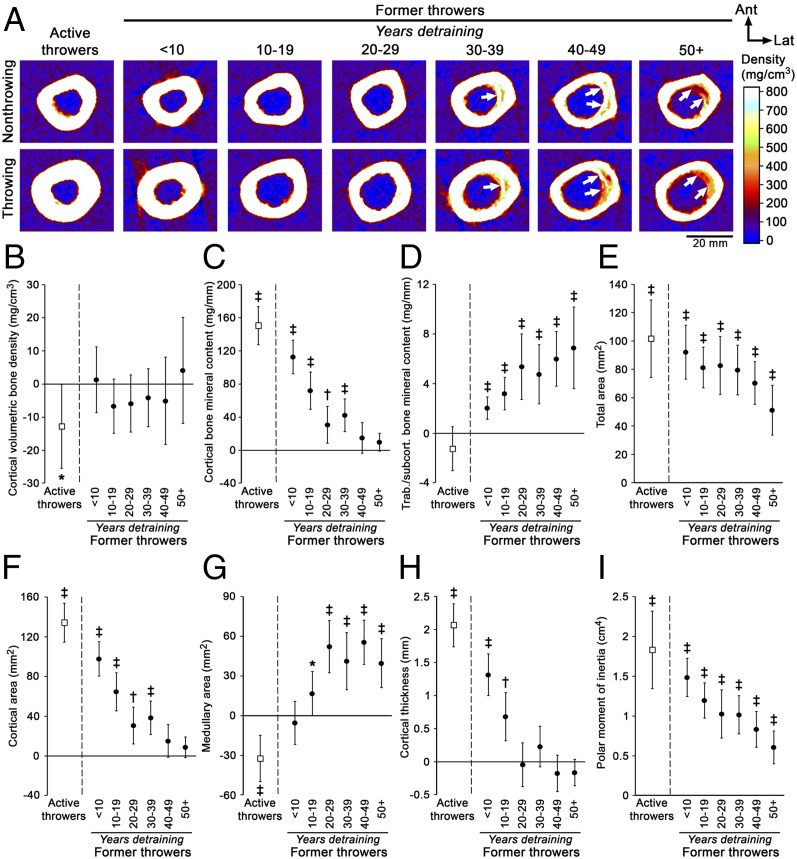
The persistence of the skeletal benefits of throwing during youth was assessed by peripheral QCT of the midshaft humerus in former throwers (MLB players who formerly played as pitcher or catcher) (n = 84) and age-matched controls (n = 86) (Table S2). When former throwers were divided into decades of years detraining (i.e., the number of years since they stopped throwing), there were no cohort differences in the age at which they started throwing, the total number of years they threw, the age of complete cessation from throwing, or the cumulative number of MLB/MiLB games played (all P > 0.05) (Table S2). There were differences in the number of MLB/MiLB innings pitched (P < 0.01), with former throwers who ceased throwing more than 50 y ago pitching more professional innings than those who ceased <10 or 10–19 y ago (all P ≤ 0.04).
To explore the lasting skeletal benefits of throwing among the different age cohorts, absolute side-to-side differences in former throwers and controls were compared to account for the influence of age-related changes in the nonthrowing/nondominant arm on the percent difference calculations. Differences in cortical bone mineral content, area, and thickness observed between the throwing and nonthrowing arms in active throwers were progressively less with each decade of detraining (Fig. 2 C, F, and H). Former throwers in the 40–49 y and 50+ y detraining groups no longer had differences in cortical bone mineral content (Fig. 2C) or area (Fig. 2 A and F) between the throwing and nonthrowing arms as compared with controls (all P ≥ 0.21). Similarly, differences in cortical thickness between the throwing and nonthrowing arms in former throwers were no longer present in the 20–29 y and subsequent detraining groups (all P ≥ 0.06) (Fig. 2 A and H). Dual-energy X-ray absorptiometry (DXA) measures supported the eventual loss of the throwing-derived benefits in bone mass, with side-to-side differences in upper extremity bone mineral content and areal bone mineral density in former throwers in the 30–39 y and subsequent detraining groups being equivalent to those in controls (Fig. S4 B and C). However, former throwers in the 50+ y detraining group did maintain 12.9% of the throwing-derived benefit in total cross-sectional bone mineral content (combined cortical and trabecular/subcortical bone mineral content) at the midshaft humerus (P = 0.02) (Fig. S4A).
Fig. 2.
Physical activity-related mechanical loading during youth conferred lifelong benefits in cortical bone size and estimated strength but not in cortical bone mass. (A) Peripheral QCT images of the midshaft humerus in representative former throwers show increased medullary expansion and cortex trabecularization (arrows) in the throwing arm with increasing years of detraining but maintenance of loading effects on overall bone cross-sectional size. (B–I) Graphs show the maintenance of the effects of physical activity during youth on cortical volumetric bone mineral density (B); cortical bone mineral content (C); trabecular/subcortical bone mineral content (D); total cross-sectional area (E); cortical cross-sectional area (F); medullary cross-sectional area (G); cortical thickness (H); and density-weighted polar moment of inertia (I). Data show the mean difference and 95% CI between the throwing and nonthrowing arms in former throwers normalized to the differences between the dominant and nondominant arms in controls. CIs greater or less than 0% indicate differences between the throwing and nonthrowing arms in throwers that are greater or less, respectively, than the differences between the dominant and nondominant arms in controls (*P < 0.05, †P < 0.01 and ‡P < 0.001, unpaired t test). Source data are provided in Table S3.
The progressive and eventual loss of the throwing-derived benefit in cortical bone mass resulted from accelerated medullary expansion and cortex trabecularization in the throwing arm (Fig. 2A). Accelerated medullary expansion was evidenced by the greater medullary area in the throwing arm of former throwers in the 10–19 y and subsequent detraining groups (all P < 0.05) (Fig. 2 A and G), whereas greater trabecular/subcortical bone mineral content in the throwing arm of former throwers in the <10 y and subsequent detraining groups provided evidence of accelerated cortex trabecularization (all P < 0.001) (Fig. 2 A and D). There were no side-to-side differences in volumetric bone mineral density of the nontrabecularized cortex between former throwers in any detraining cohort and controls (all P > 0.05) (Fig. 2B).
In contrast to the eventual loss of the benefit in cortical bone mass, former throwers in all detraining groups maintained a proportion of the throwing-derived benefit in bone total area/size (all P < 0.001) (Fig. 2 A and E). As compared with controls, former throwers in the 50+ y detraining group had a difference of 0.56 mm2 (95% CI = 0.34–0.79 mm2, P < 0.001) in total area/size between the throwing arm and the nonthrowing arm; this difference represents more than half (55.5%) of the throwing-derived benefit observed in active throwers (1.02 mm2, 95% CI = 0.74–1.29 mm2, P < 0.001) (Fig. 2 A and E). The persistent benefit of throwing on size contributed to maintenance of the throwing-derived benefit in bone strength, with former throwers in the 50+ y detraining group (0.62 mm4; 95% CI = 0.33–0.91 mm4, P < 0.001) still having more than one-third (33.7%) of the benefit in torsional strength observed in active throwers (1.83 mm4; 95% CI = 1.34–2.32 mm4, P < 0.001) (Fig. 2I). The throwing-derived benefit in bone size and strength persisted in the 50+ y detraining group despite throwing-to-nonthrowing arm differences in lean mass and range of glenohumeral external rotation being less than dominant-to-nondominant arm differences observed in controls (all P < 0.05) (Fig. S4 D and H). The lean mass data suggest that the benefits in bone size and strength conferred by physical activity during youth were maintained despite the loss of any muscle benefits.
Throwing-Related Physical Activity Continued During Aging Maintains Some of the Cortical Bone Mass and More of the Bone Strength Benefits.
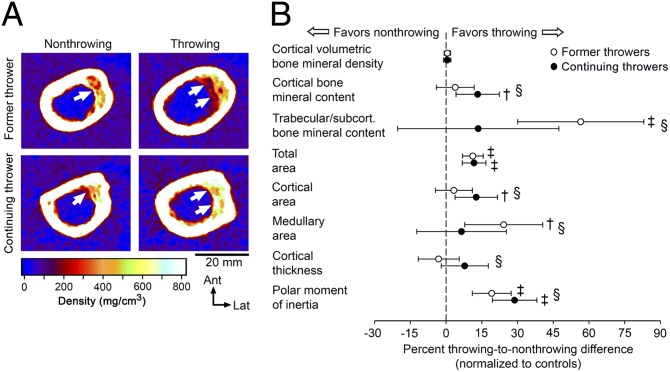
The skeletal benefits of continuing to throw during aging were assessed by peripheral QCT of the midshaft humerus in cohorts of continuing throwers (former MLB players who continued to throw for >20 y after retirement from professional throwing) (n = 10; age = 71.2 ± 3.8 y) and age-matched former throwers (n = 16; age = 72.1 ± 3.7 y) and controls (n = 18; age = 73.7 ± 3.6 y) (Table S4). Continuing and former throwers were comparable in the age at which they started throwing, the age at which they ceased professional throwing, and the cumulative number of MLB/MiLB games played and innings pitched (all P > 0.05) (Table S4). Continuing throwers threw for an additional 24.6 ± 5.4 y after ceasing professional play by participating in baseball internationally or at a lower level of competition and/or as a pitcher for batting practice or coach. Continuing throwers had ceased all forms of throwing 14.5 ± 9.0 y ago, compared with 38.8 ± 5.8 y ago in the former throwers (P < 0.001).
The side-to-side differences in cortical bone mineral content and area were greater in continuing throwers than in either former throwers or controls (all P < 0.05) (Fig. 3). These data were supported by greater side-to-side differences in the DXA measurements of the upper extremity in continuing throwers as compared with both former throwers and controls (all P < 0.05) (Fig. S5). The absolute side-to-side difference of 41.9 mg/mm (95% CI, 13.6–70.3 mg/mm, P < 0.01) in cortical bone mineral content in continuing throwers normalized to controls was approximately a quarter that observed in active throwers (150.4 mg/mm; 95% CI, 127.2–173.5 mg/mm, P < 0.001). Maintenance of some of the throwing-derived benefits in cortical bone mass in continuing throwers appeared to result from reduced endocortical bone loss and cortical trabecularization, as evidenced by the side-to-side differences in medullary area and trabecular/subcortical bone mineral content in continuing throwers being equivalent to those in controls (all P > 0.98) and less than in former throwers (all P < 0.05) (Fig. 3). Continued throwing conferred no added benefit in bone total area/size, with differences between the throwing and nonthrowing arms being comparable in continuing and former throwers (P > 0.95) (Fig. 3). The net results of less medullary area and unchanged total area in continuing throwers was greater side-to-side differences in cortical thickness as compared with former throwers (P = 0.03) and greater side-to-side differences in torsional strength as compared with both former throwers and controls (P < 0.05) (Fig. 3B). Continuing throwers (0.92 cm4, 95% CI, 0.62–1.24 cm4, P < 0.001) normalized to controls maintained 50% of the throwing-related benefit in torsional strength observed in active throwers (1.83 cm4. 95% CI, 1.34–2.32 cm4, P < 0.001), whereas former throwers maintained 38% of that benefit (0.69 cm4; 95% CI, 0.42–0.96 cm4, P < 0.001).
Fig. 3.
Continued physical activity during aging maintained a proportion of the benefits in bone mass and more of the benefits in bone strength induced during youth. (A) Peripheral QCT images of the midshaft humerii in representative individuals showed less medullary expansion and cortical trabecularization (arrows) in the throwing arm of the continuing thrower than in the throwing arm of the former thrower. (B) Cortical bone mineral content and density-weighted polar moment of inertia were greater in continuing throwers than in former throwers and controls; cortical thickness was greater in continuing throwers than in former throwers; and trabecular/subcortical bone mineral content and medullary area were smaller in continuing throwers than in former throwers. Data show the mean percent difference and 95% CI between the throwing arm and the nonthrowing arm in throwers normalized to the differences between the dominant arm and the nondominant arm in controls. CIs greater than 0% indicate greater differences between the throwing arm and the nonthrowing arm in throwers than between the dominant arm and the nondominant arm in controls (†P < 0.01; ‡P < 0.001); § indicates significant differences between continuing and former throwers (P < 0.05; one-way ANOVA with Tukey post hoc comparisons).
Discussion
The principal observation of the current work is the lifelong maintenance of the benefits in bone size and strength conferred by physical activity performed during youth. Former MLB players in their ninth decade of life retained more than half of the throwing-related benefits in bone total cross-sectional area/size and one-third of the throwing-related benefits in bone torsional strength observed in currently active MLB/MiLB players, even though their throwing activities ended more than 50 y ago and the throwing-related benefits in bone mass declining more rapidly. The benefits in size and strength conferred by physical activity are considered to be maintained lifelong, because the former throwers in the 50+ y detraining group (age = 83.8 ± 2.3 y) were older than the current life-expectancy of males in the United States (76.4 y) (25). The evidence that some of the bone-related benefits acquired through physical activity during youth persist throughout life, even after long-term return to habitual levels of loading, has important public health implications. In particular, it indicates that the old adage of “use it or lose it” is not entirely applicable to the skeleton and that physical activity during youth should be strongly encouraged for lifelong bone health. The observed greater maintenance of the benefits in bone size conferred by physical activity suggests the focus of physical activity and assessment of its effects should emphasize the optimization of bone size rather than the current paradigm of achieving a higher peak bone mass. Whether the same results hold true in females remains to be established; males and females may have differing skeletal responses to physical activity and subsequent detraining (16).
Although physical activity performed during youth had lifelong benefits related to bone size and strength, it did result in accelerated age-related changes in bone mass and structure. Benefits in cortical bone mass were lost and benefits in total (cortical plus trabecular/subcortical) bone mass declined substantially with increasing years of detraining. The accelerated loss of benefits in bone mass after the cessation of physical activity makes evolutionary sense given that humans evolved for endurance (26) and it is energy inefficient for the skeleton to maintain its mass in excess of prevailing needs. Age-related cortical bone loss is characterized principally by intracortical remodeling within the cortex adjacent to the medullary cavity (27). The remodeling thins the cortex from within by cavitation and leaves cortical remnants that look similar to trabeculae. In the current work we found that intracortical remodeling accelerated rapidly after the cessation of physical activity and was evident by greater medullary expansion and characterization of cortical bone material as trabecular/subcortical. This remodeling also was visibly evident as greater intracortical cavitation in the throwing arm of former throwers in the 30–39 y and subsequent detraining groups (Fig. 2A, arrows). The result was the rapid loss of the benefits in cortical bone mass, area, and thickness acquired through the physical activity performed in youth.
Throwing-related physical activity had a small negative effect on cortical volumetric bone mineral density in select tomographic slices of the humeral diaphysis in active throwers. The small (<3%) decrease in density is consistent with a previous report of the effects of physical activity on the skeleton (28) and possibly resulted from an accumulation of load-induced microdamage and the initiation of intracortical remodeling and/or the incomplete mineralization of load-induced new bone accrued on the periosteal and endocortical surfaces. However, the lower cortical density did not persist in any of the former thrower cohorts, indicating that a previous history of physical activity did not influence density within the remaining compact bone of the cortex during aging.
The benefits in cortical thickness declined rapidly after cessation of throwing-related physical activity and were lost within 20–29 y of detraining. However, differences in cortical thickness between the throwing arm and the nonthrowing arm in former throwers were never less than the differences between the dominant arm and the nondominant arm in controls, indicating that the rate of cortical bone thinning in the throwing arm of former throwers stabilized with ongoing detraining. One reason for the stabilization may be progressive periosteal expansion of the throwing arm during aging. There is general consensus that bones expand with age (14, 15). Periosteal expansion appeared to occur with age in the current study, with total area in the throwing and nonthrowing arms predicted using linear regression (29) as increasing by 0.72 mm2/y and 0.92 mm2/y, respectively. The slower rate of expansion in the throwing arm explains why not all of the benefit in total area/size benefit derived from physical activity during youth persisted throughout life; however, the progressive expansion in the throwing arm with advancing age raises a question regarding its stimulus. One hypothesis may be that periosteal expansion occurs during aging to compensate for age-related mechanical decay; however, this hypothesis does not appear to fit with the current data, because the throwing arm expanded even though it was mechanically superior. It is possible that the periosteal expansion in the throwing arm resulted from a need to reduce a loss of buckling resistance associated with thinning of its cortex during the initial decades of detraining or that nonmechanical stimuli are responsible. These hypotheses require further investigation.
To maximize the lifelong skeletal benefits of physical activity during youth, the current work suggests that activity should be continued during aging. Continuing to throw during aging resulted in greater maintenance of the benefits in bone strength engendered by the physical activity during youth. Continued physical activity conferred no additional benefit on bone size during aging, probably because a reduced loading stimulus was introduced to an already adapted periosteal bone surface. However, continued activity appeared to reduce the accelerated remodeling of the cortex adjacent to the medullary cavity that was observed with the complete cessation of physical activity in former throwers. Although increasing overall bone size is the most efficient means of enhancing bone strength, the seemingly lower medullary expansion and intracortical bone loss with continued throwing during aging provided a small but significant additional benefit in bone strength. These data indicate that physical activity conveys ongoing benefits by reducing structural decay, furthering evidence of the benefits in bone strength conferred by physical activity during aging (30).
Final observations of interest are the bone strains calculated in the humerus of an MLB pitcher and the magnitude of adaptation observed in currently active throwers. Throwing exposed the humerus to extreme loading. Peak tensile strains calculated in the humerus of the throwing arm (4,885 microstrain) were nearly twice those reported in the tibia during running and jumping activities (31), although they still were below the fracture threshold of cortical bone (∼8,000–10,000 microstrain). When the same throwing forces were applied to the collateral (nonthrowing) humerus, strains were elevated and peak tensile strains (18,452 microstrain) surpassed fracture thresholds. The reduced strains in the throwing arm indicated that the humerus had adapted to reduce tissue-level deformation, as demonstrated in a previous animal model (32). Throwing-related physical activity nearly doubled the torsional strength of the humerus compared with the contralateral (internal control) arm, with the humerus in the throwing arm of one individual player being more than 2.5 times stronger than the humerus in the contralateral arm (Fig. S2). These adaptation data exceed previous reports of upper extremity bilateral asymmetry induced by physical activity (12, 13, 20, 22, 33–35), establishing new levels for the plastic potential within the skeleton. Similar magnitudes of bilateral asymmetry are not expected in studies of the lower extremity because of the heightened habitual bilateral loading associated with weight-bearing in both athletes and controls. Unexpectedly, the location of highest predicted strains (proximal diaphysis) and greatest bone adaptation (distal diaphysis) did not overlap. A possible explanation for this disparity may be higher habitual loading at the upper diaphysis resulting from shoulder muscle action and an ensuing reduction in skeletal mechanosensitivity resulting from cellular accommodation (36, 37).
The current studies have a number of strengths resulting from the use of former professional baseball players as a model; however, they also have limitations. Former MLB baseball players of differing ages are considered to have minimal secular variations in throwing activities during youth and consequent adaptation at the completion of their professional careers. Although most assessed parameters of physical activity levels were equivalent among the cohorts of former throwers, former throwers in the 50+ y detraining group had thrown more professional innings than those in cohorts with fewer years of detraining. The latter difference is not considered to have introduced significant cohort variations in terms of the magnitude of adaptation induced during youth, because bone adaptation decays exponentially with repetitive loading, and former MLB players fall toward the upper end of the loading repetition axis where mild or even moderate changes in loading repetition have minimal impact on resultant skeletal adaptation. Supporting the validity of our data are the contrasting maintenance of the benefits in bone mass and size acquired through physical activity during youth and the relatively consistent pattern of bone changes among the different cohorts of former throwers. Other limitations of the current work include the modeling of strains in a single MLB player at a single time point during the throwing motion and the study of the lasting skeletal benefits of physical activity in males at only an upper extremity, cortical-rich site that is not prone to osteoporotic fracture. It is possible that conclusions would differ if the study were performed in women and at a trabecular-rich and/or lower extremity site that is prone to osteoporotic fracture.
Ultimately, the current data indicate that physical activity performed during youth confers lifelong benefits in bone size and strength, even though the cessation of activity is associated with accelerated age-related changes in bone mass and structure. The data also indicate that physical activity continued during aging can reduce age-related bone changes so as to maintain some of the benefits in bone mass and more of the benefits in bone strength acquired through physical activity during youth. Whether these benefits influence the risk of fracture at sites prone to osteoporotic fracture remains to be shown.
Methods
A full description of the methods is provided in SI Methods.
Participants.
We recruited 103 throwing athletes and 94 age-matched controls. Active throwers (n = 9) were currently competing as pitchers in MLB or MiLB (Triple A level). Former throwers (n = 84) had played in at least one game of MLB as a pitcher or catcher and had not participated more than once per month for longer than 6 mo in any one-arm-dominant activity since leaving professional baseball. Continuing throwers (n = 10) had played in at least one game of MLB as a pitcher, were aged 65–80 y, and had thrown a minimum of three times per week for at least 20 y after leaving professional baseball. Age-matched controls had participated no more than once per month for no more than 6 mo in any one-arm-dominant activities. All participants provided written informed consent, and all study procedures were approved by the Institutional Review Boards of Indiana University and St. Vincent’s Birmingham.
Strain Within the Humerus During Throwing.
A subject-specific musculoskeletal model was used to estimate strains during a fastball pitch performed by an MLB player (24). Muscle volumes within the player were obtained from 3D MR images (Siemens). Net joint torques were calculated using inverse dynamics from 3D joint angles at the shoulder and elbow, as previously described (38). Calculated muscle forces and joint reaction forces were applied as nodal point loads to a finite element model of the player’s humerus obtained from CT data (Phillips). A linear stress analysis was used to calculate the maximum principal strain at each node.
Shoulder Muscle Strength and Range of Motion.
Bilateral concentric shoulder external and internal rotation torques and range of shoulder internal and external rotation were assessed in former and continuing throwers and controls, as previously described (23).
DXA.
DXA (Hologic) was used to obtain whole-body (minus the head), hip, and lumbar spine areal bone mineral density (g/cm2). Whole-body scans also provided measures of whole-body lean mass, percent fat mass, whole-arm bone mineral content, areal bone mineral density, and lean mass.
QCT.
The bilateral humerii of active throwers and controls were imaged using a multislice helical CT scanner (Phillips). All throwers (active, former, and continuing) and controls had the midshaft humerus in both arms assessed by peripheral QCT (Stratec), as previously described (23).
Statistical Analyses.
Side-to-side differences between the throwing and nonthrowing arms in throwers were assessed by calculating absolute differences (throwing − nonthrowing) and mean percent differences [(throwing − nonthrowing)/nonthrowing × 100]. Similar analyses were performed to determine differences between the dominant and nondominant arms in controls. Throwing effects were determined by comparing the absolute or percent difference values between activity groups (throwers vs. controls).
Supplementary Material
Acknowledgments
We thank the Major League Baseball Players Alumni Association for assistance with subject recruitment; K. Buckwalter, C. Lin, and J. Shine for help in developing imaging protocols; and R. Barker and J. Richard for assisting with data processing. This work was supported by National Institutes of Health (NIH) Grant R01 AR057740 (to S.J.W.). R.K.F. was supported in part by NIH Grant K01 AR054408.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.B.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321605111/-/DCSupplemental.
References
- 1.Kannus P, et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123(1):27–31. doi: 10.7326/0003-4819-123-1-199507010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81(6):1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzoli R, Bianchi ML, Garabédian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46(2):294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Erlandson MC, et al. Higher premenarcheal bone mass in elite gymnasts is maintained into young adulthood after long-term retirement from sport: A 14-year follow-up. J Bone Miner Res. 2012;27(1):104–110. doi: 10.1002/jbmr.514. [DOI] [PubMed] [Google Scholar]
- 5.Kontulainen S, et al. Good maintenance of exercise-induced bone gain with decreased training of female tennis and squash players: A prospective 5-year follow-up study of young and old starters and controls. J Bone Miner Res. 2001;16(2):195–201. doi: 10.1359/jbmr.2001.16.2.195. [DOI] [PubMed] [Google Scholar]
- 6.Pollock NK, Laing EM, Modlesky CM, O’Connor PJ, Lewis RD. Former college artistic gymnasts maintain higher BMD: A nine-year follow-up. Osteoporos Int. 2006;17(11):1691–1697. doi: 10.1007/s00198-006-0181-3. [DOI] [PubMed] [Google Scholar]
- 7.Scerpella TA, Dowthwaite JN, Rosenbaum PF. Sustained skeletal benefit from childhood mechanical loading. Osteoporos Int. 2011;22(7):2205–2210. doi: 10.1007/s00198-010-1373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tveit M, Rosengren BE, Nilsson JA, Ahlborg HG, Karlsson MK. Bone mass following physical activity in young years: A mean 39-year prospective controlled study in men. Osteoporos Int. 2013;24(4):1389–1397. doi: 10.1007/s00198-012-2081-z. [DOI] [PubMed] [Google Scholar]
- 9.Valdimarsson O, Alborg HG, Düppe H, Nyquist F, Karlsson M. Reduced training is associated with increased loss of BMD. J Bone Miner Res. 2005;20(6):906–912. doi: 10.1359/JBMR.050107. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson MK, et al. Exercise during growth and bone mineral density and fractures in old age. Lancet. 2000;355(9202):469–470. doi: 10.1016/s0140-6736(00)82020-6. [DOI] [PubMed] [Google Scholar]
- 11.Bass SL, et al. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: A study in tennis players. J Bone Miner Res. 2002;17(12):2274–2280. doi: 10.1359/jbmr.2002.17.12.2274. [DOI] [PubMed] [Google Scholar]
- 12.Haapasalo H, et al. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: A peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone. 2000;27(3):351–357. doi: 10.1016/s8756-3282(00)00331-8. [DOI] [PubMed] [Google Scholar]
- 13.Ruff CB, Walker A, Trinkaus E. Postcranial robusticity in Homo. III: Ontogeny. Am J Phys Anthropol. 1994;93(1):35–54. doi: 10.1002/ajpa.1330930103. [DOI] [PubMed] [Google Scholar]
- 14.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003;349(4):327–334. doi: 10.1056/NEJMoa022464. [DOI] [PubMed] [Google Scholar]
- 15.Szulc P, Seeman E, Duboeuf F, Sornay-Rendu E, Delmas PD. Bone fragility: Failure of periosteal apposition to compensate for increased endocortical resorption in postmenopausal women. J Bone Miner Res. 2006;21(12):1856–1863. doi: 10.1359/jbmr.060904. [DOI] [PubMed] [Google Scholar]
- 16.Duckham RL, et al. Does physical activity in adolescence have site-specific and sex-specific benefits on young adult bone size, content, and estimated strength? J Bone Miner Res. 2014;29(2):479–486. doi: 10.1002/jbmr.2055. [DOI] [PubMed] [Google Scholar]
- 17.Kato T, et al. Adolescent exercise associated with long-term superior measures of bone geometry: A cross-sectional DXA and MRI study. Br J Sports Med. 2009;43(12):932–935. doi: 10.1136/bjsm.2008.052308. [DOI] [PubMed] [Google Scholar]
- 18.Warden SJ, Fuchs RK, Castillo AB, Nelson IR, Turner CH. Exercise when young provides lifelong benefits to bone structure and strength. J Bone Miner Res. 2007;22(2):251–259. doi: 10.1359/jbmr.061107. [DOI] [PubMed] [Google Scholar]
- 19.Warden SJ, et al. Cortical and trabecular bone benefits of mechanical loading are maintained long-term in mice independent of ovariectomy. J Bone Miner Res. doi: 10.1002/jbmr.2143. 10.1002/jbmr.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am. 1977;59(2):204–208. [PubMed] [Google Scholar]
- 21.King JW, Brelsford HJ, Tullos HS. Analysis of the pitching arm of the professional baseball pitcher. Clin Orthop Relat Res. 1969;67(67):116–123. [PubMed] [Google Scholar]
- 22.Neil JM, Schweitzer ME. Humeral cortical and trabecular changes in the throwing athlete: A quantitative computed tomography study of male college baseball players. J Comput Assist Tomogr. 2008;32(3):492–496. doi: 10.1097/RCT.0b013e31811ec72d. [DOI] [PubMed] [Google Scholar]
- 23.Warden SJ, Bogenschutz ED, Smith HD, Gutierrez AR. Throwing induces substantial torsional adaptation within the midshaft humerus of male baseball players. Bone. 2009;45(5):931–941. doi: 10.1016/j.bone.2009.07.075. [DOI] [PubMed] [Google Scholar]
- 24.Garner BA, Pandy MG. Musculoskeletal model of the upper limb based on the visible human male dataset. Comput Methods Biomech Biomed Engin. 2001;4(2):93–126. doi: 10.1080/10255840008908000. [DOI] [PubMed] [Google Scholar]
- 25.Arias E. United States life tables, 2009. Natl Vital Stat Rep. 2014;62(7):1–63. [PubMed] [Google Scholar]
- 26.Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432(7015):345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 27.Zebaze RMD, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: A cross-sectional study. Lancet. 2010;375(9727):1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- 28.Rantalainen T, Nikander R, Daly RM, Heinonen A, Sievänen H. Exercise loading and cortical bone distribution at the tibial shaft. Bone. 2011;48(4):786–791. doi: 10.1016/j.bone.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Mantila Roosa SM, Hurd AL, Xu H, Fuchs RK, Warden SJ. Age-related changes in proximal humerus bone health in healthy, white males. Osteoporos Int. 2012;23(12):2775–2783. doi: 10.1007/s00198-012-1893-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikander R, et al. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47. doi: 10.1186/1741-7015-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al Nazer R, Lanovaz J, Kawalilak C, Johnston JD, Kontulainen S. Direct in vivo strain measurements in human bone-a systematic literature review. J Biomech. 2012;45(1):27–40. doi: 10.1016/j.jbiomech.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Warden SJ, et al. Bone adaptation to a mechanical loading program significantly increases skeletal fatigue resistance. J Bone Miner Res. 2005;20(5):809–816. doi: 10.1359/JBMR.041222. [DOI] [PubMed] [Google Scholar]
- 33.Shaw CN, Stock JT. Habitual throwing and swimming correspond with upper limb diaphyseal strength and shape in modern human athletes. Am J Phys Anthropol. 2009;140(1):160–172. doi: 10.1002/ajpa.21063. [DOI] [PubMed] [Google Scholar]
- 34.Trinkaus E, Churchill SE, Ruff CB. Postcranial robusticity in Homo. II: Humeral bilateral asymmetry and bone plasticity. Am J Phys Anthropol. 1994;93(1):1–34. doi: 10.1002/ajpa.1330930102. [DOI] [PubMed] [Google Scholar]
- 35.Warden SJ. Extreme skeletal adaptation to mechanical loading. J Orthop Sports Phys Ther. 2010;40(3):188. doi: 10.2519/jospt.2010.0404. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh YF, Robling AG, Ambrosius WT, Burr DB, Turner CH. Mechanical loading of diaphyseal bone in vivo: The strain threshold for an osteogenic response varies with location. J Bone Miner Res. 2001;16(12):2291–2297. doi: 10.1359/jbmr.2001.16.12.2291. [DOI] [PubMed] [Google Scholar]
- 37.Schriefer JL, Warden SJ, Saxon LK, Robling AG, Turner CH. Cellular accommodation and the response of bone to mechanical loading. J Biomech. 2005;38(9):1838–1845. doi: 10.1016/j.jbiomech.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 38.Fleisig GS, Bolt B, Fortenbaugh D, Wilk KE, Andrews JR. Biomechanical comparison of baseball pitching and long-toss: Implications for training and rehabilitation. J Orthop Sports Phys Ther. 2011;41(5):296–303. doi: 10.2519/jospt.2011.3568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.