Abstract
Background:
Tumors are distinguished from normal tissues partly by their pronounced variability of cellular and nuclear dimensions. Therefore, such factors may be an indicator to assess whether the cells are malignant or not. Exfoliative cytology is a reliable tool in assessing such changes in the uterine cervix and has been used in the oral cavity also with success. The aims and objectives of the following study were to evaluate the malignant changes by assessing the quantitative parameters such as cytoplasmic diameter, cytoplasmic perimeter and cytoplasmic area (CD, CP, CA) and nuclear diameter, nuclear perimeter and nuclear area (ND, NP, NA) and cytoplasmic to nuclear ratio in the exfoliated cells of various subtypes of oral lichen planus (OLP) using cytomorphometry.
Materials and Methods:
Oral exfoliated cells from nineteen cases of histologically proven OLP (1 atrophic, 13 reticular, 4 erosive and 1 plaque) and ten controls with healthy mucosa were taken and stained by Feulgen-Rossenback reaction and cytomorphometric analysis was performed using an image analysis software. The parameters taken into account were CD, CP, CA and ND, NP, NA. Furthermore CA/NA was calculated. The parameters were statistically analyzed using the t-test.
Results:
Cytomorphometric analysis of all the parameters showed no significant difference between the control group and the reticular/plaque subtypes, whereas statistically significant (P < 0.05) differences was obtained between the control group and the atrophic/erosive subtypes group when compared using t-test.
Conclusions:
The cytomorphometric analysis of OLP shows that erosive/atrophic subtypes of OLP are at more risk and exfoliative cytology and cytomorphometry can be used as a tool to assess the malignant changes.
Keywords: Cytomorphometry, exfoliative cytology, oral lichen planus
INTRODUCTION
Lichen planus is a unique mucocutaneous disorder first described and named by a famous British physician named Wilson in 1869.[1] The disease apart from affecting the skin and oral mucosa, also involves hair follicles, nails, esophagus and seldom the eyes, urinary tract, nasal mucosa, larynx and genitalia.[2,3] The lesion when affecting the skin presents typically as flat topped violaceous papules affecting the wrists and ankles and also seems to affect the flexor surfaces of the extremities.[4]
The disease when it affects the oral mucosa is termed oral lichen planus (OLP). OLP usually affects 1-2% of the adult population.[4] OLP has been described as a disease of the middle aged, predominantly in adults over the age of 40 and more common in women than men in ratio 1.4:1.[5] Various etiologic factors have been described over the years for this disease including stress, foreign bodies such as restorations, trauma, bacterial and viral pathogens. The pathogenesis for this disease is considered to be a T cell mediated immune mechanism.[6] The buccal mucosa and the tongue are the most common sites where this disease manifests and usually is seen bilaterally.[4] Six clinical types of OLP lesions may be seen individually or combined: Papular, reticular, plaque type, atrophic, erosive and bullous.[2]
It is a well-known fact that OLP is considered a “potentially malignant disorder.”[7] Nevertheless, there have been various controversies regarding malignancy and OLP. One of them was addressed by Holmstrup in 1992 which was whether OLP had the inherent potential to turn to malignancy or is it the altered epithelium that makes it more susceptible to carcinogens.[8] The second controversy was regarding the percentage of OLP turning to malignancy which had been previously hampered by inconsistencies in the diagnostic criteria used for OLP. Now that it has been cleared most authors agree that 0.2-2% of OLP can turn malignant.[9,10] The third and the most important controversy is regarding the subtype of OLP that is at more risk to turn into carcinoma. Few authors claim that the erosive and atrophic forms are at more risk, whereas others feel all the subtypes are at equal risk.[11,12,13] Exfoliative cytology and cytomorphometry have been considered to be an important adjuvant for the assessment of malignant changes in the oral cavity. Quantitative parameters such as cytoplasmic diameter, cytoplasmic perimeter and cytoplasmic area (CD, CP, CA) and nuclear diameter, nuclear perimeter and nuclear area (ND, NP, NA) and cytoplasmic to nuclear ratio have been used widely for this purpose.[14,15] In this study, we have addressed the third controversy by using exfoliative cytology and cytomorphometry. We conducted this study because exfoliative cytology has been extensively used to assess the malignant potential of various potentially malignant disorders such as oral submucous fibrosis, leukoplakia and erythroplakia, but never has been successfully used to study the various subtypes of OLP.
MATERIALS AND METHODS
The present study was carried out after a clearance from the ethical committee in the University. The study comprised of nineteen patients (ten male and nine female) with clinically and histologically proven OLP consisting of the reticular [Figure 1], plaque type, atrophic and erosive [Figure 2] subtypes. A total of 10 normal, healthy individuals comprised of 5 males and 5 females served as controls. After an informed consent, buccal keratinocytes were collected from the lesional areas of patients with OLP and also from the normal appearing buccal mucosa of control group using a slightly moistened sterilized wooden spatula (Vishvesh Pvt., Ltd., Chennai, India).
Figure 1.
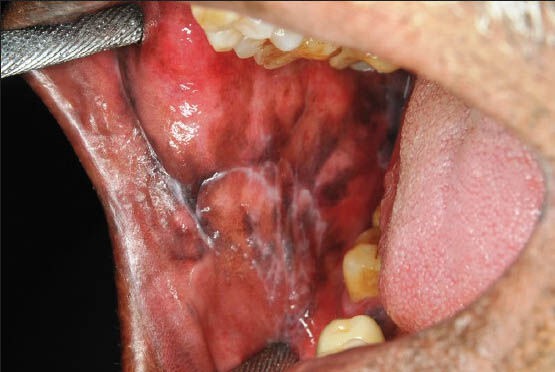
Clinical photograph of a patient showing reticular oral lichen planus in the right buccal mucosa
Figure 2.
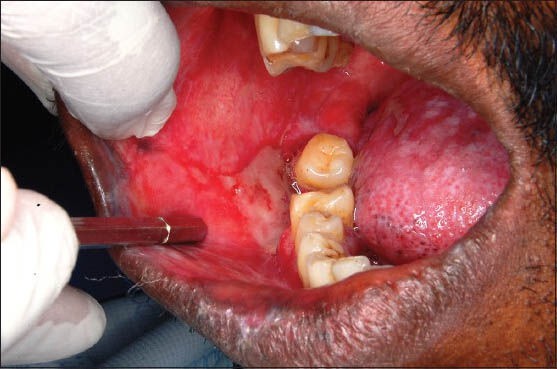
Clinical photograph of a patient showing erosive oral lichen planus in the right buccal mucosa
The smears were then transferred and spread uniformly on a clean glass slide containing 0.02 M NaCl (Coral marketing, India) which acts as a buffer and also helps in removing cell debris and bacteria. The slides containing smears were fixed in 95% alcohol for 30 min. The slides were then stained by using Feulgen reaction (Artisan feulgen kit, Dako). After reviewing, the smears were further subjected to morphometric evaluation. For each slide, 200 representative cells with distinct cellular and nuclear outlines, avoiding overlapping cells were captured as high quality photomicrographs using a Nikon 4100 digital camera (Nikon, Japan) attached to an Olympus CK trinocular microscope (Olympus, Japan). The captured images were transferred to a computer for morphometric analysis. The final image captured on the monitor had a magnification of ×400. The morphometric evaluation was carried out using image analyzer software, Image-Pro Express version 5.1 (MediaCybernetics, USA).
The morphometric parameters that were studied were CD, CP, CA [Figures 3 and 4] and ND, NP, NA [Figures 5 and 6]. Furthermore CA/NA was calculated. The 29 samples considered for the study were broadly divided into two groups. Group I (control group) consisted of normal healthy individuals. Group II (study group) consisted of the patients with OLP. The study group was further subdivided into two sub groups. Group IIa consisted of patients with reticular/plaque type OLP and Group IIb consisted to erosive/atrophic type OLP. Statistical analysis of the data was carried out using the SPSS software version 17.0 (IBM, USA) for Windows 7 (Microsoft, USA).
Figure 3.
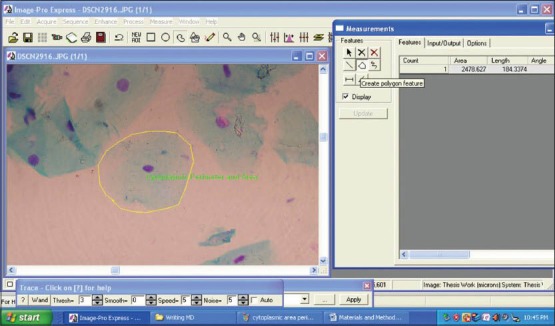
Screen-shot demonstrating the assessment of cytoplasmic perimeter and cytoplasmic area using Image Pro Express software (MediaCybernetics, USA)
Figure 4.
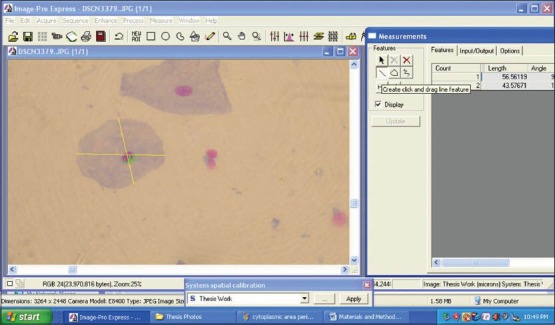
Screen-shot demonstrating the assessment of cytoplasmic diameter using Image Pro Express software (MediaCybernetics, USA)
Figure 5.
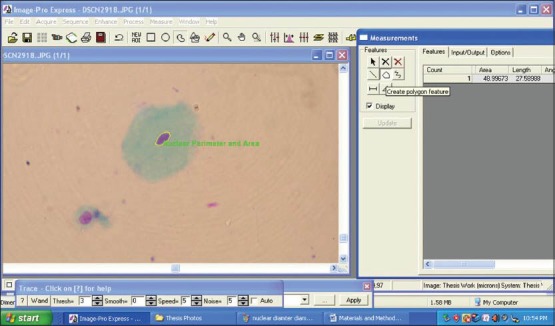
Screen-shot demonstrating the assessment of nuclear perimeter and nuclear area using Image Pro Express software (MediaCybernetics, USA)
Figure 6.
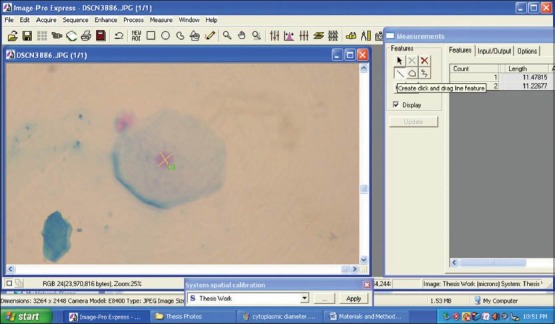
Screen-shot demonstrating the assessment of nuclear diameter using Image Pro Express software (MediaCybernetics, USA)
RESULTS
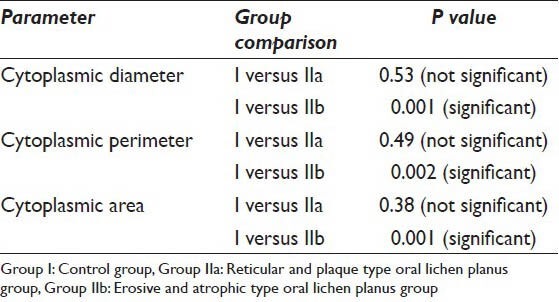
The CD, CP and CA were evaluated in the 19 samples of the study group and 10 normal individuals. The mean values of the CD in Group I was 63.15 μm whereas the mean values of the same in Group IIa and Group IIb were 62.98 μm and 52.10 μm respectively. As far as the CP is concerned the mean values of Group I, IIa and IIb were 201.11 μm, 196.24 μm and 165.88 μm respectively. When the CA in the three groups was evaluated it was found that the values were 3203.86 μm2, 3036.42 μm2 and 2126.82 μm2 in Group I, IIa and IIb respectively [Table 1]. As far as the significance is concerned the P value failed to reach significant levels (i.e. <0.05) when Group I and IIa were compared. However, P value showed that there is a significant difference in all the cytoplasmic parameters when Group I was compared with Group IIb [Table 2].
Table 1.
Descriptive statistics of all the parameters in the three groups
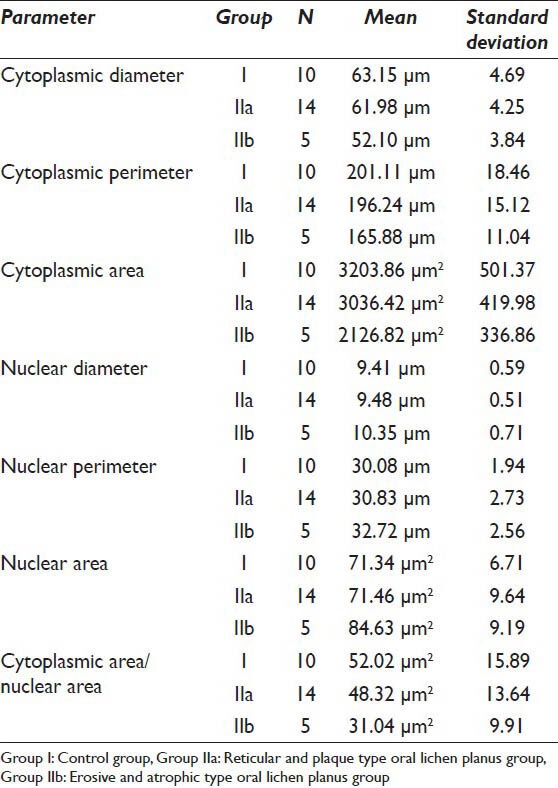
Table 2.
T-test comparing the cytoplasmic parameters between Group I and Group IIa, IIb
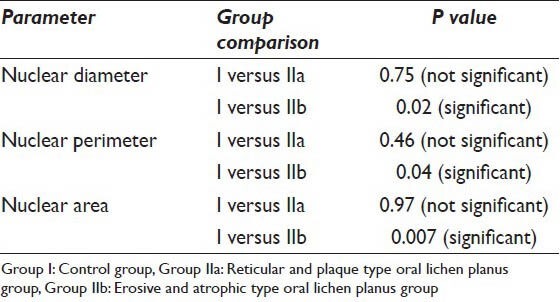
Similarly the nuclear parameters namely ND, NP and NA of the control group consisting of 10 normal individuals and the study group consisting of 19 OLP samples were compared. The mean ND and NP in the control group were 9.41 μm and 30.08 μm respectively, whereas the mean NA in this group was 71.34 μm2. The mean values of the ND in the reticular/plaque type OLP group (Group IIa) and erosive/atrophic OLP group (Group IIb) were 9.48 μm and 10.45 μm respectively. The mean NP was 30.83 μm and 32.72 μm in Group IIa and Group IIb respectively and the mean NA for Group IIa was 71.46 μm2 whereas in Group IIb it was 84.63 μm2 [Table 1]. Statistically the ND, NP and NA in Group IIb was significantly increased (P < 0.05) when compared to Group I, whereas the reticular/plaque type group showed no statistically significant difference with that of the control group consisting of normal individuals [Table 3].
Table 3.
T-test comparing the nuclear parameters between Group I and Group IIa, IIb
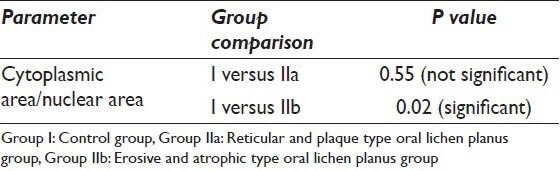
From the above values, the CA/NA was calculated and subsequently the mean was calculated for the control and study groups. The mean CA/NA for group I was 52.02 μm2. In the study groups, the mean was 48.32 μm2 and 31.04 μm2 for Group IIa and IIb respectively [Table 1]. As seen for the cytoplasmic and nuclear parameters, the P value reached statistically significant levels (P < 0.05) when Group IIb was compared with Group I and failed to reach < 0.05 when the reticular/plaque type OLP and control group were compared [Table 4].
Table 4.
T-test comparing the cytoplasmic area/nuclear area between Group I and Group IIa, IIb
DISCUSSION
OLP is a chronic autoimmune disease mediated by T lymphocytes that involves the stratified squamous epithelial tissue. The designation and description of the pathology were presented and named by the English physician Wilson.[1] Louis-Frédéric Wickham added to the description of the lesion by describing the clinical appearance as being greyish striae and dots. It was after him that the important clinical feature of OLP “Wickham striae” was named in 1895.[16,17,18]
In the present study, the cytoplasmic and nuclear parameters as well as the CA to NA ratio was compared between the control group (Group I) and the study groups (Group IIa and IIb) to assess the malignant changes in the exfoliated cells. Statistical analysis showed that there is a significant decrease in the CD, CP and CA [Table 2] in the erosive/atrophic group when compared to the normal healthy individuals. The reason that Cowpe et al.,[19] state for the reduction in CD and consequently the CP and CA to be an early indicator in a cell turning malignant is due to dysplasia. They state that when a normal cell becomes dysplastic there is reduction in the cell diameter. This statement was supported by the study done by Ramaesh et al.,[20] They found that there is reduction in the cytoplasmic parameters of the squames obtained from the lesions showing dysplasia histologically and no significant reduction in lesions lacking dysplasia. The reason for this decreased CD was thought to be because of the increased activity of cells due to which the ability of cytoplasm to mature diminishes.[21] There was statistically no significant change in the reticular/plaque OLP group when compared with the control. The reason behind this is probably the cells in these lesions were not dysplastic.
When the nuclear parameters were compared in this study, the results showed a statistically significant increase in the ND, NP and NA [Table 2] in the erosive/atrophic OLP as compared to the controls. According to the study done by Ramaesh et al.,[20] the reason they state for the increase in the ND is due to dysplasia. As the normal cells turns dysplastic and then malignant, there is a progressive increase in the ND and consequently in the NP and NA. According to Ikeguchi et al.,[22] the increase in the ND is probably due to gross changes in the number of chromosomes (aneuploidy). There was statistically no significant difference in the ND, NP and NA [Table 3] when the reticular/plaque type OLP was compared with the control group. The reason behind this is probably the cells in these lesions were not dysplastic and were not proliferating actively.
Furthermore the CA to NA was significantly reduced [Table 4] when the erosive/atrophic group was compared to the control group in this study. In the study done by Sugerman et al.,[23] they found a reduced CA to NA ratio in the erosive/atrophic OLP group when compared to the control group, but it failed to reach a statistically significant value probably due to small sample size. The reason for the smaller CA and increased NA and hence a decreased cytoplasmic to nuclear ratio seen in the squamous epithelium indicates a lesser differentiation (i.e. the basal/suprabasal cells).[24] There was statistically no significant difference in the CA to NA [Table 3] when the reticular/plaque type OLP was compared with the control group. The reason behind this is probably the cells in these lesions were highly differentiated.
It is seen in this study that there is a statistically significant difference in the cytoplasmic and nuclear parameters as well as the CA to NA ratio in the erosive/atrophic types of OLP when compared to the normal individuals and statistically no significant difference in the cytoplasmic and nuclear parameters as well the CA to NA ratio in the reticular/plaque type OLP when compared to the normal individuals. Thus these comparisons between the study groups and the control groups shows that our study is in favor of the hypothesis that erosive/atrophic OLP are at more risk of turning malignant when compared to the reticular/plaque OLP. Though a large sample size and further research is required to say anything decisively it can still be said that exfoliative cytology and cytomorphometry can be used as an adjuvant rather than as a diagnostic tool and it can also be used for mass screening and for follow-up purposes.
SUMMARY
OLP has been considered a potentially malignant disorder, though the subtypes of OLP that are at risk have not been determined conclusively. It has been shown that there is a decrease in CD, CP and CA, increase in ND, NP and NA and also a decreased CA to NA ratio in the premalignant and malignant lesions when compared to the normal. Thus these changes can be considered as early malignant changes occurring in the cells. Thus it is very important to assess the quantitative changes occurring in OLP and though computer aided cytomorphometry cannot be diagnostic, it can be used as an adjuvant or in mass screening and follow-up of patients with OLP to assess the risk status.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The author(s) declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article. CSRT, SDA, MNR, SPM and MD carried out the study, participated in the sequence alignment and drafted the manuscript. CSRT, SDA, MNR, SPM and MD participated in the design of the study and performed the statistical analysis. CSRT, SDA, MNR, SPM and MD conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) of the institution and an informed consent was taken from all the patients who participated in the study. Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system. In Before reference list have must come following three headings.
Contributor Information
Chitturi Suryaprakash Ravi Teja, Email: dr.raviteja@gmail.com.
A. Santha Devy, Email: drsantha73@gmail.com.
R. Madhavan Nirmal, Email: rmnirmal@hotmail.com.
P. M. Sunil, Email: sunil.narien@gmail.com.
M. Deepasree, Email: dr.thirusree@rediffmail.com.
REFERENCES
- 1.Wilson E. On lichen planus. J Cutan Med Dis Skin. 1869;3:117–32. [Google Scholar]
- 2.Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: Facts and controversies. Clin Dermatol. 2010;28:100–8. doi: 10.1016/j.clindermatol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Elenitas R, Murphy GF, Xu G. Autoimmune diseases of the skin. In: Elder DE, editor. Lever's Histopathology of the Skin. 10th ed. Philadelphia: Lippincott Williams and Wilkins; 2010. p. 228. [Google Scholar]
- 4.Mollaoglu N. Oral lichen planus: A review. Br J Oral Maxillofac Surg. 2000;38:370–7. doi: 10.1054/bjom.2000.0335. [DOI] [PubMed] [Google Scholar]
- 5.Lodi G, Scully C, Carrozzo M, Griffiths M, Sugerman PB, Thongprasom K. Current controversies in oral lichen planus: Report of an international consensus meeting. Part 1. Viral infections and etiopathogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:40–51. doi: 10.1016/j.tripleo.2004.06.077. [DOI] [PubMed] [Google Scholar]
- 6.Roopashree MR, Gondhalekar RV, Shashikanth MC, George J, Thippeswamy SH, Shukla A. Pathogenesis of oral lichen planus – A review. J Oral Pathol Med. 2010;39:729–34. doi: 10.1111/j.1600-0714.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- 7.George A, Sreenivasan BS, Sunil S, Varghese SS, Thomas J, Gopakumar D, et al. Potentially malignant disorders of oral cavity. Oral Maxillofac Pathol J. 2011;2:95–100. [Google Scholar]
- 8.Holmstrup P. The controversy of a premalignant potential of oral lichen planus is over. Oral Surg Oral Med Oral Pathol. 1992;73:704–6. doi: 10.1016/0030-4220(92)90014-h. [DOI] [PubMed] [Google Scholar]
- 9.van der Meij EH, Schepman KP, Smeele LE, van der Wal JE, Bezemer PD, van der Waal I. A review of the recent literature regarding malignant transformation of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:307–10. doi: 10.1016/s1079-2104(99)70033-8. [DOI] [PubMed] [Google Scholar]
- 10.Mattsson U, Jontell M, Holmstrup P. Oral lichen planus and malignant transformation: Is a recall of patients justified? Crit Rev Oral Biol Med. 2002;13:390–6. doi: 10.1177/154411130201300503. [DOI] [PubMed] [Google Scholar]
- 11.Eisen D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J Am Acad Dermatol. 2002;46:207–14. doi: 10.1067/mjd.2002.120452. [DOI] [PubMed] [Google Scholar]
- 12.Gandolfo S, Richiardi L, Carrozzo M, Broccoletti R, Carbone M, Pagano M, et al. Risk of oral squamous cell carcinoma in 402 patients with oral lichen planus: A follow-up study in an Italian population. Oral Oncol. 2004;40:77–83. doi: 10.1016/s1368-8375(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 13.Lanfranchi-Tizeira HE, Aguas SC, Sano SM. Malignant transformation of atypical oral lichen planus: A review of 32 cases. Med Oral. 2003;8:2–9. [PubMed] [Google Scholar]
- 14.Mehrotra R, Gupta A, Singh M, Ibrahim R. Application of cytology and molecular biology in diagnosing premalignant or malignant oral lesions. Mol Cancer. 2006;5:11. doi: 10.1186/1476-4598-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Mehrotra R. The role of cytology in oral lesions: A review of recent improvements. Diagn Cytopathol. 2012;40:73–83. doi: 10.1002/dc.21581. [DOI] [PubMed] [Google Scholar]
- 16.Anees MM, Szepietowski J. Oral lichen planus: A review of aetiopathogenesis, clinical manifestations, histology and treatment modalities. Dermatologia Kliniczna. 2007;9:243–8. [Google Scholar]
- 17.Canto AM, Müller H, Freitas RR, Santos PS. Oral lichen planus (OLP): Clinical and complementary diagnosis. An Bras Dermatol. 2010;85:669–75. doi: 10.1590/s0365-05962010000500010. [DOI] [PubMed] [Google Scholar]
- 18.Girish HC, Murgod S, Savita JK. Epithelial dysplasia in lichen planus. J Adv Dent Res. 2010;1:19–25. [Google Scholar]
- 19.Cowpe JG, Longmore RB, Green MW. Quantitative exfoliative cytology of abnormal oral mucosal smears. J R Soc Med. 1988;81:509–13. doi: 10.1177/014107688808100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramaesh T, Mendis BR, Ratnatunga N, Thattil RO. Cytomorphometric analysis of squames obtained from normal oral mucosa and lesions of oral leukoplakia and squamous cell carcinoma. J Oral Pathol Med. 1998;27:83–6. doi: 10.1111/j.1600-0714.1998.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 21.Khandelwal S, Solomon MC. Cytomorphological analysis of keratinocytes in oral smears from tobacco users and oral squamous cell carcinoma lesions - A histochemical approach. Int J Oral Sci. 2010;2:45–52. doi: 10.4248/IJOS10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikeguchi M, Oka S, Saito H, Kondo A, Tsujitani S, Maeta M, et al. Computerized nuclear morphometry: A new morphologic assessment for advanced gastric adenocarcinoma. Ann Surg. 1999;229:55–61. doi: 10.1097/00000658-199901000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugerman PB, Savage NW, Williams SL, Joynson OB, Daley TJ, Cowpe JG. A quantitative cytological study of lesional and non-lesional mucosa in oral lichen planus. Arch Oral Biol. 1996;41:117–20. doi: 10.1016/0003-9969(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 24.Bhavaskar RS, Soje SK, Hazarey VK, Ganvir SM. Cytomorphometric analysis for evaluation of cell diameter, nuclear diameter and micronuclei for detection of oral premalignant and malignant lesions. J Oral Biosci. 2011;53:158–69. [Google Scholar]


