Abstract
Objectives To quantify global consumption of key dietary fats and oils by country, age, and sex in 1990 and 2010.
Design Data were identified, obtained, and assessed among adults in 16 age- and sex-specific groups from dietary surveys worldwide on saturated, omega 6, seafood omega 3, plant omega 3, and trans fats, and dietary cholesterol. We included 266 surveys in adults (83% nationally representative) comprising 1 630 069 unique individuals, representing 113 of 187 countries and 82% of the global population. A multilevel hierarchical Bayesian model accounted for differences in national and regional levels of missing data, measurement incomparability, study representativeness, and sampling and modelling uncertainty.
Setting and population Global adult population, by age, sex, country, and time.
Results In 2010, global saturated fat consumption was 9.4%E (95%UI=9.2 to 9.5); country-specific intakes varied dramatically from 2.3 to 27.5%E; in 75 of 187 countries representing 61.8% of the world’s adult population, the mean intake was <10%E. Country-specific omega 6 consumption ranged from 1.2 to 12.5%E (global mean=5.9%E); corresponding range was 0.2 to 6.5%E (1.4%E) for trans fat; 97 to 440 mg/day (228 mg/day) for dietary cholesterol; 5 to 3,886 mg/day (163 mg/day) for seafood omega 3; and <100 to 5,542 mg/day (1,371 mg/day) for plant omega 3. Countries representing 52.4% of the global population had national mean intakes for omega 6 fat ≥5%E; corresponding proportions meeting optimal intakes were 0.6% for trans fat (≤0.5%E); 87.6% for dietary cholesterol (<300 mg/day); 18.9% for seafood omega 3 fat (≥250 mg/day); and 43.9% for plant omega 3 fat (≥1,100 mg/day). Trans fat intakes were generally higher at younger ages; and dietary cholesterol and seafood omega 3 fats generally higher at older ages. Intakes were similar by sex. Between 1990 and 2010, global saturated fat, dietary cholesterol, and trans fat intakes remained stable, while omega 6, seafood omega 3, and plant omega 3 fat intakes each increased.
Conclusions These novel global data on dietary fats and oils identify dramatic diversity across nations and inform policies and priorities for improving global health.
Introduction
By 2020, nearly 75% of all deaths and 60% of all disability adjusted life years worldwide will be attributable to non-communicable chronic diseases including cardiovascular diseases, type 2 diabetes, obesity, and cancers, with the largest increases and burdens in low and middle income countries rather than high income countries.1 2 Most of these burdens occur prematurely and can be prevented or delayed, making the identification and targeting of the modifiable risk factors with greatest impact on these diseases among the greatest health priorities of our time.
For decades healthcare systems, clinicians, and scientists have focused on the medical, drug treatment model of disease that highlights intermediate, downstream, metabolic risk factors as established predictors of diseases rather than fundamental root causes such as diet and lifestyle.3 The consequences of this narrow approach are evident: the global obesity epidemic and the pandemic of chronic diseases that has now swept the world. As demonstrated by the Global Burden of Diseases Study’s capstone papers,4 and as highlighted throughout the reports from the United Nations high level meeting on non-communicable disease prevention and control (http://www.who.int/nmh/events/un_ncd_summit2011/en/), diet is one of the fundamental risk factors for health, disease, and disability in the world. Indeed, given that trends in metabolic risk factors such as blood pressure, cholesterol, glucose, and body mass index are being largely driven by nutrition, suboptimal diet is the single leading modifiable cause of poor health in the world, exceeding the burdens due to tobacco and excess alcohol consumption combined.4 Eight of the top 20 individual causes of disease burden worldwide are due to poor nutrition, largely because of increased risk of chronic diseases.4
Over the past several decades, multiple diet-disease relationships, establishing either beneficial or harmful effects of specific dietary fats,5 6 7 8 9 10 11 12 have been identified and been the target of major policy programmes. And yet, the global patterns and distributions of consumption of key dietary fats and oils, as well as heterogeneity in these patterns by region, country, age, and sex, are not well established. Prior global assessments based on dietary habits at the individual level did not evaluate dietary fats and oils,13 and few countries have published nationally representative data on their consumption. Most estimates of global dietary fats and oils have relied largely on crude disappearance or expenditure data,14 15 16 17 18 which imperfectly capture consumption levels, cannot evaluate differences within the population, and often exclude key fats such as trans fats or plant omega 3 fats. Prior estimates have also not explicitly addressed missing data for certain age groups or regions, distinguished nationally representative surveys from regional or community based surveys, quantified uncertainty in the dietary estimates, or evaluated heterogeneity by age and sex. In particular, age- and sex-specific national consumption levels are essential for quantifying the impact on burdens of disease risk, which are not uniformly distributed across the population.
These limitations have together precluded valid quantitative assessment of the impact of specific dietary fats and oils on chronic diseases worldwide, of the epidemiologic nutrition transitions occurring in low and middle income nations, and of the most relevant dietary priorities in different nations. To address these issues, we identified specific dietary fats and oils with evidence for largest public health impact; characterised optimal consumption levels; and systematically investigated global dietary habits, based largely on individual-level, nationally representative surveys, and derived using comparable and consistent methods to construct a global dietary database having quantitative estimates of consumption of key dietary fats and oils, by region, country, time, age, and sex.
Methods
Study design
This work was performed by the Nutrition and Chronic Diseases Expert Group (NutriCoDE) as part of the 2010 Global Burden of Diseases, Injuries, and Risk Factors (GBD) Study.4 Our methods for identification, access, and selection of dietary risk factors and data have been reported.19 Because we used de-identified national datasets, this research was reviewed by the Harvard School of Public Health institutional human subjects committee and deemed to be exempt from human subjects research requirements. To generate valid, comparable estimates of consumption of fats and oils around the world, we used consistent methods across regions, countries, age and sex subgroups, and time to:
Identify specific dietary fats and oils with evidence for largest public health impact, based on strength of evidence for aetiological effects on coronary heart disease, stroke, type 2 diabetes, or cancers
Systematically search for nationally representative data sources from around the world on individual level dietary consumption of these fats and oils, including by age and sex
Retrieve data, including assessment of quality and representativeness, maximisation of measurement comparability and consistency, and ascertainment of missing data and uncertainty
Estimate consumption levels by region, country, age, sex, and time, accounting for missing data, incomparability of measured values, and sampling and modelling uncertainty
Characterise optimal consumption levels of each dietary fat and oil, based on observed intakes associated with lowest disease risk and observed mean national consumption levels globally, to place the observed intakes in context and enable quantification of relevant attributable disease burdens.
Identification of dietary fats and oils with largest public health impact
We reviewed the evidence for aetiological effects on chronic diseases including coronary heart disease, stroke, type 2 diabetes, and cancers, based on multiple criteria for assessing causality of diet-disease relationships.20 21 22 We required convincing or probable evidence for effects on clinical events (such as myocardial infarction) rather than simply on physiological risk factors (such as blood cholesterol concentration). The detailed results for our assessments of aetiological effects of dietary fats and oils have been reported.19 23 We identified aetiological effects of omega 6 polyunsaturated fat as a replacement for saturated fat, seafood omega 3 fats, and trans fats on coronary heart disease.6 7 8 9 10 11 12 We did not identify convincing or probable evidence for aetiological effects of these fats on stroke, diabetes, or cancers; or of total fat, monounsaturated fat, plant omega 3 fats, or dietary cholesterol (evaluated mainly through egg consumption) on coronary heart disease, stroke, type 2 diabetes, or cancers.8 21 24 25 26 27 28 29 30 31
Systematic searches for national dietary data
We performed systematic searches for individual level dietary surveys in all countries. Surveys with evidence for selection bias or measurement bias were excluded. Using standardised criteria and methods,19 we searched multiple online databases from March 2008 to September 2010 without date or language restrictions. From these searches, we identified comparably few appropriate published data sources. Thus, from March 2008 to July 2012, we also used extensive personal communications with researchers and government authorities throughout the world, including authors of published nutrition studies and nutrition authorities in a given country, inviting them to be corresponding members of the NutriCoDE group (fig 1 ). For countries lacking identified national or subnational individual-level surveys by these methods, we searched for other potential data sources, including individual level surveys from large cohorts, the WHO Global Infobase, the WHO Stepwise Approach To Surveillance (STEPS) database, and budget survey data at the household level. Given our aim to evaluate chronic diseases, we focused on data from adults (aged ≥20 years). The results of our search strategy by dietary factor, time, and region have been reported.32 A total of 266 surveys in adults representing 113 of 187 countries and 82% of the global population were identified (fig 1 ).
Fig 1 Flow diagram of systematic search for nationally representative surveys of food and nutrient intake
Data retrieval and standardisation
Data retrieval followed the 2010 Global Burden of Diseases study’s comparative risk assessment framework,33 collecting quantitative data on consumption in 16 age- and sex-specific subgroups across 21 world regions (see eTable 1 of data supplement) and two time periods (1990 and 2010). Most published or publically available dietary data were limited or not in the relevant format. For 173 surveys, 99 corresponding members provided original raw data to us or re-analysed their raw data according to our specifications, providing age and sex stratified dietary results in specified metrics and units using a standardised electronic format (appendix 1 of data supplement). Optimal and alternative metrics and units were defined for each dietary factor,19 with optimal units matching those of studies used to evaluate relationships with disease risk as well as major dietary guidelines. Based on these criteria, dietary factors were evaluated as percentage energy (saturated fat, omega 6 polyunsaturated fat, trans fat) or as mg/day standardised using the residual method34 to 2000 kcal/day (dietary cholesterol, seafood omega 3 fat, plant omega 3 fat). The surveys providing data on dietary fats and oils are listed in eTable 2 of data supplement.
For each survey, we extracted data on survey characteristics, dietary metrics, units, and mean and distribution (such as standard deviation) of consumption of each dietary fat and oil, by age and sex (eTable 2). Data were double checked for extraction errors and assessed for plausibility. We assessed survey quality by evaluating evidence for selection bias, sample representativeness, response rate, and validity of diet assessment method.19 Measurement comparability across surveys was maximised by using a standardised data analysis approach that (1) accounted for sampling strategies within the survey by including sampling weights (if available), (2) used the average of all days of dietary assessment to quantify mean intakes, (3) used a corrected population standard deviation to account for within person variation versus between person variation, (4) used standardised dietary metrics and units of measure across surveys, and (5) adjusted for total energy to reduce measurement error and account for differences in body size, metabolic efficiency, and physical activity.34
Quantification of global, regional, and national distributions
Our systematic approaches to survey identification and data retrieval identified gaps in data for certain countries, certain dietary fats or oils across countries, or time periods. Furthermore, even using the systematic data retrieval and standardisation methods described above, identified surveys and measures were not always comparable—for example, varying in representativeness, urban or rural coverage, age groups, dietary instruments, or dietary metrics. To address missing data, incomparability, and related effects on uncertainty of dietary estimates, we developed an age integrating Bayesian hierarchical imputation model (appendix 2 of data supplement).4 This model estimated the mean consumption level and its statistical uncertainty for each age, sex, country, and year stratum. For each dietary factor, primary inputs were the survey-level quantitative data, including country-, time-, age-, and sex-specific consumption levels (mean, distribution); data on the numbers of subjects in each strata; survey level indicator covariates for sampling representativeness, dietary assessment method, and type of dietary metric; and country, region (21 Global Burden of Diseases regions), and super-region (7 Global Burden of Diseases groupings of regions) random effects. Additional country-level, time-varying (year-specific) covariates, which were available in all years including 2010, included lagged distributed income35 and food disappearance data derived and standardised from United Nations Food and Agricultural Organization food disappearance balance sheets,16 including 17 nutrients or food groups and four factors derived from principal components analysis of these 17 variables. The final model covariates for each dietary risk factor are presented in table 1. The final national, regional, and global estimates were calculated as population-weighted averages of the corresponding age- and sex-specific strata. Using these methods, we quantified the consumption levels of saturated fat, omega 6 polyunsaturated fat, trans fat, dietary cholesterol, seafood omega 3 fat, and plant omega 3 fat, and among men and women in 187 countries in 1990 and 2010.
Table 1.
Data availability and covariates used for imputation of global intakes of key dietary fats and oils
| Dietary risk factor | Regions covered* | Years covered | No of surveys | No of countries (% of global adult population) | No of surveys with individual level data (No with age- and sex-specific estimates) | No of household level surveys | Covariates for imputation, each year 1990-2010† |
|---|---|---|---|---|---|---|---|
| Saturated fats | AE, APH, AS, ASE, AUS, CAR, EURC, EURE, EURW, LAC, LAT, NA, NAM, OC, SSS | 1980-2008 | 75 | 47 (70) | 75 (71) | 0 | Survey-specific: representativeness (nationally representative v subnational). Country-specific: FAO saturated fats (natural log of percentage of total calories consumed per capita per day in the form of saturated fats). |
| Omega 6 polyunsaturated fats | AE, APH, ASE, AUS, CAR, EURC, EURE, EURW, LAC, LAT, NA, NAM, SSS | 1986-2008 | 51 | 32 (47) | 51 (51) | 0 | Survey-specific: metric (optimal v secondary metric), representativeness (nationally representative v subnational). Country-specific: FAO polyunsaturated fats (natural log of percentage of total calories from polyunsaturated fats derived from cottonseed oil, rape/mustard seed oil, soybean oil, sesame seed oil, sunflower seed oil, maize germ oil, and groundnut oil consumed per capita per day), FAO factor variables 1-4. |
| Trans fats | APH, AS, CAR, EURW, LAC, LAT, NA, NAM, SSS | 1980-2009 | 56 | 23 (19) | 46 (20) | 10 | Country-specific: hydrogenated oil net ratio (ratio of hydrogenated oil to total oil crops export), total oil/fats per capita (log transformed total oils/fats per capita [from retail/food service database], in tonnes), total packaged foods per capita (log transformed total packaged foods per capita [from retail/food service database], in tonnes). |
| Dietary cholesterol | AE, APH, AS, ASE, AUS, CAR, EURC, EURE, EURW, LAC, LAS, LAT, NA, NAM, OC, SSS | 1980-2008 | 70 | 45 (53) | 70 (65) | 0 | Survey-specific: representativeness (nationally representative v subnational). Country-specific: FAO animal fats (natural log of percentage of total calories consumed per capita per day in the form of animal fats), FAO eggs (natural log of percentage of total calories consumed per capita per day in the form of eggs). |
| Seafood omega 3 fats | AE, APH, AS, ASE, AUS, CAR, EURC, EURE, EURW, LAC, LAT, NA, NAM, SSE, SSS, SSW | 1980-2008 | 109 | 57 (59) | 55 (47) | 54 | Survey-specific: metric (optimal v secondary metric), representativeness (nationally representative v subnational), diet assessment method (diet recalls/ records or FFQ v household availability/budget survey). Country-specific: FAO omega 3 polyunsaturated fats (natural log of percentage of total calories consumed per capita per day in the form of omega 3 polyunsaturated fats). |
| Plant omega 3 fats | AE, APH, CAR, EURC, EURW, LAC, LAT, NA, NAM, SSS | 1990-2007 | 28 | 21 (44) | 28 (28) | 0 | Survey-specific: representativeness (nationally representative v subnational). Country-specific: omega 6 polyunsaturated fats (natural log of percentage of total calories consumed per capita per day in the form of omega 6 polyunsaturated fats), soybean (natural log of percentage of total calories consumed per capita per day in the form of soybean and oil from soy), hydrogenated oil net ratio (ratio of hydrogenated oil to total oil crops export). |
FAO=United Nations Food and Agriculture Organization. FFQ=food frequency questionnaire
*Based on 21 Global Burden of Diseases, Injuries, and Risk Factors (GBD) study regions including APH=Asia Pacific, High Income; AC=Asia, Central; AE=Asia, East, AS=Asia, South; ASE=Asia, South East; AUS=Australasia; CAR=Caribbean, EURC=Europe, Central; EURE=Europe, Eastern; EURW=Europe, Western; LAA=Latin America, Andean; LAC=Latin America, Central; LAS=Latin America, Southern; LAT=Latin America, Tropical; NAM=North Africa/Middle East; NA=North America, High Income; OC=Oceania; SSC=Sub-Saharan Africa, Central; SSE=Sub-Saharan Africa, East; SSS=Sub-Saharan Africa, Southern; SSW=Sub-Saharan Africa, West.
†Year and country-specific covariates were based on the FAO annual Food Balance Sheets.16 A space-time smoothing procedure was used to generate a full time series of consumption estimates. Income and education were used as covariates in the space-time model to improve predictions in instances of missing data. For education, the age standardised mean number of years of education for ages ≥25 by sex as a continuous variable was used.36 For income, the estimated and normalised lag-distributed income based on the international dollar as a continuous variable was used.35 For countries that had split or merged during the time series (1990-2010), we split/merged these countries into constituent countries using a growth rate method to generate as close to a full time series as possible for all countries. The FAO covariates were used in the percentage natural logarithm form (that is, the % of total energy that is comprised of a particular food). For trans fats, the hydrogenated oil net ratio corresponded to the net amount of hydrogenated oils available for consumption in each country-year. Using the FAO data, the numerator for this ratio (that is, hydrogenated oil exports) was calculated based on exported hydrogenated oil (in kcal per capita) and exported oil crops (in kcal per capita) through space-time with lag-distributed income as a covariate. The denominator (that is, total oil crops, in kcal per capita) was calculated by adding import values to production values minus the export values, and then application of space-time model provided us the complete time series covariate. Afterward, the complete time series ratio covariate was applied to the amount of oil crops (in kcal per capita) in each country. Additional country-level time-varying (year-specific) FAO covariates used were the four factors derived from principal component analysis of 17 standardised FAO nutrients or food groups: factor 1 included red meats, animal fats, and pig meats; factor 2 included omega 3 polyunsaturated fats, omega 6 polyunsaturated fats, whole grains, nuts, and vegetables; factor 3 included fruits, legumes, and nuts; and factor 4 included sugars, stimulants, and saturated fats. For model fits see eFig 7 in data supplements.
The model included additional offset and variance components to account for differences between primary and secondary dietary metrics, national and subnational surveys, and individual and household dietary data—in each case allowing greater influence of the former. Model validity across different iterations was evaluated using cross validation, randomly omitting 10% of the raw data and comparing the imputed intakes with the original raw data. Sources of uncertainty were identified and incorporated, including from missing country data, sampling uncertainty of original data sources, and additional uncertainty associated with suboptimal metrics, subnational samples, or household level surveys. Using simulation (Monte Carlo) analyses, we drew 1000 times from the posterior distribution of each exposure for each age, sex, country, and year stratum; computed the mean exposure from the 1000 draws; and the 95% uncertainty intervals as the 2.5th and 97.5th centiles of the 1000 draws. Absolute and relative differences in exposure between 1990 and 2010 were calculated at the draw level to account for the full spectrum of uncertainty. We used Spearman correlations to evaluate interrelations between specific dietary fats and oils.
Characterisation of optimal consumption
To place observed consumption levels in context and allow separate assessment of impact on disease, for each dietary factor we characterised the optimal, yet feasible, consumption levels (table 2).19 37 This was based on both the mean observed consumption associated with lower disease risk in meta-analyses of clinical endpoints and the mean national consumption levels observed in at least two or three countries around the world. As another criterion, we also considered whether such characterised optimal consumption levels were broadly consistent with current major dietary guidelines.
Table 2.
Optimal consumption levels of key dietary fats and oils and relevant data sources*
| Dietary fats† | Related disease outcomes | Observed consumption levels associated with lowest disease risk in human studies‡¶ | Observed national mean consumption levels (top or bottom 3 countries)§ | Dietary guidelines¶ | Mean (SD) optimal consumption** |
|---|---|---|---|---|---|
| Polyunsaturated fats replacing saturated fats | Decreased CHD | Polyunsaturated fats: 14.2%E (CHD events)8 12 7.9%E (CHD deaths)8 12 Saturated fats: 4.1%E8 12 |
Top 3 countries, polyunsaturated fats: Turkey: 18.7%E Bulgaria: 12.7%E Lebanon: 9.4%E Bottom 3 countries, saturated fats: Bangladesh: 2.3%E Bahrain: 2.4%E India: 5.1%E |
<10%E for saturated fats, replacing them with monounsaturated and polyunsaturated fats | 12 (1.2)%E†† |
| Seafood omega 3 fatty acids | Decreased CHD | 250 mg/day10 | Top 3 countries: Iceland: 1189.1 mg/day Barbados: 1178.5 mg/day Japan: 994.6 mg/day |
250 mg/day | 250 (25) mg/day |
| Trans fats | Increased CHD | 0%E38 | Bottom 3 countries: Barbados: 0.2%E Finland: 0.4%E Italy: 0.5%E |
As low as possible | 0.5 (0.05)%E |
CHD=coronary heart disease. %E=percentage of total energy intake. SD=standard deviation
*For each dietary factor, the optimal consumption level was identified based both on observed levels at which lowest disease risk occurs and observed mean consumption levels in at least two or three countries around the world. We also considered whether such identified levels were consistent with major dietary guidelines.7 39
†Dietary fats for which we identified probable or convincing evidence for aetiologic effects on chronic diseases including coronary heart disease, stroke, type 2 diabetes, or cancers.19 Based on available evidence, we identified evidence for aetiologic effects on coronary heart disease of polyunsaturated fatty acids as a replacement for saturated fats; seafood omega 3 fats; and trans fats.8 10 12 38 We did not identify convincing or probable evidence for aetiological effects of these fats on stroke, diabetes, or cancers; or of total fat, monounsaturated fat, plant omega 3 fats, or dietary cholesterol (evaluated mainly through egg consumption) on coronary heart disease, stroke, type 2 diabetes, or cancers.8 21 24-31
‡Observed median consumption levels in population subgroups (top or bottom quartile or quintile) associated with lowest disease risk in meta-analyses of prospective cohort studies or randomised controlled trials.
§Observed mean national consumption levels in the top (for protective factors) or bottom (for harmful factors) three countries as identified in our global data sources. Values are adjusted for total energy and standardised to 2000 kcal/day.34
¶Recommended intake levels for a 2,000 kcal/d diet based on the US Department of Agriculture and United Nations Food and Agriculture Organization guidelines.4 5 7 39
**Because not all individuals within a population can have the same exposure level, the plausible distribution (standard deviation) of optimal consumption was calculated from the average standard deviation for all metabolic risk factors in the Global Burden of Diseases study (10% of the mean).
††Optimal consumption was based on increasing polyunsaturated fat to 12% energy as a replacement for saturated fat, based on the evidence that this specific nutrient replacement reduces risk.8 12
Results
Global consumption in 2010
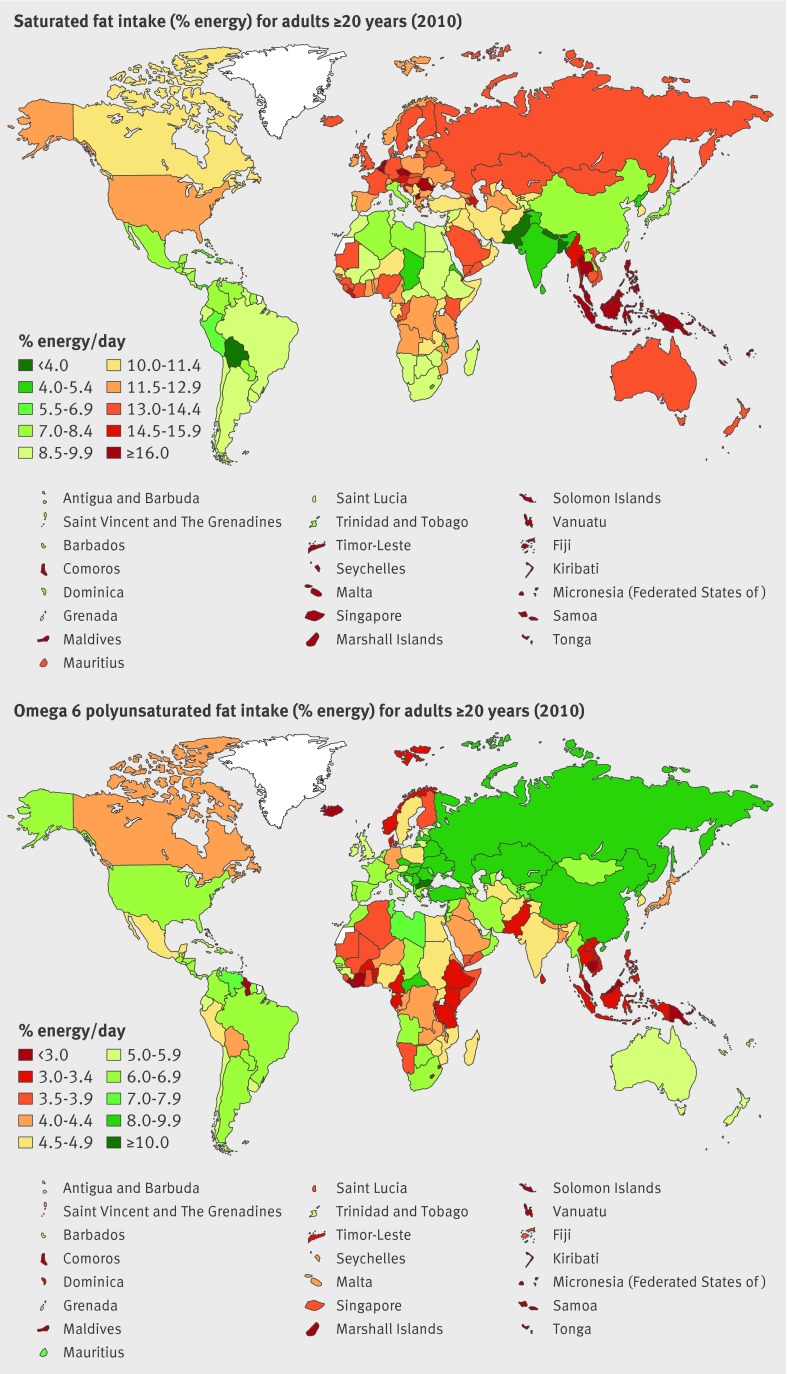
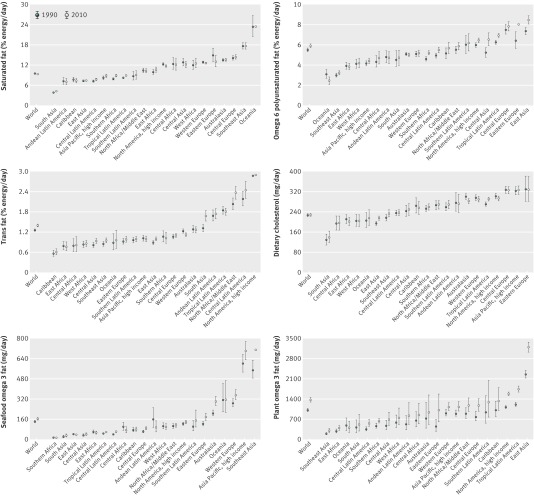
In 2010, mean global saturated fat intake in adults was 9.4% of energy intake (%E) (95% uncertainty interval 9.2%E to 9.5%E), with marked variation across regions and countries (table 3, fig 2 ). A 5.5-fold variation was identified across the 21 Global Burden of Diseases Study regions (from 4.3%E to 23.5%E), and a 12-fold variation across the 187 countries (from 2.3 to 27.5%E). Highest intakes were identified in Samoa, Kiribati, and similar palm oil producing island nations; as well as Sri Lanka, Romania, and Malaysia (eTable 3 of data supplement). Lowest intakes were in Bangladesh, Nepal, Bolivia, Bhutan, and Pakistan. In 75 of 187 countries, representing 2.73 billion adults and 61.8% of the global adult population, mean consumption was <10%E.
Table 3.
Characteristics of adult global consumption of key dietary fats and oils in 2010
| Characteristics of global consumption | Saturated fats (%E) | Omega 6 polyunsaturated fats (%E) | Trans fats (%E) | Dietary cholesterol (mg/day) | Seafood omega 3 fats (mg/day) | Plant omega 3 fats (mg/day) |
|---|---|---|---|---|---|---|
| Global mean consumption (95% UI) | 9.4 (9.2 to 9.5) | 5.9 (5.7 to 6.1) | 1.4 (1.36 to 1.44) | 228 (222 to 234) | 163 (154 to 172) | 1371 (1299 to 1465) |
| Range across 21 global regions (overall variation) | 4.3 to 23.5 (5.5-fold) | 2.5 to 8.5 (3.4-fold) | 0.6 to 2.9 (4.8-fold) | 139 to 328 (2.4-fold) | 13 to 710 (55-fold) | 302 to 3205 (10.6-fold) |
| Regions with highest levels (mean consumption) | Oceania (23.5), South East Asia (17.7), Central Europe (14.4), Australasia (13.6), Eastern Europe (13.0%) | East Asia (8.5), Eastern (8.0) and Central (7.9) Europe, Tropical Latin America (6.9), Central Asia (6.5), High-Income North America (6.5) | High-Income North America (2.9), Central (2.4), Tropical (1.8) and Andean Latin America (1.7), North Africa/Middle East (2.4) | Eastern Europe (328), High-Income Asia Pacific (326), Central Europe (326), High-Income North America (294), Tropical Latin America (291) | Southeast Asia (710), High-Income Asia Pacific (701), Western Europe (351), Oceania (315), Australasia (300) | East Asia (3205), Tropical (1742) and Southern (1288) Latin America, High-Income North America (1584), Caribbean (1331) |
| Regions with lowest levels (mean consumption) | South Asia (4.3), Andean Latin America (7.0), Caribbean (7.4), East Asia (7.4), Central Latin America (7.8) | Oceania (2.5), Southeast Asia (3.2), East (3.9), West (4.2), and Central (4.7) Sub-Saharan Africa, High-Income Asia Pacific (4.4) | Caribbean (0.6), East (0.8), Central (0.8), and West (0.9) Sub-Saharan Africa, Central (0.9) and Southeast (0.9) Asia, Oceania (1.0) | South Asia (139), Central (196), East (202), and West (205) Sub-Saharan Africa, Oceania (215) | Southern (13) and East (52) Sub-Saharan Africa, South (30), East (37), and Central (40) Asia | Southeast Asia (302), East Sub-Saharan Africa (394), Oceania (399), South Asia (514), Central Latin America (552) |
| Regions with greater statistical uncertainty | Oceania*, Eastern Europe*, Central† and West† Sub-Saharan Africa, Andean† and Southern† Latin America | South Asia†, Eastern Europe†, Southern† and Andean† Latin America, Oceania†, Central† and West† Sub-Saharan Africa, Caribbean† | Oceania†‡, East†‡, Central†‡ and Southern†‡ Sub-Saharan Africa | South Asia‡, Eastern Europe*, Oceania*, Central† and West† Sub-Saharan Africa, Andean Latin America† | Latin America†, Oceania† | South Asia†, Australasia†, Southern† Latin America, Oceania†, Eastern Europe† |
| Range across 187 countries (overall variation) | 2.3 to 27.5 (12.2-fold) | 1.2 to 12.5 (10.5-fold) | 0.2 to 6.5 (28.1-fold) | 97 to 440 (4.5-fold) | 5 to 3886 (840-fold) | 2 to 5542 (2731-fold) |
| Countries with highest levels (mean consumption) | Samoa (27.5), Kiribati (27.0), similar palm oil producing island nations (22.8 to 25.7), Sri Lanka (21.9), Romania (21.4), Malaysia (20.3) | Bulgaria (12.5), other Central European nations (8.9 to 9.9), Lebanon (9.9), Kazakhstan (8.9), Belarus (8.5) | Egypt (6.5), Pakistan (5.8), Canada (4.0), Mexico (3.6), Bahrain (3.2) | Romania (439), Algeria (402), Latvia (367), Belarus (352), Lithuania (348mg/day), Denmark (348), Paraguay (347), Japan (347), Hungary (337) | Maldives (3886), Barbados (1986), Seychelles (1291), Iceland (1229), Denmark (1225), Malaysia (988), Thailand (824), Japan (718), South Korea (708) | Jamaica (5542), China (3266), UK (2414), Tunisia (2215), Angola (2195), Canada (2085), Brazil (1747), Paraguay (1575), US (1527), Uruguay (1384), Argentina (1304) |
| Countries with lowest levels (mean consumption) | Bangladesh (2.3), Nepal (2.7), Bolivia (3.2), Bhutan (3.2), Pakistan (3.8) | Kiribati (1.2), Samoa (1.5), Vanuatu (1.5), Maldives (1.6), Sri Lanka (1.6), Solomon Islands (1.7) | Barbados (0.2), Haiti (0.4), other island nations in the Caribbean (0.5 to0.6), Ethiopia (0.6), Eritrea (0.6), other East Sub-Saharan African nations (0.6 to 0.7) | Bangladesh (97), Nepal (116), other South Asian nations (121 to 157), Rwanda (155), Burundi (163), Tajikistan (169), Ghana (169) | Zimbabwe (5), Lebanon (8), Occupied Palestinian Territory (8), Botswana (10), Guinea-Bissau (10) | Israel (2), Solomon Islands (102), Sri Lanka (106), Comoros (126), Saint Lucia (129), Philippines (131) |
| Western Europe mean consumption (95% UI) | 12.6 (12.3 to 13.9) | 5.2 (4.9 to 5.5) | 1.1 (1.1 to 1.2) | 290 (279 to 302) | 351 (314 to 393) | 1120 (1006 to 1270) |
| Western Europe range with country examples | 8.2 in Luxemburg and 9.0 in Malta to 14.7 in Belgium and 14.8 in Austria | 2.7 in Denmark and 2.9 in Iceland to 6.4 in Spain and 8.0 in Israel | 0.8 in Finland, Italy, and Malta to 1.6 in Switzerland and 2.3 in the Netherlands | 215 in Greece and 222 in Luxemburg to 333 in Austria and 348 in Denmark | 97 in Ireland and 180 in Netherlands to 1225 in Denmark and 1229 in Iceland | 2 in Israel and 300 in Denmark to 2014 in Finland and 2414 in the UK |
| US mean consumption (95% UI) | 11.8 (11.5 to 12.2) | 6.7 (6.5 to 7.0) | 2.8 (2.5 to 3.1) | 296 (284 to 306) | 141 (128 to 157) | 1527 (1456 to 1599) |
| No of countries achieving optimal mean intakes, corresponding adult population (% of global total) | <10%E§: 75 countries, 2.73bn people (61.8%) | ≥12%E§; 1 country, 6.1m people (0.1%) ≥5%E: 94 countries, 2.32bn people (52.4%) |
≤0.5%E: 12 countries, 24.43m people (0.6%) | <300 mg/day¶: 155 countries, 3.9bn people (87.6%) | ≥250 mg/day: 45 countries, 837.2m people (18.9%) | ≥0.5%E, or ≥1100 mg for a 2000 kcal/day diet**: 52 countries, 1.94bn people (43.9%) |
| No of countries not achieving optimal mean intakes, corresponding adult population (% of global total) | ≥10%E: 112 countries, 1.69bn people (38.2%) | <12%E: 186 countries, 4.42bn people (99.9%) <5%E: 93 countries, 2.1bn people (47.6%) |
>0.5%E: 175 countries, 4.42bn people (99.4%) >2.0%E: 12 countries, 643.7m people (14.6%) |
≥300 mg/day: 32 countries, 547.9m people (12.4%) | <250 mg/day: 142 countries, 3.58bn people (81.1%) <100 mg/day: 100 countries, 2.95bn people (66.8%) |
<0.5%E, or <1100 mg for a 2000 kcal/day diet: 135 countries, 2.48bn people (56.1%) <500 mg/day: 61 countries, 788.7m people (17.8%) |
UI=uncertainty interval. %E=percentage of total energy intake. bn=billion. m=million.
*Due to higher within-country statistical uncertainty in the raw data.
†Due to limited country-specific raw data on consumption levels.
‡Due to greater variation in consumption levels between countries in the region.
§Based on optimal consumption levels for polyunsaturated fats as a replacement for saturated fats.
¶We did not identify sufficient evidence to set a specific optimal intake level for preventing chronic diseases. The value here is based on recommended consumption levels in the 2010 Dietary Guidelines for Americans.7
**We did not identify sufficient evidence to set a specific optimal intake level for preventing chronic diseases. The value here is based on World Health Organization guidelines for adequate intakes.6
Fig 2 Global and regional mean consumption levels of dietary saturated fat and omega 6 polyunsaturated fat in 2010 for adults aged ≥20 years. See eTable 3 of data supplement for numerical mean estimates and uncertainty intervals
Worldwide omega 6 polyunsaturated fat mean intake was 5.9%E (table 3, fig 2 ), with approximately 3-fold variation between highest (8.5%E) and lowest (2.5%E) regions. Country-specific intake ranged from 1.2%E to 12.5%E. Highest intake was in Bulgaria, followed by other Central European nations, Lebanon, Kazakhstan, and Belarus. Lowest intake was seen in Kiribati, Samoa, and Vanuatu (eTable 3). Only one of 187 countries (Bulgaria) had intakes at or above the optimal level of 12%E. Only 94 of 187 countries had intakes at or above 5%E, representing 2.3 billion adult people and 52.4% of the world adult population. Notably, intakes of saturated fat and omega 6 fat were only modestly intercorrelated: regional r (Spearman)=−0.16, national r=−0.22.
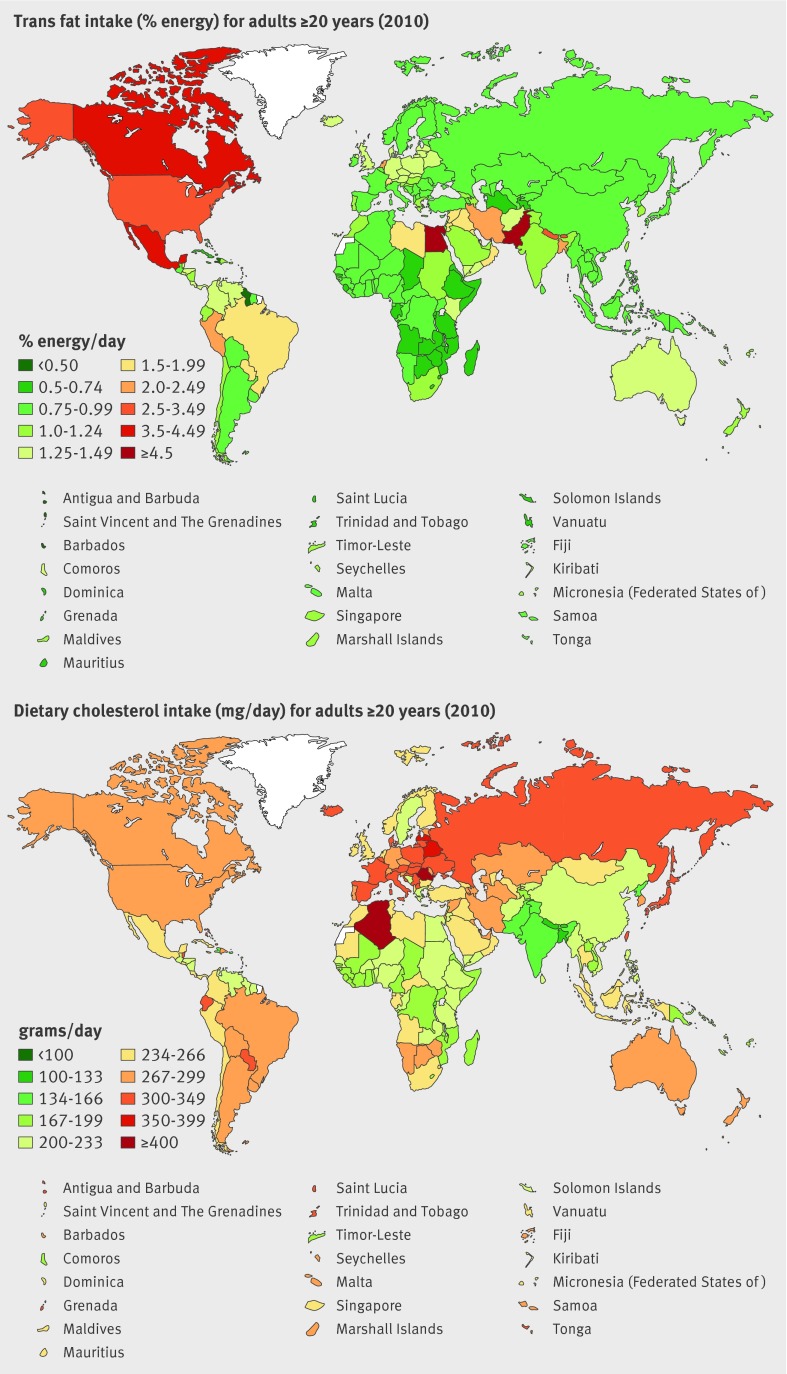
Mean global trans fat intake was 1.40%E, but with a 5-fold difference (from 0.6 to 2.9%E) across regions (table 3, fig 3 ) and a 28-fold difference (from 0.2 to 6.5%E) across countries (eTable 3). Egypt had the highest consumption, followed by Pakistan, Canada, Mexico, and Bahrain. Several island nations in the Caribbean including Barbados and Haiti had the lowest consumption, followed by East Sub-Saharan African nations such as Ethiopia and Eritrea. Only 12 of 187 countries had mean consumption ≤0.5%E. The remaining countries (99.4% of the global adult population) had higher intakes, including 12 countries with mean consumption >2.0%E. Regional and national correlations between saturated fat and trans fat were −0.25 and 0.06, respectively.
Fig 3 Global and regional mean consumption levels of dietary trans fat and cholesterol in 2010 for adults ≥20 years of age. See eTable 3 of data supplement for numerical mean estimates and uncertainty intervals
Globally, mean dietary cholesterol intake was 228 mg/day (table 3, fig 3 ). Across the Global Burden of Diseases Study regions, roughly 2.4-fold differences were identified, and across countries 4.5-fold differences (from 97 to 440 mg/day). Romania, and other Eastern European nations, such as Latvia and Belarus, as well as Algeria, Paraguay, Japan, and Hungary had highest consumption (eTable 3 of data supplement). Lowest intakes were in Bangladesh, Nepal, other South Asian nations, and East Sub-Saharan African nations such as Rwanda and Burundi. Overall, 155 of 187 countries had mean cholesterol consumption <300 mg/day, in line with current recommendations,7 representing 3.9 billion adults and 87.6 % of the world adult population. Both regionally and nationally, dietary cholesterol did not strongly correlate with saturated fat consumption (r=0.13 and 0.09, respectively).
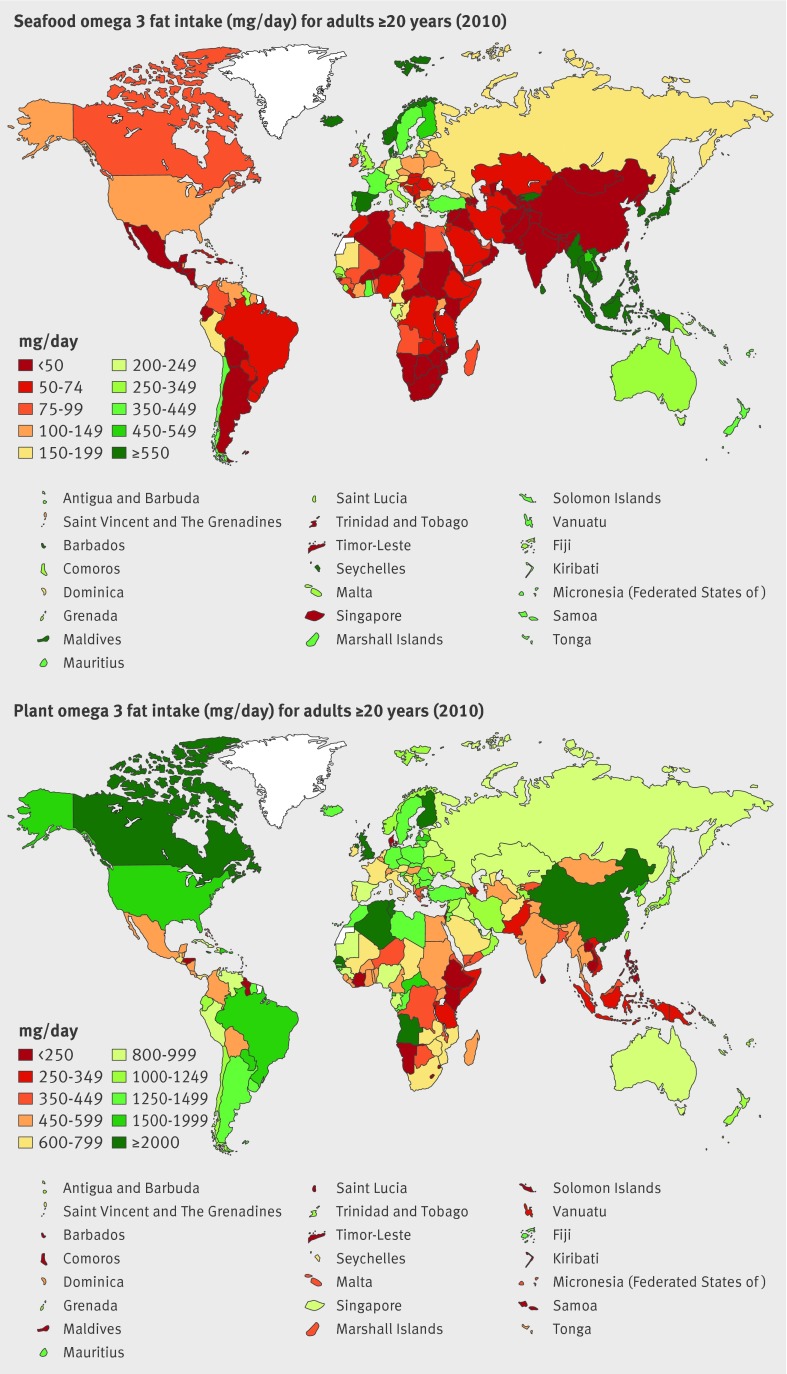
Global mean intake of seafood omega 3 fats was 163 mg/day, with tremendous regional variation (from <50 to >700 mg/day) and national variation (from 5 to 3886 mg/day) (table 3, fig 4 ). Highest intakes were identified in island nations including Maldives, Barbados, the Seychelles, and Iceland; as well as in Malaysia, Thailand, Denmark, South Korea, and Japan (eTable 3). Lowest intakes were in Zimbabwe, Lebanon, the Occupied Palestinian Territory, Botswana, and Guinea-Bissau. In 45 of 187 countries mean intakes were ≥250 mg/day, in line with current guidelines.7 Notably, 100 nations had very low mean consumption (<100 mg/day), generally in Sub-Saharan African and Asian regions as well as North Africa/Middle East, representing three billion adults and 66.8% of the world adult population.
Fig 4 Global and regional mean consumption levels of dietary seafood omega 3 fat and plant omega 3 fat in 2010 for adults ≥20 years of age. See eTable 3 of data supplement for numerical mean estimates and uncertainty intervals
Mean plant omega 3 consumption was 1371 mg/day, with a 10-fold range (302 to 3205 mg/day) across regions (table 3, fig 4 ). By country, intake ranged from <100 to >3000 mg/day (eTable 3). Highest consumption was seen in Jamaica, China, the UK, Tunisia, Angola, Senegal, Algeria, Canada, and the US. Several of these nations have substantial linseed production, such as Canada (which also has high canola production), China, and the US. Several South American nations (Brazil, Uruguay, Paraguay, Argentina) also had higher plant omega 3 consumption, potentially due to availability of chia seeds high in α-linolenic acid, or intakes of other nuts and seeds. Lowest intakes were found in Israel, the Solomon Islands, Sri Lanka, Comoros, Saint Lucia, and the Philippines. Although we did not identify sufficient evidence to set a specific optimal intake level for preventing chronic diseases, World Health Organization guidelines suggest mean population plant omega 3 consumption of ≥0.5%E, or ≥1100 mg for a 2000 kcal/day diet.6 Based on this, 52 of 187 countries met this intake. Among the 135 countries with lower consumption, 61 had intakes <500 mg/day, substantially below current recommendations, representing 800 million adults and 17.8% of the global adult population.
Differences in consumption by sex and age
Within regions and countries, consumption levels of fats and oils were generally similar by sex (eFigs 1-5, eTables 5-7 of data supplement). For example, compared with men, women generally consumed only slightly more saturated fat (roughly 0.3%E) and plant omega 3 (roughly 16 mg/day). Intakes of saturated fat, omega 6 polyunsaturated fat, and plant omega 3 were also relatively similar by age (eFig 6). Conversely, trans fat consumption varied more greatly by age with generally higher intakes in younger adults, particularly in South Asia, High-Income North America, and Central and Tropical Latin America. Dietary cholesterol also varied by age, but with higher intakes at older rather than at younger ages, especially in High-Income Asia Pacific, Central Asia, Oceania, and the Caribbean. Seafood omega 3 consumption was also generally higher among older compared with younger adults, most apparent in High-Income Asia Pacific, Southeast Asia, Australasia, Oceania, and Western Europe.
Changes in consumption between 1990 and 2010
Globally, mean saturated fat intake was stable between 1990 and 2010 (change: +0.1%E (95% uncertainty interval −0.1 to 0.3)) (fig 5 ). Intake nominally increased in 13 regions, although these increases only achieved statistical significance in Southern Sub-Saharan Africa (+0.9%E (0.5 to 1.4)), Tropical Latin America (+0.6%E (0.3 to 0.9)), Central Latin America (+0.5%E (0.1 to 1.0)), and South Asia (0.4%E (0.2 to 0.6)). Intake nominally decreased in eight regions, although none of these decreases was statistically significant, including in Eastern Europe (−1.9%E (−4.6 to 0.8)) and Central Asia (−0.7%E (−2.2 to 0.8)). Among countries, the greatest absolute increases occurred in Zambia (+4.6%E (2.3 to 7.1)), Timor-Leste (+4.4%E (0.6 to 8.5)), Georgia (+4.0%E (1.2 to 6.7)), Bosnia and Herzegovina (+4.0%E (0.9 to 7.3)), and Mauritania (+3.8%E (0.9 to 6.9)) (eTable 4 of data supplement). During this same period, Lithuania experienced the largest declines in saturated fat (−4.4%E (−5.6 to −3.1)), followed by Tajikistan (−4.1%E (−7.5 to −1.1)), Algeria (−3.8%E (−5.0 to −2.7)), Estonia (−2.8%E (−4.0 to −1.7)), and Bulgaria (−2.7%E (−4.4 to −1.1)). By country, mean intake declined by at least 2%E in only five countries and by at least 1%E in 10 countries, while it increased by at least 2%E in nine countries and by at least 1%E in 10 countries (eTable 4).
Fig 5 Global and regional mean consumption levels in 1990 and 2010 of dietary saturated fat, omega 6 polyunsaturated fat, trans fat, cholesterol, seafood omega 3 fat, and plant omega 3 fat for adults ≥20 years of age in relation to their uncertainty. See eTables 3 and 4 of data supplement for numerical mean estimates and uncertainty intervals
Mean omega 6 polyunsaturated fat intake increased by 0.5%E (0.3 to 0.8) globally (fig 5 ), including statistically significant increases in nine regions and non-significant trends in nine more. Largest increases were in Eastern Europe (+1.6%E (0.3 to 3.1)), Central Asia (+1.3%E (0.6 to 2.1)), and East Asia (+1.1%E (0.6 to 1.6)). Consumption levels remained stable in Western Europe and East Sub-Saharan Africa, and nominally decreased in Australasia, Andean Latin America, and Oceania, with a significant decrease only in the latter (−0.6 %E (−1.2 to 0.0)). Among individual nations, largest increases occurred in Eastern Europe including Latvia (+3.4%E (1.9 to 5.2)) and Belarus (+2.7%E (0.8 to 4.7)), and in Kazakhstan (+3.2%E (1.2 to 5.5)), El Salvador (+2.6%E (1.3 to 3.9)), Georgia (+2.5%E (0.9 to 4.1)) and Costa Rica (+2.3%E (0.9 to 3.7)) (eTable 4). Greatest decreases were in Malta (−1.4%E (−2.8 to −0.1)), Mauritania (−1.3%E (−2.7 to 0.0)), Mali (−1.3%E (−2.7 to 0.0)), and Denmark (−1.3%E (−2.3 to −0.3)).
Globally, mean trans fat intake remained relatively stable between 1990 and 2010 (+0.1%E (0.1 to 0.2)) (fig 5 ). Consumption increased in six regions, largest in North Africa/Middle East (+0.4%E (0.1 to 0.7)) and South Asia (+0.3%E (0.2 to 0.5)). Intakes nominally decreased in Southern Sub-Saharan Africa (−0.1%E (−0.3 to 0.2)) and Western Europe (−0.1%E (−0.2 to 0.0)). By nation, mean trans fat intakes were also relatively unchanged between 1990 and 2010, but with broader uncertainty due to more limited data across time (eTable 4). Largest increases occurred in South Asian and North African/Middle Eastern nations, including Pakistan (+1.5%E (0.9 to 2.2)), Iran (+0.6%E (0.2 to 1.0)), as well as in Costa Rica (+0.3%E (0.1 to 0.5)). Several Western European nations had larger decreases, including Norway (−0.3%E (−0.5 to −0.1)) and the Netherlands (−0.3%E (−0.5 to 0.0)).
Mean dietary cholesterol consumption was stable worldwide (global change +7 mg/day (−1 to 15)). Intake increased in East Asia (+20 mg/day (9 to 31)) and Tropical Latin America (+19 mg/day (9 to 30)), and decreased in Australasia (−18 mg/day (−34 to −2)). By nation, greatest increases were in Samoa (+58 mg/day (30 to 86)), Taiwan (+29 mg/day (12 to 45)), Barbados (+24 mg/day (2 to 48)), and Indonesia (+21 mg/day (1 to 44)) (eTable 4). Largest decreases were in Lebanon (−33 mg/day (−55 to −11)), Estonia (−33 mg/day (−60 to −8)), New Zealand (−26 mg/day (−45 to −5)), and Finland (−25 mg/day (−40 to −9)).
Worldwide seafood omega 3 consumption increased by 24 mg/day (12 to 37) (fig 5 ), including largest increases in Southeast Asia (+146 mg/day (22 to 267)), Australasia (+88 mg/day (47 to 130)), Eastern Europe (+53 mg/day (21 to 87)), and Western Europe (+53 mg/day (2 to 103)). No regions had certain decreases; non-significant decreases were seen in Andean Latin America (−65 mg/day (−160 to 20)), and Central Sub-Saharan Africa (−27 mg/day (−66 to 10)). Of 104 countries with consumption <100 mg/day in 1990, 93 remained <100 mg/day in 2010. Nationally, greatest absolute increases were in South Korea (+240 mg/day (133 to 345)), Indonesia (+187 mg/day (102 to 281)), Croatia (+110 mg/day (63 to 167)), Lithuania (+108 mg/day (67 to 150)), and Moldova (+80 mg/day (32 to 145)) (eTable 4). The largest national decreases were in North Korea (−81 mg/day (−153 to −31)), Estonia (−50 mg/day (−95 to −10)), Guinea-Bissau (−26 mg/day (−52 to −8)), and Taiwan (−25 mg/day (−35 to −16)).
Global plant omega 3 intake increased substantially, by 393 mg/day (299 to 500) (fig 5 ). Intakes increased in all regions except Oceania, which experienced a non-significant decline (−51 mg/day (−347 to 221)). Largest increases were evident in East Asia (+950 mg/day (725 to 1,173)), Eastern Europe (+570 mg/day (111 to 1,167)), Tropical Latin America (+534 mg/day (415 to 652)), High-Income North America (+467 mg/day (382 to 553)), and Central Europe (+400 mg/day (142 to 677)). Across countries, greatest increases were in Latvia (+1427 mg/day (531 to 2968)), Jamaica (+1205 mg/day (699 to 1731)), Lithuania (+1002 mg/day (350 to 2069)), China (+978 mg/day (748 to 1202)), Estonia (+840 mg/day (151 to 1899)), Canada (+809 mg/day (622 to 999)), and the UK (+723 mg/day (515 to 931)). The only statistically significant decrease was in Sweden (−238 mg/day (−395 to −97)).
Discussion
Relevance of findings for public health
This systematic investigation of individual-level dietary assessments across the world provides, for the first time, quantitative estimates of the global consumption of major dietary fats and oils by region, country, age, and sex, as well as the uncertainty in these measurements. Since suboptimal diet is the single leading cause of death and disability in the world today,4 these findings are highly relevant and of crucial interest to the global scientific community, health professionals, policy makers, and the public. The results demonstrate both similarities and substantial diversity in consumption of fats and oils across regions and nations. These findings facilitate quantitative assessment of disease burdens attributable to these dietary factors—such as by using state-transition Markov models,40 41 food impact models,42 43 44 and comparative risk assessment4 5 13 45—and inform public health and policy priorities for global, regional, and country-specific interventions. We also assessed dietary changes over the past 20 years, although some change estimates should be interpreted with caution due to more limited available data over time.
Principal findings and interpretation
At a global level, mean consumption of saturated fat (9.4%E; guideline<10%E) and dietary cholesterol (228 mg/day; guideline<300 mg/day) were in line with current recommendations or optimal intakes. Reductions of saturated fat and dietary cholesterol have been longstanding public health priorities. In 2010, national saturated fat and cholesterol intakes met recommended intakes in countries representing about 60% and 88% of the global adult population, respectively, suggesting that this public health focus has been relatively successful. Lowest intakes were identified in South and East Asia, South America, and certain Caribbean nations. Such low intakes would be beneficial for coronary heart disease, especially when accompanied by reciprocal increases in polyunsaturated fat intake,11 12 although very low saturated fat intake may increase risk of other outcomes, such as hemorrhagic stroke.46 Based on crude national availability and production estimates, saturated fat consumption in some countries decreased after the mid-20th century.47 Our results demonstrate relatively stable global and regional intakes of saturated fat and cholesterol in more recent years since 1990, except for further declines in certain Eastern European nations. These relatively stable intakes since 1990 may be attributable in part to national programmes in some countries having most of their effect on saturated fat and dietary cholesterol before 1990. For example, Finland constitutes one of the best documented examples of a community intervention that had most of its effect prior to 1990, with further (though smaller) declines seen after that.48 In addition, effectiveness of recent population-level approaches to reduce saturated fat in some countries could have been offset by increasing Westernisation of diets and cultural and social prioritisation of red meats.
Intakes of polyunsaturated fats, which have historically received less public health and policy attention than saturated fat or dietary cholesterol, were far below optimal worldwide. Intakes were lowest in several Pacific island nations with very high intakes of palm oil, creating adverse ratios of polyunsaturated to saturated fats in these nations. Low intakes were also identified in many Southeast Asian and Sub-Saharan African countries, suggesting infrequent use of healthful vegetable oils for cooking or preparing foods. In some regions such as Eastern Europe, significant increases in polyunsaturated fat and reciprocal declines in saturated fat intake were identified between 1990 and 2010, demonstrating feasibility of such broad population substitutions to reduce coronary heart disease risk. Ecological analyses based on commodity disappearance data are consistent with these findings.49 Yet, despite promising overall increases in polyunsaturated fat intakes over the past two decades, the great majority of nations consume lower than optimal levels, highlighting the need to increase public health awareness and focus on healthy vegetable oils. The absence of strong intercorrelation between polyunsaturated and saturated fat consumption (nationally, −0.22) suggests that these dietary risk factors for coronary heart disease are consumed relatively independently and should be targeted separately to reduce risk, particularly as benefits of replacing either saturated fat or carbohydrates with polyunsaturated fat may be relatively similar.11
Consistent with local cultures, highest seafood omega 3 consumption was identified in Pacific island nations, the Mediterranean basin, Iceland, South Korea, and Japan (although in the last two countries, large amounts are from salted foods, which might increase stroke and gastric cancer).21 24 50 51 Yet, 142 countries representing nearly 80% of the world’s adult population had mean seafood omega 3 intakes below 250 mg/day. Extremely low levels (often <100 mg/day) were identified in Sub-Saharan Africa, South America (except Chile), and Asian mainland nations. These findings highlight the dearth of seafood omega 3 fats in much of the world and the need for concerted public health and policy initiatives, including focus on sustainable aquaculture and fishing practices, to increase both supply and consumption. Whereas global seafood omega 3 consumption increased by about 25 mg/day between 1990 and 2010, we found that much of this was due to further increases in countries already having relatively high consumption.
In 2010, mean global consumption of plant omega 3 was 1371 mg/day. While this is consistent with current broad guidance for adequate intakes (≥1100 mg/day) and for preventing clinical deficiency, optimal intakes of plant omega 3 for reducing chronic diseases are not well established.6 28 In addition, we identified remarkable heterogeneity in intakes, with roughly 10-fold differences across regions and 61 countries having mean intakes <500 mg/day. Based on ecological evidence,52 increasing plant omega 3 consumption can reduce population coronary heart disease risk within a few years. However, definitive cardiometabolic benefits and optimal intake levels are not yet conclusively established.28 53 The extent to which the identified regional and national increases in consumption since 1990 relate to contemporaneous trends in mortality from cardiovascular disease or other diseases requires further investigation.
While several high income countries have recently established national or subnational policy efforts to reduce trans fat consumption,54 55 little prior data was available on global intakes of trans fat. Our findings suggest substantial heterogeneity across the world. Relatively few countries had mean intakes >2%E; conversely, most countries have not achieved optimal intakes of <0.5%E. Highest consumption was evident in North America, North Africa/Middle East, and South Asia, especially Pakistan. While commercial foods are a major source of trans fat in high income countries, intakes in low and middle income counties are principally derived from home and street vendor use of inexpensive partially hydrogenated cooking fats.56 57 In addition, trans fat intake can be highly skewed in certain subgroups, so that national means obscure population subsets with much higher intakes. For example, whereas mean trans fat intake in India was 1.1%E, consistent with proprietary industry data (personal communication, Mark Stavro, Bunge LLC), both the industry data as well as observations of Indian authorities suggest substantial heterogeneity in trans fat intakes across different Indian states, with several having much higher intakes.58 59 Our findings also highlight the relatively limited data availability on trans fat consumption in most nations compared with other major dietary factors. These results demonstrate the need for increased global and nation surveillance as well as consumption reduction strategies.
Interestingly, intakes of most dietary fats and oils were similar by sex, both regionally and within countries. Differences by age were evident for trans fat, with highest consumption at younger ages, perhaps because of greater consumption of processed foods. Age differences were also seen for dietary cholesterol and seafood omega 3 fats, with higher intakes at older ages. For seafood omega 3 fats, the identified age pattern could relate to a birth cohort effect—that is, maintenance of traditional diets or to adoption of healthier diets at older ages because of concerns about disease risk with aging. For cholesterol, the identified age pattern highlights the need for further investigation.
Strengths and limitations of study
Our investigation has several strengths. Systematic searches and extensive direct contacts allowed us to identify, assess, and compile, for the first time, global individual-level dietary data, largely from national studies, on major dietary fats and oils worldwide, including by age, sex, and time. Identified surveys were evaluated for eligibility, measurement comparability, and representativeness; and consistency across surveys was maximised by standardised data extraction and analyses, reinforcing validity and generalisability. Metrics and measurement units were standardised across surveys and were based on the evidence for effects on disease risk. Intakes were adjusted for total energy, reducing measurement error; and sensitivity analyses without energy adjustment were similar. We developed a hierarchical imputation model to address missing data, differences in representativeness and comparability, and related effects on statistical uncertainty. We collected data and imputed intakes using multiple surveys and covariates over time, providing inference on dietary trends.
Potential limitations should be considered. Despite comprehensive approaches to data identification and retrieval, primary data were limited for certain dietary factors, countries, and time periods: for example, more data were available for 2010 than 1990, and relatively little data were available in most Sub-Saharan African nations or for trans fats and plant omega 3 fats. Yet, our modelling methods included surveys over time within and across regions and countries, incorporated serial food balance data as covariates, and quantified resulting statistical uncertainty.
Our findings highlight the need for expanded systematic surveillance of key dietary habits globally and especially in regions with sparse data, including consideration of instrument representativeness, validity, and comparability. Our results also underscore the need for improved food composition databases in many countries, especially for trans fat and plant omega 3 fats. To maximise practicality and data retrieval from diverse global contacts, we focused on dietary fats and oils with probable or convincing evidence of aetiological effects on chronic diseases (for example, we did not gather data on monounsaturated fats, which have differing relations with risk when derived from animal versus plant sources60). Our methods incorporated data across multiple years to derive the best estimates of intakes in 1990 and 2010, which increased validity of estimates in these years but reduced the ability to detect acute changes in national intakes, such as after major policy initiatives. Because of our focus on chronic diseases, we did not collect data in children. We plan to update and expand this work in the future, to identify and incorporate new data, add new dietary factors and age groups, and refine our estimation methods, including for trends over time.
Conclusions and policy implications
Numerous epidemiological studies and several clinical trials have documented the health benefits and harms of specific dietary fats and oils. Yet, far less progress has been achieved in understanding the patterns of global consumption as well as heterogeneity by country, age, sex, and time. Our investigation, founded on individual-level, nationally representative surveys, provides a systematic and comprehensive quantitative assessment of the global consumption of key dietary fats and oils. These findings permit detailed investigation of the impact of dietary habits on disease burdens across countries, of the correlates and drivers of current dietary intakes and nutrition transitions over time, and of the impact of national policies and interventions that—intentionally or inadvertently—alter population dietary intakes. These results inform national and global efforts to alter diet, reduce disease, and improve population health. Our findings also highlight specific data gaps and provide a framework for future dietary surveillance using validated, standardised, nationally representative surveys supported by appropriate food composition data. Understanding the global patterns and impact of suboptimal dietary habits is essential to inform, implement, and evaluate specific interventions and policies to reduce disease burdens and disparities around the world.
What is already known on this topic
Suboptimal diet is the single leading modifiable cause of poor health in the world
Multiple diet-disease relationships, establishing either beneficial or harmful effects of specific dietary fats, have been identified
Most estimates of global dietary fats and oils have relied largely on crude disappearance or expenditure data, and few countries have published nationally representative data on consumption of major dietary fats and oils
What this study adds
This systematic investigation of individual-level dietary assessments across the world provides quantitative estimates of the global consumption of major dietary fats and oils by region, country, age, and sex, as well as the uncertainty in these measurements
The results demonstrate both similarities (such as between men and women) and substantial diversity (such as seafood and plant omega 3 fats) in consumption of fats and oils across regions and nations
The findings facilitate quantitative assessment of disease burdens attributable to these dietary factors and can be used to inform national and global efforts to alter diet, reduce disease, and improve population health
Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE). Core group: Dariush Mozaffarian, Harvard School of Public Health, Boston, MA, USA; Majid Ezzati, Imperial College London, London, UK; Saman Fahimi, University of Cambridge, Cambridge, UK; Shahab Khatibzadeh, Harvard School of Public Health; Renata Micha, Agricultural University of Athens, Athens, Greece, Harvard School of Public Health; John Powles, University of Cambridge; Peilin Shi, Harvard School of Public Health.
Cancer relative risks: Tim E Byers, University of Colorado, Denver, CO, USA; Edward Giovannucci, Harvard School of Public Health; Stephanie Smith-Warner, Harvard School of Public Health.
Other members: Ibrahim Elmadfa, Institute of Nutritional Sciences, University of Vienna, Vienna, Austria; Mayuree Rao, Warren Alpert Medical School of Brown University, Providence, RI, USA; Pattra Wirojratana, Harvard School of Public Health.
Dietary exposure imputation: Stephen S Lim, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, USA; Kathryn G Andrews, African Leaders Malaria Alliance, Dar es Salaam, Tanzania; Rebecca E Engell, Institute for Health Metrics and Evaluation, University of Washington.
Dietary exposures—corresponding members: Pamela A Abbott, University of Aberdeen, UK; Morteza Abdollahi, National Nutrition and Food Technology Research Institute, Iran; Enrique O Abeyá Gilardon, Ministerio de Salud, Argentina; Habibul Ahsan, University of Chicago, USA; Mohannad Abed Alfattah Al Nsour, Eastern Mediterranean Public Health Network (EMPHNET), Jordan; Suad N Al-Hooti, Kuwait Institute for Scientific Research, Kuwait; Carukshi Arambepola, Faculty of Medicine, University of Colombo, Sri Lanka; Hubert Barennes, Institut Francophone pour la Médecine Tropicale, Lao PDR; Simon Barquera, Instituto Nacional de Salud Publica (INSP), Mexico; Ana Baylin, University of Michigan, US; Wulf Becker, National Food Agency, Sweden; Peter Bjerregaard, National Institute of Public Health, University of Southern Denmark, Denmark; Lesley T Bourne, Environment and Health Research Unit, Medical Research Council, South Africa; Neville Calleja, Department of Health Information & Research, Malta; Mario V Capanzana, Food and Nutrition Research Institute, Philippines; Katia Castetbon, Institut de veille sanitaire, France; Hsing-Yi Chang, National Health Research Institutes, Taiwan; Yu Chen, New York University School of Medicine, USA; Melanie J Cowan, WHO, Switzerland; Stefaan De Henauw, Ghent University, Department of Public Health, Belgium; Eric L Ding, Harvard Medical School and Harvard School of Public Health, USA; Charmaine A Duante, Food and Nutrition Research Institute-Department of Science and Technology, Philippines; Pablo Duran, Dirección Nacional de Maternidad e Infancia, Ministerio de Salud de la Nación, Argentina; Ibrahim Elmadfa, Institute of Nutritional Sciences, University of Vienna, Austria; Heléne Enghardt Barbieri; Farshad Farzadfar, Tehran University of Medical Sciences, Iran; Dulitha N Fernando, Faculty of Medicine, University of Colombo, Sri Lanka; Aida Filipovic Hadziomeragic, Institute of Public Health of Federation of Bosnia and Herzegovina, Bosnia and Herzegovina; Regina M Fisberg, Faculty of Public Health - University of São Paulo, Brazil; Simon Forsyth; Didier Garriguet, Statistics Canada, Canada; Jean-Michel Gaspoz, Geneva University Hospitals and Faculty of Medicine of Geneva, Switzerland; Dorothy Gauci, Department of Health Information and Research, Malta; Brahmam N V Ginnela, National Institute of Nutrition, Indian Council of Medical Research, India; Idris Guessous, Geneva University Hospitals, Switzerland; Martin C Gulliford, King’s College London, UK; Wilbur Hadden; Christian Haerpfer, University of Aberdeen, UK; Daniel J Hoffman, Rutgers, State University of New Jersey, USA; Anahita Houshiar-Rad, National Nutrition and Food Technology Research Institute Shahid Beheshti University of Medical Sciences Tehran, Iran, IRIran; Inge Huybrechts, International Agency for Research on Cancer, Lyon, France; Nahla C Hwalla, American University of Beirut, Lebanon; Hajah Masni Ibrahim, Ministry of Health, Brunei; Manami Inoue, Epidemiology and Prevention Division, Research Center for Cancer Prevention and Screening, National Cancer Center, Japan; Maria D Jackson, University of the West Indies, Jamaica; Lars Johansson, Norwegian Directorate of Health, Norway; Lital Keinan-Boker, Ministry of Health, Israel; Cho-il Kim, Korea Health Industry Development Institute, Republic of Korea; Eda Koksal, Gazi University, Turkey; Hae-Jeung Lee; Yanping Li, Harvard School of Public Health, USA; Nur Indrawaty Lipoeto, Andalas University, Indonesia; Guansheng Ma, National Institute for Nutrition and Food Safety, Chinese Center for Disease Control and Prevention, China; Guadalupe L. Mangialavori, Ministerio de Salud de la Nación (National Health Ministry), Argentina; Yasuhiro Matsumura, Bunkyo University, Japan; Stephen T McGarvey, Brown University, USA; Chan Mei Fen; Gert BM Mensink, Robert Koch Institute, Germany; Rafael A Monge-Rojas, Costa Rican Institute for Research and Education and Nutrition and Health (INCIENSA), Costa Rica; Abdulrahman Obaid. Musaiger, Arab Center for Nutrition, Bahrain; Balakrishna Nagalla, National Institute of Nuyrition, Hyderabad India; Androniki Naska, Dept. of Hygiene, Epidemiology and Medical Statistics, University of Athens Medical School, Greece; Marga C Ocke, National Institute for Public Health and the Environment, Netherlands; Maciej Oltarzewski, National Food and Nutrition Institute, Poland; Philippos Orfanos, Dept of Hygiene, Epidemiology and Medical Statistics, University of Athens Medical School, Greece; Marja-Leena Ovaskainen, National Institute for Health and Welfare, Finland; Wen-Harn Pan, Division of Preventive Medicine and Health Services Research, Institute of Population Health Sciences, National Health Reserch Institutes, Taiwan; Demosthenes B Panagiotakos, Harokopio University, Greece; Gulden Ayla Pekcan, Hacettepe University Department of Nutrition and Dietetics, Turkey; Stefka Petrova, National Center of Public Health and Analyses, Bulgaria; Noppawan Piaseu, Mahidol University, Thailand; Christos Pitsavos, Athens University Medical School, Greece; Luz Gladys Posada, Universidad de Antioquia, Colombia; Leanne M Riley, WHO, Switzerland; Luz Maria Sánchez-Romero, National Institute of Public Health, Mexico; Rusidah BT Selamat, Nutrition Division, Ministry of Health Malaysia, Putrajaya, Malaysia; Sangita Sharma; Abla Mehio. Sibai, American University of Beirut- Faculty of Health Sciences, Lebanon; Rosely Sichieri, State University of Rio de Janeiro, Brazil; Chansimaly Simmala, Institut of Tropical Medecin, Laos; Laufey Steingrimsdottir, Iceland; Gillian Swan; Elżbieta Halina. Sygnowska, National Institute of Cardiology, Poland; Lucjan Szponar, National Food and Nutrition Institute, Poland; Heli Tapanainen, National Institute for Health and Welfare, Finland; Robert Templeton; Anastasia Thanopoulou, Diabetes Center, 2nd Department of Internal Medicine, National University of Athens, Hippokration General Hospital, Greece; Holmfridur Thorgeirsdóttir, Directorate of Health, Iceland; Inga Thorsdottir; Antonia Trichopoulou, Hellenic Health Foundation, Greece; Shoichiro Tsugane, National Cancer Center, Japan; Aida Turrini, National Research Institute on Food and Nutrition, Italy; Sirje Vaask, Tallinn University of Technology, Estonia; Coline van Oosterhout, National Institute for Public Health and the Environment, Netherlands; J Lennert Veerman, University of Queensland, Australia; Nowak Verena; Anna Waskiewicz, Institute of Cardiology, Department of Cardiovascular Diseases Epidemiology, Prevention and Health Promotion, Poland; Sahar Zaghloul, National Nutrition Institute, Egypt; Gábor Zajkás, National Institute of Food and Nutrition Sciences, Hungary.
This work was undertaken as part of the Global Burden of Diseases, Injuries, and Risk Factors Study. The results in this paper are prepared independently of the final estimates of the Global Burden of Diseases, Injuries, and Risk Factors study. We thank the Russia Longitudinal Monitoring Survey Phase 2, funded by the USAID and NIH (R01-HD38700), Higher School of Economics and Pension Fund of Russia, and the University of North Carolina Population Center (5 R24 HD050924) (Source: “Russia Longitudinal Monitoring survey, RLMS-HSE», conducted by HSE and ZAO “Demoscope” together with Carolina Population Center, University of North Carolina at Chapel Hill and the Institute of Sociology RAS (RLMS-HSE sites: http://www.cpc.unc.edu/projects/rlms, http://www.hse.ru/org/hse/rlms) for sharing the data with us. We thank Barbara Bowman, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA, USA; Patricia Constante Jamie, School of Public Health, University of São Paulo, Sao Paolo, Brazil; Karen Lock, London School of Hygiene and Tropical Medicine, London, UK; and Joceline Pomerleau, London School of Hygiene and Tropical Medicine, London, UK for advising and guidance on initial search strategy. We thank Louise Dekker, Jenna Golan, Shadi Kalantarian, Liesbeth Smit, and Georgina Waweru Harvard School of Public Health, Boston, MA, USA for data collection.
Contributors: All authors were responsible for study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content, and approval of final manuscript for submission. RM and SK conducted the systematic searches and data collection. RM, SK, PS, SL, SF, KGA, REE, and JP performed the data analysis. RM drafted the manuscript. RM and DM are guarantors for the study.
The study guarantors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Funding: The study was conducted as part of the Global Burden of Disease Study 2010 supported in part by the Bill & Melinda Gates Foundation.
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had access to all data sources and have responsibility for the contents of the report and the decision to submit for publication. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy, or views of WHO.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years DM reports ad hoc honoraria for scientific presentations from Bunge, Pollock Institute, and Quaker Oats; ad hoc consulting fees from Foodminds, Nutrition Impact, Amarin, Astra Zeneca, Winston & Strawn, and Life Sciences Research Organization; membership of Unilever North America Scientific Advisory Board; royalties for an online chapter on fish oil from UpToDate.
Data sharing: No additional data available.
Cite this as: BMJ 2014;348:g2272
Web Extra. Extra material supplied by the author
Appendix 1. Sample of the standardised electronic data extraction sheet.
Appendix 2. The Bayesian hierarchical model for country-level and regional estimates.
eTables 1-7. Details of world regions and countries used in study, characteristics of data sources, and consumption levels of dietary fats and oils analysed.
eFig 1. Global and regional mean consumption levels of dietary saturated fat (panel 1) and omega 6 polyunsaturated fat (panel 2) in 2010 for adult men (A) and women (B) ≥20 years of age. See eTable 5 for numerical mean estimates and uncertainty intervals.
eFig 2. Global and regional mean consumption levels of dietary trans fat (panel 1) and cholesterol (panel 2) in 2010 for adult men (A) and women (B) aged ≥20 years of age. See eTable 5 for numerical mean estimates and uncertainty intervals.
eFig 3. Global and regional mean consumption levels of dietary seafood omega 3 fat (panel 1) and plant omega 3 fat (panel 2) in 2010 for adult men (A) and women (B) ≥20 years of age. See eTable 5 for numerical mean estimates and uncertainty intervals.
eFig 4. Global and national mean dietary saturated fat (A), omega 6 polyunsaturated fat (B), and trans fat (C) (panel 1), and dietary cholesterol (A), seafood omega 3 fat (B), and plant omega 3 fat (C) (panel 2) consumption levels in 2010 for adult men and women ≥20 years of age in relation to their uncertainty. See eTables 3 and 5 for numerical mean estimates and uncertainty intervals.
eFig 5. Global and regional mean dietary saturated fat (A), omega 6 polyunsaturated fat (B), and trans fat (C) (panel 1), and dietary cholesterol (A), seafood omega 3 fat (B), and plant omega 3 fat (C) (panel 2) consumption levels in 1990 and 2010 for adult men and women ≥20 years of age in relation to their uncertainty. See eTable 6 for numerical mean estimates and uncertainty intervals.
eFig 6. Global and regional mean dietary saturated fat (A), omega 6 polyunsaturated fat (B), and trans fat (C) (panel 1), and dietary cholesterol (A), seafood omega 3 fat (B), and plant omega 3 fat (C) (panel 2) consumption levels for adults ≥20 years of age by age. Error bars for each region represent a lower side of 95% uncertainty interval (UI) for the lowest mean value and an upper side of 95% UI for the highest mean value.
eFig 7 Panel 1(A). Regional model fits for saturated fat intake. Model fits in relation to original data by region and dietary risk factor in 2010. The units on the y axis are units (% energy or mg)/day of the diet risk factor, divided by 10 000 to ensure that all exposures fit between Dismod’s expected range of [0,1]. The x axis is age of the observation, and each green bar represents a single data point. The horizontal bar of each observation indicates the age range, and the vertical bar represents the uncertainty around the point estimate, based either on the effective sample size, the standard error or the direct 95 % confidence interval in the input data. These raw data points are adjusted for study level covariates, should they be present in the model (this is indicated by red “Adjusted Data” at the bottom right of the graph). The dotted green curve with narrow vertical uncertainty lines is the prior, based on a negative binomial regression of all the data in the model (global level) with both study level and country level covariates. The solid green curve with shaded uncertainty is the population-weighted posterior which is informed by the prior and the country-specific raw data. To the right of the model fit graph are three smaller visualizations for the fixed effects (fe), random effects (re) and median adjusted relative error (MARE). The coefficients for both the random and fixed effects are in natural logarithm space on their respective x axes. The random effects also indicate the country source of the raw data. The country-level random effects sum to zero within a region; regional random effects sum to zero within a super-region and so on. The random and fixed effects are global and vary only by parameter type. The MARE plot gives an indication of the residuals which aren’t accounted for by the random or fixed effects; a larger MARE indicates a poorly specified model. Each raw data point is visualized by a separate blue square in this plot.
eFig 7 Panel 1(B). Regional model fits for omega 6 polyunsaturated fat intake. See legend for Panel 1(A) for explanation.
eFig 7 Panel 1(C). Regional model fits for trans fat intake. See legend for Panel 1(A) for explanation.
eFig 7 Panel 2(A). Regional model fits for dietary cholesterol intake. See legend for Panel 1(A) for explanation.
eFig 7 Panel 2(B). Regional model fits for seafood omega 3 fat intake. See legend for Panel 1(A) for explanation.
eFig 7 Panel 2(C). Regional model fits for plant omega 3 fat intake. See legend for Panel 1(A) for explanation.
References
- 1.World Health Organization. The world health report 1998. Life in the 21st century: a vision for all. WHO, 1998.
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Wilson PW, Kannel WB. Beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation 2008;117:3031-8. [DOI] [PubMed] [Google Scholar]
- 4.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380:2224-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Word Health Organisation. Joint FAO/WHO Expert Consultation on Fats and Fatty Acids in Human Nutrition. WHO, 2008.
- 7.Dietary Guidelines Advisory Committee. 2010 dietary guidelines for Americans. 2010. www.cnpp.usda.gov/DGAs2010-PolicyDocument.htm.
- 8.Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009;89:1425-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med 2006;354:1601-13. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296:1885-99. [DOI] [PubMed] [Google Scholar]
- 11.Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids 2010;45:893-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 2010;7:e1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lock K, Pomerleau J, Causer L, Altmann DR, McKee M. The global burden of disease attributable to low consumption of fruit and vegetables: implications for the global strategy on diet. Bulletin of the World Health Organization 2005;83:100-8. [PMC free article] [PubMed] [Google Scholar]
- 14.Elmadfa I, Meyer A, Nowak V, Hasenegger V, Putz P, Verstraeten R, et al. European Nutrition and Health Report 2009. Ann Nutr Metab 2009;55(suppl 2):1-40. [DOI] [PubMed] [Google Scholar]
- 15.Trichopoulou A, Naska A, Oikonomou E. The DAFNE databank: the past and future of monitoring the dietary habits of Europeans. J Public Health 2005;13:69-73. [Google Scholar]
- 16.Food and Agriculture Organization. Food availability data. http://faostat.fao.org/.
- 17.Murray CJL, Lopez AD, eds. Global burden of disease. A comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020. Word Health Organization, 1990.
- 18.Word Health Organisation. The global burden of disease: 2004 update. Geneva2008.
- 19.Micha R, Kalantarian S, Wirojratana P, Byers T, Danaei G, Ding EL, et al. Estimating the global and regional burden of suboptimal nutrition on chronic disease: methods and inputs to the analysis. Eur J Clin Nutr 2012;66:119-29. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation. WHO, 2003. 916: i-viii. [PubMed]
- 21.World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. AICR, 2007.
- 22.Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295-300. [PMC free article] [PubMed] [Google Scholar]
- 23.Khatibzadeh S, Micha R, Afshin A, Rao M, Yaakoob MY, Mozaffarian D. Major dietary risk factors for chronic disease: a systematic review of the current evidence for causal effects and effect sizes. Circulation 2012;125:AP060. [Google Scholar]
- 24.World Cancer Research Fund, American Institute for Cancer Research. Continuous Update Project (CUP). www.dietandcancerreport.org/cup/report_overview/index.php.
- 25.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:655-66. [DOI] [PubMed] [Google Scholar]
- 26.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 2009;169:659-69. [DOI] [PubMed] [Google Scholar]
- 27.Skeaff CM, Miller J. Dietary fat and coronary heart disease: summary of evidence from prospective cohort and randomised controlled trials. Ann Nutr Metab 2009;55:173-201. [DOI] [PubMed] [Google Scholar]
- 28.Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, et al. alpha-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr 2012;96:1262-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rong Y, Chen L, Zhu T, Song Y, Yu M, Shan Z, et al. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ 2013;346:e8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Zhou C, Zhou X, Li L. Egg consumption and risk of cardiovascular diseases and diabetes: a meta-analysis. Atherosclerosis 2013;229:524-30. [DOI] [PubMed] [Google Scholar]
- 31.Shin JY, Xun P, Nakamura Y, He K. Egg consumption in relation to risk of cardiovascular disease and diabetes: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:146-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatibzadeh S, Micha R, Elmadfa I, Kalantarian S, Powles J, Shi P, et al. Available data on food and nutrient intake around the world; Global Burden of Nutrition and Chronic Disease Expert Group. Abstract P141. American Heart Assocation/American Stroke Association EPI/NPAM Final Program and Abstracts. 2012.
- 33.GBD study. The Global Burden of Diseases, Injuries, and Risk Factors Study. Operations manual. WHO, 2008.
- 34.Willett WC. Nutritional epidemiology. 2nd ed. Oxford University Press; 1998.
- 35.James SL, Gubbins P, Murray CJ, Gakidou E. Developing a comprehensive time series of GDP per capita for 210 countries from 1950 to 2015. Population Health Metrics 2012;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gakidou E, Cowling K, Lozano R, Murray CJ. Increased educational attainment and its effect on child mortality in 175 countries between 1970 and 2009: a systematic analysis. Lancet 2010;376:959-74. [DOI] [PubMed] [Google Scholar]
- 37.Ezzati M, Lopez AD, Rodgers A, Murray CJ. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors (volumes 1 and 2). World Health Organization, 2004.
- 38.Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr 2009;63(suppl 2):S22-33. [DOI] [PubMed] [Google Scholar]
- 39.FAO. Fats and fatty acids in human nutrition. Report of an expert consultation. World Health Organization, 2010. [PubMed]
- 40.Smith-Spangler CM, Juusola JL, Enns EA, Owens DK, Garber AM. Population strategies to decrease sodium intake and the burden of cardiovascular disease: a cost-effectiveness analysis. Ann Intern Med 2010;152:481-7. [DOI] [PubMed] [Google Scholar]
- 41.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010;362:590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med 2007;356:2388-98. [DOI] [PubMed] [Google Scholar]
- 43.Capewell S, Ford ES, Croft JB, Critchley JA, Greenlund KJ, Labarthe DR. Cardiovascular risk factor trends and potential for reducing coronary heart disease mortality in the United States of America. Bulletin of the World Health Organization 2010;88:120-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huffman MD, Lloyd-Jones DM, Ning H, Labarthe DR, Guzman Castillo M, O’Flaherty M, et al. Quantifying options for reducing coronary heart disease mortality by 2020. Circulation 2013;127:2477-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collaborators UBoD. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding EL, Mozaffarian D. Optimal dietary habits for the prevention of stroke. Semin Neurol 2006;26:11-23. [DOI] [PubMed] [Google Scholar]
- 47.Kearney J. Food consumption trends and drivers. Phil Trans R Soc London. Ser B 2010;365:2793-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puska P, Vartiainen E, Tuomilehto J, Salomaa V, Nissinen A. Changes in premature deaths in Finland: successful long-term prevention of cardiovascular diseases. Bulletin of the World Health Organization 1998;76:419-25. [PMC free article] [PubMed] [Google Scholar]
- 49.Zatonski WA, Willett W. Changes in dietary fat and declining coronary heart disease in Poland: population based study. BMJ 2005;331:187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta-analysis of randomized trials. Implications for public health. J Hum Hypertens 2002;16:761-70. [DOI] [PubMed] [Google Scholar]
- 51.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zatonski W, Campos H, Willett W. Rapid declines in coronary heart disease mortality in Eastern Europe are associated with increased consumption of oils rich in alpha-linolenic acid. Eur J Epidemiol 2008;23:3-10. [DOI] [PubMed] [Google Scholar]
- 53.Wu JH, Micha R, Imamura F, Pan A, Biggs ML, Ajaz O, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr 2012;107 (suppl 2):S214-27. [DOI] [PMC free article] [PubMed]
- 54.Farley TA. The role of government in preventing excess calorie consumption: the example of New York City. JAMA. 2012;308:1093-4. [DOI] [PubMed] [Google Scholar]
- 55.Backholer K, Peeters A. Reduction of trans-fatty acids from food. JAMA 2012;308:1858-9. [DOI] [PubMed] [Google Scholar]
- 56.Mozaffarian D, Abdollahi M, Campos H, Houshiarrad A, Willett WC. Consumption of trans fats and estimated effects on coronary heart disease in Iran. Eur J Clin Nutr 2007;61:1004-10. [DOI] [PubMed] [Google Scholar]
- 57.Butt MS, Sultan MT. Levels of trans fats in diets consumed in developing economies. J AOAC Int 2009;92:1277-83. [PubMed] [Google Scholar]
- 58.Dixit S, Das M. Fatty acid composition including trans-fatty acids in edible oils and fats: probable intake in Indian population. J Food Sci 2012;77:T188-99. [DOI] [PubMed] [Google Scholar]
- 59.Downs SM, Thow AM, Ghosh-Jerath S, McNab J, Reddy KS, Leeder SR. From Denmark to Delhi: the multisectoral challenge of regulating trans fats in India. Public Health Nutr 2012:1-8. [DOI] [PMC free article] [PubMed]
- 60.Schwingshackl L, Hoffmann G. Monounsaturated fatty acids and risk of cardiovascular disease: synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012;4:1989-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Sample of the standardised electronic data extraction sheet.
Appendix 2. The Bayesian hierarchical model for country-level and regional estimates.
eTables 1-7. Details of world regions and countries used in study, characteristics of data sources, and consumption levels of dietary fats and oils analysed.
eFig 1. Global and regional mean consumption levels of dietary saturated fat (panel 1) and omega 6 polyunsaturated fat (panel 2) in 2010 for adult men (A) and women (B) ≥20 years of age. See eTable 5 for numerical mean estimates and uncertainty intervals.
eFig 2. Global and regional mean consumption levels of dietary trans fat (panel 1) and cholesterol (panel 2) in 2010 for adult men (A) and women (B) aged ≥20 years of age. See eTable 5 for numerical mean estimates and uncertainty intervals.
eFig 3. Global and regional mean consumption levels of dietary seafood omega 3 fat (panel 1) and plant omega 3 fat (panel 2) in 2010 for adult men (A) and women (B) ≥20 years of age. See eTable 5 for numerical mean estimates and uncertainty intervals.
eFig 4. Global and national mean dietary saturated fat (A), omega 6 polyunsaturated fat (B), and trans fat (C) (panel 1), and dietary cholesterol (A), seafood omega 3 fat (B), and plant omega 3 fat (C) (panel 2) consumption levels in 2010 for adult men and women ≥20 years of age in relation to their uncertainty. See eTables 3 and 5 for numerical mean estimates and uncertainty intervals.
eFig 5. Global and regional mean dietary saturated fat (A), omega 6 polyunsaturated fat (B), and trans fat (C) (panel 1), and dietary cholesterol (A), seafood omega 3 fat (B), and plant omega 3 fat (C) (panel 2) consumption levels in 1990 and 2010 for adult men and women ≥20 years of age in relation to their uncertainty. See eTable 6 for numerical mean estimates and uncertainty intervals.
eFig 6. Global and regional mean dietary saturated fat (A), omega 6 polyunsaturated fat (B), and trans fat (C) (panel 1), and dietary cholesterol (A), seafood omega 3 fat (B), and plant omega 3 fat (C) (panel 2) consumption levels for adults ≥20 years of age by age. Error bars for each region represent a lower side of 95% uncertainty interval (UI) for the lowest mean value and an upper side of 95% UI for the highest mean value.
eFig 7 Panel 1(A). Regional model fits for saturated fat intake. Model fits in relation to original data by region and dietary risk factor in 2010. The units on the y axis are units (% energy or mg)/day of the diet risk factor, divided by 10 000 to ensure that all exposures fit between Dismod’s expected range of [0,1]. The x axis is age of the observation, and each green bar represents a single data point. The horizontal bar of each observation indicates the age range, and the vertical bar represents the uncertainty around the point estimate, based either on the effective sample size, the standard error or the direct 95 % confidence interval in the input data. These raw data points are adjusted for study level covariates, should they be present in the model (this is indicated by red “Adjusted Data” at the bottom right of the graph). The dotted green curve with narrow vertical uncertainty lines is the prior, based on a negative binomial regression of all the data in the model (global level) with both study level and country level covariates. The solid green curve with shaded uncertainty is the population-weighted posterior which is informed by the prior and the country-specific raw data. To the right of the model fit graph are three smaller visualizations for the fixed effects (fe), random effects (re) and median adjusted relative error (MARE). The coefficients for both the random and fixed effects are in natural logarithm space on their respective x axes. The random effects also indicate the country source of the raw data. The country-level random effects sum to zero within a region; regional random effects sum to zero within a super-region and so on. The random and fixed effects are global and vary only by parameter type. The MARE plot gives an indication of the residuals which aren’t accounted for by the random or fixed effects; a larger MARE indicates a poorly specified model. Each raw data point is visualized by a separate blue square in this plot.
eFig 7 Panel 1(B). Regional model fits for omega 6 polyunsaturated fat intake. See legend for Panel 1(A) for explanation.
eFig 7 Panel 1(C). Regional model fits for trans fat intake. See legend for Panel 1(A) for explanation.
eFig 7 Panel 2(A). Regional model fits for dietary cholesterol intake. See legend for Panel 1(A) for explanation.
eFig 7 Panel 2(B). Regional model fits for seafood omega 3 fat intake. See legend for Panel 1(A) for explanation.
eFig 7 Panel 2(C). Regional model fits for plant omega 3 fat intake. See legend for Panel 1(A) for explanation.