Abstract
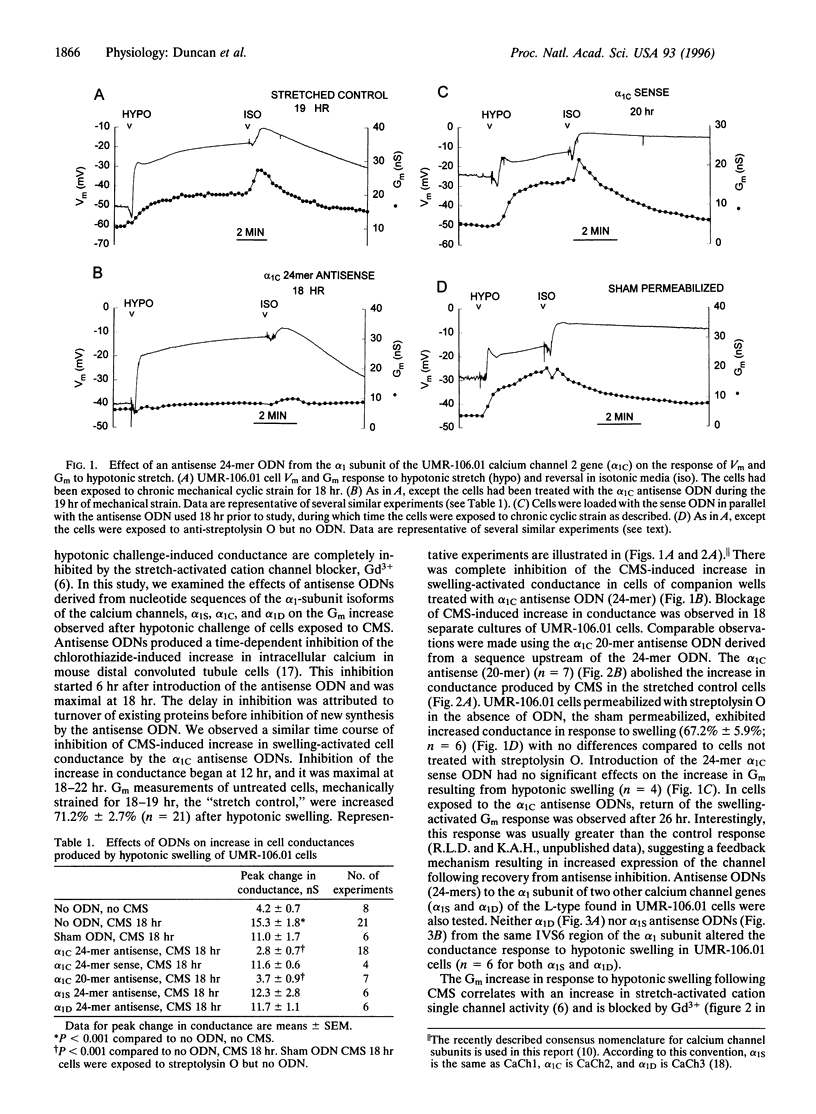
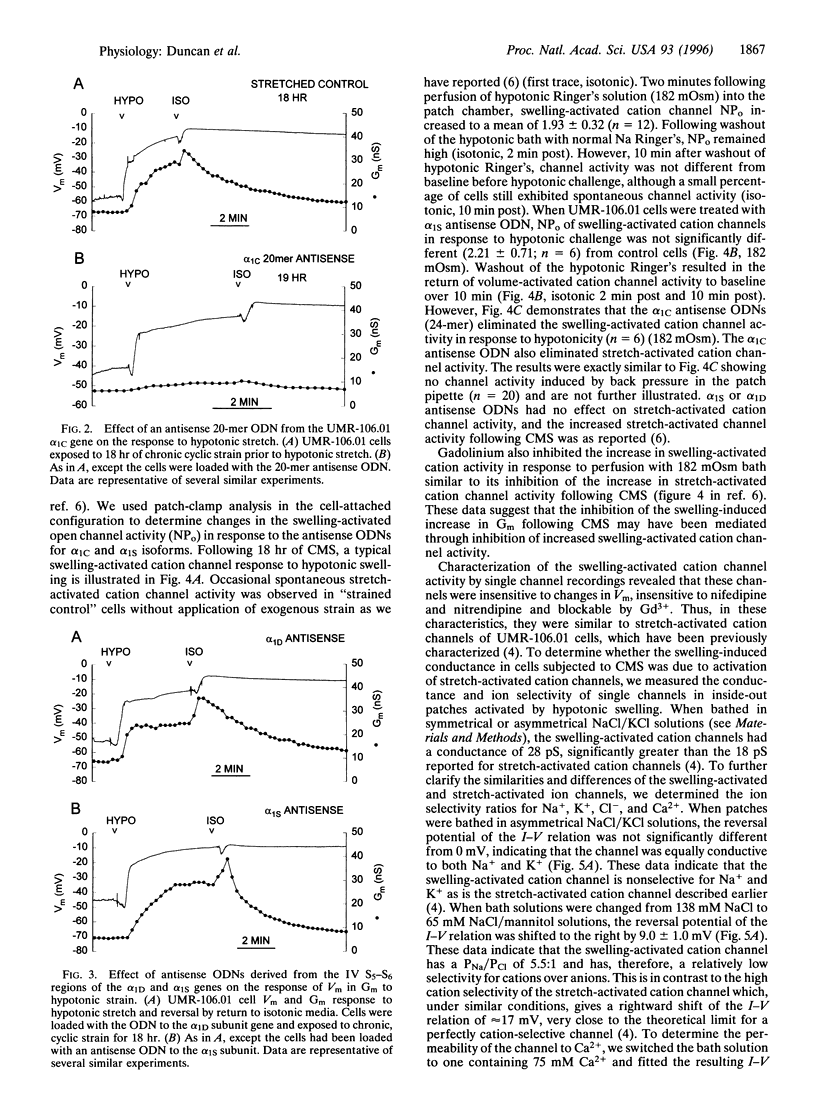
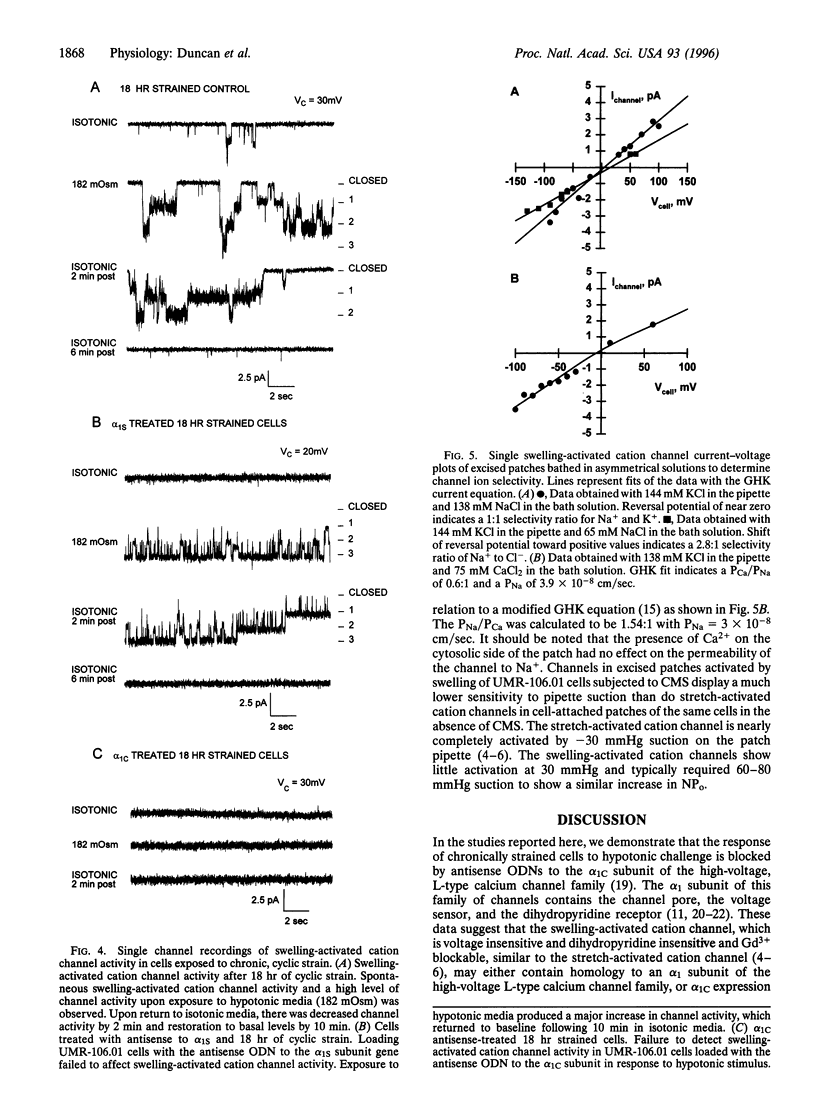
By patch-clamp analysis, we have shown that chronic, intermittent mechanical strain (CMS) increases the activity of stretch-activated cation channels of osteoblast-like UMR-106.01 cells. CMS also produces a swelling-activated whole-cell conductance (Gm) regulated by varying strain levels. We questioned whether the swelling-activated conductance was produced by stretch-activated cation channel activity. We have identified a gene involved in the increase in conductance by using antisense oligodeoxynucleotides (ODN) derived from the alpha 1-subunit genes of calcium channels found in UMR-106.01 cells (alpha1S, alpha1C, and alpha1D). We demonstrate that alpha 1C antisense ODNs abolish the increase in Gm in response to hypotonic swelling following CMS. Antisense ODNs to alpha1S and alpha1D, sense ODNs to alpha1C, and sham permeabilization had no effect on the conductance increase. In addition, during cell-attached patch-clamp studies, antisense ODNs to alpha1c completely blocked the swelling-activated and stretch-activated nonselective cation channel response to strain. Antisense ODNs to alpha1S treatment produced no effect on either swelling-activated or stretch-activated cation channel activity. There were differences in the stretch-activated and swelling-activated cation channel activity, but whether they represent different channels could not be determined from our data. Our data indicate that the alpha1C gene product is involved in the Gm and the activation of the swelling-activated cation channels induced by CMS. The possibility that swelling-activated cation channel genes are members of the calcium channel superfamily exists, but if alpha1c is not the swelling-activated cation channel itself, then its expression is required for induction of swelling-activated cation channel activity by CMS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry E. L., Gesek F. A., Froehner S. C., Friedman P. A. Multiple calcium channel transcripts in rat osteosarcoma cells: selective activation of alpha 1D isoform by parathyroid hormone. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):10914–10918. doi: 10.1073/pnas.92.24.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. F., Crooke S. T. Regulation of endothelial cell adhesion molecule expression with antisense oligonucleotides. Adv Pharmacol. 1994;28:1–43. doi: 10.1016/s1054-3589(08)60492-5. [DOI] [PubMed] [Google Scholar]
- Biel M., Ruth P., Bosse E., Hullin R., Stühmer W., Flockerzi V., Hofmann F. Primary structure and functional expression of a high voltage activated calcium channel from rabbit lung. FEBS Lett. 1990 Sep 3;269(2):409–412. doi: 10.1016/0014-5793(90)81205-3. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Campbell K. P., Catterall W. A., Harpold M. M., Hofmann F., Horne W. A., Mori Y., Schwartz A., Snutch T. P., Tanabe T. The naming of voltage-gated calcium channels. Neuron. 1994 Sep;13(3):505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Seagar M. J., Takahashi M. Molecular properties of dihydropyridine-sensitive calcium channels in skeletal muscle. J Biol Chem. 1988 Mar 15;263(8):3535–3538. [PubMed] [Google Scholar]
- Duncan R. L., Hruska K. A. Chronic, intermittent loading alters mechanosensitive channel characteristics in osteoblast-like cells. Am J Physiol. 1994 Dec;267(6 Pt 2):F909–F916. doi: 10.1152/ajprenal.1994.267.6.F909. [DOI] [PubMed] [Google Scholar]
- Duncan R. L., Hruska K. A., Misler S. Parathyroid hormone activation of stretch-activated cation channels in osteosarcoma cells (UMR-106.01). FEBS Lett. 1992 Jul 28;307(2):219–223. doi: 10.1016/0014-5793(92)80771-8. [DOI] [PubMed] [Google Scholar]
- Duncan R., Misler S. Voltage-activated and stretch-activated Ba2+ conducting channels in an osteoblast-like cell line (UMR 106). FEBS Lett. 1989 Jul 17;251(1-2):17–21. doi: 10.1016/0014-5793(89)81420-6. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Morris C. E., Horn R. Failure to elicit neuronal macroscopic mechanosensitive currents anticipated by single-channel studies. Science. 1991 Mar 8;251(4998):1246–1249. doi: 10.1126/science.1706535. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Kim H. S., Lacerda A. E., Horne W., Wei X. Y., Rampe D., Campbell K. P., Brown A. M., Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989 Jul 20;340(6230):233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E., Wei X. Y., Castellano A., Birnbaumer L. Molecular diversity of L-type calcium channels. Evidence for alternative splicing of the transcripts of three non-allelic genes. J Biol Chem. 1990 Nov 25;265(33):20430–20436. [PubMed] [Google Scholar]
- Simkin A., Ayalon J., Leichter I. Increased trabecular bone density due to bone-loading exercises in postmenopausal osteoporotic women. Calcif Tissue Int. 1987 Feb;40(2):59–63. doi: 10.1007/BF02555706. [DOI] [PubMed] [Google Scholar]
- Stein C. A. Anti-sense oligodeoxynucleotides--promises and pitfalls. Leukemia. 1992 Oct;6(10):967–974. [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Ellinor P. T., Horne W. A. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharmacol Sci. 1991 Sep;12(9):349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]


