Abstract
The feasibility and potential for the morphological and hemodynamic investigation of the heart has been increasing the use of the echocardiography in the research setting. Additionally, the development of new technologies, like the real time 3D echocardiography and speckle tracking, demands validation throughout experimental studies before being instituted in the clinical setting.
This paper aims to provide information concerning the particularities of the echocardiographic examination in quadruped mammals, targeting the experimental research.
Keywords: Echocardiography / diagnosis, Echocardiography / tendências, Animal Experimentation, Heart / physiopathology
Introduction
Considering the low cost and noninvasive nature, the portability and potential of morphological and hemodynamic investigation of the heart, the echocardiography plays an important role in the clinical and experimental research setting1.
The Swedish physician Inge Edler, considered as "The Father of the Echocardiography", was the first to use this method in the experimental research. In the early 50's, Edler demonstrated in calf hearts that the echo signals, firstly thought to be generated by left atrial anterior wall, in fact were from the anterior mitral valve leaflet2. Since then, an increasing number of experimental studies using echocardiography has been published.
Recently, Real Time 3D Echocardiography (RT3DE), Tissue Doppler Imaging (TDI) and Speckle Tracking (STE) represent novel technology tools in the early diagnosis of dyssynchrony, diastolic and systolic heart dysfunction, shedding light on scientific knowledge for clinical applications3,4. Although cardiac magnetic resonance imaging is currently considered the reference technique for ventricle volumetry and calculation of the ejection fraction, several echocardiographic parameters can provide reliable information on ventricular dimensions and volumes, besides myocardial function in daily clinical practice.
This paper aims to review the technical aspects of echocardiography in the experimental laboratory, considering its advantages and limitations according to the animal species.
The Transthoracic Echocardiography (TTE) Planes and Animal Model
Nowadays, the transthoracic echocardiographic investigation in animals employed in experimental research is favored by the technological development of the equipment; however, it is crucial to select the right frequency transducer and have the knowledge on quadruped mammals peculiarities.
According to physical principles, low-frequency transducers (2.0 - 3.5 MHz) offer smaller image resolution; nevertheless, the ultrasound beam penetration is enhanced, which allows their use in animals with a bigger body surface area5. Inversely, the high-frequency probes (4.0 - 8.0 MHz) do not enable larger penetrations, however, they are feasible for a better image resolution, being more adequate for smaller and/or younger animals6-8. Regarding rabbits and small rodents (rats, mice, and guinea pigs), in order to obtain a satisfactory image resolution, the use of higher frequency transducer (10.0 - 15.0 MHz) is needed, because of their very small size9,10. Therefore, once the frequency of the transducer is inversely proportional to the ultrasound beam penetration, the smaller the mammal, the greater the frequency of the probe.
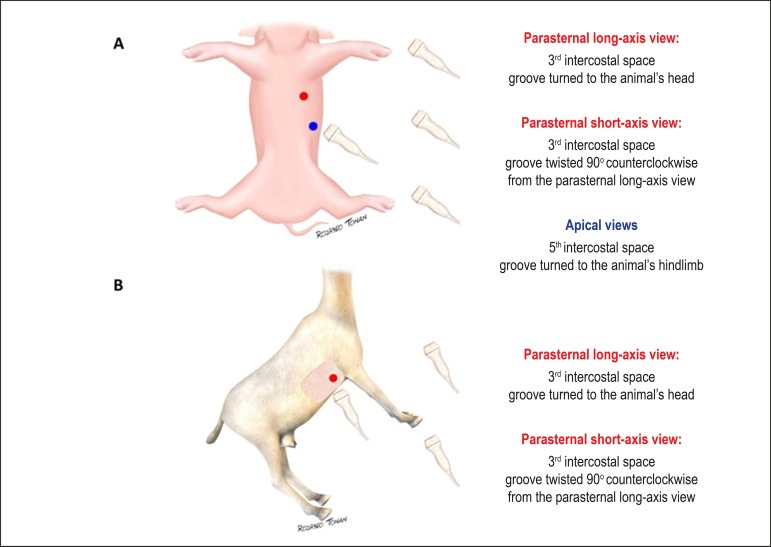
The heart of quadruped mammals present the heart located in the middle part of chest, with the apex pointed to the left; also, the chest is generally keel-shaped, allowing to obtain images from both left and right sides of the thorax, as shown below.
In order to acquire images of optimal quality, the animal should have its fur chest removed, avoiding the presence of air between the transducer and the body surface, once it represents a barrier to the echo beam penetration. Besides that, sedation or anesthesia is crucial to the accuracy of the test; choosing the proper drug and dosage, according to the species, and the appraisal of possible alterations in the cardiovascular system should be scrutinized11. The positioning varies according to the animal model: for dogs and pigs, left lateral decubitus is indicated; rabbits and little rodents should be kept in dorsal decubitus while ruminants need a customized stretcher due to their extremely kneel-shaped chest. (Figure 1)
Figure 1.

Customized stretcher for animals with kneel-shaped chest, in order to obtain the parasternal echocardiographic views.
Usually, the echocardiographic image acquisitions are the same as in humans, except for the ruminants, which do not have the apical approach. The heart of quadruped mammals, which occupies a medial position in the chest, and the apex pointed to the left and the diaphragm, confers two echocardiographic peculiarities. One of them is indeed a disadvantage: the impossibility of acquiring images of good quality through the subcostal approach, once the angle of insonation is not perpendicular to the heart (among the ruminants this is exacerbated by the presence of the pre-stomachs). The other is the feasibility of obtaining images both by left and right hemithorax.
Subsequently, a description of the approaches particularities according to the animal model; Table 1 provides a summarized guide related to each one of them and Figure 2 depicts the transducer position in relation to the chest of the animal. Of note, regarding the normal values of heart structures (diameters and volumes), they vary according to the species and size; therefore, the baseline echocardiography is focused on the myocardial function and valvular performance analysis before the experimental procedure, in order to confirm that the heart of the animals can be considered healthy.
Table 1.
Rapid guide to the echocardiographic examination in animal cardiovascular research
| Animal | Transducer Frequency (MHz) | Animal Position | Main Echocardiographic Planes | Main Limitations |
| Rabbits | ||||
| 4.0 - 8.0 (jovens) | Customized stretcher | |||
| 4.0 - 8.0 (jovens) | ||||
| 4.0 - 8.0 (jovens) |
PLAX: paraesternal long-axis view.
Figure 2.
Position of the transducer in relation to the chest of the animal in left (A) and right (B) hemithorax approach.
Left parasternal approach
Feasible to obtain in dogs, pigs, rabbits and little rodents, at the third intercostal space.
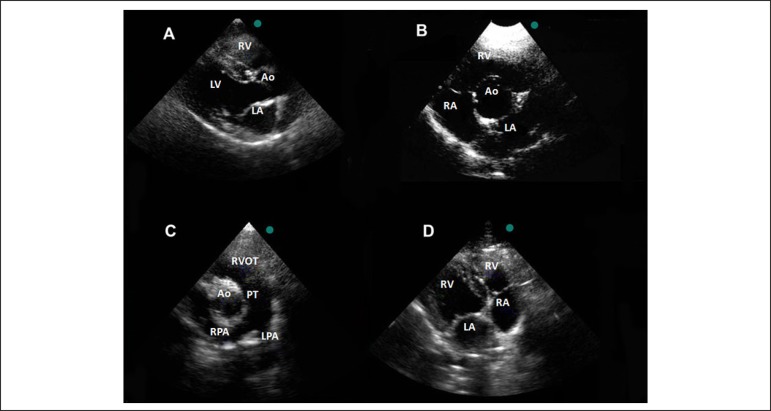
Parasternal longitudinal plane (Figure 3A): allows visualization of the right ventricle (RV), left ventricle (LV), left ventricular outflow tract (LVOT), aorta (Ao) and aortic valve (AV), mitral valve (MV) and left atrium (LA).
Figure 3.
Echocardiographic views from the left parasternal approach in a dog. Parasternal long-axis view (A). Parasternal short-axis view at the basal level (B and C) Long-axis view of the right ventricular inflow tract (D). RV: right ventricle; LV: left ventricle; Ao: aorta; LA: left atrium; RA: right atrium; PT: pulmonary trunk; RPA: right pulmonary artery; LPA: left pulmonary artery.
Parasternal short-axis view: at the basal level it is observed the AV in the middle, the RV, right atrium (RA), tricuspid valve (TV) and the LA (Figure 3B). Tilting the probe towards the animal's head, it is possible to obtain images of the right ventricular outflow tract (RVOT), pulmonary valve (PV) and pulmonary trunk (PT), the latter winging in the right and left pulmonary branches (Figure 3C). The short axes at the MV and papillary muscles level generally are not well visualized in this approach, because of the obliquely positioned heart.
Long-axis view of the right ventricular inflow tract (Figure 3D).
Right parasternal approach
Achievable in all animal models, at the third intercostal level. Of note, regarding the ruminants, this is the only reliable transthoracic echocardiography approach, allowing measurements that would not be feasible by the conventional views, as in the case of right ventricular mass calculation, where the long-axis view of the right ventricular inflow tract act as a surrogate for the apical 4-chamber view12.
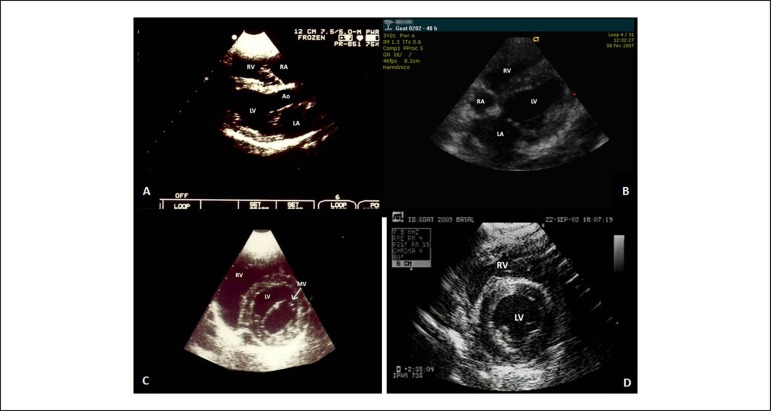
Parasternal longitudinal view (Figure 4A). In this view it is possible to visualize the same structures assessed through the left parasternal longitudinal approach (Ao, LA, LV and RV), besides the RA.
Figure 4.
Echocardiographic views from the right parasternal approach in a dog (A and C) and a goat (C and D). Parasternal long-axis view (A). Left and right ventricular inflow tract long-axis view (B). Parasternal short-axis view at the baseline (C) and at the papillary muscles level (D).MV: mitral valve; RV: right ventricle; LV: left ventricle; Ao: aorta; LA: left atrium; RA: right atrium.
Left and right ventricular inflow tract long-axis view (Figure 4B) As mentioned above, this view might be used as a substitute for the apical view; however, care must be taken when considering the true apical region, which cannot be evaluated by this approach.
Parasternal short-axis view: obtained at the basal, MV (Figure 4C), and papillary muscles level (Figure 4D), by tilting the transducer from the most anterior position in relation to the heart (basal regions, similar to the left parasternal short-axis view, Figure 3B), passing by the MV level to a most posterior one, in order to assess the papillary muscles (by tilting the transducer towards the animal's forelimbs).
Apical approach
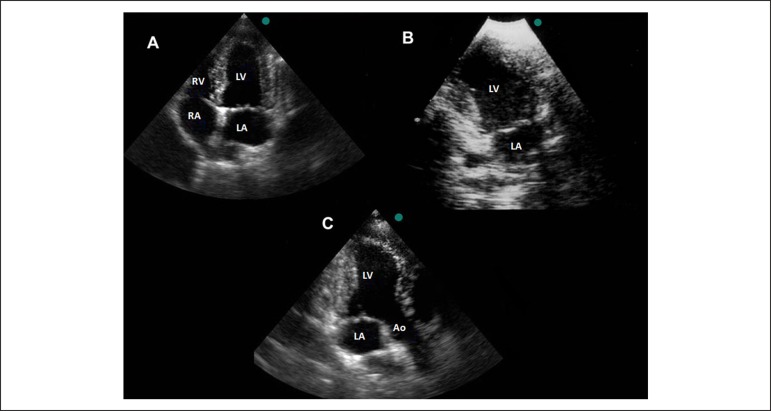
Possible to obtain in dogs, pigs, rabbits and little rodents, at the fifth intercostal space, in the left hemithorax, at the ictus cordis (Figure 5). This plane is essential to perform the TDI analysis, since the Doppler sample volume has to be parallel to the myocardial wall13.
Figure 5.
Apical views acquisition in a dog. A) Apical 4-chamber view; B) Apical 2-chamber view; C) Apical long-axis view. LV: left ventricle; Ao: aorta; LA: left atrium; RA: right atrium; RV: right ventricle.
The Transesophageal Echocardiography (TEE)
The TEE in the experimental lab is proving to be a reliable tool when TTE does not provide adequate images or when it is necessary to assess the heart structures during surgical procedure14. It is feasible in a variety of animal models and, again, the drug used to achieve anesthesia must be chosen according to the species14-19.
The images are very similar to those obtained in humans, composed by a transgastric view and three additional views within the esophagus: cranial, middle and caudal.
Transgastric
This view is very limiting, only approaching the LV in the short axis view.
Caudal Esophagus
Short axis view: provides images of the right and left ventricles at the MV level; soft anti-flexion movements allow the approach of the heart base, at the AV level, similar to the parasternal short axis view in the TTE.
Long axis view: in this plane it is possible to obtain images of the LV, LVOT and MV; rotating the probe counterclockwise allows imaging the left ventricular inflow tract, RA, atrial septum, and caval veins.
Middle Esophagus
Four-chamber view (Figure 6).
Figure 6.
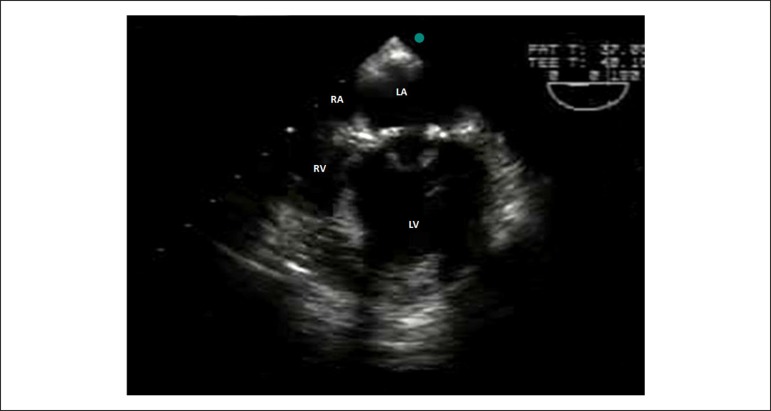
Transesophageal echocardiography in a dog. Note the bioprosthetic valve in the mitral valve position. LV: left ventricle; LA: left atrium; RA: right atrium; RV: right ventricle.
Long axis view: provides imaging of the LA, LV and aorta, like in the TTE apical longitudinal view.
Cranial Esophagus
Short axis view: aortic arch, LVOT and pulmonary valve.
Long axis view: the centrally-oriented beam allows imaging the ascending aorta and the LVOT; the clockwise rotation of the transducer enables imaging the RVOT and the PT.
Novel Technology
Over the past few decades, there has been a rapid and increasing development of novel technology and, usually, those tools need to be tested experimentally before they are introduced in the clinical scenario.
Contrast echocardiography aims to improve the endocardial border delineation and, associated with the stress echocardiography, it can be employed in the research setting for myocardial perfusion assessment20.
Real time 3D echocardiography, 2D and 3D STE are promising novel tools for the evaluation and early diagnosis of cardiac diseases21-24. The experimental validation of these techniques was important to support their use in the clinical setting, mainly considering the STE, once there is no true gold standard that might be applied with this purpose to humans: considering the torsional mechanics, the elegant study of Helle Valle et al25, as well as the assays of Langeland et al26, Amundsen et al27, and Seo et al28 regarding to the 2D and 3D myocardial strain, the technique was validated against sonomicrometry, which demands open chest surgery and the insertion of crystals in the myocardium. Table 2 shows the correlation and agreement between the gold standard and STE in these trials. However, in order to obtain reliable data, it is very important to proceed with the inhalation anesthesia to induce respiratory apnea while acquiring the images, once both the RT3DE and STE have their accuracy affected by the respiratory movements of chest. Another relevant issue concerns the suitable preset, since low temporal resolution, represented by the frame rate, can also interfere with the results.22.
Table 2.
Experimental validation of speckle tracking against sonomicrometry
| (Reference #) | ||
| Twist | 0.94 | |
| Longitudinal Strain | 0.80 | |
| 2D STE (27) | Longitudinal Strain | 0.90 |
| 3D STE (28) | Area Strain | 0.87 |
2D STE: two-dimensional speckle tracking; 3D STE: three-dimensional speckle tracking.
Final Considerations
According to the best of the authors' knowledge, the first systematic studies employing the echocardiography in the experimental laboratory were performed in the 1960's. Lategola, in 1966, through a miniaturized ultrasonic transducer placed inside the aorta of dogs, evaluated the stroke volume invasively, in a prototype that, few years later, would be clinically applied, by means of the transthoracic Continuous Wave Doppler29. Two years later, Christiensen and Bonte showed, in dogs, the accuracy of the echocardiography to detect pericardial effusion. Since then, its importance has been increasing in the experimental investigation scenario, seeking for clinical applications30.
Unlike using other technologies, like magnetic resonance imaging, relevant information can be easily obtained by the echocardiography approach. According to the authors' experience, the method is useful to follow-up myocardial hypertrophy and failure31,32,12.
The development of novel technologies, such as the ultrasound biomicroscopy, which uses frequencies from 30 to 100 MHz, allows the assessment of tiny structures - like the mice fetal heart in the uterus33-35. Certainly, it will be of extremely importance to shed light on issues of the cardiovascular system and to the development of microsurgeries in small animals. It represents a very appealing option in experimental research, since those animals are easily manipulated, have a better cost-benefit ratio, and can be maintained in small areas, enabling study larger samples in a shorter period of time.
Footnotes
Author contributions
Conception and design of the research: Abduch MCD, Aiello VD; Acquisition of data, Analysis and interpretation of the data and Critical revision of the manuscript for intellectual content: Abduch MCD, Assad RS, Aiello VD; Writing of the manuscript: Abduch MCD, Assad RS, Mathias Jr. W, Aiello VD.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any post-graduation program.
Acknowledgement
We would like to thank Rodrigo Tonan for his technical assistance with the Figures.
References
- 1.Krishnamoorthy VK, Sengupta PP, Gentile F, Khanderia BK. History of echocardiography and its future applications in medicine. Crit Care Med. 2007;35(8) Suppl:S309–S313. doi: 10.1097/01.CCM.0000270240.97375.DE. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Goyal A. The origin of echocardiography: a tribute to Inge Edler . Tex Heart Inst J. 2007;34(4):431–438. [PMC free article] [PubMed] [Google Scholar]
- 3.Parlakian A, Charvet C, Escoubet B, Mericskay M, Molkentin JD, Gary-Bobo G, et al. Temporally controlled onset of dilated cardiomyopathy through disruption of the SRF gene in adult heart. Circulation. 2005;112(19):2930–2939. doi: 10.1161/CIRCULATIONAHA.105.533778. [DOI] [PubMed] [Google Scholar]
- 4.Popovic ZB, Benejam C, Bian J, Mal N, Drinko J, Lee K, et al. Speckle-tracking echocardiography correctly identifies segmental left ventricular dysfunction induced by scarring in a rat model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;292(6):H2809–H2816. doi: 10.1152/ajpheart.01176.2006. [DOI] [PubMed] [Google Scholar]
- 5.Zacà V, Brewer R, Khanal S, Imai M, Jiang A, Wang M, et al. Left atrial reverse remodeling in dogs with moderate and advanced heart failure treated with a passive mechanical containment device: an echocardiographic study. J Card Fail. 2007;13(4):312–317. doi: 10.1016/j.cardfail.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas WP. Two-dimensional, real-time echocardiography in the dog: technique and anatomic validation. Veterinary Radiology. 1984;25(2):50–64. [Google Scholar]
- 7.Gwathmey JK, Nakao S, Come PC, Abelmann WH. Echocardiographic assessment of cardiac chamber size and functional performance in swine. Am J Vet Res. 1989;50(2):192–197. [PubMed] [Google Scholar]
- 8.Dias CA, Assad RS, Caneo LF, Abduch MC, Aiello VD, Dias AR, et al. Reversible pulmonary trunk banding II: an experimental model for rapid pulmonary ventricular hypertrophy. J Thorac Cardiovasc Surg. 2002;124(5):999–1006. doi: 10.1067/mtc.2002.124234. [DOI] [PubMed] [Google Scholar]
- 9.Hanton G, Eder V, Rochefort G, Bonner P, Hyvelin JM. Echocardiography, a non-invasive method for the assessment of cardiac function and morphology in preclinical drug toxicology and safety pharmacology. Expert Opin Drug Metab Toxicol. 2008;4(6):681–696. doi: 10.1517/17425255.4.6.681. [DOI] [PubMed] [Google Scholar]
- 10.Scherrer-Crosbie M, Thibault HB. Echocardiography in translational research: of mice and men. J Am Soc Echocardiogr. 2008;21(10):1083–1092. doi: 10.1016/j.echo.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtenberger M, Ko J. Anesthesia and analgesia for small mammals and birds. Vet Clin North Am Exot Anim Pract. 2007;10(2):293–315. doi: 10.1016/j.cvex.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Abduch MC, Assad RS, Rodriguez MQ, Valente AS, Andrade JL, Demarchi LM, et al. Reversible pulmonary trunk banding III: assessment of myocardial adaptive mechanisms - contribution of cell proliferation . J Thorac Cardiovasc Surg. 2007;133(6):1510–1516. doi: 10.1016/j.jtcvs.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Ng AC, Thomas L, Leung DY. Tissue Doppler echocardiography. Minerva Cardioangiol. 2010;58(3):357–378. [PubMed] [Google Scholar]
- 14.Ren JF, Schwartzman D, Lighty GW, Jr, Menz VV, Michele JJ, Li KS, et al. Multiplane transesophageal and intracardiac echocardiography in large swine: imaging technique, normal values, and research applications. Echocardiography. 1997;14(2):135–148. doi: 10.1111/j.1540-8175.1997.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 15.Loyer C, Thomas WP. Biplane transesophageal echocardiography in the dog: technique, anatomy and imaging planes. Veterinary Radiology & Ultrasound. 1995;36(3):212–226. [Google Scholar]
- 16.Lucas CM, Van der Veen FH, Cheriex EC, Lorusso R, Havenith M, Penn OC, et al. Long-term follow-up (12 to 35 weeks) after dynamic cardiomyoplasty. J Am Coll Cardiol. 1993;22(3):758–767. doi: 10.1016/0735-1097(93)90188-7. [DOI] [PubMed] [Google Scholar]
- 17.Medeiros A, Rolim NP, Oliveira RS, Rosa KT, Mattos KC, Casarini DE, et al. Exercise training delays cardiac dysfunction and prevents calcium handling abnormalities in sympathetic hyperactivity-induced heart failure mice. J Appl Physiol. 2008;104(1):103–109. doi: 10.1152/japplphysiol.00493.2007. [DOI] [PubMed] [Google Scholar]
- 18.dos Santos L, Santos AA, Gonçalves GA, Krieger JE, Tucci PJ. Bone marrow cell therapy prevents infarct expansion and improves border zone remodeling after coronary occlusion in rats. Int J Cardiol. 2010;145(1):34–39. doi: 10.1016/j.ijcard.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Soltysinska E, Olesen SP, Osadchii OE. Myocardial structural, contractile and electrophysiological changes in guinea-pig heart failure model induced by chronic sympathetic activation. Exp Physiol. 2011;96(7):647–663. doi: 10.1113/expphysiol.2011.058503. [DOI] [PubMed] [Google Scholar]
- 20.Dourado PM, Tsutsui JM, Mathias W, Jr, Andrade JL, da Luz PL, Chagas AC. Evaluation of stunned and infarcted canine myocardium by real time myocardial contrast echocardiography. Braz J Med Biol Res. 2003;36(11):1501–1509. doi: 10.1590/s0100-879x2003001100009. [DOI] [PubMed] [Google Scholar]
- 21.Mor-Avi V, Lang RM. The use of real-time dimensional echocardiography for the quantification of left ventricular volumes and function. Curr Opin Cardiol. 2009;24(5):402–409. doi: 10.1097/HCO.0b013e32832cbb8a. [DOI] [PubMed] [Google Scholar]
- 22.Notomi Y, Lysyansky P, Setser RM, Shiota T, Popovic ZB, Martin-Miklovic MG, et al. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Col Cardiol. 2005;45(12):2034–2041. doi: 10.1016/j.jacc.2005.02.082. [DOI] [PubMed] [Google Scholar]
- 23.Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17(6):630–633. doi: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Edvardsen T, Helle-Valle T, Smiseth OA. Systolic dysfunction in heart failure with normal ejection fraction: speckle tracking echocardiography . Prog Cardiovasc Dis. 2006;49(3):207–214. doi: 10.1016/j.pcad.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Helle-Valle T, Crosby J, Edvardsen T, Lyseggen E, Amudsen BH, Smith HJ, et al. New noninvasive method for assessment of left ventricular rotation. Speckle tracking echocardiography . Circulation. 2005;112(20):3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- 26.Langeland S, D'Hooge J, Wouters PF, Leather HA, Claus P, Bijnens B, et al. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation. 2005;112(14):2157–2162. doi: 10.1161/CIRCULATIONAHA.105.554006. [DOI] [PubMed] [Google Scholar]
- 27.Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography. J Am Coll Cardiol. 2006;47(4):789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 28.Seo Y, Ishizu T, Enomoto Y, Sugimori H, Aonuma K. Endocardial surface area tracking for assessment of regional LV wall deformation with 3D speckle tracking imaging. JACC Cardiovasc Imaging. 2011;4(4):358–365. doi: 10.1016/j.jcmg.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Lategola MT. Ultrasonic echocardiography - a new vista. J Okla State Med Assoc. 1966;59(5):208–212. [PubMed] [Google Scholar]
- 30.Christensen EE, Bonte FJ. The relative accuracy of echocardiography, intravenous CO2 studies, and blood-pool scanning in detecting pericardial effusions in dogs. Radiology. 1968;91(2):265–270. doi: 10.1148/91.2.265. [DOI] [PubMed] [Google Scholar]
- 31.Miana LA, Assad RS, Abduch MC, Gomes GS, Nogueira AR, Oliveira FS, et al. Intermittent systolic overload promotes better myocardial performance in adult animals. Arq Bras Cardiol. 2010;95(3):364–372. doi: 10.1590/s0066-782x2010005000105. [DOI] [PubMed] [Google Scholar]
- 32.Thomaz PG, Assad RS, Abduch MC, Marques E, Aiello VD, Stolf NA. Assessment of a new experimental model of isolated right ventricular failure. Artif Organs. 2009;33(3):258–265. doi: 10.1111/j.1525-1594.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 33.Foster FS, Pavilin CJ, Harasiewicz KA, Christopher DA, Turnbull DH. Advances in ultrasound biomicroscopy. Ultrasound Med Biol. 2000;26(1):1–27. doi: 10.1016/s0301-5629(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 34.Turnbull DH, Bloomfield TS, Baldwin HS, Foster FS, Joyner AL. Ultrasound backscatter microscope analysis of early mouse embryonic brain development. Proc Natl Acad Sci USA. 1995;92(6):2239–2243. doi: 10.1073/pnas.92.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McVeigh ER. Emerging imaging techniques. Circ Res. 2006;98(7):879–886. doi: 10.1161/01.RES.0000216870.73358.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]