Abstract
Hypoxic ischemic encephalopathy is a serious condition affecting newborn infants which can result in death and disability. There is now strong clinical evidence that moderate post-asphyxial total body cooling or hypothermia in full term neonates results in long-term neuroprotection, allowing us to proclaim this innovative therapy as “standard of care.” The treatment is a time-critical emergency and should be started within 6 hours after the insult. Such requires optimal collaboration among local hospitals, transport teams and the closest neonatal intensive care unit. The technique is only safe when applied according to published clinical trial protocols, and with admission of these patients to a neonatal intensive care unit. Future studies should be aimed at optimizing the onset, duration, and depth of hypothermia. Combination of hypothermia and drugs may further improve neuroprotection in asphyxiated full term neonates.
Keywords: Newborn, asphyxia, hypothermia, aEEG, HIE
Background
Asphyxia is the term commonly used to denote an acute or chronic interruption of placental blood flow during labour. Its impact on fetal cerebral and systemic blood flow with subsequent multiple organ failure may be substantial. One of the most distressing sights in neonatology remains that of the term infant with moderate-to-severe hypoxic-ischemic encephalopathy (HIE) as a consequence of profound perinatal asphyxia (1 to 2 per 1,000 term live births).
Despite improvements in perinatal practice and neonatal care in recent decades, the incidence of long-term neurological sequelae, such as cerebral palsy, has remained essentially unchanged. Such is due to the fact that, until recently, resuscitative and post-resuscitative management was restricted to supportive intensive care, such as correction of hemodynamic and pulmonary disturbances (hypotension, hypoventilation), correction of metabolic disturbances (glucose, calcium, magnesium, electrolytes), treatment of seizures, and monitoring for other organ system dysfunction. Intravenous pharmacological neuroprotective therapy has always been attractive because of its rapidity and ease of administration. A potential limitation to pharmacological therapy is that therapeutic concentrations must be rapidly achieved throughout the brain tissue. The blood-brain-barrier has always posed a potential limitation to the effectiveness of neuroprotective drug therapy.
Having completed several feasibility studies, three large clinical trials have recently demonstrated that moderate prolonged hypothermia for term neonates with perinatal asphyxia can be an effective neuroprotective strategy (Gluckman et al., 2005; Shankaran et al., 2005; Azzopardi et al., 2009). The National Institute for health and Clinical Excellence (NICE, United Kingdom), as well as the International Liaison Committee on Resuscitation guidelines (ILCOR) recently supported a full clinical implementation of hypothermia within term asphyxiated newborns.
Hypoxic-Ischemic Encephalopathy (HIE)
There is currently no clear diagnostic test for encephalopathy due to hypoxia-ischemia. Events that can lead to HIE include a history of placental abruption, uterine rupture, amniotic fluid embolism, tight nuchal cord, cord prolapse/avulsion, maternal haemorrhage or cardio-respiratory arrest, severe and sustained fetal bradycardia, and prolonged labour. Most infants who suffer encephalopathy do not have an obvious cause.
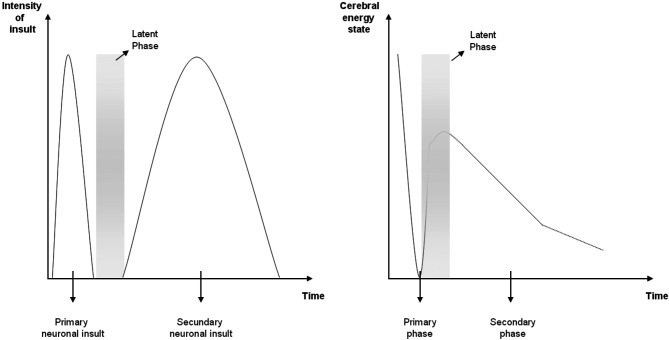
Despite the rapid growth in our understanding of the cellular and molecular mechanisms underlying neuronal death following hypoxia–ischemia, the development of clinically feasible neuroprotective treatments has depended on the use of experimental/ animal models. Considerable evidence suggests that injury to the newborn brain is not a single event occurring at, or just after the insult. Rather, it is an evolving process that leads to cell death until well after the initial insult (Fig. 1):
Fig. 1. Three phases during an asphyxiating insult in the term newborn infant. In neonatal animals, secondary energy failure occurs between 24 to 48 hours after hypoxia-ischemia.
1. The actual episode of hypoxia-ischemia is called “the primary phase” of cell injury. During this phase of energy failure, one observes reduced cerebral concentrations of high-energy phosphorylated compounds such as adenosine triphosphate (ATP) and phosphor-creatine. This energy failure results in hypoxic depolarization of cells, loss of membrane ionic homeostasis leading to severe cytotoxic oedema, as well as accumulation of excitatory amino acids (excito-toxins).
2. Following cerebral reperfusion, i.e. the restoration of cerebral circulation and energy state during resuscitation, the cytotoxic oedema may resolve over approximately 30 to 60 minutes, with a partial recovery of cerebral oxidative metabolism in “the latent phase”. Although its duration is not precisely known for human infants, animal data suggests that this latent phase lasts in the order of hours.
3. Approximately 6 to 15 hours later, the infant may further deteriorate. This so-called “secondary phase” of energy failure may last several days and is likely to involve multiple patho-physiologic processes such as a further release of excitatory amino acids, free radical formation, a parallel rise in intracerebral lactate, induction of apoptosis, and inflammatory activation, leading to delayed onset of seizures (secondary cytotoxic oedema).
The existence of a time window of recovering cerebral energetics (i.e. the latent phase) led to the introduction of a new therapeutic intervention (i.e. hypothermia) following resuscitation, with the aim to reduce secondary energy failure after an acute ischemic event. Note that we remain uncertain as to how much hypothermia may be effective in situations where chronic antenatal hypoxia/ischemia contributes to the insult.
3 Hypothermia: clinical trials and mechanism
More than 6 years have passed since the results were published of 3 randomised controlled trials, which individually and collectively (n = 767 term neonates) demonstrated the efficacy of hypothermia for the treatment of perinatal HIE in term infants (Gluckman et al., 2005; Shankaran et al., 2005; Azzopardi et al., 2009). All patients were randomized within 6 hours of age, and half of them were cooled for 72 hours, followed by rewarming at a rate of 0.5°C/hour. A meta-analysis of the 3 trials (Edwards et al., 2010) estimated a risk ratio of 0.81 (95% confidence interval, 0.71- 0.93; P = .002) for the combined rate of death and severe disability, with a number needed to treat of 9. The meta-analysis also showed that hypothermia resulted in an increase of normal survival, i.e. survival without cerebral palsy and with mental development index and psychomotor developmental index > 84 and normal vision and hearing (risk ratio, 1.53; 95% CI, 1.22-1.93; P < .001), with a number needed to treat of 9.
Modest hypothermia is a non-specific neuroprotective therapy. It reduces the extent of brain injury via at least the following three mechanisms:
1. Firstly, hypothermia results in a graded reduction in cerebral metabolism that slows cell depolarization, reduces accumulation of excito-toxic neurotransmitters, and suppresses oxygen free radical release as well as lipid peroxidation of cell membranes.
2. Secondly, more and more data suggest that hypothermia has a particular role in suppressing apoptotic processes in the developing brain (i.e. programmed cell death), most likely via inhibition of the caspase enzymes, i.e. a large family of enzymes that amplify the intra-cytoplasmic phase of apoptosis after ischemia.
3. Thirdly, there is good evidence that cooling can suppress the release of pro-inflammatory cytokines and interleukins, reducing direct neurotoxicity, such via suppression of microglial activation.
Clinical use of hypothermia: criteria for selection of patients
Hypothermia is increasingly being validated using MRI brain scanning, especially diffusion weighted imaging, facilitating early identification of injury. The treatment clearly results in less severe cortical and deep gray nuclear injury (Bonifacio et al., 2011). Many countries and individual hospitals have therefore now introduced hypothermia as a standard of care for term asphyxiated infants.
Based on the available evidence and the known gaps in our knowledge at the current time, therapeutic hypothermia, if offered, should be executed using a published protocol. Table I lists the inclusion criteria for the application of hypothermia in term infants, born within Flanders and the Netherlands. It is agreed within this protocol that the process of active cooling till 33.5-34°C must be started prior to 6 hours after birth.
Table I. Inclusion criteria for total body hypothermia (Flanders, the Netherlands). Authors: F Groenendael in collaboration with the ‘Vlaams Nederlandse Werkgroep Neonatale Neurologie’.
| – ≥ 36 weeks post menstrual age |
| – AND asphyxia • Apgar ≤ 5 at 5 minutes • OR resuscitation/ventilation during 10 minutes after birth • OR pH < 7.0 and BE > 16 mmol/L (1 hour after birth) Cord blood or arterial/central venous blood 1 hour after birth • OR lactate > 10.0 mmol/L Cord blood or arterial/central venous blood 1 hour after birth |
| – AND encephalopathy • Thompson score > 7 between 1 and 3 hours of age (see table II) |
| – AND starting time of hypothermia < 6 hours after birth |
The criteria further include a pH ≤ 7.0, which reflects acidosis below which end organ dysfunction is detected in infants with fetal distress and newborn depression. However, isolated pathologic fetal acidemia does not accurately identify newborns with HIE, as unanticipated acidemia in stable neonates can be associated with induction of labour.
In term infants, neurological signs following acute asphyxia have been characterized by Sarnat and Sarnat in order to delineate three grades of encephalopathy, i.e. mild (grade I), moderate (grade II), or severe (grade III) HIE (Sarnat and Sarnat, 1976). Neonates with grade I encephalopathy have a normal outcome, grade II encephalopathy results in 25% adverse outcome, and grade III in 50-100% adverse outcome. However, this scoring system is to be used at 24 hours after birth and implies EEG changes. New clinical scoring systems have been developed to assess the severity of neonatal encephalopathy, such as the Thompson score (Thompson et al., 1997; Table II): a Thompson score of ≥ 7 suggests a moderate to severe clinical encephalopathy.
Table II. Thompson score.
| Score 0 | Score 1 | Score 2 | Score 3 | |
| Tone | Normal | Hyper | Hypo | Flaccid |
| Consciousness | Normal | Hyper alert | Lethargic | Comatose |
| Fits | None | Infreq < 3/day | Frequent > 2/day | |
| Posture | Normal | Fisting, cycling | Strong distal flexion | Decerebrate |
| Moro | Normal | Partial | Absent | |
| Grasp | Normal | Poor | Absent | IPPV (apnoea) |
| Suck | Normal | Poor | Absent + bites | |
| Respiration | Normal | Hyperventilation | Brief apnoea | |
| Fontanel | Normal | Full, not tense | Tense |
Cerebral function monitoring (i.e. a single channel, amplitude integrated EEG, aEEG) is useful to detect neonates with a high risk of adverse outcome after perinatal asphyxia, and can also be useful to detect subclinical convulsions. Pharmacologial treatment of subclinical status epilepticus in peripartal asphyxia may also improve the prognosis of the patient.
Methods of hypothermia
To provide adequate neuroprotection with minimal risk of systemic adverse effects, ideally the brain only should be cooled. In the multi-center Cool Cap Study (Gluckman et al., 2005), involving n = 243 infants with moderate to severe HIE, the scalp and underlying brain tissue were selectively cooled using a CoolCap®, whilst the body was warmed with an overhead heater. However, in view of a temperature gradient between the cerebral cortex and the deep grey nuclei, i.e. structures that are often affected in acute asphyxia, mild systemic hypothermia (34.5°C) was aimed for in order to limit the steepness of the intra-cerebral gradient. This technique is clearly impractical for routine practice.
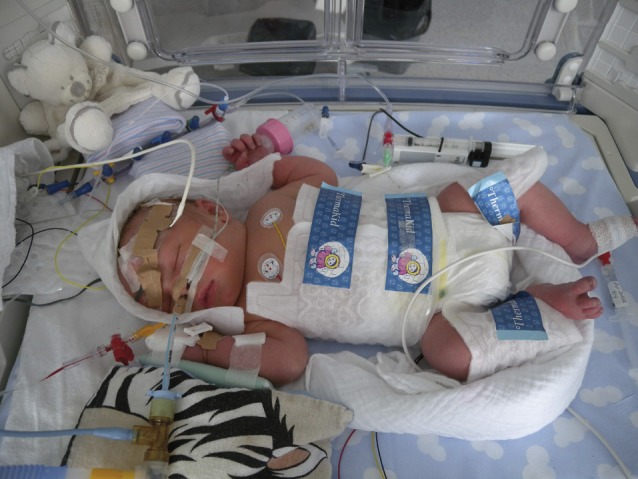
A second (Shankaran et al., 2005) and third (Azzopardi et al., 2009) large clinical trial successfully used whole body hypothermia, with a target temperature between 33 and 34°C. Although seemingly simple to implement, the lowering and maintenance of the core temperature during 72 hours is complex. Different commercial devices are now in use for whole body hypothermia, with servo-controlled systems aimed at reducing fluctuations in body temperature, being most desirable. Such systems are also helpful in avoiding hyperthermia during rewarming after hypothermia (Fig. 2).
Fig. 2. Clinical setting of hypothermia. Total body cooling using the Criticool device.
Specifics of clinical care during hypothermia
Cardio-Pulmonary issues
After asphyxia, hypotension and pulmonary hypertension are frequent clinical findings. Mild hypothermia in itself does not further compromise this cardio-pulmonary instability:
– A temporary physiological reduction in left ventricular cardiac output is seen in some neonates, which disappears during re-warming. Compensatory adjustments in systemic vascular resistance aim to maintain the infant’s blood pressure within normal range. If insufficient, then inotropic support may be added.
– Sinus bradycardia and prolongation of the QT interval are usually noted. Hence, a heart rate within the normal range may indicate stress and a need for deeper sedation.
– Increased pulmonary vascular resistance (i.e. transient increase in tricuspid regurgitation) can incidentally be seen. However, the multicenter trials of hypothermia (Azzopardi et al., 2009; Gluckman et al., 2005; Shankaran et al., 2005) do not report an increase in the incidence of death due to persistent pulmonary hypertension. If pulmonary hypertension occurs, then nitric oxide can be used, but hyperoxia should be avoided.
– When lowering the body temperature, blood is more viscous and the solubility of the gases in the blood increases; for example, in vivo values at 33.5°C of PaCO2 are approximately 0.83 the value read at 37°C. Correcting for temperature may result in an increase in PaCO2 with a resultant increase in cerebral blood flow, whereas not correcting may result in the opposite effect, i.e. hypocapnia-induced vasoconstriction.
– During hypothermia, there may be an increased risk of endotracheal tube obstruction due to sticky secretions. Avoid such by setting the temperature of the humidifier at 37°C.
Drugs
– Stress during cooling should be avoided, since it may reduce the neuroprotective effects of hypothermia. Therefore (dia)morphine should be given to neonates during hypothermia.
– The dosage of (dia)morphine, as well as other drugs such as anticonvulsants and aminoglycosides, should be adjusted during hypothermia, in order to avoid toxic levels due to hypoxic-ischemic injury of liver and kidneys.
Haematology
–nThrombocytopenia (< 150 × 109 /L) can be noted during hypothermia. However, major coagulation disorders and haemorrhage are not reported more often.
Temperature issues
–nContinuous rectal temperature monitoring is imperative during hypothermia, since active cooling without monitoring of the core temperature carries a risk of overcooling (Kendall et al., 2010). Skin temperature should not be used, as there is a wide discrepancy between skin and rectal temperatures, possibly due to cutaneous vasoconstriction and environmental temperature.
–nOn rewarming (0.5°C/h) special attention should be paid to avoid hyperthermia (> 38°C), since hyperthermia may decrease neuroprotective effects; therefore, it is advisable to keep the rectal probe in place for 24 hours after rewarming, aiming for a rectally measured temperature of 36.0-36.5°C, along with keeping the infant’s head uncovered to allow for natural selective head cooling.
–nGeneralised edema is often seen after severe asphyxia. Avoid decubitus and subcutaneous fat necrosis during hypothermia.
Research directions & future challenges
Several gaps in our knowledge of hypothermia as a neuroprotective strategy for HIE remain. These gaps are summarised below.
1. What is the optimal duration of hypothermia?
Compared to human adults, where a cardiac arrest can be treated with 12 or 24 hours of hypothermia, the uncertain timing of the asphyxia insult in newborns supports a longer (72 hours) duration of treatment.
The optimal duration of hypothermia in newborns may however vary strongly according to the aetiology of the asphyxia process (acute versus chronic).
2. What is the optimal depth of hypothermia?
There appears to be a critical depth of cerebral hypothermia (32 to 34°C) required for effective neuronal rescue. At even lower temperatures, neuroprotection is lost, suggesting a trade-off between the adverse systemic effects of cooling, which increase markedly below 32°C, and the potential cerebral benefit.
The adverse systemic effects accounting for the impaired effectiveness of cooling < 32°C in newborn infants are not known. However, animal experiments indicate a significantly impaired cardiac output resulting in refractory arterial hypotension.
3. Can we safely initiate hypothermia beyond 6 hours of age in term infants?
The sequence of initial stabilization and close evaluation delays the initiation of neuroprotective treatments, but probably results in a more accurate neurologic assessment.
In general, there is a decreasing efficacy of neuroprotective treatment the further into the therapeutic window treatment is started. However, evidence suggests that HIE related brain injury continues beyond the 6-hours therapeutic window. Note that the NICHD is currently randomizing encephalopathic infants aged 6-24 hours to normothermia or hypothermia.
4. Is hypothermia for late preterms with HIE (32 to 36 weeks gestation) possible?
Anecdotal evidence has been published on the use of hypothermia in moderately preterm and also very low birth weight infants. For example, Hall et al. (2010) subjected 15 premature infants (27 weeks gestation) with necrotizing enterocolitis and multi-organ failure to systemic cooling (48 hours at 35.5°C, 34.5°C, or 33.5°C) before, throughout, and after surgery. Compared with historical controls, these infants had no increase in mortality, bleeding, infection, or need for inotropic support. The statement that any degree of hypothermia is deleterious for premature infants is thus not fully supported.
However, in the absence of randomized trials in preterm infants, comparing the effects of different core temperatures at different gestational ages, one should not use hypothermia as a routine practice in preterm infants.
5. Do we systematically need aEEG to recruit infants for hypothermia?
Many researchers suggest that aEEG is not necessary for selecting infants towards therapeutic cooling, since most patients with a poor outcome can be identified using metabolic and clinical entry criteria.
On the other hand, aEEG at < 6 hours, coupled with an early neurological examination enhances the prediction of term infants at risk for persistent encephalopathy (Shalak et al., 2003). The role of aEEG in recruiting infants for hypothermia thus needs to be further assessed and refined.
6. Should we cool mild cases of asphyxia?
In the 3 clinical hypothermia trials, infants with mild asphyxia were excluded; however, there are more and more reports supporting the association between mild asphyxia and a subsequent poorer cognitive outcome at late childhood.
7. What are the pharmacokinetics of drugs used during hypothermia?
Excretion of many drugs and their metabolites can be slowed down by hypothermia. Moreover, failure of liver and kidney clearance due to HIE injury could exacerbate toxicity. For example, potentially toxic serum concentrations of morphine may be seen with moderate hypothermia and infusion rates > 10 microg/kg per hour (Roka et al., 2008). Therapeutic drug monitoring is thus mandatory for particular drugs.
Attention must also be directed to those pharmacological components that might trigger neurodegeneration in the developing brain, and thus counteract the neuroprotective effects of hypothermia.
8. Is passive cooling prior to transport safe?
The duration of the latent phase between primary and secondary energy failure is inversely proportional to the severity of the primary asphyxial insult (Iwata et al., 2007). Thus, the therapeutic window may be shorter than 6 hours in severe asphyxia insults. Passive cooling started within the referring unit whilst waiting for the arrival of the transfer team, may thus have an important role. Such can be achieved by switching off any active warming device (radiant heater or incubator), opening the port holes of the incubator and nursing the baby naked (apart from a nappy), whilst monitoring the infant’s rectal temperature.
A number of combined passive and active cooling strategies have been described to use during transport. However, there is a clear need for validated passive cooling protocols, in order to avoid overcooling.
9. Should we combine hypothermia with other neuroprotective agents (the so-called “hypothermia plus” studies or “adjunctive interventions”)?
Hypothermia alone cannot provide complete protection or stimulate the cerebral repair that is necessary for normal neurodevelopmental outcome. Drugs added during or after hypothermia in order to extend the therapeutic window or to provide long-lasting synergistic protection, are needed.
Some pharmacologic agents currently being investigated in conjunction with hypothermia include
– erythropoietin, which reduces excitotoxicity, NO production, apoptosis and inflammation (Aydin et al., 2003);
– topiramate, which reduces glutamate excitotoxicity (Filippi et al., 2010);
– melatonin, which decreases oxidative stress through free radical scavenging (Carloni et al., 2008);
– xenon, which acts as a NMDA-receptor antagonist (Dingley et al., 2006).
10. When is the best timing for prognostic neuro-imaging?
– Myelin within the posterior limb of the internal capsule (PLIC) should be visible from 37 weeks’ gestation (high signal intensity (SI) on T1 and low SI on T2). Loss of a normal SI within the PLIC can be seen following perinatal asphyxia, but such may take 1-2 days to evolve, and can be missed if the MRI scan is done too early. In reality, a MRI scan is performed on day 4 or 5 (i.e. after rewarming), which is an optimal timing to look for possible changes in SI within the PLIC. Such SI abnormality has a 100% positive predictive value for an abnormal outcome at 1 year of age (Rutherford et al., 1998).
– Further studies using advanced imaging techniques with early serial imaging are needed to determine the best timing for imaging within this population.
Conclusion
Newborn neuroprotection remains a major health care priority, given the human suffering and enormous financial cost caused by perinatal brain damage. With the advent of hypothermia as a standard therapy for term moderate-to-severe HIE, there is now hope for protection of the brain. As the therapeutic window after asphyxia is short (6 hours), hypothermia can only be successful within the circumstances of early identification of high-risk term infants as well as rapid referral to a cooling centre.
References
- Aydin A, Genc K, Akhisaroglu M. Erythropoietin exerts neuroprotective effect in neonatal rat model of hypoxic-ischemic brain injury. Brain Dev. 2003;25:494–498. doi: 10.1016/s0387-7604(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Azzopardi D, Strohm B, Edwards AD. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94:F260–264. doi: 10.1136/adc.2008.146977. [DOI] [PubMed] [Google Scholar]
- Bonifacio SL, Glass HC, Vanderpluym J. Perinatal events and early magnetic resonance imaging in therapeutic hypothermia. J Pediatr. 2011;158 doi: 10.1016/j.jpeds.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni S, Perrone S, Buonocore G. Melatonin protects from the long-term consequences of a neonatal hypoxic-ischemic brain injury in rats. J Pineal Res. 2008;44:157–164. doi: 10.1111/j.1600-079X.2007.00503.x. [DOI] [PubMed] [Google Scholar]
- Dingley J, Tooley J, Porter H. Xenon provides short-term neuroprotection in neonatal rats when administered after hypoxiaischemia. Stroke. 2006;37:501–506. doi: 10.1161/01.STR.0000198867.31134.ac. [DOI] [PubMed] [Google Scholar]
- Edwards AD, Brocklehurst P, Gunn AJ. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;(340) doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi L, Poggi C, la Marca G. Oral topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia: a safety study. J Pediatr. 2010;157:361–366. doi: 10.1016/j.jpeds.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Wyatt JS, Azzopardi D. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;(365):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Hall NJ, Eaton S, Peters MJ. Mild controlled hypothermia in preterm neonates with advanced necrotizing enterocolitis. Pediatrics. 2010;125:e1–9. doi: 10.1542/peds.2008-3211. [DOI] [PubMed] [Google Scholar]
- Iwata O, Iwata S, Thornton JS. “Therapeutic time window” duration decreases with increasing severity of cerebral hypoxia-ischaemia under normothermia and delayed hypothermia in newborn piglets. Brain Res. 2007;(1154):173–180. doi: 10.1016/j.brainres.2007.03.083. [DOI] [PubMed] [Google Scholar]
- Kendall GS, Kapetanakis A, Ratnavel N. Cooling on Retrieval Study Group. Passive cooling for initiation of therapeutic hypothermia in neonatal encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:F408–412. doi: 10.1136/adc.2010.187211. [DOI] [PubMed] [Google Scholar]
- Roka A, Melinda KT, Vásárhelyi B. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics. 2008;121:e844–849. doi: 10.1542/peds.2007-1987. [DOI] [PubMed] [Google Scholar]
- Rutherford MA, Pennock JM, Counsell SJ. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics. 1998;102:323–328. doi: 10.1542/peds.102.2.323. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- Shalak LF, Laptook AR, Velaphi SC. Amplitude integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics. 2003;111:351–357. doi: 10.1542/peds.111.2.351. [DOI] [PubMed] [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Thompson CM, Puterman AS, Linley LL. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86:757–761. doi: 10.1111/j.1651-2227.1997.tb08581.x. [DOI] [PubMed] [Google Scholar]