Abstract
Noise is omnipresent in biomedical systems and signals. Conventional views assume that its presence is detrimental to systems’ performance and accuracy. Hence, various analytic approaches and instrumentation have been designed to remove noise. On the contrary, recent contributions have shown that noise can play a beneficial role in biomedical systems. The results of this literature review indicate that noise is an essential part of biomedical systems and often plays a fundamental role in the performance of these systems. Furthermore, in preliminary work, noise has demonstrated therapeutic potential to alleviate the effects of various diseases. Further research into the role of noise and its applications in medicine is likely to lead to novel approaches to the treatment of diseases and prevention of disability.
Keywords: Noise, physiology, medicine
1 Introduction
Albert Einstein discovered noise accidentally in 1905, when he observed that atoms move according to the Brownian molecular motion [1]. Following his discovery, numerous descriptions of physical and biological systems have made incidental reference to noise, without recognizing its essential contribution. Noise is often regarded as an unwanted component or disturbance to a system, even though it has a tremendous impact on many aspects of science and technology [1], including medicine and biology. A typical example for such a statement is a field of engineering called signal processing. On one hand, many signal processing algorithms have been designed to remove noise from a system, since greater noise levels are associated with degraded performance of algorithms. On the other hand, noise has been shown to enhance system performance in many areas of signal processing including stochastic optimization techniques, genetic algorithms, dithering, just to name a few. Similarly, another concept called stochastic resonance (SR), first proposed in 1981 (e.g., [2], [3]), describes a positive impact of noise in nonlinear systems. SR refers to the fact that at an optimal level of input noise, signal detection is enhanced [4], [5]. SR is observed in both man-made and naturally occurring nonlinear systems [6]. For example, paddlefish were shown to use SR to locate and capture prey, implicating this phenomenon in animal behavior [7]. Also, small noisy input can influence the firing patterns of squid axons [8], enhance breathing stability in pre-term infants [9], improve postural control in human aging, stroke or peripheral neuropathy [10], [11], and stabilize gait in elderly people with recurrent falls [12].
The intent of this manuscript is to inform researchers from multiple scientific disciplines that noise (i.e., stochastic processes) is a critical component of many biological and physiological systems that may be exploited in the future to develop interventions for the prevention and treatment of diseases. In other words, this manuscript is a crossover between a review paper and a position paper and as such is meant to initiate further discussions about the role of stochastic processes in modeling of physiological systems.
1.1 Search criteria
To gather previous contributions cited in this manuscript, we utilized PubMed and Google Scholar to find manuscript published in English using a variety of search terms (e.g., “noise physiology,” “noise medicine,” “noise brain,” “noise aging”). These search terms yielded thousands of manuscripts and we focused only on representative publications from several fields. Extensive coverage of all topics is beyond the scope of this paper, since excellent extensive reviews of each have been previously published (e.g., [6], [13]).
1.2 Organization of the paper
The paper is organized as follows: Section 2 introduces various stochastic processes considered in biomedical systems, while also describing the physiological meaning of these processes. Section 3 discusses the important role of noise in fundamental biomedical systems. In Section 4, we discuss several translational applications of noise to treat diseases, while in Section 5, we provide concluding remarks along with an outline of possible future directions.
2 Noise and variability in physiological systems
2.1 Description of noise
By definition, noise is a stochastic process with specific spectral characteristics. While many different stochastic processes exist, we consider here the most common types discussed in the literature. White noise is a stochastic process characterized by equal energy over all frequencies. In mathematical terms, its power spectral density is equal to:
| (1) |
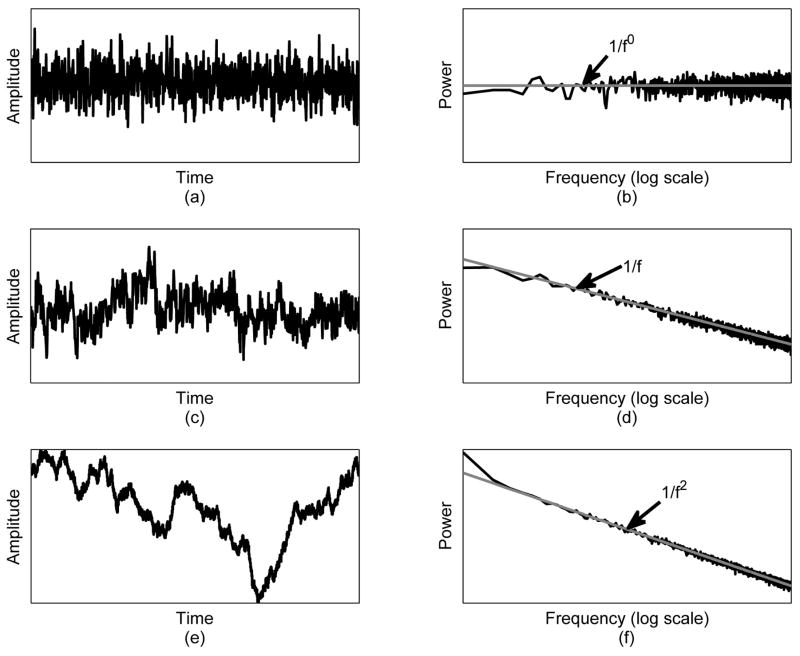
where Cw is a constant. The name “white” stems from the fact its power spectral density is the same at all frequencies in an analogy to the frequency spectrum of white light. A time-domain realization of the white noise is depicted in Fig. 1(a), while its power spectral density is depicted in Fig. 1(b).
Figure 1.
Examples of various noise processes: (a) white noise; (b) power spectral density of the white noise (grey line denotes the theoretical value, which is a constant for all frequencies); (c) pink noise; (d) power spectral density of the pink noise (the grey line denotes the theoretical value, which is C/f); (e) Brownian noise; (f) power spectral density of the Brownian noise (the grey line denotes the theoretical value, which is C/f2).
Pink noise (also called fractal or 1/f noise) is a stochastic process suitable for modeling evolutionary or developmental systems characterized by equal energy per octave as depicted in Fig 1(d) [14]. The power spectral density of pink noise is roughly inversely proportional to frequency [14]:
| (2) |
where Cf is a constant and 0 < α < 2. 1/f noise is a stochastic process between white noise (1/f0) and red (Brownian) noise (1/f2); hence, the name pink noise. Pink or fractal noise is found in numerous biological and physiological processes, including the organization of neural networks, Purkinje fibers in the heart, the vascular tree, bronchial tree, and bone trabeculae, as well as electroencephalographic rhythms, heart rate variability, and respiratory intervals [15], [16]. The omnipresence of pink noise in many diverse applications has led researchers to speculate that there exists some profound law of nature that applies to all nonequilibrium systems and results in such noise [14].
Because pink or fractal noise arises from the interaction of multiple physiologic or biologic control systems operating over different scales in time or space, it may confer system resiliency, adaptability, and structural integrity. For example, the structural (e.g. bone trabeculae) or functional (e.g., heart rate control) networks that generate such noise retain their integrity or functional ability if individual components are lost or interrupted. This fractal network organization also enables a system to adapt to stress by drawing on specific components and fine tuning its response to overcome a given perturbation [16].
Brownian or red noise is a stochastic process whose power spectral density, as depicted in Fig. 1(f), is defined as:
| (3) |
where Cb is a constant. Mathematically, the Brownian noise can be defined as the integral of the white noise.
There are other types of noise specific to certain applications (e.g., blue noise, diotic noise, and dichotic noise). However, the extensive coverage of these topics is beyond the scope of the current manuscript.
2.2 Variability in health and psychosocial functions
Physiology teaches us that healthy systems are self-regulated to reduce variability and maintain physiologic constancy [17]. However, that is not the case in reality. Small amounts of noise, as depicted in Fig. 2(a), can have a very beneficial role in physiological systems (e.g., [9], [10], [15], [16]). Also, the non-linear interactions of multiple regulatory systems and environmental influences operating over different time scales produce highly variable “noisy” behaviours in physiological processes that are far from constant [18]. For example, the normal human heartbeat fluctuates in a complex stochastic manner [17], and can be modeled as a 1/f process (e.g., [19]).
Figure 2.
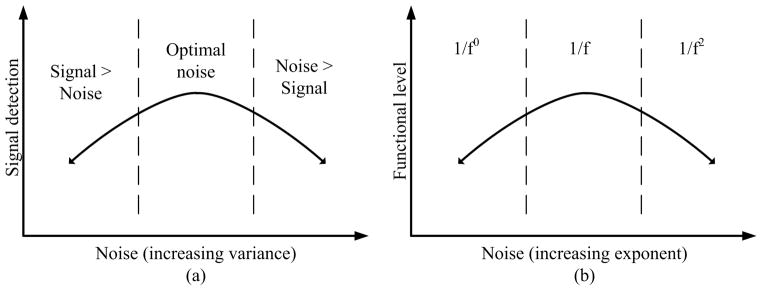
A dual role of noise in biomedical systems: (a) an optimal noise level can have beneficial effects on signal detection, while any other noise levels will degrade the system performance; (b normative physiological signals can be modeled as 1/f processes, while a change in their stochastic properties to either white (1/f0) or Brownian (1/f2) processes due to aging or diseases is associated with functional loss.
On the contrary, the stochastic properties of the heartbeat time series degrade in subjects at high risk of sudden death (e.g., congestive heart failure patients) becoming more characteristic of white noise. This situation is depicted in Fig. 2(b), where a deviation from 1/f noise can result in reduced functional capacity and the onset of disease. Similar counterintuitive results have been obtained in other fields. For example, gene expression can be thought of as a stochastic process [18], [20]. Stochastic gene variations can have both beneficial and harmful roles. Different patterns of gene expression can influence the stress response, metabolism, development, the cell cycle, circadian rhythms, and aging [18]. Therefore, elucidating the stochastic mechanisms involved in physiologic control systems and complex signaling networks is emerging as a major challenge in the postgenomic era [17].
3 The role of noise in fundamental biomedical systems
A number of studies elucidating the fundamental mechanisms of biological systems suggest that noise is an “essential ingredient” in these systems, without which they cannot function. For example, noise plays an important role in molecular transitions or interactions that control cellular behavior (e.g., how cells acquire fate) [21]. Furthermore, several mathematical models used to describe biological processes require a noise term to adequately model the behaviour of these processes.
3.1 Cells, genetics/gene networks
Cellular processes, such as transcription and translation, chromatin remodeling and pathway-specific regulation, are sources of stochastic events leading to cell-to-cell variability [18], [22]. In fact, cellular behaviour varies in clonal cell populations despite their development in identical environments [23].
Stochastic processes can have a dual role in these systems. One point of view is that the stochasticity obstructs the efficient functioning of cellular processes [24]. The accuracy of cellular processes, such as the circadian oscillator, is limited by noise in gene expression [20]. Noise can interfere with the operation of engineered genetic circuits [25] and cell-to-cell variability can be reduced by engineering a circuit with negative feedback [26]. There is also evidence that aging is associated with increased randomness in gene expression [18]. For example, cell type-specific gene expressions in individual murine cardiac myocytes [27], murine muscle tissues [28] and C. elegans [29] become increasingly stochastic as the organism ages. While the mechanisms underlying these stochastic phenomena are still unclear, the process of aging may be dependent on the effects of stochastic gene expression [18]. Another interpretation of these observations is that changes in gene expression with aging are associated with a shift from the more adaptive 1/f or fractal-like noise, to more random or white noise-like behavior that cannot adapt to the metabolic demands of the aged cell. This notion will need experimental validation.
The second point of view is that noise might have beneficial properties [30]. For example, living cells usually acquire their fate deterministically by virtue of their lineage or their proximity to an inductive signal from another cell. However, a cell can choose to differentiate stochastically without apparent regard to environment or history [31]. This random behavior can arise from significant stochastic fluctuations (i.e., noise) in cellular components and biochemical reactions [30], [32]. Additionally, differences in the micro-environments inhabited by individual cells and pre-existing heterogeneity propagated to subsequent cell generations can be sources of such cell-to-cell variations [33]. Cell-to-cell variability is thus a complex function of regulation of gene expression and the regulatory and biochemical networks in which the gene products are embedded [30].
3.2 Neural systems
Neuronal networks are known to have noisy, heterogeneous and compact structures [34], [35]. There are two points of view regarding the role of noise in these networks. One point of view argues that noise lowers the signal-to-noise ratio causing the performance of these networks to degrade. The second point of view states that noise reduces spike-timing precision and therefore, the information rate is lowered. However, noise can play important and constructive roles for the amplification of information transfer in neuronal networks [36], [37].
Stochastic variations are an essential part of the nervous system [37], and the effects of these variations in dynamical neurobiological systems have been studied extensively for both single neurons and neural networks. Pioneering works by Derksen and Verveen in 1966 [38] and by Katz and Miledi in 1970 [39] were the first to establish the probabilistic behavior of neurons in the central nervous system [34]. Derksen and Verveen investigated the role of membrane noise in the probabilistic behavior of neurons in the central nervous system [38], while Katz and Miledi studied signal fluctuations associated with ACh receptor-mediated muscle depolarization [39]. The foundations set by these two groups were later applied to experimental data to gain an insight into the nature of transmembrane-conductance changes and information processing in the brain [34]. Subsequent publications showed that intracellular recordings of cortical neurons consistently display highly complex and irregular activity due to an intense and sustained discharge of presynaptic neurons in the cortical network [36].
In addition to synaptic noise, the stochastic activity of ion channels is another significant source of noise in the nervous system. For example, thermal agitation causes voltage-gated ion channels in neuronal membranes to fluctuate randomly between conducting and nonconducting states inducing noisy membrane currents and subthreshold voltage fluctuations [40], [41]. It is now understood that channel noise affects spike-timing reliability, action potential dynamics, signal detection, the tuning properties of the cell and overall has important effects on neuronal information processing capabilities [36], [40], [41]. Lastly, while noise often leads to increased responsiveness in the nervous system, empirical data and neuronal models demonstrate that noise can also subdue or turn off repetitive neuronal activity [8], [42].
3.3 Brain functions
Noise generated in the brain may influence brain behavior. Noise is generated in the brain by random spike firing times of neurons [13]. By influencing the variability of the firing of neurons, noise may influence decision-making, memory, and the stability of short-term memory and attention [43]. Furthermore, cognitive operations are also affected by stochasticity in N-methyl-D-aspartate activated receptors, which affect the stability of short-term memory and attention, and in alterations of gamma-amino-butyric acid receptor activated synaptic ion channel conductances which are predicted to influence how likely the system is to jump incorrectly into a pathological state of high activity [13]. Similarly, in motor learning, the brain uses movement errors to adjust planning of future movements. This physiologically plausible strategy is optimally tuned to the properties of motor noise, and likely underlies learning in many motor tasks [44].
Overall, noise in the brain promotes decision-making, creativity and the shifting of attention to new tasks [13]. The presence of stochastic brain variations (e.g., due to stochastic variations in spiking of neurons and in synaptic transmissions) is helping investigators and clinicians to understand pathological brain stability states, such as schizophrenia and obsessive-compulsive disorder. One notion is that there is a range of stability states in different individuals. Instability (e.g., due to random firing of neurons) contributes to the symptoms of schizophrenia [45], while too much stability contributes to the symptoms of obsessive-compulsive disorder [45]. Of potentially great importance is that by having a model that is based on the ion channel conductances affected by different neurotransmitters, it is becoming possible to make predictions about what could be favorable combinations of treatments for particular disorders [45].
3.4 Human visual and auditory perception
Noise is omnipresent in sensory systems, ranging from the emission of neurotransmitters from the presynaptic membrane to the behavioral results in visual and auditory experiments (e.g., [6], [46], [47], [48], [49], [50], [51]). For example, a recent study suggested that the addition of an appropriate amount of external noise can improve the perception of an “uncertain” visual signal that is difficult to detect [52], [53]. Figure 3 depicts how adding an appropriate amount of noise improves image contrast and then degrades as we add too much noise [54]. Noise of a particular magnitude (i.e., the SR effect) also tends to enhance visually evoked responses in electroencephalography (e.g., [55]) and magnetoencephalography studies (e.g., [56]). Similarly, in [57], the authors showed that a certain amount of noise reduced the pedestal effect, i.e., the improved detectability of a grating in the presence of a low-contrast masking grating. Their results supported the idea that a single mechanism underlies the pedestal effect and stochastic resonance in contrast perception [57]. 1/f noise is also effective in driving hallucinatory pattern formation as shown in [58], where the authors explored the relationship between ordinary stimulus-controlled pattern perception and the autonomous hallucinatory geometrical pattern formation that occurs for unstructured visual stimulation (e.g., empty-field icker). Similar results were observed in human hearing experiments [4].
Figure 3.
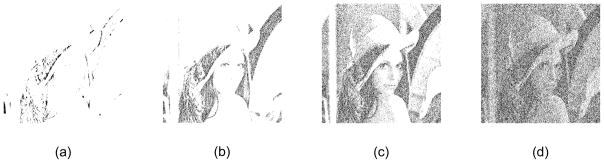
Nonmonotonic effects of noise on visual perception. Consider the popular “Lenna” (Lenna, [image online] Available at: <www.lenna.org> [Accessed 22 March 2012]) image used in the image processing community. We processed the image, I, to obtain I1 = Φ{(I + ξ) − T} where Φ{i} = 1 for an input pixel value i ∈ [0, 1] and Φ{i} = 0 otherwise; the threshold, T, is 0.12; and ξ is zero-mean uniform noise with the standard deviation, σ. Please refer to [54] for more information. Image contrast improves as we increase σ, but then decreases past a certain value: (a) σ = 0; (b) σ = 0.25; (c) σ = 0.834; and (d) σ = 2.2.
3.5 Models of biological systems that require noise
Noise is a necessary component even in modeling of certain biomedical systems. In some cases, noise has a specific physiological meaning, while in others, limited knowledge about the systems under investigation yielded creation of a noise category to capture variability observed in experimental data. The next few subsections briey cover some of the most well-known models requiring a noise term in order to adequately describe a function of a biomedical system. Although there are many more mathematical models that require a noise term in order to accurately model the phenomenon under consideration, it is beyond the scope of this manuscript to review all these different models.
3.5.1 Hodgkin-Huxley model
The Hodgkin-Huxley model, one of the most important models in biomedicine, describes membrane potential, activation of Na and K currents, and inactivation of Na current [59]. Specifically, the Hodgkin-Huxley model describes the spiking behavior and refractory properties of neurons and serves as a paradigm for spiking neurons based on the nonlinear conductance of ion channels [60]. The model is given by four nonlinear coupled equations, one for the membrane potential V, and three for gating variables m, n, and h:
| (4) |
| (5) |
| (6) |
| (7) |
where m∞, h∞, n∞, τm, τh, τn represent the saturation values and the relaxation times of the gating variables. The membrane potential is driven by three types of currents: ionic current Iion, external stimulus current Iext, and synaptic current Isyn. Iion is related to the gating variables of m, n, h and describes the ionic transport through the membrane:
| (8) |
where VNa, VK, Vl are the corresponding reversal potentials and the constants gNa, gK, and gl are the maximal conductances for ion and leakage channels. Iext is the external stimulus usually serving as a bifurcation parameter of the system. Isyn is the sum of the current inputs from all synapses connected to the other neurons and can be modeled as:
| (9) |
where ξ(t) is Gaussian white noise, and σ and τd are the intensity and the correlation time of the synaptic noise, respectively [60].
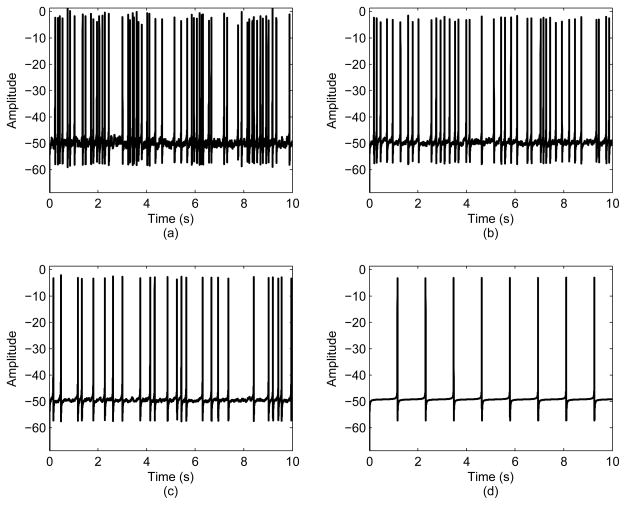
Fig. 4 examines the effects of varying σ on the membrane potential, V. As σ decreases, the potentials become highly regular as depicted in Fig. 4(d). This shows that without noise in the organism, the human body would be a highly deterministic system, and would not be able to account for any changes in the environment.
Figure 4.
The effect of varying σ on the membrane potential, V: (a) σ = 0.2; (b) σ = 0.1; (c) σ = 0.05; (d) σ = 0.
The Hodgkin-Huxley-type models are important not only because their parameters are biophysically meaningful and measurable, but also because they allow us to investigate questions related to synaptic integration, dendritic cable filtering, effects of dendritic morphology, the interplay between ionic currents, and other issues related to single cell dynamics [59] and there are extensions to various other fields such as cardiology (e.g., [61]) in the literature.
3.5.2 Fitz Hugh-Nagumo model
The Fitz Hugh-Nagumo model is a simple but representative example of excitable systems that occur in application ranging from kinetics of chemical reactions and solid-state physics to biomedical processes [62]. Originally it was suggested for the description of nerve pulses [62], but it found its applications in other fields as well. The equations are:
| (10) |
| (11) |
where ε ≪ 1 is a small parameter allowing one to separate all fast and slow motions; the parameter α governs the character of solutions; and the parameter σ governs the amplitude of the noisy external force ξ assumed to be additive white Gaussian noise with zero mean [62].
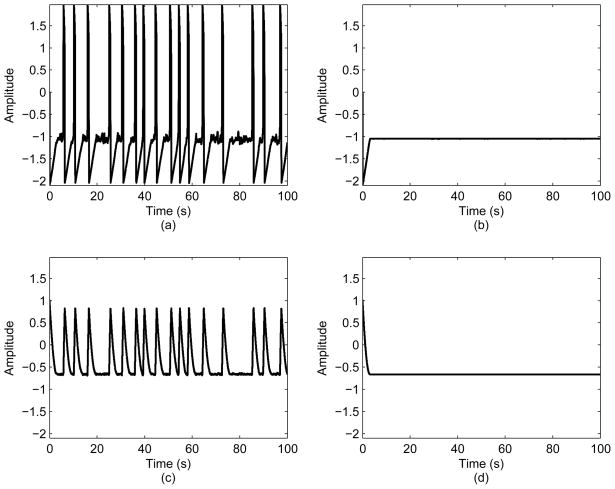
Similarly as the Hodgkin-Huxley model, the Fitz Hugh-Nagumo model is sensitive to the magnitude of σ as depicted in Fig. 5. The presence of noise is necessary in order for the model to accurately represent a biomedical process.
Figure 5.
The effect of varying σ on the Fitz Hugh-Nagumo model: (a) the variable x for σ = 0.25; (b) the variable x for σ = 0; (c) the variable y for σ = 0.25; (d) the variable y for σ = 0.
3.5.3 Cancer risk modeling
Cancer is stimulated by successive somatic mutations [63]. Here, we briefly review a stochastic model for the computation of cancer risks based on the hypothesis of two successive mutations [63]. The model assumes that cells likely to mutate will divide over the lifetime of the tissue. Next, the number of type 1 mutation cells produced over the lifetime of the tissue is distributed according to the Poisson distribution with mean μ. The branching process begins with the appearance of the first type 1 cell. This type 1 cell may die with probability 1 − p1. The second option is that the type 1 cell divides in two type 1 cells with probability p1. At each division of a type 1 cell, there is a probability p2 for each daughter cell to be a type 2 cell. The probability that a branching process started by a single type 1 cell eventually gives birth to at least one type 2 cell may be computed exactly:
| (12) |
The number of type 1 branching processes that eventually produce at least one type 2 cell is given by the Poisson distribution with mean μ
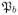 (p1, p2). Hence, the probability that cancer will occur in a particular tissue is given by
(p1, p2). Hence, the probability that cancer will occur in a particular tissue is given by
| (13) |
The parameter μ is crucial in this model as shown in Figure 6, as its value will dictate the shape of the probability density function of cancer. For small values of μ, the probability of cancer is almost negligible, even when the probability of type 1 cell branching increases past 50%. However, as we increase the mean number of first mutations to μ = 100, the probability of cancer becomes 100%, as the probability of type 1 cell branching increases past 50%.
Figure 6.
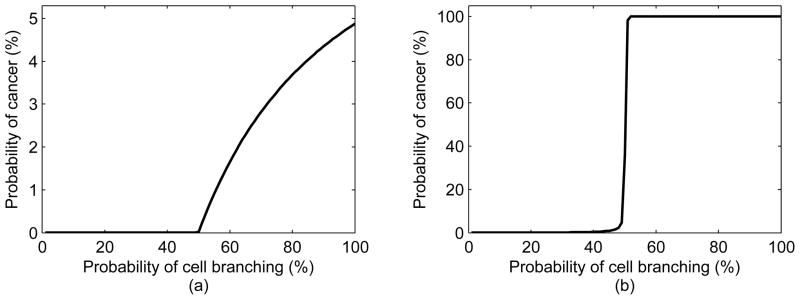
The effect of varying μ on the probability of cancer over lifetime: (a) μ = 0.01 and p2 = 10−5; (b) μ = 100 and p2 = 10−5. Please note that the y-axes are scaled differently (a) and (b).
4 Clinical and therapeutic application of noise
Here, we present several applications of noise to enhance health. Also, we briefly discuss how noise (i.e., stochastic processes) can be used to model the effects of aging or social networks.
4.1 Noise-based devices
Noise-based bioengineering techniques and medical devices can play an important role for treating diseases and enhancing health overall. From a clinical standpoint, noise-based techniques and devices have been used to enhance signal detection in patients with significant sensory deficits, such as older adults [64], [65], patients with diabetic neuropathy [66], patients with stroke [67], or profoundly deaf people receiving speech cues by direct electrical stimulation of the cochlear nerve [68]. Nose-based devices have been used to increase tactile sensations [69], [70] to help post-partum women achieve higher pelvic oor muscle activation [71], or to alleviate postural instability due to ankle sprains [72], [73] and lower back pain [74]. Noise-based solutions can even be used for the enhancement of brain-to-computer interfaces [75].
Noise-based devices, such as randomly vibrating shoe insoles [10] that apply noise during specific activities or throughout the day, may enable people to overcome functional difficulties due to age-related sensory loss [11], [64], [76], [77], [78], [79]. Furthermore, noise-based mechanical ventilators can improve gas exchange and could have a significant effect on morbidity by breaking the chain of injury propagation in acute lung injury [80]. These devices could potentially reduce the morbidity associated with various health issues, such as sensory loss and postural instability in elderly and disabled people or to help stroke patients and individuals with muscle and joint injuries in rehabilitation activities [64]. Noise-based techniques could potentially accelerate a patient’s rehabilitation. In this regard, the ultimate realization of a noise-based device may be one that provides durable benefit that lasts long after the device is removed [76].
4.2 Cognition
Noise has detrimental effects on cognitive performance due to the competition for attentional resources between the distracting and the target stimuli. This has been observed for a wide variety of tasks and stimuli as well as in different participant populations [81], [82]. However, recent empirical evidence suggests that noise can also improve central processing and cognitive performance. For example, auditory noise enhanced the speed of arithmetic computations [83] and recall on visual memory tasks [84]. Thus, adding a moderate level of noise to the input of the information processing system can increase its signal-to-noise output. On the other hand, adding too little or too much noise attenuates performance [82]. This is consistent with the phenomenon of SR. Noise exerted a positive effect on cognitive performance for patients with the attention deficit hyperactivity disorder, indicating that these subjects need more noise than controls for optimal cognitive performance [82]. The Moderate Brain Arousal model suggests that noise in the environment introduces internal noise into the neural system through the perceptual system. This noise induces SR in the neurotransmitter systems and makes noise beneficial for cognitive performance [81]. Similarly, a recent experiment showed that background noise had opposing effects on inattentive and attentive children. While it enhanced performance for the former group, background noise deteriorated performance for latter group. Background noise also reduced episodic memory differences between these two groups of school children. This suggests that cognitive performance can be moderated by external background white noise stimulation in a non-clinical group of inattentive participants [82]. However, one should be aware that these stochastic resonance effects were not always present [50].
4.3 Aging
As described above, many healthy physiologic processes exhibit stochastic variations due to multiple regulatory influences operating over different time scales. These influences include biochemical pathways, opening and closing of ion chambers, feed-forward and feed-back loops, temperature fluctuations, circadian rhythms and environmental changes. Together, they produce pink noise in the output signal as is evident in healthy heart rate, blood pressure, respiratory rate, electroencephalographic potentials, or center-of-pressure time series [15], [16]. These noise signals lose their 1/f characteristics with aging and disease due to the degradation of various control mechanisms and their interactions, becoming more white or Brownian. As a result, the organism loses resiliency or adaptive capacity [15], [16].
This has been demonstrated in the postural control system by examining the body’s center-of-pressure (COP) excursions while standing on a force plate [85]. Under normal circumstances, the COP time series exhibits 1/f behaviour, characteristic of pink noise. However, with the loss of vision, sensation in the feet, or both, there is a progressive loss of complexity and long-range correlations in the data. As a result, the individual has more difficulty adapting to a superimposed cognitive task (e.g., counting backwards while standing) and postural sway increases [85].
Similarly, anatomic structures lose fractal-like architecture with aging, leading to a loss of functionality. This is evident in degeneration and loss of connectivity of the bone trabecular network leading to osteoporosis and fractures; the breakdown of fractal-like alveoli in the lungs leading to emphysema; and the disruption of the collagen matrix in the dermis leading to skin fragility and hemorrhage. In addition, age-related diseases such as the Alzheimer’s (e.g., [86]) or Parkinson’s (e.g., [87]) diseases affect the stochastic variations of physiological variables. For example, the insole forces during the freezing of gait in patients with the Parkinson’s disease have been shown to have stochastic behaviour similar to a Brownian process [87]. Furthermore, Parkinson’s patients lose the noisy, fractal-like physiologic tremor of the normal motor control system and develop a highly periodic tremor, which is characteristic of their disease. Fortunately, there is evidence that noise can be restored in at least the postural control system by exploiting the phenomenon of SR. When subsensory vibratory white noise was applied to the soles of the feet in healthy elderly subjects while standing on a force plate, the fractal-like multiscale complexity of COP displacements increased to values similar to those seen in young subjects [65]. This intriguing finding supports the notion that noise is an important component of a healthy, and highly functional, postural control system.
4.4 Social networks
Noise has also been shown to play role in the social sciences. For example, the psychic structure long known as the “self” is best conceptualized as a dynamical stochastic system [88]. Among the various topics addressed in this field, it has been found that models for opinion formation in a society exhibit a rich variety of nonlinear behavior, such as phase transitions and critical phenomena, stochastic resonance, chaos, and bistability [89]. In fact, the existence of SR in a model of opinion formation yields the appealing implication that there is an optimal noise level for a population to respond to an external “fashion” modulation. Lower noise intensities lead to the dominance of the majority’s opinion, irrespective of external influences, while sufficiently stronger random fluctuations prevent the formation of a definite collective opinion [90].
5 Conclusion and future directions
Since the recognition of noise at the beginning of the twentieth century, the prevalent view in most fields is that noise degrades system performance and most real-life events do not exhibit noise-like behavior. In this manuscript, we reviewed several biomedical fields where noise plays a constructive role and in some cases is necessary for a biomedical system to function properly. Such a constructive behavior is particularly obvious in systems that depend on the complex interactions of many different components operating on different time scales (i.e., nonlinear systems). Therefore, most of the research efforts have been geared towards:
understanding the sources of stochastic fluctuations in biomedical systems and possible advantages and/or adverse consequences of these fluctuations on the systems;
understanding why and how these systems have become robust in their noisy environments; and
how we can use noise to develop treatments and enhance human health.
Further development of noise-based devices or treatments in biomedicine depends on available computational and experimental tools that will answer questions about the the origins of noise in physiological systems and the mechanisms by which noise affects their function. On the computational side, we need to develop more sophisticated algorithms that are capable of simultaneously extracting important stochastic and deterministic variations from the system and handle huge amounts of data. Also, the software applications needed for the understanding of noise in biomedical/physiological systems are almost non-existent. Most computational investigations are carried out using custom-made functions or toolboxes via commercially available packages such as MATLAB (MathWorks, Natick, MA, USA) or SAS (SAS Institute, Cary, NC, USA). On the experimental side, we need to develop experiments and tools that can characterize the noise behavior in systems. The ultimate goal for these advances is to achieve full stochastic resolution over different scales and systems. However, a plan for wide dissemination of data acquired in these experiments should be embedded in these projects to accelerate advances in noise physiology. We anticipate that a limited number of laboratories will have necessary monetary, equipment and staff resources needed to carry some of these sophisticated experiments.
Noise is potentially a very powerful tool in physiology and medicine. We hope this paper will catalyze further research and applications of noise to improve human health and ameliorate diseases.
Acknowledgments
The work of Ervin Sejdić was supported by the Melvin First Young Investigator’s Award from the Institute for Aging Research at Hebrew Senior Life, Boston, MA, USA. His work was also supported by the University of Pittsburgh. Lewis A. Lipsitz was supported by grant R37-AGO25037 from the National Institute on Aging, Bethesda, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ervin Sejdić, Email: esejdic@ieee.org.
Lewis A. Lipsitz, Email: lipsitz@hsl.harvard.edu.
References
- 1.Cohen L. The history of noise. IEEE Signal Processing Magazine. 2005 Nov;22(6):20–45. [Google Scholar]
- 2.Benzi R, Sutera A, Vulpiani A. The mechanism of stochastic resonance. Journal of Physics A: Mathematical and General. 1981 Nov;14(11):L453–L457. [Google Scholar]
- 3.Benzi R, Parisi G, Sutera A, Vulpiani A. Stochastic resonance in climatic change. Tellus. 1982 Feb;34(1):10–16. [Google Scholar]
- 4.Tanaka K, Kawakatsu M, Nemoto I. Stochastic resonance in auditory steady-state responses in a magnetoencephalogram. Clinical Neurophysiology. 2008 Sep;119(9):2104–2110. doi: 10.1016/j.clinph.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh K, Sarkar S, Bhaumik K. A possible mechanism of stochastic resonance in the light of an extra-classical receptive field model of retinal ganglion cells. Biological Cybernetics. 2009 May;100:351–359. doi: 10.1007/s00422-009-0306-9. [DOI] [PubMed] [Google Scholar]
- 6.Moss F, Ward LM, Sannita WG. Stochastic resonance and sensory information processing: a tutorial and review of application. Clinical Neurophysiology. 2004 Feb;115(2):267–281. doi: 10.1016/j.clinph.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Collins JJ. Biophysics: Fishing for function in noise. Nature. 1999 Nov;402(6759):241–242. doi: 10.1038/46179. [DOI] [PubMed] [Google Scholar]
- 8.Paydarfar D, Forger DB, Clay JR. Noisy inputs and the induction of on-off switching behavior in a neuronal pacemaker. Journal of Neurophysiology. 2006 Dec;96(6):3338–3348. doi: 10.1152/jn.00486.2006. [DOI] [PubMed] [Google Scholar]
- 9.Bloch-Salisbury E, Indic P, Bednarek F, Paydarfar D. Stabilizing immature breathing patterns of preterm infants using stochastic mechanosensory stimulation. Journal of Applied Physiology. 2009 Oct;107(4):1017–1027. doi: 10.1152/japplphysiol.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Priplata AA, Niemi JB, Harry JD, Lipsitz LA, Collins JJ. Vibrating insoles and balance control in elderly people. The Lancet. 2003 Oct;362(9390):1123–1124. doi: 10.1016/S0140-6736(03)14470-4. [DOI] [PubMed] [Google Scholar]
- 11.Priplata AA, Patritti BL, Niemi JB, Hughes R, Gravelle DC, Lipsitz LA, Veves A, Stein J, Bonato P, Collins JJ. Noise-enhanced balance control in patients with diabetes and patients with stroke. Annals of Neurology. 2006 Jan;59(1):4–12. doi: 10.1002/ana.20670. [DOI] [PubMed] [Google Scholar]
- 12.Galica AM, Kang HG, Priplata AA, D’Andrea SE, Starobinets OV, Sorond FA, Cupples LA, Lipsitz LA. Subsensory vibrations to the feet reduce gait variability in elderly fallers. Gait and Posture. 2009 Oct;30(3):383–387. doi: 10.1016/j.gaitpost.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deco G, Rolls ET, Romo R. Stochastic dynamics as a principle of brain function. Progress in Neurobiology. 2009 May;88(1):1–16. doi: 10.1016/j.pneurobio.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Keshner MS. 1/f noise. Proceedings of the IEEE. 1982 Mar;70(3):212–218. [Google Scholar]
- 15.Lipsitz LA, Goldberger AL. Loss of “complexity” and aging. JAMA: The Journal of the American Medical Association. 1992 Apr;267(13):1806–1809. [PubMed] [Google Scholar]
- 16.Lipsitz LA. Dynamics of stability: The physiologic basis of functional health and frailty. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002 Mar;57(3):B115–B125. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 17.Goldberger AL, Amaral LAN, Hausdorff JM, Ivanov PC, Peng CK, Stanley HE. Fractal dynamics in physiology: Alterations with disease and aging. Proceedings of the National Academy of Sciences of the United States of America. 2002 Feb;99:2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raj A, van Oudenaarden A. Nature, nurture, or chance: Stochastic gene expression and its consequences. Cell. 2008 Oct;135(2):216–226. doi: 10.1016/j.cell.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng CK, Havlin S, Hausdorff JM, Mietus JE, Stanley HE, Goldberger AL. Fractal mechanisms and heart rate dynamics: Long-range correlations and their breakdown with disease. Journal of Electrocardiology. 1995;28:59–65. doi: 10.1016/s0022-0736(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 20.Raser JM, O’Shea EK. Noise in gene expression: Origins, consequences, and control. Science. 2005 Sep;309(5743):2010–2013. doi: 10.1126/science.1105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson ML, Cox CD, Allen MS, McCollum JM, Dar RD, Karig DK, Cooke JF. Noise in biological circuits. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009 Mar-Apr;1(2):214–225. doi: 10.1002/wnan.22. [DOI] [PubMed] [Google Scholar]
- 22.Ptitsyn A. Stochastic resonance reveals “pilot light” expression in mammalian genes. PLoS ONE. 2008 Mar;3(3):e1842-1–6. doi: 10.1371/journal.pone.0001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar-Even A, Paulsson J, Maheshri N, Carmi M, O’Shea E, Pilpel Y, Barkai N. Noise in protein expression scales with natural protein abundance. Nature Genetics. 2006 Jun;38(6):636–643. doi: 10.1038/ng1807. [DOI] [PubMed] [Google Scholar]
- 24.Maheshri N, O’Shea EK. Living with noisy genes: How cells function reliably with inherent variability in gene expression. Annual Review of Biophysics and Biomolecular Structure. 2007 Jun;36(1):413–434. doi: 10.1146/annurev.biophys.36.040306.132705. [DOI] [PubMed] [Google Scholar]
- 25.Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000 Jan;403(6767):335–338. doi: 10.1038/35002125. [DOI] [PubMed] [Google Scholar]
- 26.Becskei A, Serrano L. Engineering stability in gene networks by autoregulation. Nature. 2000 Apr;405(6786):590–593. doi: 10.1038/35014651. [DOI] [PubMed] [Google Scholar]
- 27.Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dolle MET, Calder RB, Chisholm GB, Pollock BH, Klein CA, Vijg J. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006 Jun;441(7096):1011–1014. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- 28.Newlands S, Levitt LK, Robinson CS, Karpf ABC, Hodgson VR, Wade RP, Hardeman EC. Transcription occurs in pulses in muscle fibers. Genes and Development. 1998 Sep;12(17):2748–2758. doi: 10.1101/gad.12.17.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of caenorhabditis elegans. Nature Genetics. 2005 Jul;37(8):894–898. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson CJ, Surette MG. Individuality in bacteria. Annual Review of Genetics. 2008 Dec;42(1):253–268. doi: 10.1146/annurev.genet.42.110807.091601. [DOI] [PubMed] [Google Scholar]
- 31.Losick R, Desplan C. Stochasticity and cell fate. Science. 2008 Apr;320(5872):65–68. doi: 10.1126/science.1147888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eldar A, Elowitz MB. Functional roles for noise in genetic circuits. Nature. 2010 Sep;467(7312):167–173. doi: 10.1038/nature09326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Locke JCW, Elowitz MB. Using movies to analyse gene circuit dynamics in single cells. Nature Reviews Microbiology. 2009 May;7(5):383–392. doi: 10.1038/nrmicro2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traynelis SF, Jaramillo F. Getting the most out of noise in the central nervous system. Trends in Neurosciences. 1998 Apr;21(4):137–145. doi: 10.1016/s0166-2236(98)01238-7. [DOI] [PubMed] [Google Scholar]
- 35.Goltsev AV, de Abreu FV, Dorogovtsev SN, Mendes JFF. Stochastic cellular automata model of neural networks. Physical Review E. 2010 Jun;81(6):061 921-1–9. doi: 10.1103/PhysRevE.81.061921. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Wang J, Hu W. Effects of chemical synapses on the enhancement of signal propagation in coupled neurons near the canard regime. Physical Review E. 2007 Oct;76(4):041 902-1–6. doi: 10.1103/PhysRevE.76.041902. [DOI] [PubMed] [Google Scholar]
- 37.Butson CR, Clark GA. Random noise paradoxically improves light-intensity encoding in Hermissenda photoreceptor network. Journal of Neurophysiology. 2008 Jan;99(1):146–154. doi: 10.1152/jn.01247.2006. [DOI] [PubMed] [Google Scholar]
- 38.Derksen HE, Verveen AA. Fluctuations of resting neural membrane potential. Science. 1966 Mar;151(3716):1388–1389. doi: 10.1126/science.151.3716.1388. [DOI] [PubMed] [Google Scholar]
- 39.Katz B, Miledi R. Membrane noise produced by acetylcholine. Nature. 1970 Jun;226(5249):962–963. doi: 10.1038/226962a0. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Yu L, Qin SM. Detection of subthreshold pulses in neurons with channel noise. Physical Review E. 2008 Nov;78(5):051 909-1–11. doi: 10.1103/PhysRevE.78.051909. [DOI] [PubMed] [Google Scholar]
- 41.Gmez C. Numerical exploration of the influence of neural noise on the psychometric function at low stimulation intensity levels. Journal of Biosciences. 2008 Dec;33(5):743–753. doi: 10.1007/s12038-008-0094-8. [DOI] [PubMed] [Google Scholar]
- 42.Gutkin B, Jost J, Tuckwell H. Inhibition of rhythmic neural spiking by noise: the occurrence of a minimum in activity with increasing noise. Naturwissenschaften. 2009 Sep;96(9):1091–1097. doi: 10.1007/s00114-009-0570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deco G, Rolls ET. Decision-making and Weber’s law: a neurophysiological model. European Journal of Neuroscience. 2006 Aug;24(3):901–916. doi: 10.1111/j.1460-9568.2006.04940.x. [DOI] [PubMed] [Google Scholar]
- 44.van Beers RJ. Motor learning is optimally tuned to the properties of motor noise. Neruon. 2009 Aug;63(3):406–417. doi: 10.1016/j.neuron.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nature Reviews Neuroscience. 2008 Sep;9(9):696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- 46.Simonotto E, Riani M, Seife C, Roberts M, Twitty J, Moss F. Visual perception of stochastic resonance. Physical Review Letters. 1997 Feb;78(6):1186–1189. [Google Scholar]
- 47.Sasaki H, Todorokihara M, Ishida T, Miyachi J, Kitamura T, Aoki R. Effect of noise on the contrast detection threshold in visual perception. Neuroscience Letters. 2006 Nov;408(2):94–97. doi: 10.1016/j.neulet.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 48.Manjarrez E, Mendez I, Martinez L, Flores A, Mirasso CR. Effects of auditory noise on the psychophysical detection of visual signals: Cross-modal stochastic resonance. Neuroscience Letters. 2007 Mar;415(3):231–236. doi: 10.1016/j.neulet.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 49.Zeng FG, Fu QJ, Morse R. Human hearing enhanced by noise. Brain Research. 2000 Jun;869(1–2):251–255. doi: 10.1016/s0006-8993(00)02475-6. [DOI] [PubMed] [Google Scholar]
- 50.Aihara T, Kitajo K, Nozaki D, Yamamoto Y. Internal noise determines external stochastic resonance in visual perception. Vision Research. 2008 Jun;48(14):1569–1573. doi: 10.1016/j.visres.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 51.Shepherd D, Hautus MJ. Evidence of stochastic resonance in an auditory discrimination task may reflect response bias. Attention, Perception, & Psychophysics. 2009 Nov;71(8):1931–1940. doi: 10.3758/APP.71.8.1931. [DOI] [PubMed] [Google Scholar]
- 52.Sasaki H, Sakane S, Ishida T, Todorokihara M, Kitamura T, Aoki R. Suprathreshold stochastic resonance in visual signal detection. Behavioural Brain Research. 2008 Nov;193(1):152–155. doi: 10.1016/j.bbr.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Kontsevich LL, Tyler CW. What makes Mona Lisa smile? Vision Research. 2004 Jun;44(13):1493–1498. doi: 10.1016/j.visres.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 54.Mitaim S, Kosko B. Adaptive stochastic resonance in noisy neurons based on mutual information. IEEE Transactions on Neural Networks. 2004 Nov;15(6):1526–1540. doi: 10.1109/TNN.2004.826218. [DOI] [PubMed] [Google Scholar]
- 55.Mori T, Kai S. Noise-induced entrainment and stochastic resonance in human brain waves. Physical Review Letters. 2002 May;88(21):218 101-1–4. doi: 10.1103/PhysRevLett.88.218101. [DOI] [PubMed] [Google Scholar]
- 56.Stufflebeam SM, Poeppel D, Roberts TP. Temporal encoding in auditory evoked neuromagnetic fields: stochastic resonance. Neuroreport. 2008 Dec;11(18):4081–4085. doi: 10.1097/00001756-200012180-00034. [DOI] [PubMed] [Google Scholar]
- 57.Goris RLT, Wagemans J, Wichmann FA. Modelling contrast discrimination data suggest both the pedestal effect and stochastic resonance to be caused by the same mechanism. Journal of Vision. 2008 Nov;8(15):17-1–21. doi: 10.1167/8.15.17. [DOI] [PubMed] [Google Scholar]
- 58.Billock VA, Tsou BH. Neural interactions between flicker-induced self-organized visual hallucinations and physical stimuli. Proceedings of the National Academy of Sciences. 2007 May;104(20):8490–8495. doi: 10.1073/pnas.0610813104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Izhikevich EM. Which model to use for cortical spiking neurons? IEEE Transactions on Neural Networks. 2004 Sep;15(5):1063–1070. doi: 10.1109/TNN.2004.832719. [DOI] [PubMed] [Google Scholar]
- 60.Lee SG, Neiman A, Kim S. Coherence resonance in a Hodgkin-Huxley neuron. Physical Review E. 1998 Mar;57(3):3292–3297. [Google Scholar]
- 61.Noble D. From the Hodgkin-Huxley axon to the virtual heart. The Journal of Physiology. 2007 Apr;580(1):15–22. doi: 10.1113/jphysiol.2006.119370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pikovsky AS, Kurths J. Coherence resonance in a noise-driven excitable system. Physical Review Letters. 1997 Feb;78(5):775–778. [Google Scholar]
- 63.Schinazi RB. A stochastic model for cancer risk. Genetics. 2006 Sep;174(1):545–547. doi: 10.1534/genetics.106.059295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Priplata A, Niemi J, Salen M, Harry J, Lipsitz LA, Collins JJ. Noise-enhanced human balance control. Physical Review Letters. 2002 Nov;89(23):238 101-1–4. doi: 10.1103/PhysRevLett.89.238101. [DOI] [PubMed] [Google Scholar]
- 65.Costa M, Priplata AA, Lipsitz LA, Wu Z, Huang NE, Goldberger AL, Peng CK. Noise and poise: Enhancement of postural complexity in the elderly with a stochastic-resonance-based therapy. Europhysics Letters. 2007 Mar;77(6):68 008-1–5. doi: 10.1209/0295-5075/77/68008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khaodhiar L, Niemi JB, Earnest R, Lima C, Harry JD, Veves A. Enhancing sensation in diabetic neuropathic foot with mec–hanical noise. Diabetes Care. 2003 Dec;26(12):3280–3283. doi: 10.2337/diacare.26.12.3280. [DOI] [PubMed] [Google Scholar]
- 67.Stein J, Hughes R, D’Andrea S, Therrien B, Niemi J, Krebs K, Langone L, Harry J. Stochastic resonance stimulation for upper limb rehabilitation poststroke. American Journal of Physical Medicine and Rehabilitation. 2010 Sep;89(9):697–705. doi: 10.1097/PHM.0b013e3181ec9aa8. [DOI] [PubMed] [Google Scholar]
- 68.Morse RP, Evans EF. Enhancement of vowel coding for cochlear implants by addition of noise. Nature Medicine. 1996 Aug;2(8):928–932. doi: 10.1038/nm0896-928. [DOI] [PubMed] [Google Scholar]
- 69.Collins JJ, Imhoff TT, Grigg P. Noise-enhanced tactile sensation. Nature. 1996 Oct;383(6603):770. doi: 10.1038/383770a0. [DOI] [PubMed] [Google Scholar]
- 70.Perez CA, Cohnb TE, Medina LE, Donoso JR. Coincidence-enhanced stochastic resonance: experimental evidence challenges the psychophysical theory behind stochastic resonance. Neuroscience Letters. 2007 Aug;424(1):31–35. doi: 10.1016/j.neulet.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 71.Lauper M, Kuhn A, Gerber R, Luginbhl H, Radlinger L. Pelvic floor stimulation: What are the good vibrations? Neurourology and Urodynamics. 2009 Jun;28(5):405–410. doi: 10.1002/nau.20669. [DOI] [PubMed] [Google Scholar]
- 72.Ross S, Arnold B, Blackburn JT, Brown C, Guskiewicz K. Enhanced balance associated with coordination training with stochastic resonance stimulation in subjects with functional ankle instability: an experimental trial. Journal of NeuroEngineering and Rehabilitation. 2007;4(1):47-1–8. doi: 10.1186/1743-0003-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ross SE. Noise-enhanced postural stability in subjects with functional ankle instability. British Journal of Sports Medicine. 2007 Oct;41(10):656–659. doi: 10.1136/bjsm.2006.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reeves NP, Cholewicki J, Lee AS, Mysliwiec LW. The effects of stochastic resonance stimulation on spine proprioception and postural control in chronic low back pain patients. Spine. 2009 Feb;34(4):316–321. doi: 10.1097/BRS.0b013e3181971e09. [DOI] [PubMed] [Google Scholar]
- 75.Lin CCK, Ju MS, Hsu CW, Sun YN. Applying stochastic resonance to magnify μ and β wave suppression. Computers in Biology and Medicine. 2008 Oct;38(10):1068–1075. doi: 10.1016/j.compbiomed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Collins JJ, Priplata AA, Gravelle DC, Niemi J, Harry J, Lipsitz LA. Noise-enhanced human sensorimotor function. IEEE Engineering in Medicine and Biology Magazine. 2003 Mar-Apr;22(2):76–83. doi: 10.1109/memb.2003.1195700. [DOI] [PubMed] [Google Scholar]
- 77.Gravelle DC, Laughton CA, Dhruv NT, Katdare KD, Niemi JB, Lipsitz LA, Collins JJ. Noise-enhanced balance control in older adults. Neuroreport. 2002 Oct;13(15):1853–1856. doi: 10.1097/00001756-200210280-00004. [DOI] [PubMed] [Google Scholar]
- 78.Liu W, Lipsitz LA, Montero-Odasso M, Bean J, Kerrigan DC, Collins JJ. Noise-enhanced vibrotactile sensitivity in older adults, patients with stroke, and patients with diabetic neuropathy. Archives of Physical Medicine and Rehabilitation. 2002 Feb;83(2):171–176. doi: 10.1053/apmr.2002.28025. [DOI] [PubMed] [Google Scholar]
- 79.Cloutier R, Horr S, Niemi JB, D’Andrea S, Lima C, Harry JD, Veves A. Prolonged mechanical noise restores tactile sense in diabetic neuropathic patients. The International Journal of Lower Extremity Wounds. 2009 Mar;8(1):6–10. doi: 10.1177/1534734608330522. [DOI] [PubMed] [Google Scholar]
- 80.Suki B, Alencar AM, Sujeer MK, Lutchen KR, Collins JJ, Andrade JS, Ingenito EP, Zapperi S, Stanley HE. Life-support system benefits from noise. Nature. 1998 May;393(6681):127–128. doi: 10.1038/30130. [DOI] [PubMed] [Google Scholar]
- 81.Soderlund G, Sikstrom S, Smart A. Listen to the noise: noise is beneficial for cognitive performance in ADHD. Journal of Child Psychology and Psychiatry. 2007 Aug;48(8):840847. doi: 10.1111/j.1469-7610.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 82.Soderlund G, Sikstrom S, Loftesnes J, Sonuga-Barke E. The effects of background white noise on memory performance in inattentive school children. Behavioral and Brain Functions. 2010;6(1):55-1–10. doi: 10.1186/1744-9081-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Usher M, Feingold M. Stochastic resonance in the speed of memory retrieval. Biological Cybernetics. 2000 Nov;83(6):L11–L16. doi: 10.1007/PL00007974. [DOI] [PubMed] [Google Scholar]
- 84.Wilkinson D, Nicholls S, Pattenden C, Kilduff P, Milberg W. Galvanic vestibular stimulation speeds visual memory recall. Experimental Brain Research. 2008 Aug;189(2):243–248. doi: 10.1007/s00221-008-1463-0. [DOI] [PubMed] [Google Scholar]
- 85.Manor B, Costa MD, Hu K, Newton E, Starobinets O, Kang HG, Peng CK, Novak V, Lipsitz LA. Physiological complexity and system adaptability: evidence from postural control dynamics of older adults. Journal of Applied Physiology. 2010 Dec;109(6):1786–1791. doi: 10.1152/japplphysiol.00390.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maxim V, Sendur L, Fadili J, Suckling J, Gould R, Howard R, Bullmore E. Fractional gaussian noise, functional MRI and Alzheimer’s disease. NeuroImage. 2005 Mar;25(1):141–158. doi: 10.1016/j.neuroimage.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 87.Hausdorff JM, Balash Y, Giladi N. Time series analysis of leg movements during freezing of gait in Parkinson’s disease: akinesia, rhyme or reason? Physica A: Statistical Mechanics and its Applications. 2003 Apr;321(3–4):565–570. [Google Scholar]
- 88.Marks-Tarlow T. The self as a dynamical system. Nonlinear Dynamics, Psychology, and Life Sciences. 1999 Oct;3(4):311–345. [Google Scholar]
- 89.Bordogna CM, Albano EV. Dynamic behavior of a social model for opinion formation. Physical Review E. 2007 Dec;76(6):061 125-1–6. doi: 10.1103/PhysRevE.76.061125. [DOI] [PubMed] [Google Scholar]
- 90.Kuperman M, Zanette D. Stochastic resonance in a model of opinion formation on small-world networks. The European Physical Journal B - Condensed Matter and Complex Systems. 2002 Apr;26(3):387–391. [Google Scholar]