Abstract
Connectivity analyses have become increasingly important in functional imaging. When used to describe the functional anatomy of a specific behavior, these analyses are generally applied to a subset of the data that demonstrate significant differences when experimental conditions are contrasted. Such data reduction is sub-optimal for a systems approach as it assumes that all data that survive the statistical contrast filter are related to the behavior and that none of the filtered data have a significant function. When such data filtering is applied to speech and language tasks, the resulting functional anatomy rarely reflects the brain lateralization established in over a century and a half of clinical studies. A two-step performance-based connectivity analysis is described in which the first step uses multiple linear regression to establish a direct relationship between regional brain activity and a measure of performance. The second step uses partial correlations to examine the functional relationships between the predictor regions and other brain regions. When applied to regional cerebral blood flow data obtained with positron emission tomography during a speech production task, the results demonstrate left lateralization of motor control areas, thalamic involvement in repetition rate, and auditory cortical suppression, all consistent with clinical observations. The integration of performance measures into the earliest stages of image analysis without reliance on data filtering based on decomposition may provide a path towards convergence with traditional descriptions of functional anatomy based on clinical studies.
Keywords: Functional connectivity, Functional imaging, Positron emission tomography, Speech, Inferior frontal gyrus, Caudate nucleus
1. Introduction
Connectivity is becoming an increasingly important concept in functional imaging (Rowe, 2010). This approach to imaging data can be traced to earlier strategies that were based on pair-wise correlations and covariance analyses (Clark et al., 1984, 1985, 1986; Horwitz et al., 1984; Metter et al., 1984; Moeller et al., 1987). Unlike analytic approaches that focus simply on focal or multi-focal activations, functional connectivity strategies are better suited to systems-level analyses (Sidtis, 2007a,b; Rowe, 2010). However, generally speaking, functional connectivity analyses operate solely within the brain data. The resulting statistical structure is assumed to reflect the mental processes associated with a behavioral task (Friston et al., 1993, 1995), or the lack of one (i.e., the default mode). Connectivity in the brain data is typically sought in the subset of data that survive a filtering procedure based on contrasting two or more imaging conditions. This contrast filter passes only those data in which there are significant differences between experimental conditions, that is, activation (Friston et al., 1993).
An important comment about analytic goals is necessary. Applying signal processing approaches to brain image data sets to uncover connectivity is unfortunately not always consonant, either in intent or outcome, with the traditional search for brain-behavior relationships developed in the study of brain damage, neurosurgical interventions, and neurologic disease over the past 150 years. There are many reasons for this. Cerebrovascular responses are, at best, indirect measures of mental processes, reflecting the multiply determined functions of the vascular matrix (Drake and Iadecola, 2007) and not simply task-related neuronal activity, narrowly considered. Consequently, each of the factors affecting the vascular matrix can affect statistical relationships in the brain data. For example, cerebral blood flow is influenced by vasoactive agents such as the widely distributed excitatory neurotransmitter glutamate, the inhibitory neurotransmitter GABA, and the catecholamine dopamine, which are not restricted to producing local effects, as well as by the functions of subcortical nuclei (Golanov et al., 2000; 2001; Underwood et al., 1994; Sidtis et al., 2009). While the statistical structure in an image data set may reflect the state of the brain during the study, the observed structure may not have the desired specificity for the targeted behavior (e.g., Sidtis et al. 2003). When the brain data are filtered by examining only the results of a statistical contrast of two or more conditions, additional problems enter the picture. For example, it has been shown that image subtractions in speech tasks produce data that have little relationship to the functional anatomy of speech production established over a long history of lesion studies (Sidtis et al., 1999; 2003). The discrepancies between lesion and functional imaging studies have been discussed by others as well (e.g., Fellows, 2005; Van Lancker Sidtis, 2007). Further, important regions can disappear following a statistical contrast filter because of the influence of task context (Sidtis et al., 2004; 2006), and the amplitude of a region's signal level does not necessarily reflect its functional significance, either before or after a task contrast (Sidtis, 2007b). Not all brain areas identified by a behavioral contrast will be related to performance on the task under study, some important areas are eliminated by the contrast, and the scale properties of the brain responses do not necessarily reflect functional significance.
There is a conceptual difficulty in seeking structure within the brain data alone that over-rides other potential problems. That is, the properties of vascular responses are not fully understood nor can these responses be uniformly attributed to a single source. Although we generally have an appreciation of the ways in which we can design a behavioral decomposition study to obtain simple measures like accuracy or reaction time, the brain's responses are spatially and temporally complex. More importantly, the “psychometric properties” of response measures like cerebral blood flow or BOLD are not understood from either a neurobehavioral or neurophysiological perspective. From a psychometric point of view, each brain region could be thought of as having multiple response characteristics that are neither consistent across tasks nor across other brain regions. Brain responses, including activations, do not act in a reflexive, stimulus-response fashion (Sidtis et al., 2004). The assumption that the responses of complex brain systems can be decomposed in the same fashion as a choice reaction time1 is ambitious. As a simple example, both repetitive single articulatory gestures and sustained vowel productions have more brain activity, as measured by blood flow, than a motorically more complicated task of repeating a series of syllables that includes both vowel and articulatory components (Sidtis et al., 1999).
An alternative to the independent analysis of image data is an approach that has been referred to as performance-based analysis (Sidtis et al., 2003; Sidtis, 2007b). The key features of this approach are minimal pre-processing (data are normalized for global effects), no elimination of brain data2 by significance testing for “activation” or “deactivation,” and most importantly, the incorporation of a behavioral measure obtained during the acquisition of the functional image to explore the brain data. Although this does not reduce the complexity of the vascular responses, it does have the potential for establishing a more direct relationship between these responses and behavior.
The initial applications of this approach used multiple linear regression to identify a linear combination of brain regions that predicted syllable repetition rate. Syllable rate was measured for a speech task performed during scanning and was used as the dependent variable in a multiple linear regression analysis. A set of pre-defined regions of interest were used as independent variables and were entered, retained, or rejected in the regression model using a step-wise procedure.
With this linear regression procedure, repetition rates in normal speakers were modeled as a combination of increased blood flow in the left inferior gyrus region and a decrease in the head of the right caudate nucleus. The laterality of these predictive regions reflected the laterality observed in lesion studies in spite of the fact that group mean data demonstrated left/right symmetry for these regions. This cortical-subcortical relationship with syllable repetition rate was replicated in subjects with hereditary spinocerebellar ataxia (Sidtis et al., 2006), and was shown to persist over a two year period of disease progression (Sidtis et al., 2010).
While this cortical-subcortical relationship with speech rate was found in normal and neurological populations, it has been argued that the current sensitivity of functional imaging technologies, and more likely the insensitivity of cerebral blood flow to small but significant changes in activity in highly specialized neurological systems, only provide a view of the “tip of the iceburg” when trying to describe complex neurological systems (Sidtis et al., 2006; Sidtis 2007b). This point was made in the results obtained from the subjects with cerebellar ataxia, where the syllable rate prediction involved not only the left inferior gyrus region and right caudate, but also regions in the left superior temporal gyrus and the right cerebellum. It is clear from clinical studies of neurological disease that the cerebellum is involved in speech production (Ackermann et al., 1992; Amarenco et al., 1993; Urban et al., 2001; 2003), and changes in speech following post-lingual deafness demonstrate the need for auditory feedback (Waldstein, 1990), but neither the cerebellum nor superior temporal regions were found to be predictors in the normal data. It was suggested that in the ataxic subjects, cerebellar disease stressed the production system, serving as a contrast agent to reveal more of the normal speech production network.
In the present paper, an extension of the original multiple linear regression approach to performance-based analysis is proposed. With the current high level of sophistication in signal processing, this approach is admittedly elementary and may appear nostalgic for the early days of functional imaging. However, the actual statistical procedures are probably less important than the strategy of having the behavior drive the image analysis without the constraint of activation/deactivation filters. In this approach, it is the relationship with behavior rather than the result of an image contrast that identifies the functionally significant brain areas. While the use of regions-of-interest reduces the brain data compared to voxel-based analyses, 2 the number of regions can be hypothesis driven or can be expanded to encompass the whole brain. However, the identification of regions is not determined by the presence of a statistical difference between two or more dynamic neurological states, which may or may not represent a neurobiologically significant decomposition of a complex behavior.
The aim of the present approach is to pursue the “tip of the iceburg” below the waterline. This is done by first identifying brain regions associated with a performance measure using the multiple linear regression previously described. These regions are considered to have a primary relationship with the target behavior. The expansion of this procedure involves a second step, which examines the relationships between the brain regions with a primary association with performance and other brain areas not identified in the regression procedure (secondary relationships). The second step uses partial correlation method in which the effects of the contralateral region homologous to a primary region are controlled for in the correlation. So for example, the relationships between the left inferior gyrus and other regions are examined controlling for the effects of the right inferior frontal gyrus to enhance the specificity of the functional connectivity of the left inferior frontal gyrus.
It should be noted that this procedure was developed using PET data, and an important element of the preliminary processing is an intra- and inter-subject normalization for global blood flow effects (Sidtis et al., 2003). This step may be especially important in accurately characterizing sertain clinical populations (Sidtis et al., 2009). However, we have observed the same reciprocal relationship between the inferior frontal gyrus and the caudate nucleus during speech using fMRI (Sidtis et al., 1999b). In principle, with the appropriate normalization, image intensity in fMRI data could be handled in the same way. Finally, by keeping the analytic approach simple, it is also believed that a better appreciation of the vascular response properties of specific brain areas (i.e., their “psychometric properties”) can be developed in the context of known vascular physiology.
2. Materials and methods
H215O positron emission tomography (PET) data from 13 right-handed, native speakers of English were used in this study. They have been described previously, as have the scanning procedures and region of interest analysis (Sidtis et al., 1999; 2003). All subjects provided informed consent to the protocol approved by the Institutional Review Board of the University of Minnesota Medical School. Briefly, subjects were instructed to repeat the syllable sequence /pa-ta-ka/ as quickly as possible on a single breath. This was initiated approximately 15 sec prior to the detection of brain activity by the scanner and repeated for 60 sec. during the scan. Each subject performed four scans while producing syllable repetitions.
A set of 22 regions of interest (11 left-right pairs) were extracted from each scan. A threshold was applied to each region so that voxels corresponding to the highest 25% of values in the brain volume were used to calculate a mean for each region for each subject. All region-of-interest values were normalized for global effects by multiplying them by the ratio of the highest global value in the data set divided by the global value for the scan from which the region was extracted.
The first phase of this analysis used a multiple linear regression with a stepwise selection procedure to determine if a linear combination of regional blood flow values could predict syllable repetition rate. The set of 22 regions of interest were entered as independent variables in a multiple linear regression analysis to predict speech rate as the dependent measure. A stepwise analysis was conducted with the following criteria: Probability of F to enter (0.05), probability of F to remove (0.10), and tolerance (0.01) (SPSS, 1997). Of the 22 regions entered as independent measures, two regions (left inferior frontal gyrus, right caudate nucleus), with an inverse relationship to each other, predicted speech rate in normal and ataxic speakers (Sidtis et al., 2003; 2006; 2010).
The second phase of this analysis represents the new element of this approach. A partial correlation procedure was used to investigate possible relationships between these predictor regions and the remaining regions in the data set. A partial correlation technique was used to control for the influence of the contralateral homologous brain region since functional laterality is a significant factor in the neurological organization of speech and language. In these data, 10 of the 11 left/right pairs of homologous regions were positively correlated (mean r = 0.58). The sole exception was the transverse temporal region, which did not demonstrate a left/right correlation. A probability level less than 0.01 was considered significant for reporting the results of the partial correlations.
3. Results
As previously reported, the first phase of this analysis demonstrated that syllable repetition rates are predicted by a linear combination of increased blood flow in the left inferior frontal region and decreased flow in the right caudate nucleus [F(2,49) = 10.26; = p < 0.001]. This relationship is depicted in Figure 1, which includes the unstandardized regression coefficients and standard errors for these regions.
Figure 1.
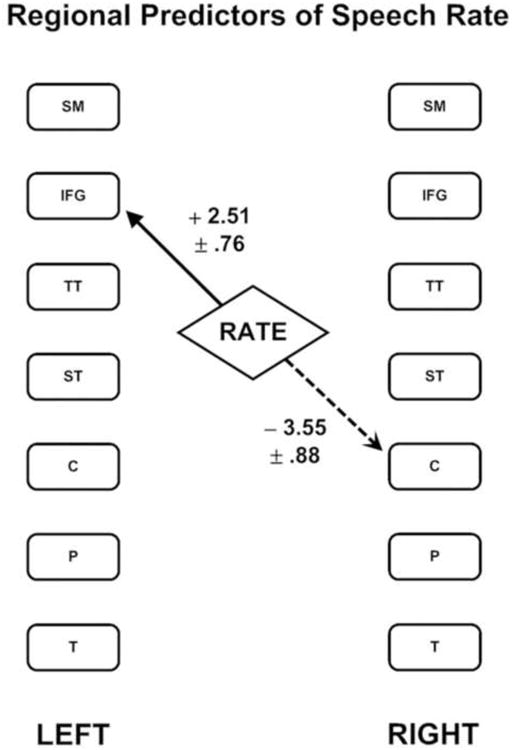
The two brain regions that combine to predict syllable repetition rate as identified by multiple linear regression using a stepwise selection process. The solid line represents a positive regression weight while the dashed line represents a negative regression weight. The values listed are the unstandardized regression coefficients plus or minus one standard error. The following regions are represented: sensori-motor cortex (SM); inferior frontal gyrus (IFG); transverse temporal cortex (TT); superior temporal cortex (ST); caudate nucleus head (C); putamen (P); thalamus (T).
In the second phase of this analysis, the left inferior frontal and caudate nucleus regions were subjected to separate partial correlation procedures with the remaining brain regions, controlling for the respective contralateral homologue region. The partial correlations between the left inferior frontal region and the remaining regions, controlling for the right inferior frontal region, revealed significant positive correlations with the left caudate [r = 0.48; p < 0.001] and putamen [r = 0.5; p < 0.001], and the right transverse temporal region [r = 0.38; p = 0.006]. The left inferior frontal region was also negatively correlated with the left thalamus [r = - 0.49; p < 0.001]. These relationships are depicted in Figure 2.
Figure 2.
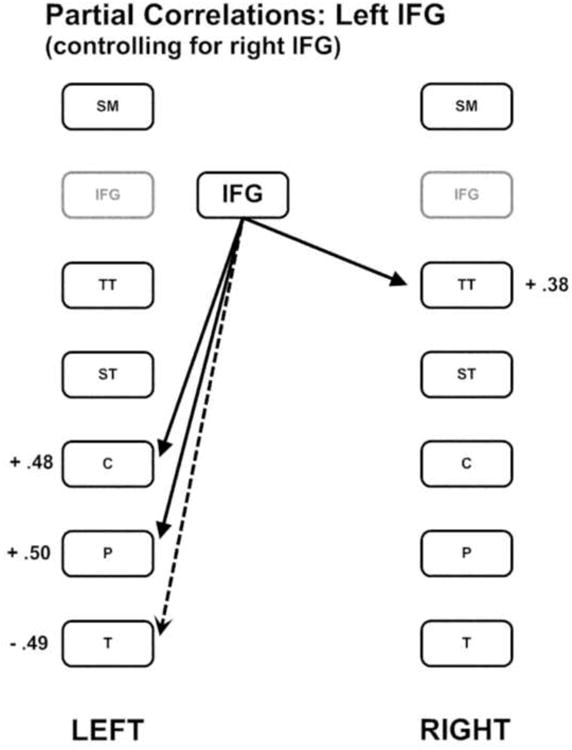
The pattern of partial correlations between the left inferior frontal gyrus region and the remaining brain regions controlling for the right inferior frontal gyrus region. Positive correlations are indicated by solid lines and negative correlations are indicated by dashed lines.
The second regional predictor of rate was the right caudate nucleus. The partial correlations between the right caudate nucleus and the remaining regions, controlling for the left caudate nucleus, revealed significant positive correlations with the right inferior frontal region [r = 0.53; p < 0.001], putamen [r = 0.66; p < 0.001], and superior temporal region [r = 0.60; p < 0.001]. The right caudate nucleus was also positively correlated with the left superior temporal [r = 0.51; p < 0.001] and transverse temporal [r = 0.39; p = 0.004] regions, and negatively correlated with the left sensori-motor strip [r = - 0.52; p < 0.001]. These relationships are depicted in Figure 3. The relationships between regional activity and syllable repetition rate established in the first and second phases of this analysis are depicted in Figure 4. Because the flow in the right caudate nucleus is inversely related with syllable rate, regions positively correlated with the caudate are represented as negatively related to syllable rate.
Figure 3.
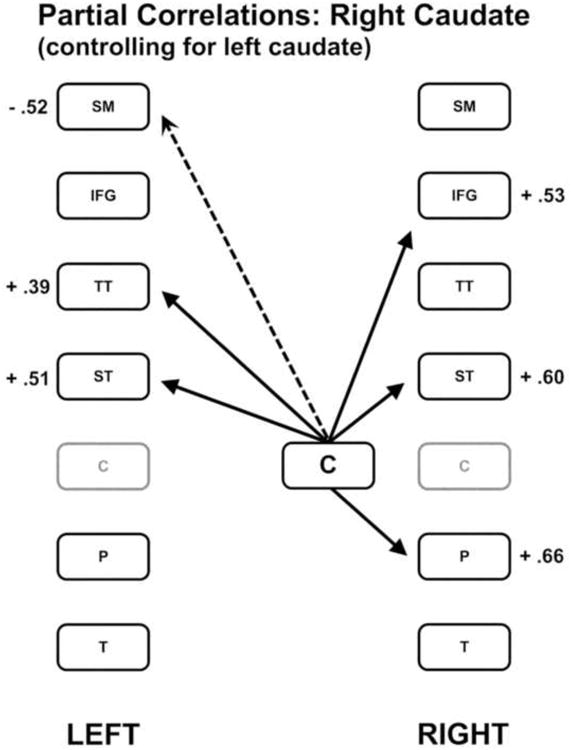
The pattern of partial correlations between the right caudate nucleus region and the remaining brain regions controlling for the left caudate nucleus region. Positive correlations are indicated by solid lines and negative correlations are indicated by dashed lines.
Figure 4.
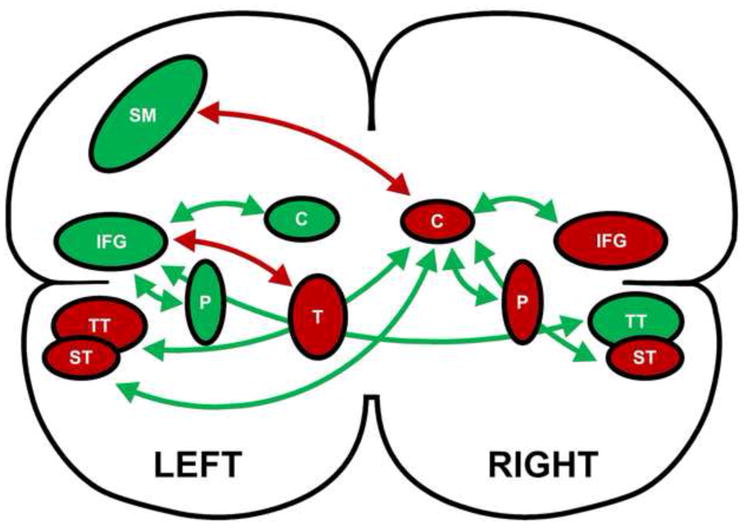
Schematic depiction of the inter-relationships among brain regions and their relationships to speech rate. The arrows represent the partial correlations among brain regions, with green depicting positive correlations and red depicting negative correlations. The green brain regions have a positive relationship with speech rate while the red regions have a negative relationship with speech rate. The regions are as follows: inferior frontal gyrus (IFG); sensori-motor cortex (SM); transverse temporal cortex (TT); superior temporal cortex (ST); caudate nucleus (C); putamen (P); and thalamus (T). The left inferior frontal gyrus and the right caudate nucleus have a primary association with speech rate while the other brain regions have a secondary relationship.
4. Discussion
In a regional cerebral blood flow data set marked by bilateral symmetry (Sidtis et al., 1999), a performance-based analysis identified a cortical-subcortical relationship consistent with the results of lesion studies, which have documented the asymmetrical representation of speech and language in the brain for over a century and a half. An additional connectivity analysis, originating with the predictive regions and implemented using a partial correlation technique that controlled for the association between homologous contralateral regions, revealed a more detailed estimation of regional cerebral blood flow changes associated with syllable repetition. Neither the mechanism nor the significance of the correlations between blood flow in the homologous left and right hemisphere regions is known. However, in the case of progressive ataxia, blood flow increases in the left inferior frontal region suggested compensation for cerebellar decline while decreases in the right inferior frontal region did not (Sidtis et al., 2010).
As noted, the present performance-based functional connectivity analysis detected functional relationships between speaking and lateralized brain areas that are consistent with lesion studies, in spite of the fact that the group mean data for these regions were bilaterally symmetrical. It has been reported previously that subtraction of a non-speaking resting state does not yield data that predict speaking rate in the multiple linear regression model, nor does the result of a covariance analysis (Sidtis et al., 2003). This is due, in part, to the observation that the resting state is significantly influenced by adjacent task conditions (Sidtis et al., 2004; Sidtis 2007). Whereas neither contrasting tasks (Sidtis et al., 1999) nor rest states identify regions that predict speech rate, functionally relevant data can be identified when a behavioral measure rather than a task contrast is used.
As with the original performance-based analysis, the brain regions identified by this two phase approach are consistent with the results of lesion studies. The left sided regions that represent cortical-striatal motor control areas demonstrated a positive relationship between blood flow and syllable repetition rate (sensori-motor strip, inferior frontal gyrus, caudate, putamen). With the exception of the right sensori-motor strip, which was not correlated with either predictor, the homologous regions on the right side showed a negative relationship with rate (inferior frontal gyrus, caudate, putamen). This asymmetry in favor of left hemisphere regions is consistent with the well-established lateralization of speech and language in the right-handed population (Davis and Wada, 1978; Loring et al., 1992). It should be emphasized that this asymmetry is neither present in mean blood flow values nor in values in which other tasks or resting states were subtracted (Sidtis et al., 1999; 2007b).
Clinical observations have also demonstrated that the thalamus has a role in the control of speech rate. Stimulation of the dominant thalamus can produce speech slowing (e.g., Schaltenbrand, 1975; Mateer, 1978) while bilateral ablation of this structure can produce pathologically rapid speech (Canter and Van Lancker, 1985). The relationship between syllable repetition rate and left thalamic blood flow in the present analysis is consistent with these clinical observations if we view the reduction in blood flow as reflecting neurophysiological function that is opposite of that which occurs with electrical stimulation. Increased thalamic involvement in speech production appears to slow the process, so that it would be expected that increased rate would be associated with reduced thalamic involvement. In the case of bilateral thalamotomy, any regulating effect on the speed of syllable production by this structure may be lost.
The negative relationship between blood flow in the superior temporal region and repetition rate is consistent with a well-documented electrophysiological response: suppression of auditory cortical activity during vocal production. First described in the squirrel monkey (Müller-Preuss and Ploog, 1981) and later in human neurosurgical subjects (Creutzfeldt, Ojemann, and Lettich, 1989), electrophysiological and magnetoencephalographic recordings demonstrate that activity in the superior temporal gyrus is reduced when vocalization is produced compared to when vocalization is simply heard (Curio et al., 2000; Houde et al., 2002; Heinks-Maldonado et al., 2005; Aliu et al., 2008). Figure 4 indicates that the more anterior regions of the superior temporal gyrus have a negative association with speech rate, consistent with the auditory suppression phenomenon. The more posterior region, the transverse temporal region, was negatively associated with rate in the language dominant left side but positively associated with rate in the right side. This lateralization is also consistent with the electrophysiological and magnetoencephalographic data (Curio et al., 2000; Houde et al., 2002; Heinks-Maldonado et al., 2005).
5. Conclusions
A framework for a connectivity analysis is described based on primary associations between a performance measure and regional blood flow, and secondary associations between predictor regions and other regions in the data set. In data remarkable for bilateral symmetry during speech production, the performance-based analysis identified laterality for cortical-motor and striatal regions consistent with lesion studies. The relationship between thalamic blood flow and syllable rate was also consistent with clinical observations. Finally, the relationship between blood flow in the superior temporal gyrus regions and syllable rate was consistent with the auditory cortical suppression effect observed in magnetoencephalographic and electrophysiological data. While sophisticated pattern analytic approaches are providing insights into the statistical structure in brain data, the performance-based approach appears to provide a more direct link with behavior, without the assumptions required for identifying “activations,” or the reliance on contrasts that assume an unconstrained ability to decompose brain signals. It is another of the many approaches to connectivity analysis (Horwitz, 2003) that extends beyond the brain data. Finally, by reducing the level of abstraction of the blood flow (or BOLD) signals, the vascular responses during functional imaging studies may more easily placed in the broader context of cerebrovascular physiology.
Highlights.
Speaking rate is modeled by blood flow in two brain regions
Functional connectivity with these regions is established
Brain-behavior relationships reflect hemispheric lateralization for language
Involvement of sub-cortical structures is described
Cortical auditory suppression is observed
Acknowledgments
The comments of D. Van Lancker Sidtis are gratefully acknowledged. This work was supported by a grant from the NIDCD RO1 DC007658. Support was provided for analytic development and preparation of this manuscript.
Footnotes
Decomposition of behavioral measures like reaction time is not without significant difficulty as well. Donders (1869) notion of “pure insertion,” upon which subtraction techniques are based, was rejected by investigators by the early 1900s (Ach, 1905; Boring, 1929; Woodworth, 1938). More recently, Van Zandt and Ratcliff (1995) demonstrated that the decomposition of reaction times into constituent components does not yield unique solutions.
In our work, the data are reduced by using regions of interest (ROIs). A threshold is also applied to each ROI to take an average of the upper percentage of voxels, to approximate gray matter cerebral blood flow. The use of thresholded ROIs also has the advantage of improved tolerance of individual differences in functional anatomy without image smoothing. The measurement is always made within a brain region (e.g., inferior frontal region), but the distribution of the threshold signal need not correspond exactly from subject to subject.
The widely used Statistical Parametric Mapping (SPM) toolbox (Friston et al., 1995) provides for linear regression in defining the design matrix. However, SPM has overwhelmingly been used to identify activations, and behavioral correlates are generally identified for brain data that survive the activation/deactivation filter (e.g., Friston et al., 1993).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ach N. Über die Willenstätigkeit und das Denken. Göttingen: Vandenhoeck & Ruprecht; 1905. [Google Scholar]
- Ackermann H, Vogel M, Peterson D, Poremba M. Speech deficits in ischaemic cerebellar lesions. Neurology. 1992;239(4):223–227. doi: 10.1007/BF00839144. [DOI] [PubMed] [Google Scholar]
- Aliu SO, Houde JF, Nagarajan S. Motor-induced suppression of the auditory cortex. J Cogn Neurosci. 2008;21(4):791–802. doi: 10.1162/jocn.2009.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarenco P, Rosengart A, DeWitt LD, Pessin MS, Caplan LR. Anterior inferior cerebellar artery territory infarcts. Arch Neurol. 1993;50(2):154–161. doi: 10.1001/archneur.1993.00540020032014. [DOI] [PubMed] [Google Scholar]
- Boring EG. A History of Experimental Psychology. New York: The Century Co; 1929. [Google Scholar]
- Canter GJ, Van Lancker DR. Disturbances of the temporal organization of speech following bilateral thalamic surgery in a patient with Parkinson's disease. J Commun Disord. 1985;18:329–349. doi: 10.1016/0021-9924(85)90024-3. [DOI] [PubMed] [Google Scholar]
- Clark C, Kessler R, Buchsbaum M, Margolin R, Holcomb H. Correlational methods for determining coupling of regional glucose metabolism: a pilot study. Biol Psych. 1984;19:663–678. [PubMed] [Google Scholar]
- Clark C, Carson R, Kessler R, Margolin R, Buchsbaum M, DeLisi L, King C, Cohen R. Alternative statistical models for the examination of clinical positron emission tomography/fluorodeoxyglucose data. J Cereb Blood Flow Metab. 1985;5:142–150. doi: 10.1038/jcbfm.1985.18. [DOI] [PubMed] [Google Scholar]
- Clark CM, Hayden MR, Stoessl AJ, Martin WRW. Regression model for predicting dissociations of regional cerebral glucose metabolism in individuals at risk for Huntington's disease. J Cereb Blood Flow Metab. 1986;6:756–762. doi: 10.1038/jcbfm.1986.132. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O, Ojemann G, Lettich E. Neuronal activity in the human lateral temporal lobe II Responses to the subjects own voice. Exp Brain Res. 1989;77(3):476–489. doi: 10.1007/BF00249601. [DOI] [PubMed] [Google Scholar]
- Curio G, Neuloh G, Numminen J, Jousmäki Hari. Speaking modifies voice-evoked activity in the human auditory cortex. Human Brain Mapp. 2000;9:183–191. doi: 10.1002/(SICI)1097-0193(200004)9:4<183::AID-HBM1>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102(2):141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Davis AE, Wada JA. Speech dominance and handedness in the normal human. Brain Lang. 1978;5(1):42–55. doi: 10.1016/0093-934x(78)90006-8. [DOI] [PubMed] [Google Scholar]
- Donders FC. Over de snelheid van psychische processen. Nederlandsch Archief voor Genees- en Natuurkunde. 1869;4:117–145. [Google Scholar]; Koster WG, translator. On the speed of mental processes. Acta Psychol. 1969;30:412–431. doi: 10.1016/0001-6918(69)90065-1. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH. Method matters: an empirical study of impact in cognitive science. J Cogn Neurosci. 2005;17(6):850–858. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ. Functional connectivity: The principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapp. 1995;2:189–210. [Google Scholar]
- Golanov EV, Christensen JR, Reiss DJ. Neurons of a limited subthalamic area mediate elevations in cortical cerebral blood flow evoked by hypoxia and excitation of neurons of the rostral ventrolateral medulla. J Neurosci. 2001;21(11):4032–4041. doi: 10.1523/JNEUROSCI.21-11-04032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golanov EV, Ruggiero DA, Reiss DJ. A brainstem area mediating cerebrovascular and EEG responses to hypoxic excitation of rostral ventrolateral medulla in rat. J Physiol. 2000;529(2):413–429. doi: 10.1111/j.1469-7793.2000.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Mathalon DH, Gray M, Ford JM. Fine-tuning of auditory cortex during speech productions. Psychophysiology. 2005;42(2):180–190. doi: 10.1111/j.1469-8986.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- Horwitz B. The elusive concept of brain connectivity. Neuroimage. 2003;19:466–470. doi: 10.1016/s1053-8119(03)00112-5. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Duara R, Rapoport SI. Intercorrelations of glucose metabolic rates between brain regions: Application to healthy males in a state of reduced sensory input. J Cereb Blood Flow Metab. 1984;4:484–499. doi: 10.1038/jcbfm.1984.73. [DOI] [PubMed] [Google Scholar]
- Houde JF, Nagarajen SS, Sekihara K, Merzenich MM. Modulation of the auditory cortex during speech: An MEG study. J Cogn Neurosci. 2002;14(8):1125–1138. doi: 10.1162/089892902760807140. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Lee GP, King DW. Amobarbital Effects and Lateralized Brain Function. New York: Springer-Verlag; 1992. [Google Scholar]
- Mateer C. Asymmetric effects of thalamic stimulation on rate of speech. Neuropsychologia. 1978;16:497–499. doi: 10.1016/0028-3932(78)90073-8. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Riege WH, Kuhl DE, Phelps ME. Cerebral metabolic relationships for selected brain regions in healthy adults. J Cereb Blood Flow Metabol. 1984;4:1–7. doi: 10.1038/jcbfm.1984.1. [DOI] [PubMed] [Google Scholar]
- Moeller JR, Strother SC, Sidtis JJ, Rottenberg DA. The scaled subprofile model: A statistical approach to the analysis of functional patterns of brain metabolism in positron emission tomographic/fluorodeoxyglucose data. J Cereb Blood Flow Metabol. 1987;7:649–658. doi: 10.1038/jcbfm.1987.118. [DOI] [PubMed] [Google Scholar]
- Müller-Preuss P, Ploog D. Inhibition of auditory cortical neurons during phonation. Brain Res. 1981;215(1-2):61–76. doi: 10.1016/0006-8993(81)90491-1. [DOI] [PubMed] [Google Scholar]
- Rowe JB. Connectivity analysis is essential to understanding neurological disorders. Front Syst Neurosci. 2010;4:e144. doi: 10.3389/fnsys.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaltenbrand G. The effects on speech and language of stereotactical stimulation in thalamus and corpus callosum. Brain Lang. 1975;2:70–77. doi: 10.1016/s0093-934x(75)80055-1. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ. Activation: Will hot spots get the hot field in hot water? Brain Lang. 2007a;102(2):127–129. doi: 10.1016/j.bandl.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidtis JJ. Some problems for representations of brain organization based on activation. Brain Lang. 2007b;102(2):130–140. doi: 10.1016/j.bandl.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidtis JJ, Gomez C, Groshong A, Strother SC, Rottenberg DA. Mapping cerebral blood flow during speech production in hereditary ataxia. Neuroimage. 2006;31(1):246–254. doi: 10.1016/j.neuroimage.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Liu H, Anderson JH, Truwit C. Functional magnetic resonance imaging during speech: bilateral flow increases in inferior frontal regions are coupled with flow decreases in the caudate and putamen. Society for Neuroscience Abstracts. 1999b;25:375. 1999. [Google Scholar]
- Sidtis JJ, Strother SC, Anderson JR, Rottenberg DA. Are brain functions really additive? Neuroimage. 1999a;9(5):490–496. doi: 10.1006/nimg.1999.0423. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Strother SC, Groshong A, Rottenberg DA, Gomez C. Longitudinal cerebral blood flow changes during speech in hereditary ataxia. Brain Lang. 2010;114(1):43–51. doi: 10.1016/j.bandl.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidtis JJ, Strother SC, Rottenberg DA. Predicting performance from functional imaging data: Methods matter. Neuroimage. 2003;20(2):615–624. doi: 10.1016/S1053-8119(03)00349-5. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Strother SC, Rottenberg DA. The effect of set on the resting state in functional imaging: A role for the striatum? Neuroimage. 2004;22(3):1407–1413. doi: 10.1016/j.neuroimage.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Tagliati M, Sidtis D, Dhawan V, Eidelberg D. Globally increased cerebral blood flow (CBF) during high-frequency deep brain stimulation of the subthalamic nucleus (STN-DBS) in Parkinson's disease (PD) Mov Disord. 2009;24:S473. [Google Scholar]
- SPSS 7.5 for Windows. SPSS Inc.; Chicago: [Google Scholar]
- Underwood MD, Iadecola C, Reis DJ. Lesions of the rostral ventrolateral medulla reduce the cerebrovascular response to hypoxia. Brain Res. 1994;635(1-2):217–223. doi: 10.1016/0006-8993(94)91442-7. [DOI] [PubMed] [Google Scholar]
- Urban PP, Wicht S, Vukurevic G, Fitzek C, Stoeter P, Massinger C, Hopf HC. Dysarthria in acute ischemic stroke. Neurology. 2001;56(8):1021–1027. doi: 10.1212/wnl.56.8.1021. [DOI] [PubMed] [Google Scholar]
- Urban PP, Marx J, Hunsche S, Gawehn J, Vukurevic G, Wicht S, Massinger C, Stoeter P, Hopf HC. Cerebellar speech representation. Arch Neurol. 2003;60(7):965–972. doi: 10.1001/archneur.60.7.965. [DOI] [PubMed] [Google Scholar]
- Van Lancker Sidtis D. Does functional neuroimaging solve the questions of neurolinguistics? Brain Lang. 2007;102(2):130–140. doi: 10.1016/j.bandl.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Van Zandt T, Ratcliff R. Statistical mimicking of reaction time data: single process models, parameter variability, and mixtures. Psychonomic Bull Rev. 1995;2:20–54. doi: 10.3758/BF03214411. [DOI] [PubMed] [Google Scholar]
- Waldstein RS. Effects of postlingual deafness on speech production: Implications for the role of auditory feedback. J Acoust Soc Am. 1990;88(5):2099–2114. doi: 10.1121/1.400107. [DOI] [PubMed] [Google Scholar]
- Woodworth RS. Experimental Psychology. New York: Henry Holt and Company; 1938. [Google Scholar]


