We recently reported that a proto-type thrombopoietin (TPO) receptor agonist, SB-559457, is toxic to acute myeloid leukemia (AML) cell lines and primary AML cells.1 SB-559457 is a nonpeptidyl, hydrazone class, TPO receptor agonist similar to the Food and Drug Administration approved TPO mimetic, Eltrombopag (E). Here, we confirm that E itself is toxic to AML cell lines and primary AML cells, and initiate studies to understand the drug’s mechanism of action in the inhibition of AML cell survival. Myeloproliferative leukemia virus proto-oncogene c-Mpl is the gene encoding TPO-receptor, a member of the hematopoietic growth factor receptor superfamily. TPO and c-Mpl are both necessary for the proliferation and differentiation of megakaryocytes and production of platelets. E also binds to c-Mpl through a defined transmembrane domain, and activates c-Mpl-dependent signaling in cell lines and primary normal CD34+ stem and progenitor cells.2 It is hypothesized that these effects are necessary for the clinical activity of E in stimulating platelet production. c-Mpl is expressed in some myeloid leukemia cells, but its function in AML is not defined. Some reports have stated that TPO activates signaling pathways in primary AML cells, but this is not consistently observed.3,4 In this paper, we studied the role of c-Mpl in the toxic effects of the hydrazone class of TPO-receptor agonists on AML cells.
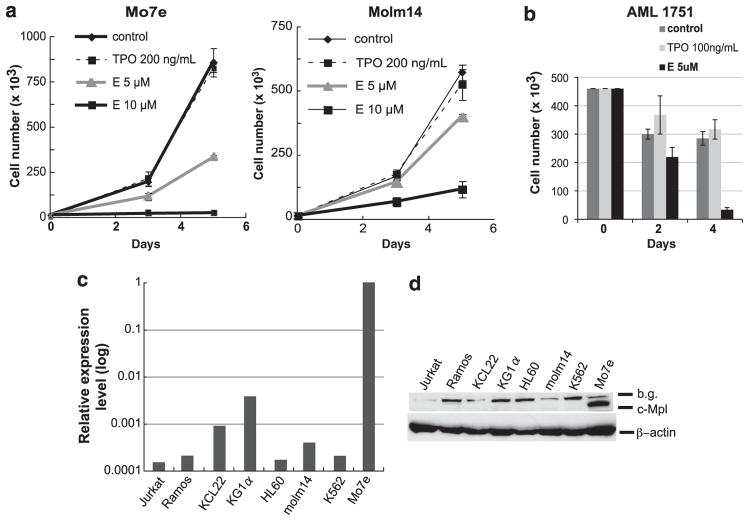
First, we tested whether E has a similar effect on AML cell lines as SB-559457 by performing cell growth analysis. Myeloid leukemia cell lines, KCL22, KG1a, HL60, Molm14, K562 and Mo7e, were cultured in their respective optimal culture medium. Cells were seeded into 96-well culture plates and treated with increasing concentrations of E or recombinant human TPO (rhTPO) as a control. The growth curves demonstrated that E inhibited cell proliferation of all tested cell lines in a dose-dependent manner (Figure 1a; representative data are shown). Although the drug sensitivity varied, proliferation of all tested leukemia cell lines were inhibited with 2.5 to 10 μM of E, which is similar to the cell growth inhibition observed with SB-559457.1 Second, to determine whether E inhibits the growth of primary AML cells, we cultured primary leukemia cells from AML patients without or with E/rhTPO and studied their survival. All tested primary AML samples (n = 14) treated with E show significant decreases in cell number compared with controls (Figure 1b; data from a representative patient, sample characteristics provided in Supplementary Table 1). Our experiments, which were performed in a liquid culture system in the presence of 2% fetal bovine serum, revealed that E at 5 μM concentration was highly toxic to all tested AML samples (n = 14). These data indicate that E inhibits the growth and survival of primary AML cells in addition to AML cell lines. Of note, analysis of caspase and poly(ADP-ribose) polymerase cleavage demonstrated that E induced caspase activation and poly(ADP-ribose) polymerase cleavage, consistent with an apoptotic mechanism of E-induced toxicity, which in turn is consistent with the previously described effects of SB559457 (data not shown).1
Figure 1.
(a) Cell growth analyses of Mo7e and Molm14. A total number of 1000 to 20 000 cells in 250 μl culture medium were seeded in triplicate wells in a 96-well plate and treated with E (5 or 10 μM, supplied by GlaxoSmithKline, Collegeville, PA, USA) or rhTPO (200 ng/ml, R&D Systems, Minneapolis, MN, USA) as a control. The numbers of viable cells were counted using a hemocytometer with trypan blue exclusion on days 3 and 5. Experiments were repeated at least three times with consistent results. Data are shown as the mean±s.d. Mo7e cell line requires cytokines (TPO or granulocyte-macrophage colony-stimulating factor (GM-CSF)) for its proliferation and is maintained with 5 ng/ml of GM-CSF (Immunex Corporation, Seattle, WA, USA). (b) Cell growth analyses of primary AML cells. The proliferation assays from a representative primary AML sample are shown. Primary samples were obtained from the Stem Cell and Xenograft Core facility at the University of Pennsylvania School of Medicine. All samples were collected in accordance to the federal and University guidelines, and provided to us pathologically annotated without patient personal information. Available clinical information about AML’s is provided in Supplementary Table 1. A total number of 100 000 to 450 000 cells were cultured in the presence of E (5 μM), rhTPO (100 ng/ml) or in medium alone. The numbers of viable cells were counted on indicated days and data are shown as the mean±s.d. of three replicates. (c) Relative c-Mpl mRNA expression levels in various leukemia cell lines; Jurkat, Ramos, KCL22, KG1α, HL60, Molm14, K562 and Mo7e. qRT–PCR using a SYBR Green reagent was performed using β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as reference genes to normalize mRNA expression in different cell lines. The sequences of primers are as follows. c-Mpl, Forward: 5′-TTTCTCCCGAACATTTGAGG-3′; reverse: 5′-GTGCAGCGGAAAGAAGAGAC-3′. β-Actin, Forward: 5′-AAACTGGAACGGTGAAGGTG-3′; reverse: 5′-AGAGAAGTGGGGTGGCTTTT-3′. GAPDH, Forward: 5′-GACAGTCAGCCGCATCTTCTT-3′; reverse: 5′-CCAATACGACCAAATCCGTTGA-3′. The two-step PCR method was performed according to the following protocol: Enzyme activation at 95 °C, 10 min, 40 cycles of denaturing step at 95 °C for 15 s and annealing/extension step at 60 °C for 1 min. c-Mpl mRNA expression in Mo7e is denoted as 1, and the data are presented on a log scale. (d) c-Mpl protein expression in leukemia cell lines. Cell pellets of each cell line were lysed in ice-cold lysis buffer (50 mM Tris–HCl (pH 7.6), 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% NP-40) containing a cocktail of phosphatase inhibitors and protease inhibitors (Pierce, Rockford, IL, USA). Protein lysates were separated using SDS-polyacrylamide gel electrophoresis and were transferred onto polyvinylidene fluoride membrane. Transferred proteins were probed with the following primary antibodies and horseradish peroxidase (HRP)-conjugated secondary antibodies: anti-c-Mpl antibody (Millipore, Billerica, MA, USA, no.06-944), anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO, USA), HRP -conjugated anti-rabbit immunoglobulinG (IgG; Cell Signaling, Danvers, MA, USA, no.7074) and HRP-conjugated anti-mouse IgG (Cell Signaling, no.7076). Protein–antibody signals were detected using enhanced chemiluminescence detection reagent (GE Healthcare, Piscataway, NJ, USA).
To investigate whether E inhibits the proliferation of myeloid leukemia cells without a correlation to c-Mpl expression, we initially focused on AML cell lines. We studied several myeloid and lymphoid leukemic cell lines, which are not known to express c-Mpl (Jurkat, Ramos, KCL22, KG1a, HL-60, Molm-14 and K562 cells), and one c-Mpl highly expressing megakaryocytic leukemia cell line, Mo7e (Figure 1c, d). First, we analyzed c-Mpl expression using quantitative real-time PCR (qRT–PCR) and western blot methods. We confirmed that the Mo7e cell line expresses high levels of c-Mpl. To validate relative expression analysis, qRT–PCR was performed using two different reference genes. The qRT–PCR results showed that relative c-Mpl mRNA expression levels in all tested cell lines were less than 1% when compared with that in Mo7e cells (Figure 1c). Second, we performed western blot analyses to evaluate c-Mpl protein expression (Figure 1d). A very strong c-Mpl signal was detected in Mo7e cells, however, no signal was detected in lysates from the other cell lines. Taken together, these data confirm the c-Mpl expression in Mo7e cells, but show no evidence of c-Mpl expression in other cell lines. Of note, inhibition of cell growth by E in Mo7e cells, which express c-Mpl, is similar to E-mediated cell growth inhibition in other cell lines that do not have detectable c-Mpl expression (Figure 1a; data not shown), which suggests that E inhibition of leukemic growth may be independent of c-Mpl expression.
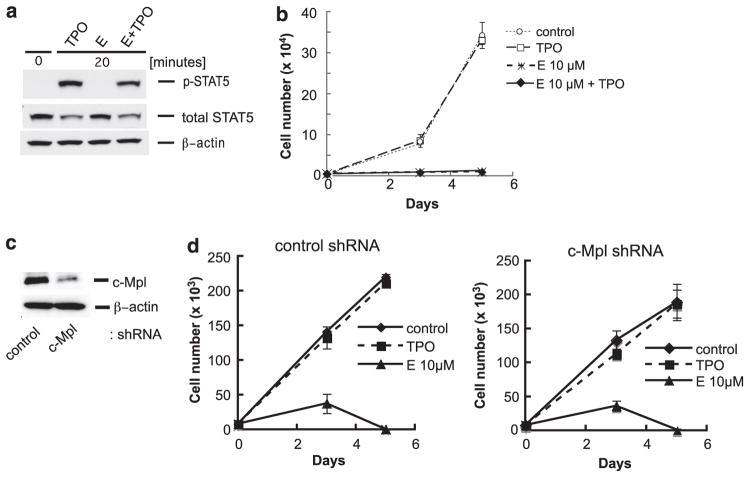
In multipotent hematopoietic progenitor cells, binding of TPO to c-Mpl initiates activation of signaling pathways such as Jak2/STAT5, Ras/Raf/MAPK and PI3K-Akt signaling pathways, which lead to proliferation and maturation of megakaryocytes and platelet production.5–12 To determine whether E activates c-Mpl-dependent signaling in myeloid leukemia cells, we evaluated phosphorylation of STAT5 by E or rhTPO using Mo7e cells. After 24 h starvation of serum and cytokines, Mo7e cells were stimulated with E (10 μM) or rhTPO (100 ng/ml). Activation of STAT5 by rhTPO peaked between 10 and 20 min (Figure 2a; data not shown). Interestingly, phosphorylated (p)-STAT5 was not detected in E-treated Mo7e cells. Stimulation tests were also done with lower and higher dose of E (5 and 30 μM), however, no signal of p-STAT5 was detected with either concentration. To investigate whether c-Mpl activation by rhTPO will rescue cells from the E effect, we costimulated Mo7e cells with E and rhTPO simultaneously and determined cell growth and STAT5 activation. Our data demonstrated that the combination of E and rhTPO activated STAT5 to the same level as rhTPO alone (Figure 2a), but did not rescue the cells from death caused by E (Figure 2b). Combined, these experiments further demonstrate that the E effect on AML cell survival is independent of c-Mpl signaling.
Figure 2.
(a) Western blot analyses to detect p-STAT5 in Mo7e cells. After 24 h starvation of serum and cytokines, Mo7e cells were stimulated with E (10 μM) alone, rhTPO (100 ng/ml) alone or with combination of E and rhTPO for 20 min. Western blot was performed as described in Figure 1 using the following antibodies; anti-phosphorylated STAT5 (Tyr694) antibody and anti-STAT5 antibody (nos 9356 and 9363, respectively, Cell Signaling). (b) Cell growth analyses (drug competition assay) of Mo7e cells after treatment of E (5 μM or 10 μM) alone, rhTPO (200 ng/ml) alone, or combination of E and rhTPO. Cell culture conditions, methods, and analyses were performed as described in Figure 1. Data are shown as the mean±s.d. Experiment was repeated at least three times with consistent results. (c) Western blot of c-Mpl protein expression in Mo7e cells without and with the c-Mpl knockdown. The targeted sequence was selected from the previous report15 and shRNA constructs were designed with two base modifications in the sense strand. The c-Mpl small interfering RNA (siRNA) targeting sequence and control siRNA sequence were as follows: c-Mpl: 5′-gcacctctgggtgaagaatgt-3′, control: 5′-ctttatacgtagtcataag-3′. The shRNA constructs were cloned into the HIUG lentiviral vector, which was kindly provided by Dr EJ. Brown in the University of Pennsylvania, and the lentivirus clones were stably transduced into Mo7e cells. The western blot was performed as described in Figure 1, and protein–antibody signals detected were quantified using ImageQuant TL software (GE Healthcare, Piscataway, NJ, USA). (d) Cell growth analyses in Mo7e cells without and with the c-Mpl knockdown. Knockingdown of c-Mpl did not change E’s inhibitory effect. The manipulated control- and c-Mpl knocked-down Mo7e were cultured in the same conditions as described in Figure 1. After treatment with rhTPO (200 ng/ml) or E (10 μM), the number of viable cells in triplicate wells were counted on days 3 and 5. Cell growth assay data are shown as the mean±s.d.
We also investigated the influence of reduced c-Mpl expression on E’s effect in Mo7e cells, which express high levels of c-Mpl. We silenced c-Mpl expression using a lentiviral vector carrying small hairpin RNA (shRNA) directed against c-Mpl and established a stable cell line. Continued expression of the short hairpin construct was tracked using green fluorescent protein, which is coexpressed from this construct. Stably transduced cells containing control or c-Mpl shRNA were green fluorescent protein positive in 82.9% and 97.4%, respectively, as determined by flow cytometry analyses (data not shown). c-Mpl expression levels were analyzed with qRT–PCR and western blot. The qRT–PCR analyses revealed that c-Mpl shRNA-transduced Mo7e (shRNA-Mo7e) cells expressed 54% less c-Mpl mRNA compared with the control shRNA-transduced Mo7e (control-Mo7e) (data not shown). Western blot analyses showed that c-Mpl protein expression in shRNA-Mo7e was 33.2±4.4% (mean±s.d.) compared with that in control-Mo7e (Figure 2c). Using these manipulated Mo7e cells, cell proliferation assays were performed after treatment with E or rhTPO. The cell growth curves demonstrated that proliferation of shRNA-Mo7e cells was inhibited by E to the same extent as in the parental cell line (Figure 2d). Thus, even in a cell line that endogenously expressed c-Mpl, E inhibition of survival is independent of c-Mpl expression.
Because E inhibits leukemic cell line growth, but does not inhibit normal hematopoietic stem cell growth,2 we questioned whether E might be a general inhibitor of malignant cell growth. Therefore, we assessed the effect of E in other cancer cell lines. First, we performed cell growth assays of E-treated leukemia/lymphoma cell lines, Jurkat and Ramos cells. Surprisingly, E inhibited cell proliferation of Jurkat and Ramos as well as in myeloid cell lines (data not shown). We also prepared two breast cancer cell lines, MCF7 and T47D, and used the XTT assay (Cell Proliferation Kit II, Roche, Indianapolis, IN, USA) optimal to these adherent cells for drug sensitivity assays. MCF7 and T47D cells were incubated in optimal medium in a 96-well culture plate and the XTT assay was performed 96 h after treatment with E (10, 20 and 30 μM), or with rhTPO (200 ng/ml) as a control. The data were analyzed by calculating the percentage of treated viable cells relative to non-treated viable cells. The XTT assay showed that cell proliferation of Etreated cells was inhibited dose-dependently in both MCF7 and T47D with similar toxicity. In MCF7 cells, the cell proliferation was 63.5±0.98, 46.6±3.10 and 23.0±2.54% (mean±s.d.) when treated with 10, 20 and 30 μM of E, respectively (data not shown). These results suggest that E may be a general inhibitor of cancer cell growth through a c-Mpl-independent mechanism. According to previous papers reporting pharmacokinetics in healthy volunteers and idiopathic thrombocytopenic purpura patients receiving 75 mg once daily, an approved maximum dose for idiopathic thrombocytopenic purpura patients, the mean maximum plasma concentrations (Cmax) were 7.3 and 12.7 μg/ml,13,14 which suggests that tested concentrations of E in AML (2.5–10 μM) in this paper are physiologically achievable (10 μM = 5.56 μg per ml free drug).
In summary, we demonstrated that E inhibits myeloid leukemia cell lines regardless of c-Mpl expression. This inhibitory effect was not changed by exogenously added rhTPO or silencing of c-Mpl expression in Mo7e cells. E also inhibits the proliferation of non-AML cell lines such as Jurkat, Ramos, MCF7 and T47D. Taken together, we conclude that E inhibits AML cell proliferation independent of c-Mpl expression. E is a potential drug used for cancer patients under myelosuppresive chemotherapy to support megakaryopoiesis and simultaneously inhibit tumor cell growth. Further interpretation of the mechanism of E’s cytotoxic effect on AML cells is now under investigation.
Supplementary Material
Acknowledgments
We thank Connie Erickson-Miller for helpful comments and Dr Susan Shetzline for critical reading of the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
CONFLICT OF INTEREST
This work was supported by the National Cancer Institute grant 1R21CA153018-01, and by support from GlaxoSmithKline.
References
- 1.Kalota A, Gewirtz AM. A prototype nonpeptidyl, hydrazone class, thrombopoietin receptor agonist, SB-559457, is toxic to primary human myeloid leukemia cells. Blood. 2010;115:89–93. doi: 10.1182/blood-2009-06-227751. [DOI] [PubMed] [Google Scholar]
- 2.Erickson-Miller CL, Delorme E, Tian SS, Hopson CB, Landis AJ, Valoret EI, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27:424–430. doi: 10.1634/stemcells.2008-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeshita A, Shinjo K, Izumi M, Ling P, Nakamura S, Naito K, et al. Quantitative expression of thrombopoietin receptor on leukaemia cells from patients with acute myeloid leukaemia and acute lymphoblastic leukaemia. Br J Haematol. 1998;100:283–290. doi: 10.1046/j.1365-2141.1998.00558.x. [DOI] [PubMed] [Google Scholar]
- 4.Corazza F, Hermans C, D’Hondt S, Ferster A, Kentos A, Benoit Y, et al. Circulating thrombopoietin as an in vivo growth factor for blast cells in acute myeloid leukemia. Blood. 2006;107:2525–2530. doi: 10.1182/blood-2005-06-2552. [DOI] [PubMed] [Google Scholar]
- 5.Gurney AL, Wong SC, Henzel WJ, de Sauvage FJ. Distinct regions of c-Mpl cytoplasmic domain are coupled to the JAK-STAT signal transduction pathway and Shc phosphorylation. Proc Natl Acad Sci USA. 1995;92:5292–5296. doi: 10.1073/pnas.92.12.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezumi Y, Takayama H, Thrombopoietin Okuma M. c-Mpl ligand, induces tyrosine phosphorylation of Tyk2, JAK2, and STAT3, and enhances agonists-induced aggregation in platelets in vitro. FEBS Lett. 1995;374:48–52. doi: 10.1016/0014-5793(95)01072-m. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Kirito K, Kashii Y, Uchida M, Watanabe T, Endo H, et al. Forkhead family transcription factor FKHRL1 is expressed in human megakaryocytes. Regulation of cell cycling as a downstream molecule of thrombopoietin signaling. J Biol Chem. 2001;276:15082–15089. doi: 10.1074/jbc.M007958200. [DOI] [PubMed] [Google Scholar]
- 8.Drachman JG, Griffin JD, Kaushansky K. The c-Mpl ligand (thrombopoietin) stimulates tyrosine phosphorylation of Jak2, Shc, and c-Mpl. J Biol Chem. 1995;270:4979–4982. doi: 10.1074/jbc.270.10.4979. [DOI] [PubMed] [Google Scholar]
- 9.Bacon CM, Tortolani PJ, Shimosaka A, Rees RC, Longo DL, O’Shea JJ. Thrombopoietin (TPO) induces tyrosine phosphorylation and activation of STAT5 and STAT3. FEBS Lett. 1995;370:63–68. doi: 10.1016/0014-5793(95)00796-c. [DOI] [PubMed] [Google Scholar]
- 10.Tortolani PJ, Johnston JA, Bacon CM, McVicar DW, Shimosaka A, Linnekin D, et al. Thrombopoietin induces tyrosine phosphorylation and activation of the Janus kinase, JAK2. Blood. 1995;85:3444–3451. [PubMed] [Google Scholar]
- 11.Sattler M, Durstin MA, Frank DA, Okuda K, Kaushansky K, Salgia R, et al. The thrombopoietin receptor c-MPL activates JAK2 and TYK2 tyrosine kinases. Exp Hematol. 1995;23:1040–1048. [PubMed] [Google Scholar]
- 12.Yamada M, Komatsu N, Okada K, Kato T, Miyazaki H, Miura Y. Thrombopoietin induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in a human thrombopoietin-dependent cell line. Biochem Biophys Res Commun. 1995;217:230–237. doi: 10.1006/bbrc.1995.2768. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins JM, Williams D, Deng Y, Uhl J, Kitchen V, Collins D, et al. Phase 1 clinical study of eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist. Blood. 2007;109:4739–4741. doi: 10.1182/blood-2006-11-057968. [DOI] [PubMed] [Google Scholar]
- 14.Gibiansky E, Zhang J, Williams D, Wang Z, Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol. 2011;51:842–856. doi: 10.1177/0091270010375427. [DOI] [PubMed] [Google Scholar]
- 15.Pang SF, Li XK, Zhang Q, Yang F, Xu P. Interference RNA (RNAi)-based silencing of endogenous thrombopoietin receptor (Mpl) in Dami cells resulted in decreased hNUDC-mediated megakaryocyte proliferation and differentiation. Exp Cell Res. 2009;315:3563–3573. doi: 10.1016/j.yexcr.2009.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.