Abstract
Background
While it is recognized that pulmonary hysteresis can influence the effects of positive end-expiratory pressure (PEEP), the extent to which expansion of previously opened (vs. newly opening) peripheral airspaces contribute to increased lung volume is not known.
Methods
Following a recruitment maneuver, rats were ventilated with constant tidal volumes and imaged during ascending and descending ramps of PEEP.
Results
We estimated peripheral airspace dimensions by measuring the apparent diffusion coefficient (ADC) of 3He in 10 rats. In a separate group (n = 5) undergoing a similar protocol, we used computerized tomography to quantify lung volume. Hysteresis was confirmed by larger end-inspiratory lung volume (mean ± SD; all PEEP levels included): 8.4 ± 2.8 versus 6.8 ± 2.0 mL (P < 0.001) and dynamic compliance: 0.52 ± 0.12 versus 0.42 ± 0.09 mL/cmH2O (P < 0.001) during descending versus ascending PEEP ramps. ADC increased with PEEP but it was smaller during the descending versus ascending ramps for corresponding levels of PEEP: 0.168 ± 0.019 versus 0.183 ± 0.019 cm2/s (P < 0.001). ADC was smaller in the posterior versus anterior lung regions, but the effect of PEEP and hysteresis on ADC was greater in the posterior regions.
Conclusions
Our results suggest that in healthy lungs, larger lung volumes due to hysteresis are associated with smaller individual airspaces. This may be explained by opening of previously non-aerated peripheral airspaces, rather than expansion of those already aerated. Setting PEEP on a descending ramp may minimize distension of individual airspaces.
INTRODUCTION
Mechanical ventilation causes lung injury associated with tissue stress and deformation but may be lessened by use of lower tidal volume (VT) and optimal positive end-expiratory pressure (PEEP).1 While the impact of VT is established,2 optimizing PEEP is less well understood.3 Hysteresis - an energy dissipating mechanism - is characterized by greater lung volume at a given distending pressure (e.g., PEEP) during deflation vs. during inflation, and may be apparent when the level of PEEP is increased or decreased.4 While hysteresis is considered in terms of overall elasticity, the relationship among altered PEEP, hysteresis and airway dimensions is not understood.5 Early morphometric studies in healthy lungs6, 7 indicated that hysteresis may result from volume-dependent alterations in surfactant8 or tissue viscoelasticity properties,9 but other studies reported that individual airspace dimensions were unchanged10 or decreased11 despite larger overall lung volume (i.e., alveolar recruitment12 — the sustained opening of previously collapsed or under-ventilated airspaces). Although recruitment characterizes the response to PEEP in lungs with atelectasis and lung injury,4 evidence suggests that it contributes to lung expansion also in healthy lungs.13
Hyperpolarized gas magnetic resonance imaging (HPMRI) provides both in vivo and non-invasive estimates of the dimensional properties of small airspaces,14 and is accomplished by measuring the apparent diffusion coefficient (ADC) of hyperpolarized 3He gas within the ventilated parenchyma.15 ADC estimates the dimensions of peripheral airspaces by measuring the space available for diffusion. Using this approach, we previously reported that in the injured16 and atelectatic lung,17 the mean dimensions of ventilated airspaces was decreased following recruitment. However, the relationships among altered PEEP, hysteresis and airspace dimensions are not known in normal lungs during anesthesia. We hypothesized that hysteresis is associated with smaller ADC despite larger lung volume during descending versus ascending PEEP, due to increased numbers of newly aerated airspaces rather than further expansion of already aerated units.
MATERIALS AND METHODS
Studies were performed on male Sprague-Dawley rats (n = 15; 400 ± 50 g), following approval by the local Institutional Animal Care and Use Committee (Philadelphia, Pennsylvania). The experimental protocol is described in full detail in Methods, Supplemental Digital Content 1. Briefly, general anesthesia and paralysis were induced and maintained with intraperitoneal pentobarbital and intravenous pancuronium bromide, the trachea intubated and airway pressure, heart rate, and peripheral oxygen saturation levels measured.16, 17 Normothermia was maintained.
Experimental Outline
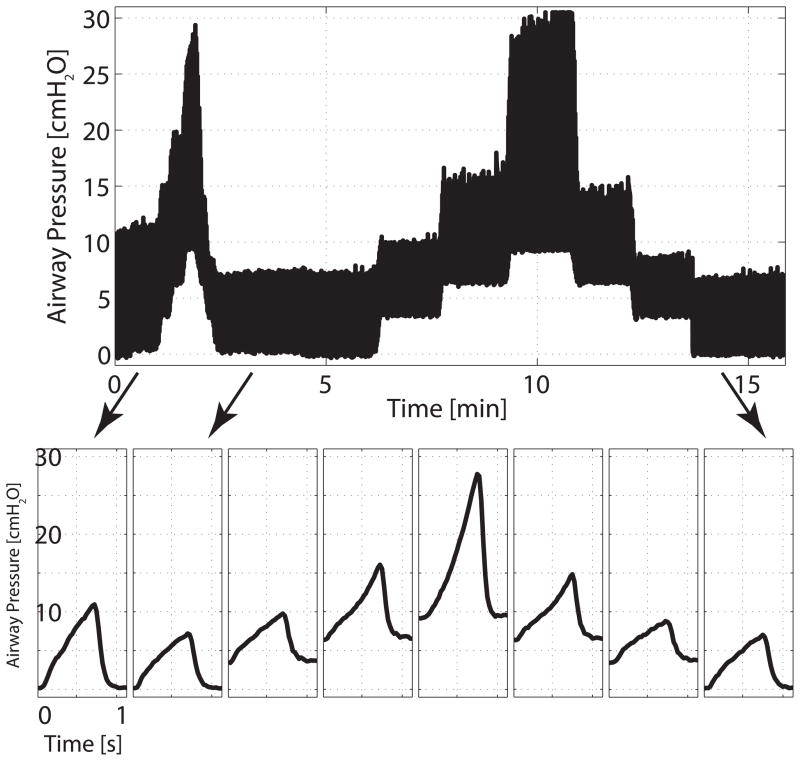
All rats were ventilated in the supine position with a magnetic resonance imaging (MRI)-compatible small animal ventilator,18 with the following initial ventilator settings (VT 10 mL/kg, PEEP 0 cmH2O, FiO2 0.21, rate 53 min−1), which were shown to guarantee normal gas exchange in pilot experiments. To standardize lung volume history, an alveolar recruitment maneuver was performed prior to the first image acquisition. Ten animals underwent HPMRI in order to measure the ADC of 3He during ascending and descending PEEP trials; this was designed to illustrate hysteresis in a lung inflation-deflation cycle. After baseline measurements, PEEP was increased from 0 to 9 cmH2O (in 3 cmH2O increments) and returned to baseline in the reverse process (fig. 1). To quantify lung volume hysteresis and recruitment, computerized tomography (CT) was performed in place of HPMRI on a separate group of five animals undergoing similar ventilator protocols. In HPMRI rats, each PEEP level was maintained for a 90-s period before ADC measurement (in order to minimize 3He gas depolarization). For the animals that received CT, each PEEP level was maintained for an additional 8 min to permit adequate image acquisition. Peak inspiratory pressure (PIP) and dynamic compliance [Cdyn = VT/(PIP − PEEP)] were measured at the end of each PEEP period in all animals. The ADC measurements obtained at PEEP zero in five animals of the HPMRI group have been used as ventilated, noninjured group in a previously published experiment.16 After the last set of measurements, animals were euthanized by lethal pentobarbital injection.
Fig. 1.
Representative trend of airway pressures during positive end-expiratory pressure ramps and imaging series. Positive end-expiratory pressure was increased and decreased between 0 and 9 cm H2O in 3 cm H2O steps. Each positive end-expiratory pressure level was maintained for 90 s before image acquisitions.
HPMRI
MRI was performed using a diffusion-weighted gradient echo pulse sequence, and the ADC acquisition is detailed in Supplemental Digital Content 1, as previously described.17 Briefly, animals were placed supine in the MRI scanner and in a radiofrequency coil that was tuned to the 3He resonance frequency. End-inspiratory images were obtained in a 20 mm thick subcardiac axial slice, following ventilation with a mixture of hyperpolarized 3He and oxygen.15,17. 3He gas had been previously hyperpolarized to approximately 30% over 14 h.19 Each ADC acquisition was obtained during a single breath hold and consisted of multiple diffusion-weighted images, each corresponding to a different diffusion-sensitizing gradient. Images were analyzed by fitting the time evolution of each pixel signal intensity to a standard equation, to yield maps of regional ADC values. Maps were subdivided in three horizontal bins of equal thickness in the upper to lower direction, to study the vertical distribution of ADC signal. Single-breath 3He spin density images were also acquired.
CT Imaging
High-resolution, end-inspiration CT scans were acquired using a micro-CT scanner, and were reconstructed to three-dimensional whole lung maps with 100 μm isotropic resolution. Quantitative analysis of CT density used established methods to quantify end-inspiratory lung gas volume (EILV),20 atelectasis, and recruitment21 at different levels of PEEP.
Statistical Analysis
Mean, standard deviation, and skewness of ADC were calculated for each imaged HPMRI slice as previously described.17 Group mean and standard deviation of all the computed quantities were calculated. Repeated measurements two-way ANOVA was conducted to examine the main effects of changes in PEEP (between 0 and 6 cmH2O) in ascending vs. descending ramps on tested variables. Post-hoc comparisons (paired-t tests with Bonferroni correction for multiple comparisons) were conducted to test differences between individual ascending or descending PEEP values. The area-under the curve method for serial measurement analysis22 was used to test (by paired t-test) for differences between ascending and descending ramps. P < 0.05 (for two-tailed hypothesis) was considered statistically significant. Statistical analysis was performed using “R” (R Foundation for Statistical Computing; Vienna Austria*) applications developed in the authors’ laboratory.
RESULTS
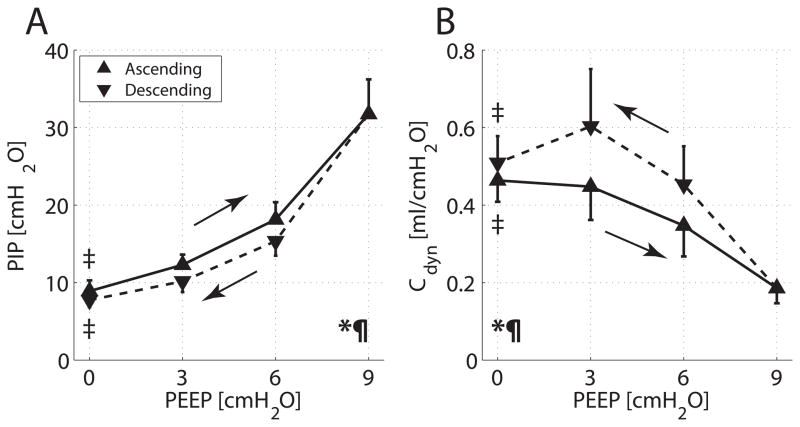
Ascending PEEP ramps were associated with increasing PIP (fig. 2A) and decreasing Cdyn (fig. 2B). PIP was significantly lower —and Cdyn higher— during descending vs. ascending PEEP (P < 0.001). PIP and Cdyn values in response to PEEP were comparable in the CT and HPMRI experiments.
Fig. 2.
Group averages and SD for (A) peak inspiratory pressure (PIP) versus positive end-expiratory pressure (PEEP) and (B) dynamic compliance (Cdyn) versus PEEP. Values obtained during ascending and descending PEEP ramps are displayed with solid and dashed lines, respectively. Two-way ANOVA for changing PEEP: *P < 0.001; for type of ramp (ascending vs. descending): P < 0.001. Paired t test for PEEP 0 versus 9 cm H2O: ‡P < 0.001. The area under the curve subtended by PIP was larger in the ascending than in the descending PEEP ramp (P < 0.001); the area under the curve subtended by Cdyn was larger with descending PEEP (P < 0.001).
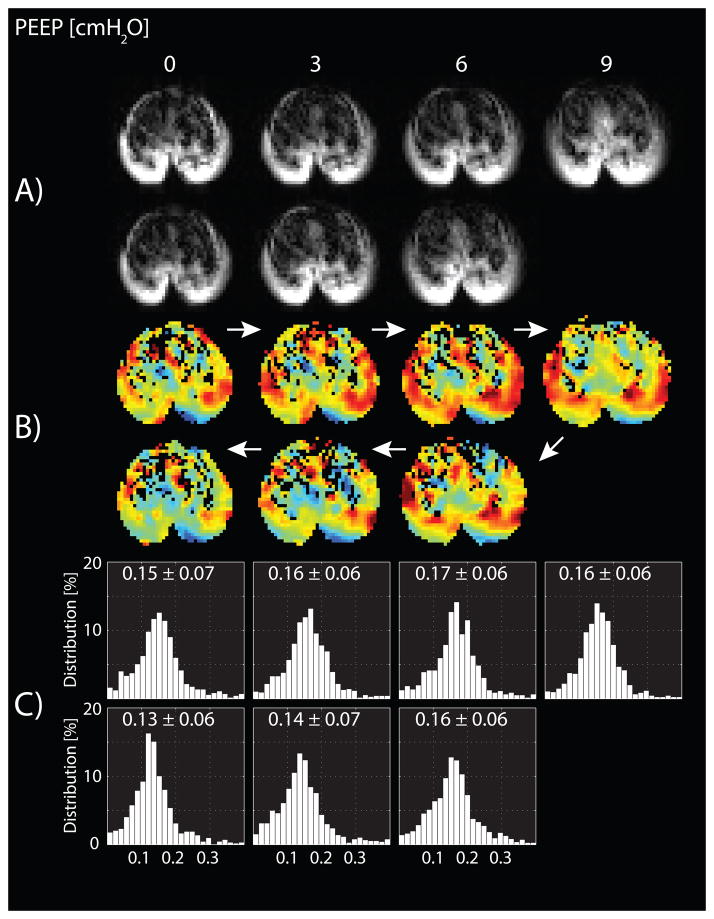
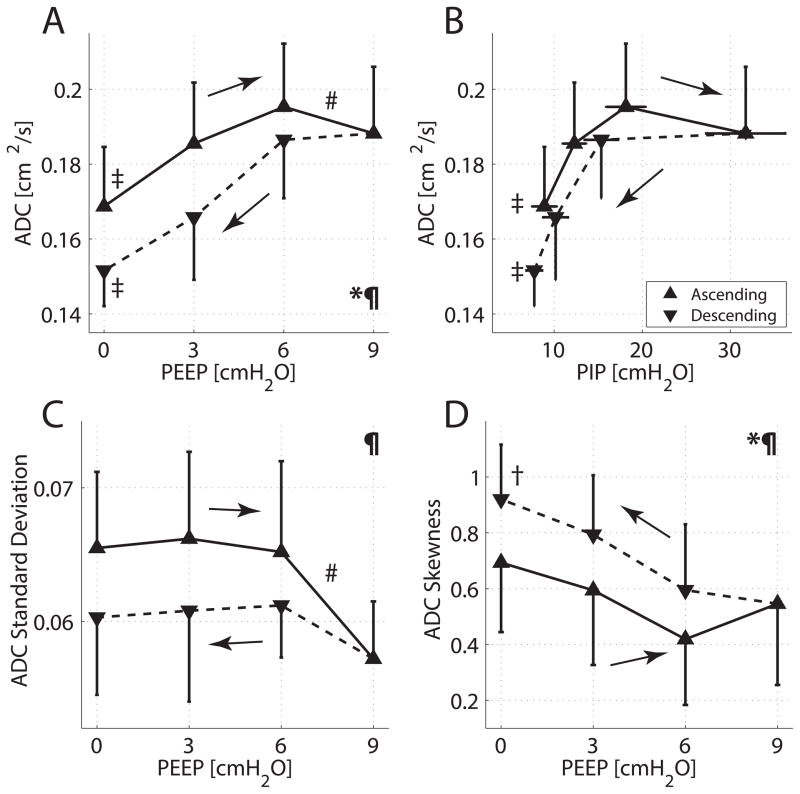
ADC maps and frequency distributions were obtained from a representative animal during PEEP trials (fig. 3). ADC increased with ascending PEEP and decreased with descending PEEP; however, the ADC values during descending PEEP were lower than on the ascending PEEP ramp. The variation of the mean ± SD for the ADC values is plotted (fig. 4A), as a function of both ascending and descending PEEP ramps (and in fig. 4B as a function of PIP). Repeated measures two-way ANOVA confirmed that the effects on ADC of the type of PEEP ramp (descending vs. ascending) and of changing PEEP level were both significant (P < 0.001). A PEEP increase from zero to 6 cmH2O resulted in an increase of 15.8% in mean ADC (fig. 4A, P < 0.001). However, further increase in PEEP from 6 to 9 cmH2O resulted in a small drop in mean ADC (3.6%; P < 0.01), as well as signal attenuation (fig. 3). ADC decreased monotonically as PEEP was lowered (fig. 4A).
Fig. 3.
Representative (A) axial magnetic resonance images, (B) axial apparent diffusion coefficient maps, and (C) apparent diffusion coefficient frequency distribution histograms obtained from a ventilated rat during ascending and descending positive end-expiratory pressure (PEEP) ramps between 0 and 9 cm H2O in 3 cm H2O steps.
Fig. 4.
Group averages and SD for: (A) 3He apparent diffusion coefficient (ADC) versus positive end-expiratory pressure (PEEP); (B) ADC versus peak inspiratory pressure (PIP); (C) ADC SD versus PEEP; and (D) skewness of ADC frequency distribution versus PEEP. Values obtained during both ascending and descending PEEP ramps are displayed with solid and dashed lines, respectively. Two-way ANOVA for changing PEEP: *P < 0.001; for type of ramp (ascending versus descending): P < 0.001. Paired t tests for PEEP 0 versus 9 cm H2O: ‡P < 0.001, †P < 0.01; PEEP 6 versus 9 cm H2O: #P < 0.01. The area under the curve subtended by ADC was larger in the ascending than in the descending PEEP ramps (P < 0.001). Area under the curve comparisons between ascending and descending PEEP ramps were significant also for ADC SD (P < 0.05) and for ADC skewness (P < 0.01).
The area under the ADC-PEEP curve had higher values during ascending vs. descending PEEP ramps (fig. 4A), as with the ADC-PIP relationship (fig. 4B), although the degree of hysteresis was smaller (i.e., lower PIP values) during decreasing PEEP (fig 2A). The group statistics for standard deviation (fig. 4C) and skewness (fig. 4D) of the ADC frequency distribution are shown for each HPMRI image. The lower standard deviation and increased skewness observed in the descending (vs. ascending) PEEP ramps indicated a more homogeneous distribution of ADC (i.e., a larger contribution from smaller airspaces), in the descending PEEP ramp.
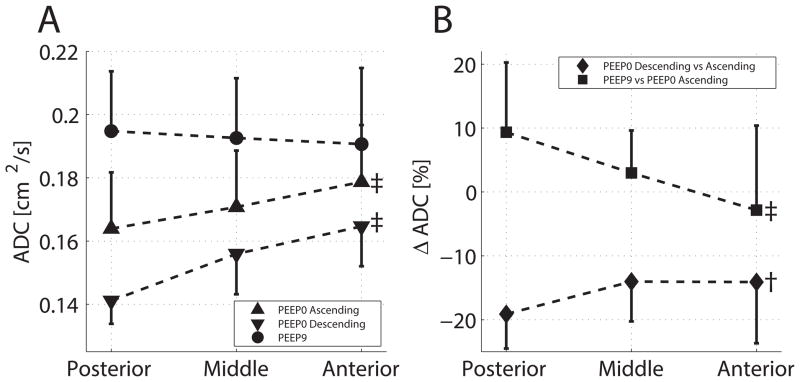
We detected vertical changes in ADC (fig. 5): the mean ADC increased from the lower to the upper bin levels at PEEP 0 cmH2O (but not at PEEP 9 cmH2O; fig. 5A). The effects of PEEP were more in the lower bin (i.e., larger difference of ADC at PEEP 9 vs. 0 cmH2O; fig. 5B), as was the case with hysteresis (i.e., lower ADC in descending vs. ascending ramp at PEEP 0 cmH2O).
Fig. 5.
(A) Vertical distribution of average values and SD of apparent diffusion coefficient (ADC) in three horizontal bins of equal size at positive end-expiratory pressure (PEEP) 0 cm H2O (ascending and descending ramp) and PEEP 9 cm H2O. (B) Percent change of ADC going from PEEP 0 cm H2O to PEEP 9 cm H2O (ascending ramp) and between ascending and descending ramps at PEEP 0 cm H2O. Effect of bin level (ANOVA): ‡P < 0.01 and †P < 0.05.
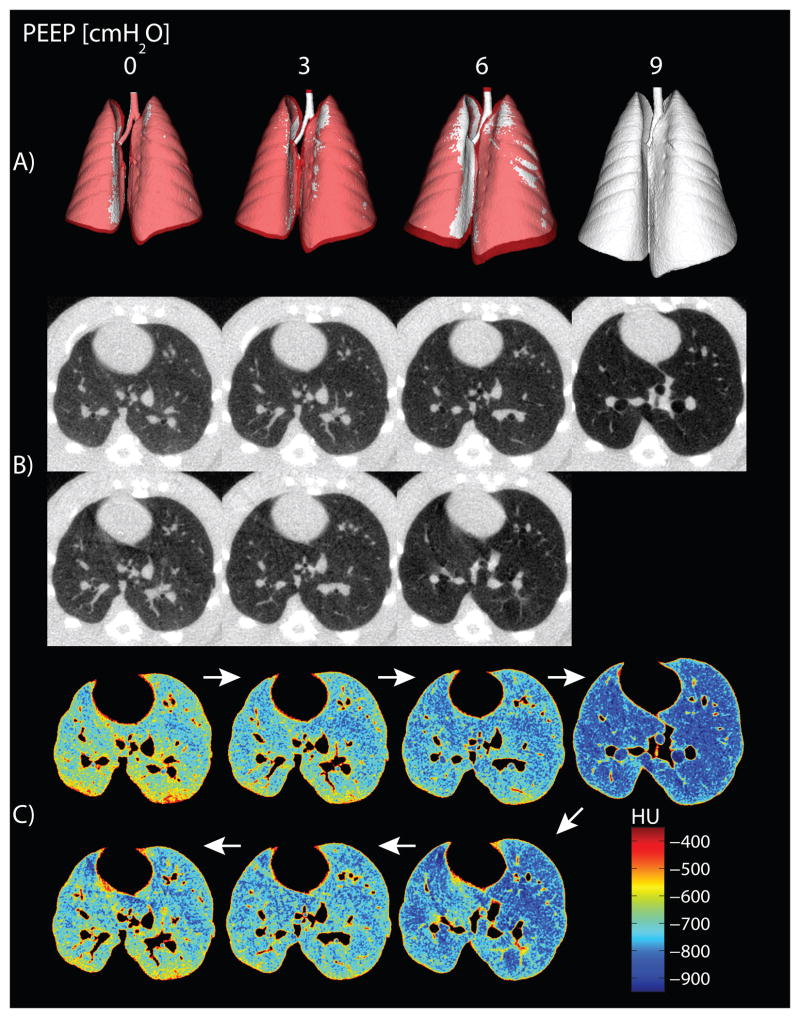
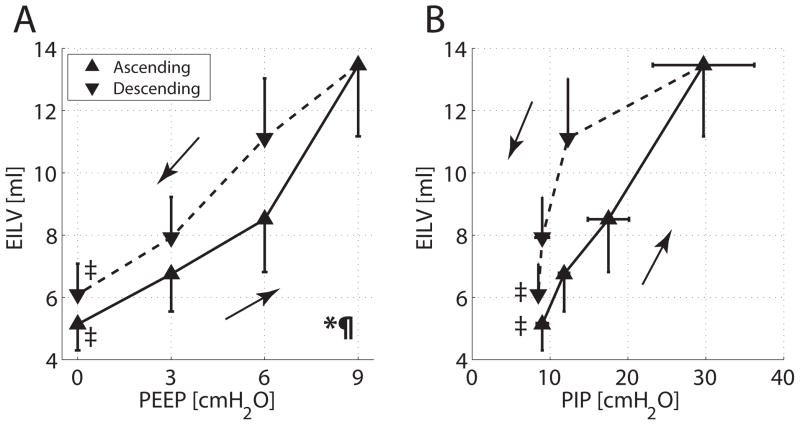
Whole lung CT image reconstruction indicated a smaller total lung volume in ascending versus descending PEEP (fig. 6A). The unprocessed axial CT images are shown in figure 6B, (color post-thresholding maps of the same slices are in fig. 6C). Hysteresis was evident by greater EILV (fig. 7A) and lower PIP levels (fig. 7B) during descending versus ascending PEEP ramps.
Fig. 6.
(A) Tridimensional reconstructions of the whole lung, obtained by automatic segmentation and thresholding of computerized tomography images (excluding Hounsfield units [HU] greater than −200), are shown for all levels of positive end-expiratory pressure (PEEP) in a representative rat. Images obtained during ascending and descending PEEP are superimposed on each other and color-coded: white for increasing PEEP and red for decreasing PEEP. Lung dimensions during descending PEEP were larger than those measured in the ascending ramp, except for the areas colored in white, where they overlapped. (B) Axial computerized tomography slices obtained in the same animal at all PEEP levels are shown. Images were obtained shortly after a recruitment maneuver, and no significant atelectasis is visible. (C) Postthresholding maps of the same computerized tomography slices show exclusion of major blood vessels and nonpulmonary tissue only.
Fig. 7.
Group averages and SD for (A) end-inspiratory lung volume (EILV) versus positive end-expiratory pressure (PEEP); (B) EILV versus peak inspiratory pressure (PIP). Values obtained during both ascending and descending PEEP ramps are displayed with solid and dashed lines, respectively. Two-way ANOVA for changing PEEP: *P < 0.001; for type of ramp (ascending vs. descending): P < 0.001. Paired t test for PEEP 0 versus 9 cm H2O: ‡P < 0.001. The area under the curve subtended by EILV was larger in the descending than in the ascending PEEP ramp (P < 0.01).
Quantitative analysis of CT density (see table 1, Supplemental Digital Content 2,) demonstrated a small amount (<0.5% of total lung weight) of nonaerated lung tissue, suggesting that recruitment before imaging minimized atelectasis. However, a quota of poorly aerated tissue was observed at PEEP 0 cmH2O (less at PEEP 9 cmH2O) and is consistent with recruitment of poorly aerated airspaces. Hyperinflated tissue was quantitatively small and detected only at PEEP 9 cmH2O. CT also demonstrated a vertical distribution of densities (increasing upper to lower), with a larger response to PEEP in the lower regions (see fig. 1, Supplemental Digital Content 3).
DISCUSSION
In this study of diffusion HPMRI in healthy anesthetized animals, larger lung volumes corresponding to pulmonary hysteresis were associated with smaller aerated airspaces, especially in dependent lung. We16, 17 and others23 previously observed decreased airspace dimensions after recruitment in atelectatic17 and injured16, 23 lungs. The current data advance previous findings and suggest that hysteresis in the healthy lung reflects opening of previously nonaerated airspaces rather than further expansion of those already aerated. We utilized ADC because it permits noninvasive, serial quantification of airspace geometry in vivo, has advantages over standard morphometric approaches (i.e., tissue handling, restricted visualization),24, 25 and is in good agreement with established morphometric measures.15, 26
The current study protocol resulted in hysteresis by the sequential increase and decrease of airway pressure (fig. 1); under these conditions hysteresis appeared to be associated with de novo aeration rather than further expansion of already aerated airspaces. In fact, the lower ADC during descending PEEP ramps indicates smaller airspaces in the descending vs. the ascending ramp (fig. 4A, B).
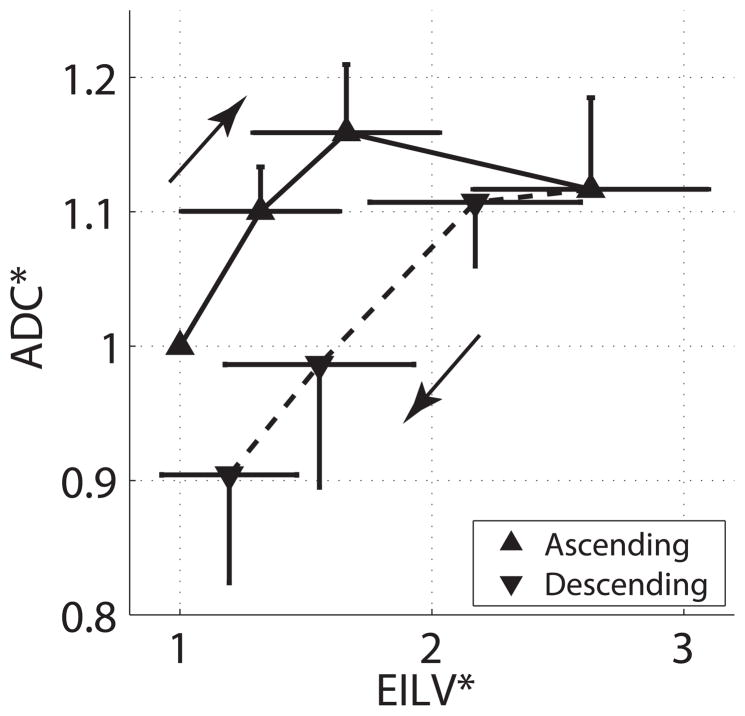
The study puts local airspace volume (from ADC) and overall lung volume (EILV, from CT scan) into perspective: the airspace hysteresis is in the opposite direction to that of the EILV (fig. 7A, B). Indeed, plot of the fractional changes from baseline of ADC versus EILV (fig. 8), demonstrates smaller individual airspaces and larger global lung volumes, in the descending vs. ascending PEEP ramp. Although these data contrast with morphometric studies reporting alveolar dilatation associated with lung inflation and hysteresis,6, 7 they are supported by aerosol deposition studies indicating greater numbers of smaller alveoli in descending versus ascending limbs of an inflation-deflation cycle.11 In addition, the current data indicate decreased dispersion and increased skewness (i.e., favoring smaller values) of the frequency distribution of ADC (fig. 4C, D); such data are more consistent with opening of smaller (not further expansion of already ventilated) airspaces.17, 27
Fig. 8.
Relative values of apparent diffusion coefficient (ADC*) and end-inspiratory lung volume (EILV*) expressed as a fraction of their baseline values at positive end-expiratory pressure (PEEP) 0 cm H2O (ascending ramp) and plotted versus each other at each level of ascending and descending PEEP. EILV and ADC were obtained in different groups of animals at matching experimental conditions. Error bars indicate SD for ADC (vertical bars) and for EILV (horizontal bars).
We observed a vertical distribution of ADC (fig. 5A), which likely reflects the known vertical gradients of lung aeration associated with gravity and compressive factors.28 Thus, airspace expansion by PEEP (fig. 5B) was more evident in the lower regions. Poor dependent aeration may also explain why the effect of hysteresis on ADC and airspace reopening was more prominent in lower lung.
The current study differs from our previous study of ADC16, 17 in that the lungs were uninjured, recruitment maneuvers were performed and supplemental oxygen was avoided. While frank atelectasis was present in previous studies,16, 17 the degree of atelectasis in the current study was minimal based on qualitative (fig. 6B) and quantitative CT (table 1, Supplemental Digital Content 2). Thus the precise nature of the subsequently recruited airspaces is unclear, but the data suggest hypoventilated units and microatelectasis.29 While alveolar dimensions in healthy rats range between 40 and 100 μm7, deaerated alveoli occupy considerable less space30 and, when sparsely distributed, they are below the 100 μm resolution of our CT (see also fig. 1, Supplemental Digital Content 4). Furthermore, recruitment may be integral to normal inflation, as suggested by 3He lung morphometry (discriminates alveolar vs. alveolar duct dimensions at varying lung volume)31 and intravital microscopy13.
We observed a biphasic response of ADC to increasing PEEP (fig. 4A). These findings are similar to those reported using ex vivo laser scanning confocal microscopy during inflation of healthy mouse lungs,32 where mean alveolar size increased up to a threshold airway pressure (25 cmH2O) and thereafter decreased. While this could represent redistribution of gas from previously inflated to newly open units,11, 23, 27 it could be due to opening of smaller alveoli.32 Recruitment is consistent with reduced compliance associated with increasing PEEP (fig. 2B), which may reflect regional lung tissue compression33 or progressive tissue stiffening due to increased airway pressure (demonstrated by MRI34). Tissue stretch may not be countered by an increased number of open airspaces, and thereby result in lower overall lung compliance.
The current data may have implications for development of ventilator-associated lung injury, which is a major concern in patients with injured lungs2 and is a potential risk in ventilated surgical patients without overtly injured lungs.35 While multiple studies indicate that PEEP can attenuate experimental lung injury,36, 37 the clinical impact has been far less;3 thus further insights into potential mechanisms of protection by PEEP are key. The current findings that recruitment and PEEP decrease airspace distension, coupled with recent indications that lung recruitment in anesthetized patients may improve postoperative lung function,38 may together suggest that techniques enabling titration of recruitment (or PEEP) against airspace micromechanics may ultimately help to translate the experimental benefits of PEEP into clinical practice. In the interim, setting PEEP on a descending ramp after recruitment —shown to optimize gas exchange and lung aeration in healthy lungs39— may be a reasonable choice for lung protection during surgical anesthesia.
There are limitations to extrapolation of the current work. First, there are important limitations to extrapolation in terms of lung size and thoracic compliance. Mechanical ventilation, particularly at zero PEEP, does not correspond to the state of inflation of healthy, spontaneously breathing subjects. However, the experimental setup was comparable to those in similar experimental models that have provided important insights,36, 37, 40, 41 and such ventilator settings are required to ensure adequate gas exchange. Although not recommended, survey data indicate that comparable ventilator settings (i.e., high VT, zero PEEP) continue to be used in anesthetized patients.42 Second, the relationship among ADC, hysteresis and altered PEEP may be altered by the onset of more significant atelectasis, in case of prolonged monotonous ventilation with low PEEP;40 however, atelectasis would likely augment ADC hysteresis - the use of a recruitment maneuver aimed to mitigate any such augmentation of ADC.17 Furthermore, the findings reflect similar investigations in injured and atelectatic lungs,16,17 as well as alternative models assessed by traditional morphometry.10, 43
Technical issues are important in this work. For example, ADC cannot distinguish between alveoli and alveolar ducts.31 The different image acquisition times may have affected the comparison between ADC versus EILV, as PEEP was maintained for shorter interval during HPMRI than during CT —necessary to limit signal loss from 3He repolarization. Nonetheless, any impact of these different time-frames would likely be minimal in the current model because of the absence of injury and stable airspaces, as well as previous experience in healthy rats17 indicating stability of ADC during such a timescale. In addition, the similar values of PIP and Cdyn in the two groups further confirm comparable conditions.
Finally, the anesthesia employed was intraperitoneal pentobarbital, and it is not known whether a different anesthetic (i.e., inhaled) would have different effects. We believe that this is unlikely as there are minimal effects of anesthetic type on intrinsic lung mechanics (except, perhaps in bronchospasm), whereas propensity to progressive atelectasis seems to be more determined by the impact on respiratory muscle tone activity than by choice of anesthetic.44
Our results suggest that in healthy lungs, larger lung volumes due to hysteresis are associated with smaller individual airspace dimensions. This may be explained by opening of previously nonaerated peripheral airspaces, rather than expansion of those already aerated. Setting PEEP on a descending ramp minimizes distension of individual airspaces and may potentially attenuate propensity to lung injury.
Supplementary Material
Final Box Summary.
What we already know about this topic
Hysteresis of the lung occurs because of the increased energy required for opening the lung during inspiration.
What this article tells us that is new
In healthy rats, larger lung volumes due to hysteresis were associated with smaller individual airspace dimensions, suggesting opening of previously nonaerated peripheral airspaces, rather than expansion of already opened airspaces.
Acknowledgments
Funding: this work was supported by National Institutes of Health (Bethesda, Maryland) grants R01-HL064741 and R01-HL077241. Dr Cereda is supported by a grant (MRTG-BS-2/15/2013-Cereda) from the Foundation for Anesthesia Education and Research (Rochester, Minnesota) and the Society of Critical Care Anesthesiologists (Park Ridge, Illinois).
The authors would like to acknowledge the support of Small Animal Imaging Facility at the Department of Radiology, University of Pennsylvania (Philadelphia, Pennsylvania).
Footnotes
http://www.R-project.org; last accessed July 31, 2013.
Conflicts of interest: The authors declare no competing interests
References
- 1.Dreyfuss D, Saumon G. Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 2.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA. 2010;303:865–73. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 4.Hickling KG. Best compliance during a decremental, but not incremental, positive end-expiratory pressure trial is related to open-lung positive end-expiratory pressure: A mathematical model of acute respiratory distress syndrome lungs. Am J Respir Crit Care Med. 2001;163:69–78. doi: 10.1164/ajrccm.163.1.9905084. [DOI] [PubMed] [Google Scholar]
- 5.Escolar JD, Escolar A. Lung hysteresis: A morphological view. Histol Histopathol. 2004;19:159–66. doi: 10.14670/HH-19.159. [DOI] [PubMed] [Google Scholar]
- 6.Storey WF, Staub NC. Ventilation of terminal air units. J Appl Physiol. 1962;17:391–7. doi: 10.1152/jappl.1962.17.3.391. [DOI] [PubMed] [Google Scholar]
- 7.Mercer RR, Laco JM, Crapo JD. Three-dimensional reconstruction of alveoli in the rat lung for pressure-volume relationships. J Appl Physiol. 1987;62:1480–7. doi: 10.1152/jappl.1987.62.4.1480. [DOI] [PubMed] [Google Scholar]
- 8.Bachofen H, Hildebrandt J, Bachofen M. Pressure-volume curves of air- and liquid-filled excised lungs-surface tension in situ. J Appl Physiol. 1970;29:422–31. doi: 10.1152/jappl.1970.29.4.422. [DOI] [PubMed] [Google Scholar]
- 9.Brewer KK, Sakai H, Alencar AM, Majumdar A, Arold SP, Lutchen KR, Ingenito EP, Suki B. Lung and alveolar wall elastic and hysteretic behavior in rats: Effects of in vivo elastase treatment. J Appl Physiol. 2003;95:1926–36. doi: 10.1152/japplphysiol.00102.2003. [DOI] [PubMed] [Google Scholar]
- 10.Escolar JD, Escolar MA, Guzman J, Roques M. Pressure volume curve and alveolar recruitment/de-recruitment: A morphometric model of the respiratory cycle. Histol Histopathol. 2002;17:383–92. doi: 10.14670/HH-17.383. [DOI] [PubMed] [Google Scholar]
- 11.Smaldone GC, Mitzner W, Itoh H. Role of alveolar recruitment in lung inflation: Influence on pressure-volume hysteresis. J Appl Physiol. 1983;55:1321–32. doi: 10.1152/jappl.1983.55.4.1321. [DOI] [PubMed] [Google Scholar]
- 12.Cheng W, DeLong DS, Franz GN, Petsonk EL, Frazer DG. Contribution of opening and closing of lung units to lung hysteresis. Respir Physiol. 1995;102:205–15. doi: 10.1016/0034-5687(95)00055-0. [DOI] [PubMed] [Google Scholar]
- 13.Carney DE, Bredenberg CE, Schiller HJ, Picone AL, McCann UG, Gatto LA, Bailey G, Fillinger M, Nieman GF. The mechanism of lung volume change during mechanical ventilation. Am J Respir Crit Care Med. 1999;160:1697–702. [PubMed] [Google Scholar]
- 14.Tanoli TS, Woods JC, Conradi MS, Bae KT, Gierada DS, Hogg JC, Cooper JD, Yablonskiy DA. In vivo lung morphometry with hyperpolarized 3He diffusion MRI in canines with induced emphysema: Disease progression and comparison with computed tomography. J Appl Physiol. 2007;102:477–84. doi: 10.1152/japplphysiol.00397.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii M, Emami K, Xin Y, Barulic A, Kotzer CJ, Logan GA, Chia E, Macduffie-Woodburn JP, Zhu J, Pickup S, Kuzma N, Kadlecek S, Podolin PL, Rizi RR. Regional functional-structure relationships in lungs of an elastase murine model of emphysema. J Appl Physiol. 2011;112:135–48. doi: 10.1152/japplphysiol.01181.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cereda M, Emami K, Xin Y, Kadlecek S, Kuzma NN, Mongkolwisetwara P, Profka H, Pickup S, Ishii M, Kavanagh BP, Deutschman CS, Rizi RR. Imaging the interaction of atelectasis and overdistension in surfactant-depleted lungs. Crit Care Med. 2013;41:527–35. doi: 10.1097/CCM.0b013e31826ab1f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cereda M, Emami K, Kadlecek S, Xin Y, Mongkolwisetwara P, Profka H, Barulic A, Pickup S, Mansson S, Wollmer P, Ishii M, Deutschman CS, Rizi RR. Quantitative imaging of alveolar recruitment with hyperpolarized gas MRI during mechanical ventilation. J Appl Physiol. 2011;110:499–511. doi: 10.1152/japplphysiol.00841.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spector ZZ, Emami K, Fischer MC, Zhu J, Ishii M, Yu J, Kadlecek S, Driehuys B, Panettieri RA, Lipson DA, Gefter W, Shrager J, Rizi RR. A small animal model of regional alveolar ventilation using HP 3He MRI1. Acad Radiol. 2004;11:1171–9. doi: 10.1016/j.acra.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Walker TG, Happer W. Spin-exchange optical pumping of noble-gas nuclei. Rev Mod Phys. 1997;69:629–42. [Google Scholar]
- 20.Denison DM, Morgan MD, Millar AB. Estimation of regional gas and tissue volumes of the lung in supine man using computed tomography. Thorax. 1986;41:620–8. doi: 10.1136/thx.41.8.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164:1701–11. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- 22.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–5. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachofen H, Gehr P, Weibel ER. Alterations of mechanical properties and morphology in excised rabbit lungs rinsed with a detergent. J Appl Physiol. 1979;47:1002–10. doi: 10.1152/jappl.1979.47.5.1002. [DOI] [PubMed] [Google Scholar]
- 24.Hsia CC, Hyde DM, Ochs M, Weibel ER ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure: An official research policy statement of the American Thoracic Society/European Respiratory Society. Standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smaldone GC, Mitzner W. Unresolved mysteries. J Appl Physiol. 2012;113:1945–7. doi: 10.1152/japplphysiol.00545.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods JC, Choong CK, Yablonskiy DA, Bentley J, Wong J, Pierce JA, Cooper JD, Macklem PT, Conradi MS, Hogg JC. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med. 2006;56:1293–300. doi: 10.1002/mrm.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lum H, Huang I, Mitzner W. Morphological evidence for alveolar recruitment during inflation at high transpulmonary pressure. J Appl Physiol. 1990;68:2280–6. doi: 10.1152/jappl.1990.68.6.2280. [DOI] [PubMed] [Google Scholar]
- 28.Hubmayr RD, Walters BJ, Chevalier PA, Rodarte JR, Olson LE. Topographical distribution of regional lung volume in anesthetized dogs. J Appl Physiol. 1983;54:1048–56. doi: 10.1152/jappl.1983.54.4.1048. [DOI] [PubMed] [Google Scholar]
- 29.Reber A, Engberg G, Sporre B, Kviele L, Rothen HU, Wegenius G, Nylund U, Hedenstierna G. Volumetric analysis of aeration in the lungs during general anaesthesia. Br J Anaesth. 1996;76:760–6. doi: 10.1093/bja/76.6.760. [DOI] [PubMed] [Google Scholar]
- 30.Gil J, Weibel ER. Morphological study of pressure-volume hysteresis in rat lungs fixed by vascular perfusion. Respir Physiol. 1972;15:190–213. doi: 10.1016/0034-5687(72)90098-9. [DOI] [PubMed] [Google Scholar]
- 31.Hajari AJ, Yablonskiy DA, Sukstanskii AL, Quirk JD, Conradi MS, Woods JC. Morphometric changes in the human pulmonary acinus during inflation. J Appl Physiol. 2012;112:937–43. doi: 10.1152/japplphysiol.00768.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Namati E, Thiesse J, de Ryk J, McLennan G. Alveolar dynamics during respiration: Are the pores of Kohn a pathway to recruitment? Am J Respir Cell Mol Biol. 2008;38:572–8. doi: 10.1165/rcmb.2007-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaczka DW, Cao K, Christensen GE, Bates JH, Simon BA. Analysis of regional mechanics in canine lung injury using forced oscillations and 3D image registration. Ann Biomed Eng. 2011;39:1112–24. doi: 10.1007/s10439-010-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGee KP, Mariappan YK, Hubmayr RD, Carter RE, Bao Z, Levin DL, Manduca A, Ehman RL. Magnetic resonance assessment of parenchymal elasticity in normal and edematous, ventilator injured lung. J Appl Physiol. 2012;113:666–76. doi: 10.1152/japplphysiol.01628.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, Rana R, St Sauver JL, Lymp JF, Afessa B, Hubmayr RD. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–24. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 36.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149:1327–34. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 37.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–52. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Severgnini P, Selmo G, Lanza C, Chiesa A, Frigerio A, Bacuzzi A, Dionigi G, Novario R, Gregoretti C, de Abreu MG, Schultz MJ, Jaber S, Futier E, Chiaranda M, Pelosi P. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118:1307–21. doi: 10.1097/ALN.0b013e31829102de. [DOI] [PubMed] [Google Scholar]
- 39.Carvalho AR, Jandre FC, Pino AV, Bozza FA, Salluh JI, Rodrigues R, Soares JH, Giannella-Neto A. Effects of descending positive end-expiratory pressure on lung mechanics and aeration in healthy anaesthetized piglets. Crit Care. 2006;10:R122. doi: 10.1186/cc5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duggan M, McCaul CL, McNamara PJ, Engelberts D, Ackerley C, Kavanagh BP. Atelectasis causes vascular leak and lethal right ventricular failure in uninjured rat lungs. Am J Respir Crit Care Med. 2003;167:1633–40. doi: 10.1164/rccm.200210-1215OC. [DOI] [PubMed] [Google Scholar]
- 41.Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, McKerlie C, Post M, McLoughlin P, Kavanagh BP. Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med. 2006;174:279–89. doi: 10.1164/rccm.200506-1006OC. [DOI] [PubMed] [Google Scholar]
- 42.Jaber S, Coisel Y, Chanques G, Futier E, Constantin JM, Michelet P, Beaussier M, Lefrant JY, Allaouchiche B, Capdevila X, Marret E. A multicentre observational study of intra-operative ventilatory management during general anaesthesia: Tidal volumes and relation to body weight. Anaesthesia. 2012;67:999–1008. doi: 10.1111/j.1365-2044.2012.07218.x. [DOI] [PubMed] [Google Scholar]
- 43.Silva MF, Zin WA, Saldiva PH. Airspace configuration at different transpulmonary pressures in normal and paraquat-induced lung injury in rats. Am J Respir Crit Care Med. 1998;158:1230–4. doi: 10.1164/ajrccm.158.4.9710067. [DOI] [PubMed] [Google Scholar]
- 44.Hedenstierna G, Tokics L, Lundquist H, Andersson T, Strandberg A, Brismar B. Phrenic nerve stimulation during halothane anesthesia. Effects of atelectasis. Anesthesiology. 1994;80:751–60. doi: 10.1097/00000542-199404000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.