Abstract
Converging evidence from clinical, preclinical, neuroimaging, and genetic research implicates dopamine neurotransmission in the pathophysiology of attention deficit hyperactivity disorder (ADHD). The in vivo neuroreceptor imaging evidence also suggests alterations in the dopamine system in ADHD; however, the nature and behavioral significance of those have not yet been established. Here, we investigated striatal dopaminergic function in ADHD using [11C]raclopride PET with a d-amphetamine challenge. We also examined the relationship of striatal dopamine responses to ADHD symptoms and neurocognitive function. A total of 15 treatment-free, noncomorbid adult males with ADHD (age: 29.87±8.65) and 18 healthy male controls (age: 25.44±6.77) underwent two PET scans: one following a lactose placebo and the other following d-amphetamine (0.3 mg/kg, p.o.), administered double blind and in random order counterbalanced across groups. In a separate session without a drug, participants performed a battery of neurocognitive tests. Relative to the healthy controls, the ADHD patients, as a group, showed greater d-amphetamine-induced decreases in striatal [11C]raclopride binding and performed more poorly on measures of response inhibition. Across groups, a greater magnitude of d-amphetamine-induced change in [11C]raclopride binding potential was associated with poorer performance on measures of response inhibition and ADHD symptoms. Our findings suggest an augmented striatal dopaminergic response in treatment-naive ADHD. Though in contrast to results of a previous study, this finding appears consistent with a model proposing exaggerated phasic dopamine release in ADHD. A susceptibility to increased phasic dopamine responsivity may contribute to such characteristics of ADHD as poor inhibition and impulsivity.
Keywords: ADHD, dopamine, PET, [11C]raclopride, d-amphetamine, executive functions
INTRODUCTION
Converging indirect evidence from clinical, preclinical, neuroimaging, and genetic research implicates catecholamine, particularly dopamine (DA), neurotransmission in the pathophysiology of attention deficit hyperactivity disorder (ADHD) (Faraone and Mick, 2010; Sontag et al, 2010; Spencer et al, 1996). The nature of this putative DA dysfunction remains to be elucidated. It could involve one or more presynaptic (eg, synthesis, release, reuptake) or postsynaptic (eg, receptor density, receptor affinity, metabolism) system alterations. Based on the clinical efficacy of stimulants that augment extracellular catecholamine levels, some have hypothesized that ADHD symptoms result from insufficient extracellular DA levels, that is, a hypoactive DA system (Volkow et al, 2005). Another model proposes that ADHD symptoms reflect low tonic striatal DA activity coupled with elevated stimulus-evoked phasic DA release (Grace, 2001). According to this model, stimulants confer their therapeutic effects by elevating DA tone that helps attenuate phasic DA release.
More direct measures of DA transmission in ADHD using molecular neuroimaging have produced inconsistent findings. For example, both increases and decreases have been reported for D2/D3 receptor availability and for dopamine transporter (DAT) binding in ADHD patients vs controls (Lou et al, 2004; Spencer et al, 2005; Spencer et al, 2013; Volkow et al, 2007a; Volkow et al, 2009). Only one study has examined DA release in untreated ADHD (Volkow et al, 2007b). Using a methylphenidate challenge, this study found lower DA release in the caudate of adult patients than controls. Together, the findings suggest widespread DA system dysfunctions in ADHD, but the direction of these effects remains unclear.
Neurocognitive concomitants of putative striatal DA alterations in ADHD would presumably include executive dysfunction—a key aspect of the cognitive and behavioral profile of ADHD. Executive functions rely on frontal–striatal circuitry (Alexander et al, 1990), and functional imaging studies have linked reduced activation in frontal–striatal circuitry in ADHD to performance deficits on executive function tasks (Dickstein et al, 2006). However, few studies to date have linked molecular imaging measures of striatal DA function to neurocognitive performance in ADHD (Lou et al, 2004; Rosa-Neto et al, 2005).
In this study, we measured striatal DA responses in treatment-naive men with ADHD and healthy controls to a challenge dose of d-amphetamine (d-amph) using PET with [11C]raclopride. This well-established technique yields an index of tracer binding potential (BPND) that is proportional to the number of available D2/D3 receptors (Laruelle, 2000). The signal is attenuated by drug-induced increases in extracellular DA concentrations, providing an index of DA release. We predicted that d-amph-evoked DA release and baseline receptor availability in the striatum would differ significantly between ADHD subjects and healthy controls. As molecular imaging findings in ADHD have been inconsistent, and preclinical models of hyperactivity, impulsivity, and poorly regulated executive function have been linked to both increases and decreases in DA (Sontag et al, 2010), group differences and behavior associations in either direction were considered of interest.
PATIENTS AND METHODS
Participants
A total of 15 adult men with ADHD (5 combined subtype; 10 predominantly inattentive subtype) and 18 adult male healthy controls completed the study and were included in the analyses. Four participants completed the study but could not be included: three for poor PET data quality (two ADHD), and one (a control) because of a structural anomaly in striatum on MR. Two additional participants began but did not complete the study because of equipment failure.
The diagnosis of ADHD (DSM-IV-TR) required the presence of at least 6/9 inattention symptoms (with or without 6/9 hyperactivity/impulsivity symptoms) since childhood; diagnosis was ascertained by one of the research psychiatrists (CB, LH, and RJ). Symptoms of ADHD (Table 1) were measured as continuous variables in both groups using the Conners' Adult ADHD Rating Scale (CAARS) (Conners et al, 1999).
Table 1. Sample Characteristics.
| Controls (n=18) | ADHD (n=15) | Test statistic (d.f.) | p | |
|---|---|---|---|---|
| Age (SD) | 25.44 (6.77) | 29.87 (8.65) | U(33)=99.50 | 0.20 |
| Estimated full scale IQ (SD) | 116.83 (16.07) | 107.13 (12.78) | U(27)=51.00 | 0.06 |
| Abbreviated WAIS-IIIa | 124.25 (14.70) | 109.44 (15.16) | U(17)=17.00 | 0.07 |
| Abbreviated WAIS-Rb | 102.00 (1.63) | 103.67 (8.12) | U(10)=11.50 | 0.91 |
| Years of education (SD) | 17.11 (3.32) | 16.20 (3.63) | t(31)=0.75 | 0.46 |
| CAARS t-scores (SD) | ||||
| Inattention/memory problems | 43.77 (7.41) | 74.00 (10.49) | t(26)=8.67 | <0.0005* |
| Hyperactivity/restlessness | 43.76 (6.08) | 62.27 (12.93) | t(21.79)=4.82 | <0.0005* |
| Impulsivity/emotional lability | 42.92 (9.42) | 58.53 (11.28) | t(26)=3.94 | 0.001* |
| Problems with self-concept | 43.08 (5.89) | 63.07 (7.64) | t(26)=7.66 | <0.0005* |
| DSM-IV inattention | 48.73 (12.49) | 84.4 (8.73) | t(26)=8.85 | <0.0005* |
| DSM-IV hyperactivity | 44.69 (8.54) | 68.13 (14.48) | t(26)=5.11 | <0.0005* |
| DSM-IV total | 46.69 (11.64) | 81.47 (10.30) | t(26)=8.39 | <0.0005* |
| ADHD index | 42.00 (8.45) | 66.86 (8.74) | t(26)=7.63 | <0.0005* |
| BDI at intake (SD) | 1.53 (2.00) | 6.04 (3.86) | U(31)=23.50 | <0.0005* |
| Recreational drug use history | ||||
| Stimulants: no. of lifetime uses (SD) | 0.06 (0.24) | 0.36 (0.74) | U(32)=105.00 | 0.17 |
| Marijuana: no. of lifetime uses (SD) | 18.00 (33.08) | 49.27 (90.07) | U(32)=119.50 | 0.22 |
| Nicotine: no. of lifetime uses (SD) | 1359.06 (3677.02) | 1614.13 (5171.99) | U(33)=128.00 | 0.81 |
| No. of smokers | 1 | 1 | ||
Group differences: *p⩽0.05.
Wechsler Adult Intelligence Scale–Revised (WAIS-R) (n=9) (Reynolds et al, 1983).
Wechsler Adult Intelligence Scale–III (WAIS-III) (n=9) (Pilgrim et al, 1999).
Participants underwent a Structured Clinical Interview for DSM-IV Axis I disorders (SCID I (First et al, 1996)) and were excluded for any current or past history of Axis I disorder, other than ADHD. However, 2/15 ADHD participants reported a single mild depressive episode occurring for ⩾2 years in the past. Other exclusion criteria were: a first-degree relative with a history of substance dependence; current use of psychotropic medications; a Beck Depression Inventory (BDI) (Beck and Steer, 1987) score >13, an estimated IQ <80; a neurological history; a reported history of a head injury with loss of consciousness for >5 min; a history of any physical disorder (eg, cardiovascular) contradictory to participation as per comprehensive physical exam; and a positive toxicology screen (cocaine, opiates, phencyclidine, barbiturates, benzodiazepines, Δ9-tetrahydrocannabinol, amphetamines) as per the Triage Drugs of Abuse urine test (Biosite, San Diego, CA). Controls were excluded for a reported ADHD diagnosis in a first-degree relative.
All ADHD participants were stimulant treatment naive except one who, 2 years before his participation, underwent a 6-month methylphenidate trial. Excluding his data did not change the results. Lifetime stimulant exposure did not exceed two uses for any other participants.
The study was carried out in accordance with the Declaration of Helsinki and was approved by the Research Ethics Board of the Montreal Neurological Institute. All participants gave written informed consent.
Procedure
Participants underwent two [11C]raclopride PET scans, one following a lactose placebo and the other following 0.3 mg/kg p.o. of d-amph; capsule administration occurred 60 min before tracer injection and was double blind, randomized, and counterbalanced. PET scans occurred at least 3 days apart. Before each scan, participants were asked to abstain from food, caffeine, and smoking for 4 h and from alcohol for 24 h. A structural magnetic resonance image (MRI) was obtained on a separate day. Participants completed the neurocognitive battery a minimum of 24 h from the time of either PET scan to avoid any drug carryover effects. Toxicology screening occurred on the initial screening interview and before both PET scans and neurocognitive testing.
Neuroimaging
Participants were scanned on a Siemens ECAT HR+ PET scanner (CTI/Siemens, Knoxville, TN) with lead septa removed (63-slice coverage), with a maximum resolution 4.2-mm, full width at half maximum (FWHM) in the center of the field of view. Attenuation correction was performed using a 12-min 68Ga transmission scan immediately before tracer injection. The emission scan started simultaneously with the injection of [11C]raclopride, as an i.v. bolus, and data were acquired for 60 min in 26 time frames of progressively longer duration. Tracer doses did not differ significantly between scans for either group (controls: placebo=7.25±1.74, d-amph=7.41±1.55, p=0.46; ADHD: placebo=6.44±0.74, d-amph=6.59±.29, p=0.37), but were marginally higher across scans for controls (controls=7.33±1.63; ADHD=6.51±0.56, p=0.06). The [11C]raclopride-specific activity, sampled for 10/66 scans, ranged from 335 to 925 Ci/mmol, with the injected dose ranging from 2.34 to 6.46 μg (3.77±1.55). Vital signs were monitored and blood samples for plasma amphetamine collected just before capsule administration, at the time of tracer administration, mid-scan, and at the end of scan. Ratings of subjective drug (or placebo) effects were also obtained (Supplementary Table S4).
High-resolution (1 mm) T1-weighted MRIs were obtained on a 1.5-Tesla Siemens scanner, using gradient echo pulse sequence (TR=22 ms, TE=9.2 ms, flip angle=30°, FOV=256 mm, and matrix 256 × 256) for co-registration to the PET images.
Neurocognitive Battery
A subset of participants (14 ADHD and 12 controls) completed a battery of neurocognitive tests, tapping functions associated with medium to large effect sizes for ADHD in meta-analyses (Nigg, 2005), and suggested to be in part mediated by catecholamine systems (Allman et al, 2010; Aron et al, 2003; Leyton et al, 2007). The battery included two response inhibition tasks: the Stop Signal Paradigm (Logan et al, 1984) and the antisaccade task (Hallett, 1978). The Stop Signal Paradigm assesses inhibition by measuring the time required to stop a planned response, that is, stop signal reaction time (SSRT). An auditory stop signal instructs participants to withhold responses on 25% of trials in a choice reaction time task. SSRT measures how early the stop signal must occur relative to a participant's mean response time in order for responses to be successfully withheld.
The antisaccade task measures inhibitory oculomotor control (Hallett, 1978). It requires withholding a reflexive saccade to a suddenly appearing peripheral target and, instead, generating a saccade to its mirror location (ie, an antisaccade). Two measures of inhibitory function were derived from this task: % reflexive saccades toward the target (error rate) and % anticipatory saccades (latency ⩽80 ms) that reflect impulsive responding when waiting for the peripheral target to appear.
The battery also included tasks of working memory, planning, motor speed and cognitive flexibility, and responsivity to reward and punishment. We will subsequently focus only on those tasks that revealed significant differences between the groups; a detailed description of the battery and the results appears in Supplementary Materials and Methods and Supplementary Table S1.
Analyses
MR volumes were corrected for image intensity nonuniformity (Sled et al, 1998) and transformed into the Montreal Neurological Institute (MNI) space using automated feature matching to the MNI305 template (Collins et al, 1994).
The PET images were reconstructed using a 6-mm FWHM Hanning filter and corrected for motion (Costes et al, 2009). Parametric images were generated by calculating [11C]raclopride binding potential values (BPND) (Innis et al, 2007) at each voxel using a simplified reference tissue compartmental model (SRTM) with cerebellum as the reference tissue with a very low density of D2/D3 receptors (Gunn et al, 1997; Lammertsma and Hume, 1996). BPND is a function of the estimated concentration of available D2/D3 receptors (BAvail), the dissociation constant of the radiotracer from D2/D3 receptors (KD), and the free fraction of the nonspecifically bound tracer in the brain (FND): BPND=FND × (BAvail/KD).
As previously described (Boileau et al, 2006), mean BPND values from each individual parametric image were extracted from regions of interest (ROIs) delineated on each individual MRI. The ROIs were based on the functional organization of the striatum (Martinez et al, 2003): limbic (LST, includes ventral striatum), associative (AST, includes precommissural dorsal caudate, precommissural dorsal putamen, postcommissural caudate), and sensorimotor (SMST, includes postcommissural putamen). Mean BPND values were corrected for partial volume effects (Aston et al, 2002). ΔBPND values were calculated as (BPND placebo−BPND d-amph)/BPND placebo × 100 and used for intergroup statistical analysis in SPSS 17.
To examine specific loci of d-amph-induced change in BP in each group, a voxel-wise statistical mapping method was utilized to determine the t-statistic associated with the change in BPND between the d-amph and the placebo conditions (Aston et al, 2000). Voxels of statistically significant change had t-values of ⩾3.8 that corresponded to p<0.05 based on the random field theory, considering the search volume of the striatum, the reconstructed image resolution of 8 mm at FWHM, and correction for multiple comparisons (Aston et al, 2000; Worsley et al, 1996).
Plasma amphetamine concentrations were analyzed using combined gas chromatography–mass spectrometry (GC–MS) (Asghar et al, 2002). Area under the curve (AUC) was calculated for each participant to reflect plasma amphetamine concentrations over all measurement points.
Behavioral data were analyzed statistically using SPSS 17. Extreme values outside 3 SDs of the mean for a given variable were Winsorized (replaced by the value of their nearest neighbor) (Dixon and Yuen, 1974). Appropriate transformations were used to normalize the distribution for some measures, and analyses were carried out on the transformed data.
RESULTS
Participants
ADHD participants did not differ significantly from controls on demographic variables. Estimated IQ was marginally higher in controls (p=0.06; Table 1). Although no participant was clinically depressed, the mean BDI score at intake was significantly higher in the ADHD group than controls (p<0.0005).
d-Amph-Induced Change in D2/D3 Binding
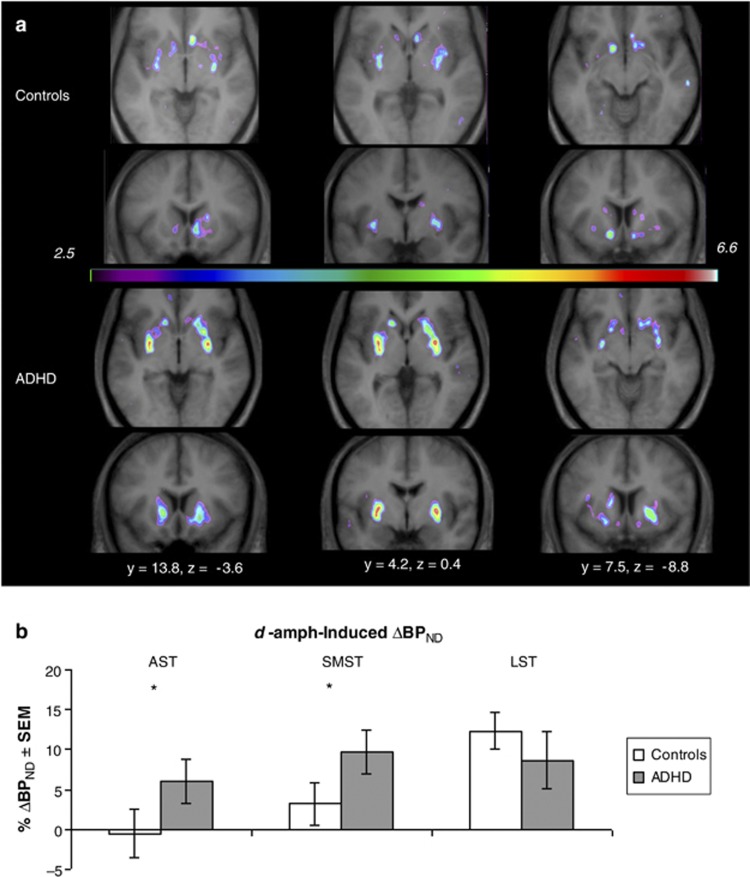
In the ROI analysis, ΔBPND values across three ROIs were significantly correlated with intake BDI scores in the ADHD group (rs=−0.68; p=0.007), but not in controls (rs=0.23; p=0.37). Intake BDI was therefore entered as a covariate in the two-way group × ROI mixed ANCOVA on the ΔBPNDs. One ADHD and one control participant were missing intake BDI scores; their missing values were replaced by the mean of their respective groups. Excluding those participants produced the same results. The ANCOVA revealed a significant group × ROI interaction (F(1.32, 39.70)=4.07; p=0.04). Bonferroni adjusted pairwise comparisons showed greater magnitude ΔBPND in the ADHD group than controls in AST (controls: −0.57% ADHD: 6.08% F(1, 30)=4.24, p=0.05) and SMST (controls: 3.25% ADHD: 9.68% F(1, 30)=4.73, p=0.04), but not in LST (controls: 12.34% ADHD: 8.62% F(1, 30)=0.02, p=0.82) (Figure 1b). There were no other main effects or interactions.
Figure 1.
The d-amph-induced change in [11C]raclopride binding. (a) Parametric t-maps showing d-amph-induced decreases in [11C]raclopride BPND for the control (top) and ADHD (bottom) groups. t>3.8 equivalent to p⩽0.05 based on random field theory. (b) Mean d-amph-induced decreases in [11C]raclopride BPND extracted from three predetermined ROIs: associative (AST), sensorimotor (SMST), and limbic (LST). The p-values indicate the significance of the between-group differences in % change in BPND in each region: *p⩽0.05. More positive values indicate larger d-amph-induced BPND decreases (greater DA release).
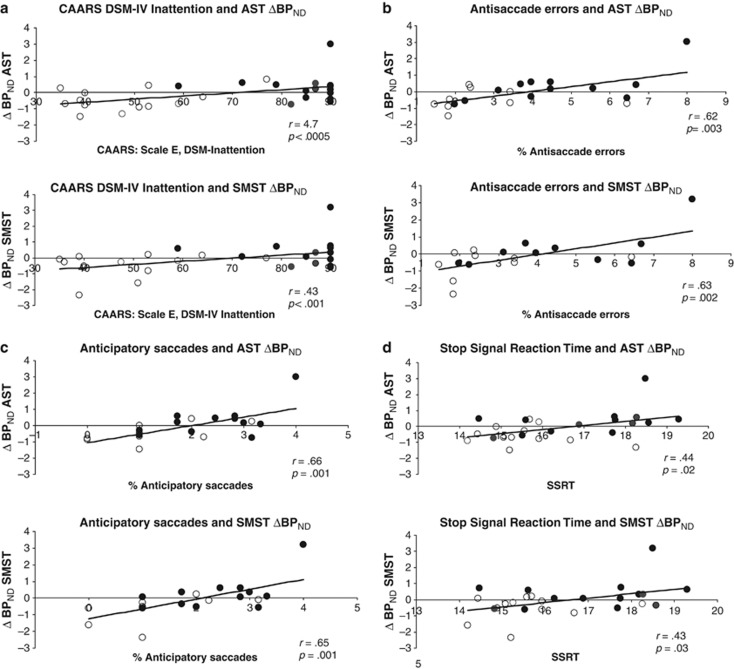
To explore the relationship between ΔBPND and ADHD symptoms, we performed semipartial correlations between ΔBPNDs and scores on two subscales of the CAARS, the DSM-IV Symptoms of Inattention and the DSM-IV Symptoms of Hyperactivity–Impulsivity subscales. Analyses were conducted across groups and co-varied intake BDI scores. Significance threshold was 0.008 (Bonferroni-corrected to keep ‘family-wise' error rate at 0.05). More pronounced ΔBPND decreases in AST and SMST were associated with higher inattention scores (AST: r(33)=0.47, p<0.0005; SMST: r(33)=0.43; p=0.001) (Figure 2a). Although regression diagnostics suggested a possible ‘lever' (leverage values: ⩾0.47; standardized DF βs: ⩾0.40), nonparametric correlations between inattention scores and residualized ΔBPNDs with intake BDI ‘removed' yielded the same results (AST: Rho=0.43, p=0.02; SMST: Rho=0.40, p=0.03). ΔBPNDs were not related to hyperactivity scores (all rs(33)<0.27; ps>0.17). LST ΔBPNDs were not significantly correlated with ADHD symptoms (rs(33)<0.16; ps>0.29).
Figure 2.
Associations of ΔBPND with ADHD symptoms on the CAARS and response inhibition. Associations between ΔBPND and (a) CAARS DSM-IV Inattention symptoms; (b) % antisaccade errors; (c) % anticipatory saccades; and (d) stop signal reaction times. Open symbols represent controls and filled symbols represent ADHD participants. CAARS DSM-IV Inattention t-scores and square root transformed values for neurocognitive performance appear on the x axis; residualized ΔBPND values, removing the effect of intake BDI, appear on the y axis. More positive values represent greater d-amph-induced BPND decreases (greater DA release). Note that the correlations are not driven by the ADHD participant in the upper right of the graphs and remain significant with nonparametric analyses.
We also examined BPND on the placebo day. A group × ROI mixed ANOVA revealed a significant group × ROI interaction (F(1.70, 52.80)=4.67, p=0.02), indicating a different pattern of D2/D3 binding across ROIs as a function of group (Table 2).
Table 2. D2/D3 Binding Potential (BPND) in Striatal ROIs.
|
Controls |
ADHD |
|||||
|---|---|---|---|---|---|---|
| Placebo | d-Amph | Placebo vs d-amph | Placebo | d-Amph | Placebo vs d-amph | |
| AST (SD) | 3.00 (0.41) | 3.00 (0.49) | t(17)=−0.04; p=0.97 | 3.25 (0.35) | 3.04 (0.43) | t(14)=2.15; p<0.05 |
| SMST (SD) | 3.53 (0.47) | 3.41 (0.54) | t(17)=1.37; p=0.19 | 3.76 (0.49) | 3.37 (0.48) | t(14)=3.24; p<0.006 |
| LST (SD) | 3.26 (0.39) | 2.86 (0.47) | t(17)=5.32; p<0.0005 | 3.16 (0.53) | 2.85 (0.43) | t(14)=2.36; p<0.03 |
| AST vs LSTa | t(17)=3.52; p=0.003 | t(17)=1.34; p=0.12 | t(14)=0.81; p=0.43 | t(14)=2.31; p=0.04 | ||
| SMST vs LSTa | t(17)=2.86; p=0.01 | t(17)=5.87; p<0.005 | t(14)=5.21; p=0.005 | t(14)=5.16; p<0.005 | ||
| AST vs SMST | t(17)=9.11; p<0.0005 | t(17)=6.14; p<0.005 | t(14)=5.95; p<0.0005 | t(14)=4.87; p<0.005 | ||
Abbreviations: AST, associative ROI; LST, limbic ROI; SMST, sensorimotor ROI.
BPND values for control and ADHD groups on placebo and d-amph. Regional pattern of D2/D3 binding on placebo differed as a function of Group, as described by statistics provided in the last three rows.
The group × ROI interaction was driven by group differences in binding discrepancies between AST vs LST (F(1, 31)=7.20; p=0.01) and between SMST vs LST (F(1, 31)=4.86; p=0.04); pairwise comparisons among regions are Bonferroni corrected. Regional BPND on d-amph did not differ significantly as a function of group (ps>0.45).
The loci of significant DA release for each group are presented using voxel-wise t-maps in Figure 1 and Supplementary Table S2.
Neurocognitive Performance
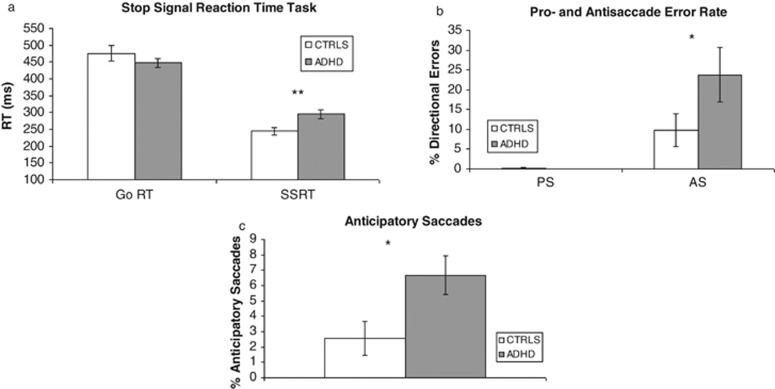
In the Stop Signal Paradigm, a two-way mixed ANOVA revealed a significant group × reaction time (RT) type (Go RT vs Stop Signal RT) interaction (F(1, 24)=5.83, p=0.02), reflecting longer SSRTs in the ADHD participants than controls (t(24)=3.0; p=0.008), but no group difference on Go RTs (Figure 3).
Figure 3.
Neurocognitive performance. (a) Stop Signal Paradigm performance. Go RT, Go Reaction Time; SSRT, Stop Signal Reaction Time. (b) Antisaccade and prosaccade error rate. PS, prosaccade; AS, antisaccade. (c) Anticipatory saccades across the pro- and antisaccade blocks. *p<0.05; **p<0.01.
Independent sample t-tests revealed that relative to controls, ADHD participants made significantly more antisaccade (AS) errors (t(19)=2.36; p=0.03). ADHD participants also made significantly more anticipatory responses during the fixation period on both prosaccade (the control condition, where the participant follows the target) and antisaccade trial blocks (t(19)=2.67; p=0.02) (Figure 3). For saccade latency, a two-way group × saccade (antisaccade vs prosaccade) ANOVA revealed a trend for a group × saccade interaction (F(1, 17)=4.34; p=0.053), indicating a larger difference between prosaccade and antisaccade latencies in ADHD participants (70 ms) than controls (48 ms).
d-Amph-Induced ΔBPND and Response Inhibition
We evaluated associations between ΔBPND and response inhibition measures that distinguished between the groups using semipartial correlations that controlled for the effect of intake BDI on ΔBPND. Significance threshold was set at 0.0055 to keep family-wise error rate at 0.05 (3 regions, 3 inhibition measures). Larger ΔBPNDs in AST and SMST were associated with higher proportions of antisaccade errors (AST: r(18)=0.62; p=0.003; SMST: r(18)=0.63; p=0.002) and anticipatory saccades (AST: r(18)=0.66; p(18)=0.001; SMST: r(18)=0.65; p=0.001). Correlations for ΔBPND in LST were at trend level (AS errors: r(18)=0.44; p=0.05; anticipatory saccades: r(18)=0.39; p=0.08). Longer SSRTs showed trend-level associations with larger ΔBPNDs in AST and SMST (AST: r(23)=0.44; p=0.02; SMST: r(23)=0.43; p=0.03) (Figure 2b). Because regression diagnostics suggested a possible ‘lever' (leverage values: ⩾0.47; Standardized DF βs: ⩾0.36), confirmatory nonparametric correlations were conducted using residualized ΔBPND values with intake BDI ‘removed'. Significant associations remained between both antisaccade variables and ΔBPND in AST (AS errors: Rho(19)=0.60; p=0.004; anticipatory saccades: Rho(19)=0.58; p=0.005) and SMST (AS errors: Rho(19)=0.59; p=0.005; anticipatory saccades: Rho(19)=0.58; p=0.006). The trend associations between SSRT and ΔBPND in these regions also persisted (AST(24): Rho=−0.45; p=0.02; SMST: Rho(24)=0.39; p=0.05). For LST, the trends were not significant with nonparametric analyses (AS errors: Rho(19)=0.32; p=0.16; anticipatory saccades: Rho(19)=0.31; p=0.18).
Plasma Amphetamine
The peak plasma concentrations following d-amph administration were 14.66±12.35 ng/ml for controls and 15.16±13.72 ng/ml for ADHD participants. AUC values did not differ between groups (ADHD: 27.50±30.4; controls: 23.02±25.30) (t(27)=0.44, p=0.66) or correlate with ΔBPND in any ROI (ps>0.36). No amphetamine was detected in plasma on the placebo day or before drug administration on the d-amph day.
DISCUSSION
This study provides evidence of an augmented DA response to an amphetamine challenge in the associative (controls: −0.57% ADHD: 6.08%) and sensorimotor (controls: 3.25% ADHD: 9.68%) striatal regions in treatment-naive adults with ADHD. Across groups, more pronounced d-amph-induced DA responses in these regions were associated with higher self-reported levels of inattention and poorer performance on tests of response inhibition. The two groups did not differ on plasma d-amph levels or baseline DA receptor availability.
Although the physiological relevance of an increased d-amph-induced ΔBPND in ADHD participants is uncertain, it could signal an augmented phasic DA release. In nonanesthetized, nonrestrained, behaving animals, phasic DA release contributes most to the overall increases in extracellular DA in response to amphetamine (Daberkow et al, 2013). Our findings would then appear to be in line with one model of DA function in ADHD that postulates augmented phasic DA release ensuing from abnormally low striatal DA tone (Grace, 2001). Exocytotic DA release is not the sole mechanism contributing to ΔBPND; DA efflux through reverse transport might also affect BPND. To the extent that it does, augmented ΔBPND in the ADHD group could signal altered DAT function, higher DAT density, higher number of DA neurons, or greater presynaptic DA stores. The associations between the magnitude of the DA response and levels of inattention across groups suggest that levels of synaptic DA may modulate attentional function in healthy and ADHD individuals. Notably, the augmented ΔBPND in ADHD participants is unlikely to have resulted from stimulant sensitization (Boileau et al, 2006): the ADHD participants were stimulant treatment naive (except one subject) and the majority had no prior stimulant exposure.
We observed significant differences in DA response between ADHD participants and controls in associative and sensorimotor ROIs but not in limbic striatum. These ROIs are based on the topography of functionally distinct cortico–striatal–thalamo–cortical circuits in non-human primates (Haber, 2010). According to a recent delineation of striatal subregions based on corticostriatal connectivity in humans (Tziortzi et al, 2013), our peak voxels of DA release in the AST map onto loci connected with prefrontal executive cortical areas; peak voxels in SMST map onto loci connected with executive and premoter regions. Thus, it is plausible that the group differences in DA release in these striatal regions are linked with executive and motor control, but not with motivation and reward.
Across both groups, greater d-amph-induced DA release in associative and sensorimotor ROIs was associated with poorer performance on tasks of response inhibition; the ADHD group had impaired performance of these tasks. This finding is consistent with previous research in animals and humans that has implicated striatal DA neurotransmission in response inhibition and impulsivity (Buckholtz et al, 2010; Dalley et al, 2007; Rosa-Neto et al, 2005). Some of these findings have suggested that overstimulation of striatal DA receptors by DA is associated with disinhibition: trait impulsivity has been positively associated with amph-induced striatal DA release in healthy controls (Buckholtz et al, 2010); in adolescents with ADHD, poorer performance off drug on a behavioral measure of impulsivity and inattention has been associated with greater methylphenidate-induced DA responses (Rosa-Neto et al, 2005). The association of an amplified DA release to response inhibition could be mediated by activation of striatal D1 receptors in the direct pathway of the basal ganglia circuits that could decrease discharge of the globus pallidus internal and substantia nigra pars reticulata, in turn disinhibiting thalamic nuclei and the superior colliculus (Alexander et al, 1990; Hikosaka et al, 2000), thus causing weaker inhibition of skeletomotor and oculomotor circuits. Alternatively, the association could be mediated via striatal D2 receptors in the indirect pathway, as D2 stimulation is believed to prevent inhibitory learning (Dagher, 2012).
Our results are at variance with one previous report of a blunted methylphenidate-induced DA response and lower placebo BPND levels in the caudate of adults with ADHD relative to healthy controls (Volkow et al, 2007b). Although placebo BPND values in the two studies were very similar for controls, they were ≈11% lower for the ADHD participants in the study of Volkow et al (2007b) than here. Differences in methodology could have contributed to the discrepancy in findings. All of our participants were male; the previous study included females with a trend for a higher proportion of females in the ADHD than control group. Sex differences in striatal DA receptor binding, DA release, and ADHD-relevant personality traits have been reported (Munro et al, 2006; Pohjalainen et al, 1998; Riccardi et al, 2006). Another possible difference is previous substance use. Although the study of Volkow et al (2007b) excluded individuals who met criteria for substance abuse or dependence, the paper did not report on the amount of subclinical recreational substance use. Even ‘casual' drug use is associated with a blunted DA response to amph (Casey et al, 2013); group differences on this variable could affect ΔBPND. Finally, probes for the dopamine system differed between studies: methylphenidate and d-amph augment extracellular DA levels through different mechanisms, and the route of administration (oral vs intravenous) and the dose also differed.
Interpretation of the present data should be considered in light of the following. (1) We interpret the association between disinhibition and striatal ΔBPND as reflecting a meaningful relationship of stable traits. However, these variables were estimated at different times and only once in each participant. Nonetheless, high test–retest reliability of our inhibition measures (Ettinger et al, 2003; Logan et al, 1984) as well as of ΔBPND to d-amph (Kegeles et al, 1999) supports the interpretation that dopaminergic dysfunction is related to response inhibition deficits in ADHD. (2) Our sample size had power to detect only associations of a medium to large effect size, given the Bonferroni correction for multiple comparisons. Larger studies may find associations of ΔBPND with other aspects of neurocognition that were not significant here. In addition, there is some inherent instability in correlations found in smaller samples. (3) The magnitude of d-amph-induced decreases in [11C]raclopride binding in the control group is at the lower end of the range of ≈10–20% observed in previous studies using a similar dose of oral d-amph. However, the difference between groups in ΔBPND that we observed here cannot be attributed to group differences in dose or to a lack of responsiveness to the drug on the part of the controls. Plasma amphetamine levels were nearly identical between the groups, and the subjective and cardiovascular responses of controls were significant and similar in magnitude to those observed in ADHD participants (Supplementary Tables S3 and S4). (4) Because the sample here was exclusively male, findings cannot be generalized to females.
In conclusion, this study provides evidence of an augmented striatal DA response to an amphetamine challenge in treatment-naive men with ADHD. Whether an enhanced striatal DA responsivity underlies response inhibition deficits and impulsivity in ADHD remains a matter of conjecture, but the indirect evidence presented here supports this possibility.
FUNDING AND DISCLOSURE
RJ sits on the advisory boards and speakers' bureaus of Pfizer Canada and Janssen Ortho Canada; he has received grant funding from them and from AstraZeneca. He has received honoraria from Janssen Ortho Canada for CME presentations and royalties for Henry Stewart talks. LH has received research support, served on advisory boards, and has been a speaker for Eli Lilly, Glaxo Smith/Kline, Ortho Janssen, Purdue, and Shire; GBB has received grant funding from Pfizer and SinoVeda Canada. The other authors declare no conflict of interest.
Acknowledgments
We thank P Lajeix for ascertaining diagnosis on an ADHD subject; Y Goto for critical discussion and reading of the findings; A Perna for contribution to participant evaluation; K Auclair and F Durand for nursing support; R Fukasawa, G Sauchuck, and S Mattei for technical assistance at the PET unit; D Jolly and M Kovacevic for preparation of radiotracers; G Rauw for technical assistance in plasma amphetamine determinations; and K Larcher, SM Cox, and K Welfeld for assistance in PET image analysis. This study was funded by Canadian Institutes of Health Research grant number 77728 to CB and ML.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
These data were presented in part at the 47th and 49th (2010) Annual Meetings of the American College of Neuropsychopharmacology at Scottsdale AZ (December 2008) and Miami FL (December 2010), respectively.
Supplementary Material
References
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, ‘prefrontal' and ‘limbic' functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Allman A-A, Benkelfat C, Durand F, Sibon I, Dagher A, Leyton M, et al. Effect of d-amphetamine on inhibition and motor planning as a function of baseline performance. Psychopharmacology. 2010;211:423–433. doi: 10.1007/s00213-010-1912-x. [DOI] [PubMed] [Google Scholar]
- Aron AR, Dowson JH, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2003;54:1465–1468. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- Asghar SJ, Baker GB, Rauw GA, Silverstone PH. A rapid method of determining amphetamine in plasma samples using pentafluorobenzenesulfonyl chloride and electron-capture gas chromatography. J Pharmacol Toxicol Methods. 2002;46:111–115. doi: 10.1016/s1056-8719(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Aston J, Gunn R, Worsley K, Ma Y, Evans AC, Dagher A. A statistical method for the analysis of positron emission tomopgraphy neuroreceptor ligand data. Neuroimage. 2000;12:245–256. doi: 10.1006/nimg.2000.0620. [DOI] [PubMed] [Google Scholar]
- Aston JA, Cunningham VJ, Asselin MC, Hammers A, Evans AC, Gunn RN. Positron emission tomography partial volume correction: estimation and algorithms. J Cereb Blood Flow Metab. 2002;22:1019–1034. doi: 10.1097/00004647-200208000-00014. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA.1993Manual for the Revised Beck Depression InventoryPsychological Corporation: San Antonio, TX.
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KF, Benkelfat C, Cherkasova MV, Baker GB, Dagher A, Leyton M.2013Reduced dopamine response to amphetamine in subjects at ultra-high risk for addiction Biol Psychiatrye-pub ahead of print 16 October 2013 doi: 10.1016/j.biopsych.2013.08.033 [DOI] [PubMed]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Conners CK, Erhart D, Sparrow E. Conners' Adult ADHD Rating Scales. Technical Manual. Multi-Health Systems: New York; 1999. [Google Scholar]
- Costes N, Dagher A, Larcher K, Evans AC, Collins DL, Reilhac A. Motion correction of multi-frame PET data in neuroreceptor mapping: Simulation based validation. Neuroimage. 2009;47:1496–1505. doi: 10.1016/j.neuroimage.2009.05.052. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME, et al. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci. 2013;33:452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher A. Addiction as aberrant learning-evidence from Parkinson's disease. Addiction. 2012;107:248–250. doi: 10.1111/j.1360-0443.2011.03624.x. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Dixon W, Yuen K. Trimming and winsorization: a review. Stat Papers. 1974;15:157–170. [Google Scholar]
- Ettinger U, Kumari V, Crawford TJ, Davis RE, Sharma T, Corr PJ. Reliability of smooth pursuit, fixation, and saccadic eye movements. Psychophysiology. 2003;40:1–9. doi: 10.1111/1469-8986.00063. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 2010;33:159–180. doi: 10.1016/j.psc.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research Department, New York State Psychiatric Institute: New York; 1996. [Google Scholar]
- Grace AA.2001Psychostimulant actions on dopamine and limbic system function: relevance to the pathophysiology and treatment of ADHDIn: Solanto M, Arnsten AF, Castellanos FX (eds)Stimulant Drugs and ADHD: Basic and Clinical Neuroscience Oxford University Press: New York; 134–157. [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Haber SN.2010Convergence of limbic, cognitive, and motor cortico-striatal circuits with dopamine pathways in the primate brainIn: Iversen L, Iversen SD, Dunnett SB, Bjorklund A (eds)Dopamine Handbook Oxford University Press: Oxford; 38–48. [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Zea-Ponce Y, Abi-Dargham A, Rodenhiser J, Wang T, Weiss R, et al. Stability of [123I]IBZM SPECT measurement of amphetamine-induced striatal dopamine release in humans. Synapse. 1999;31:302–308. doi: 10.1002/(SICI)1098-2396(19990315)31:4<302::AID-SYN9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4 (3 Pt 1:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Leyton M, aan het Rot M, Booij L, Baker GB, Young SN, Benkelfat C. Mood-elevating effects of d-amphetamine and incentive salience: the effect of acute dopamine precursor depletion. J Psychiatry Neurosci. 2007;32:129–136. [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Lou HC, Rosa P, Pryds O, Karrebaek H, Lunding J, Cumming P, et al. ADHD: increased dopamine receptor availability linked to attention deficit and low neonatal cerebral blood flow. Dev Med Child Neurol. 2004;46:179–183. doi: 10.1017/s0012162204000313. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Munro CA, McCaul ME, Wong DF, Oswald LM, Zhou Y, Brasic J, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the sate of the field and salient challenges for the coming decade. Biol Psychiatry. 2005;57:1424. doi: 10.1016/j.biopsych.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Pilgrim BM, Meyers JE, Bayless J, Whetstone MM. Validity of the Ward seven-subtest WAIS-III short form in a neuropsychological population. Appl Neuropsychol. 1999;6:243–246. doi: 10.1207/s15324826an0604_7. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Någren K, Syvälahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. Am J Psychiatry. 1998;155:768–773. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Willson VL, Clark PL. A four-test short-form of the WAIS-R for clinical screening. Clin Neuropsychol. 1983;5:111–116. [Google Scholar]
- Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, et al. Sex differences in amphetamine-induced displacement of [18F]fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry. 2006;163:1639–1641. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Lou HC, Cumming P, Pryds O, Karrebaek H, Lunding J, et al. Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention deficit hyperactivity disorder. Neuroimage. 2005;25:868. doi: 10.1016/j.neuroimage.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sontag TA, Tucha O, Walitza S, Lange KW. Animal models of attention deficit/hyperactivity disorder (ADHD): a critical review. Atten Defic Hyperact Disord. 2010;2:1–20. doi: 10.1007/s12402-010-0019-x. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Madras B, Faraone S, Dougherty D, Bonab A, et al. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: a focus on the dopamine transporter. Biol Psychiatry. 2005;57:1293–1300. doi: 10.1016/j.biopsych.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Harding M, O'Donnell D, Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adoles Psychiatry. 1996;35:409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Faraone SV, Madras BK, Bonab AA, Dougherty DD, et al. Functional genomics of attention-deficit/hyperactivity disorder (ADHD) risk alleles on dopamine transporter binding in ADHD and healthy control subjects. Biol Psychiatry. 2013;74:84–89. doi: 10.1016/j.biopsych.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi AC, Haber SN, Searle GE, Tsoumpas C, Long CJ, Shotbolt P, et al. 2013Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography Cereb Cortexe-pub ahead of print, 2 January 2013 doi: 10.1093/cercor/bhs397 [DOI] [PMC free article] [PubMed]
- Volkow ND, Wang G-J, Newcorn J, Fowler JS, Telang F, Solanto MV, et al. Brain dopamine transporter levels in treatment and drug naïve adults with ADHD. Neuroimage. 2007;34:1182. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.