Abstract
The acquired enamel pellicle (AEP) is important for minimizing the abrasion caused by parafunctional conditions as they occur, for instance, during bruxism. It is a remarkable feature of the AEP that a protein/peptide film can provide enough protection in normofunction to prevent teeth from abrasion and wear. Despite its obvious critical role in the protection of tooth surfaces, the essential adhesion features of AEP proteins on the enamel surface are poorly characterized. The objective of this study was to measure the adhesion force between histatin 5, a primary AEP component, and hydroxyapatite (HA) surfaces. Both biotinylated histatin 5 and biotinylated human serum albumin were allowed to adsorb to streptavidin-coated silica microspheres attached to atomic force microscope (AFM) cantilevers. A multimode AFM with a Nanoscope IIIa controller was used to measure the adhesion force between protein-functionalized silica microspheres attached to cantilever tips and the HA surface. The imaging was performed in tapping mode with a Si3N4 AFM cantilever, while the adhesion forces were measured in AFM contact mode. A collection of force-distance curves (~3,000/replicate) was obtained to generate histograms from which the adhesion forces between histatin 5 or albumin and the HA surface were measured. We found that histatin 5 exhibited stronger adhesion forces (90% >1.830 nN) to the HA surface than did albumin (90% > 0.282 nN). This study presents an objective approach to adhesion force measurements between histatin 5 and HA, and provides the experimental basis for measuring the same parameters for other AEP constituents. Such knowledge will help in the design of synthetic proteins and peptides with preventive and therapeutic benefits for tooth enamel.
Keywords: histatin, proteomics, atomic force microscopy, oral biofilm, mass spectrometry, saliva
Introduction
Histatins are a group of low-molecular-weight cationic salivary proteins that are secreted by the major and minor salivary glands (Oppenheim et al., 1986; Siqueira et al., 2008). Histatins possess a high affinity to hydroxyapatite (HA), resulting in their selective adherence to the enamel surface (Hay, 1975; Oppenheim et al., 1986, 1988; Jensen et al., 1992). As a result of being one of the first salivary proteins to adhere to enamel, they are among the proteins responsible for initiating the development of the acquired enamel pellicle (AEP), a protein integument formed in vivo as a result of the selective adsorption process to the enamel surface in the oral cavity (Siqueira et al., 2012).
It is important to develop an understanding of protein-surface interactions within the oral cavity, since many of the adsorbent proteins have important biological functions (Kambara and Norde, 1995). Understanding the adsorption of histatins, particularly histatin 5 (H5), is important because H5 exhibits antimicrobial activity against the opportunistic yeast and pathogenic bacteria present in the oral cavity (Pollock et al., 1984; Oppenheim et al., 1988; Gyurko et al., 2001). In addition, understanding the adhesive properties of proteins with high affinity for HA would facilitate the design of therapeutic treatments comprised of natural or synthetic proteins. Although histatins have been found to have high affinity to HA (Oppenheim et al., 1988; Jensen et al., 1992), the exact strength of adhesion forces under dynamic conditions between H5 and HA has not yet been established.
Atomic force microscopy (AFM) is a powerful tool used for imaging surfaces at nanometer scales. This is achieved by placing a pointed probe attached to a weak cantilever spring in contact with a sample surface and measuring the minute deflections of the cantilever as the probe is moved laterally along the surface (Binnig et al., 1986). Forces between the tip and the surface of the sample are responsible for causing the cantilever to deflect, and a detector measures the cantilever deflection as the tip scans the sample. A contact AFM image of the surface topography is typically obtained by mapping the vertical displacement of the sample required to maintain a constant force. AFM is also able to explore the structure-characterization of proteins at the molecular scale under physiological conditions (Hoh et al., 1991; Karrasch et al., 1993; Muller et al., 1995). This feature facilitates the study of conformational changes in proteins upon their adsorption (Holland and Marchant, 2000; Dufrêne, 2003; Agnihotri and Siedlecki, 2004; Toworfe et al., 2004; Toscano and Santore, 2006). Moreover, AFM has been used for the quantitative measurement of adhesive forces between an adsorbent and an adsorbate (Postollec et al., 2006; van der Mei et al., 2008; Wessel et al., 2014). This approach is accomplished by measuring the pull-off force needed to separate the AFM tip from the substrate surface, such as HA (Bowen et al., 1998; Fang et al., 2000; Pelin et al., 2012). This feature has been used to analyze adhesion between an AFM cantilever and a variety of micro-organisms (Fang et al., 2000; Vadillo-Rodriguez et al., 2004; Vadillo-Rodriguez and Logan, 2006). AFM, therefore, represents a promising tool for development of the fundamental information of adhesion forces between pellicle precursor proteins and enamel surfaces.
The major focus of this study was to measure the adhesion forces between a well-established AEP component (H5) and HA. Analysis of the data generated will form the foundation for AFM to be successfully used to advance our knowledge of forces at the molecular level of this important interface between the enamel surface and AEP proteins.
Materials & Methods
Biotinylation of Proteins
H5 (protein purity > 95%; GenScript, Piscataway, NJ, USA) and human serum albumin (Fisher Scientific, Rochester, NY, USA) were each re-suspended in water (final protein concentration of 100 µM). EZ-Link Sulfo-NHS-SS-Biotin (Pierce Biotechnology, Rockford, IL, USA) was brought to room temperature and dissolved in distilled water to a final concentration of 10 mM. Biotin was added to each protein solution at 20-fold molar excess, and the mixture was incubated at room temperature for 60 min. To remove excess biotin, the solution was passed through a de-salting spin column (3 kDa; Nanosep®, Pall Corporation, Port Washington, NY, USA) and centrifuged at 4,000 x g for 10 min. The remaining solution was collected, and the flow-through fluid in the column was discarded. This was repeated a total of 3 times to obtain a purified biotinylated protein sample, which was immediately placed on ice.
The HABA assay (4′-hydroxyazobenzene-2-carboxylic acid; Pierce Biotechnology, Rockford, IL, USA) was performed to quantify biotinylation and the level of biotin incorporation and to determine the molar ratio of biotin to protein. The biotinylated protein sample (either H5 or albumin) was added to a HABA/Avidin mixture, and the absorbance was read at 500 nm by means of a Bio-Rad iMark™ spectrophotometer (Bio-Rad Laboratories Inc, Hercules, CA, USA). To calculate the biotin/protein ratios, we entered the values of the absorbance readings of HABA/Avidin and biotinylated protein/HABA/Avidin, along with the molecular weight and concentration of each protein, into the HABA Calculator (Thermo Scientific, Pierce Biotechnology). The Beer Lambert Law (Beer’s Law) was applied using the known variables to provide an estimate of moles of biotin per mole of protein.
Customized Cantilever System
To obtain accurate adhesion force measurements between H5 and the HA surface, we developed a cantilever system consisting of a Si3N4 AFM cantilever (NP-S, Bruker AFM Probes, Camarillo, CA, USA) with an attached silica microsphere containing the biotinylated protein to be analyzed. Silica microspheres of ~5 µm diameter pre-conjugated with streptavidin (ProActive® Microspheres, Bangs Laboratories, Fishers, IN, USA) were acclimated to room temperature prior to use. A suspension of 0.5 mg/mL of microspheres was washed by vortexing for 20 sec, centrifuged at 1,200 x g for 15 min, and re-suspended in a 10x volume of phosphate buffered saline. The supernatant was discarded, and the washing step was repeated 3 times. The pellet obtained (containing microspheres) was re-suspended in a 100-µM solution of either histatin 5 or albumin biotinylated proteins. This was followed by incubation at room temperature for 30 min under gentle mixing. Following incubation, the microspheres were washed once again and then dried for 2 hr on silicon substrates.
Once dry, the microspheres were individually attached to the AFM cantilevers by means of an Araldite 10-min two-component epoxy resin (Araldite®, Huntsman Advanced Materials, Basel, Switzerland), as described in previous studies (Ong and Sokolov, 2007; Lee et al., 2009). For each cantilever system, the attachment of a single bead to the cantilever was confirmed by optical microscopy (400x magnification).
In addition, 6 cantilever systems were used for SEM microscopy (and not re-used for AFM measurements). They were secured on 12-mm SEM carbon adhesive tabs and platinum-coated (Denton Vacuum Desk II, Denton Vacuum Inc., Moorestown, NJ, USA). The cantilever system was then observed under scanning electron microscopy (SEM; LEO 1540XB Field Emission SEM, Carl Zeiss SMT AG, Oberkochen, Germany) at a detector beam energy of 3 kV to confirm that the microspheres were successfully attached to the AFM cantilever, and that the Araldite was not covering the microspheres.
Substrate Preparation
HA discs of 5-mm diameter and 2-mm thickness (Hitemco Medical, Old Bethpage, NY, USA) were used as the substrate. Their surfaces were polished with aluminum oxide papers (320-, 400-, and 600-grit) to standardize surface characteristics (SR) and to mimic the natural enamel surface in the oral cavity (Botta et al., 2009). Discs were then cleaned by sonication in distilled water 3 times for 20 min each to obtain clean discs for adhesion measurements.
Adhesion Measurements
In this study, we opted for using streptavidin-biotin conjugates, since the biochemical reaction between streptavidin and biotin provides a strong and stable link. Our preliminary experiments demonstrated that this bond is reliable for AFM measurements (data not shown). HA surfaces were imaged in a fluid cell containing distilled water in AFM contact mode with Si3N4 cantilevers. Adhesion between the cantilevers containing protein-functionalized silica microspheres and the HA surface was measured by force spectroscopy, in which the cantilever is brought into contact with, then retracted from, the sample surface. Following each adhesion measurement, the cantilever system was examined by optical microscopy to confirm that the microsphere remained attached to the cantilever.
Adhesion measurements to the HA surfaces were obtained for 2 treatments with microspheres functionalized with either biotinylated H5 or biotinylated albumin. The control was a microsphere pre-conjugated with streptavidin (containing no protein of interest). Adhesion measurements were made for each condition on 3 HA discs, with a new cantilever system used for each disc. The spring constant of each cantilever was calibrated by the thermal noise method. For each HA disc, adhesion measurements were obtained on 3 different 10-µm x 10-µm areas, each subdivided into a matrix of 32 × 32 points, for a total of 1,024 force-distance curves for each area. This provided about 3,000 force-distance curve acquisitions per disc.
Once all adhesion measurements were obtained, each force curve was examined by means of custom IGOR Pro macros (Wavemetrics, Lake Oswego, OR, USA). Erroneous force-distance curves were removed, and maximum adhesion forces were measured for the remainder. In total, close to 9,000 adhesion measurements for each treatment were plotted as histograms, depicting the range of adhesive forces (nN) measured between H5 or albumin and HA surfaces.
Results
Biotinylation results from the HABA assay indicate that the mole/mole ratio of biotin/protein was 0.73 for H5 and 0.71 for albumin, which demonstrated efficient biotinylation. SEM and AFM micrographs of the HA surfaces revealed a visually smooth surface for adhesion measurements (Fig. 1).
Figure 1.
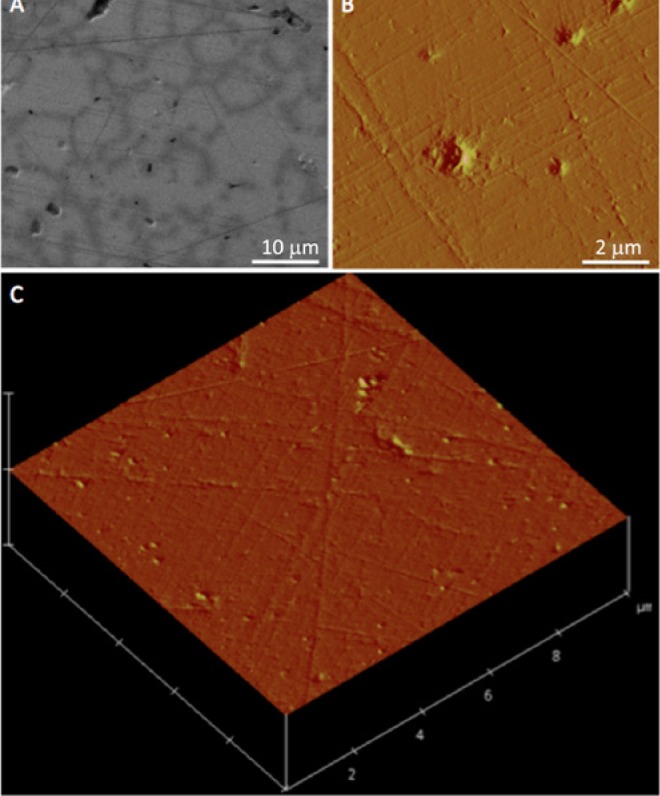
The surface topography of the HA disc, viewed by SEM, AFM, and AFM in a three-dimensional surface representation (A, B, C, respectively), reveals a smooth surface amenable for adhesion measurements.
SEM examination of the cantilever systems showed successful attachment of the microsphere to the cantilever (Fig. 2). In addition, SEM confirmed that the microsphere remained intact even following adhesion measurements (Appendix Fig.).
Figure 2.
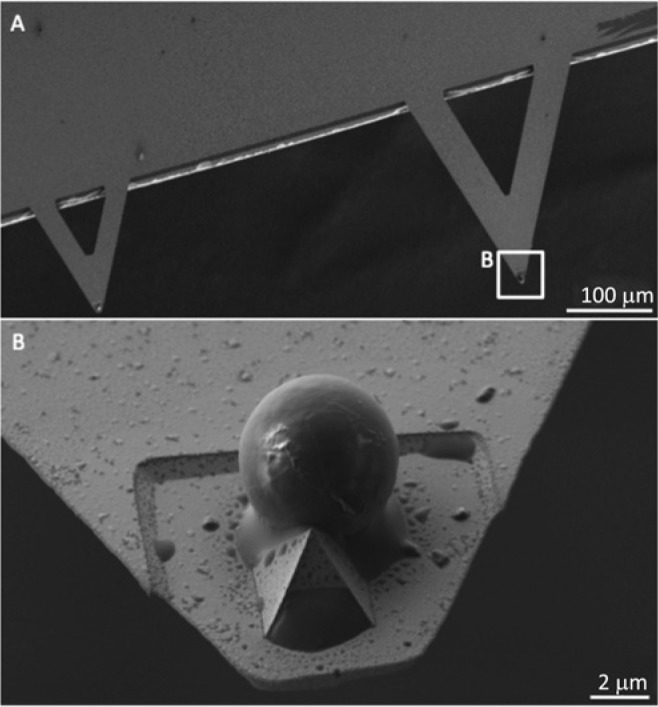
Customized AFM cantilever by a Silica microsphere.(A) Scanning electron micrograph of a customized AFM cantilever. (B) Silica microsphere (~5 µm) attached to the AFM cantilever tip by Araldite® epoxy resin.
As an example, a typical force-distance curve describing a single approach-retract cycle with an H5-functionalized AFM cantilever and a HA surface is illustrated in Fig. 3. As the AFM tip approached the sample (approach curve), the sample height (height between the cantilever tip and HA surface) decreased. When the AFM tip made contact with the HA surface, the cantilever deflected (left-hand side of Fig. 3). When the AFM tip retracted, the cantilever experienced negative forces due to adhesion (retraction curve). The tip eventually overcame the adhesive forces and lost contact with the surface. The adhesion force was measured as the maximum negative force achieved before the 2 surfaces were separated. A collection of force-distance curves (~9,000/replicate) was used to formulate histograms that yielded adhesion forces between the HA surface and H5, albumin, and control (Fig. 4). As can be seen, H5 exhibited stronger adhesion to the HA surface relative to albumin and the control. All of the histograms showed a non-Gaussian distribution. Therefore, the decision was made to characterize the results by determining the 90th percentile of the forces, demonstrating the force relative to which 90% of the non-zero adhesion measurements were higher. We found that this value was > 0.570 nN for the control (streptavidin), > 0.282 nN for albumin, and > 1.830 nN for H5. In addition, a median value for each tested condition was calculated. Values of 4 nN, 3 nN, and 8 nN were observed for control (streptavidin), albumin, and H5, respectively (Fig. 4).
Figure 3.
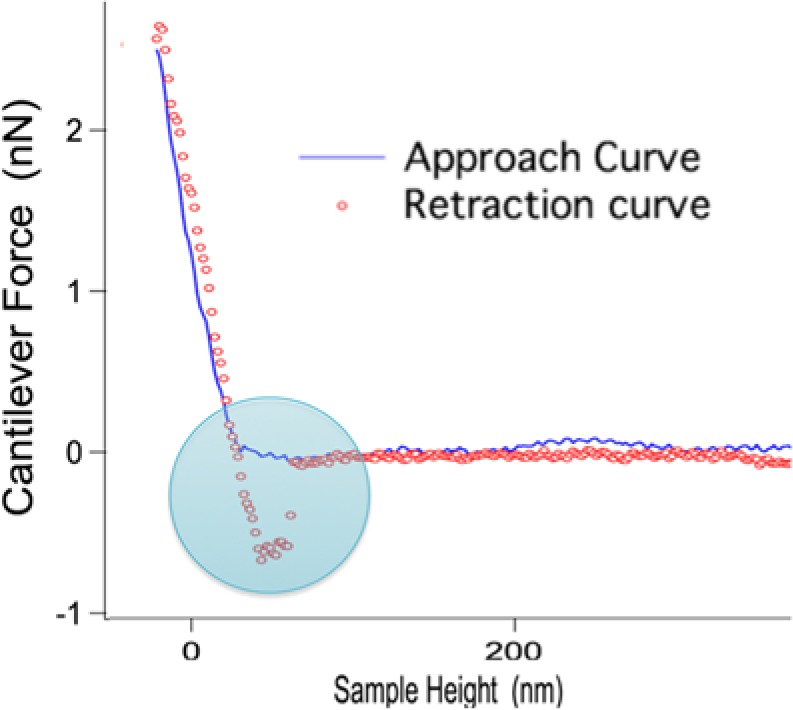
A typical force curve used to calculate the adhesion force between a tested protein-functionalized AFM tip and the HA surface. The blue line represents the approach of the AFM cantilever linked with specific salivary protein to the surface, while the red dotted line represents the retraction of the AFM cantilever linked with specific salivary protein from the surface. The circled area represents the adhesion force variation between the approach and retraction curves. Note: As the AFM tip approaches the sample (approach curve, blue line), the sample height (height between the cantilever tip and HA surface) decreases. The initial contact between the AFM tip and the surface results in the attraction of the tip toward the surface via, for example, van der Waals forces. When the AFM tip makes contact with the HA surface (with a constant force), there is an increase in force resulting in cantilever deflection (due to stiffness of the surface). During the retraction phase, the AFM tip retracts and tries to break contact with the surface (retraction curve, red line). Adhesion forces between the surface and AFM tip attempt to prevent the tip retraction, but the tip eventually overcomes the adhesive forces. The adhesion force measured is ultimately the force required to detach the AFM tip from the surface by quantifying the difference in the approach and retract curves at the point when the 2 surfaces are separated.
Figure 4.
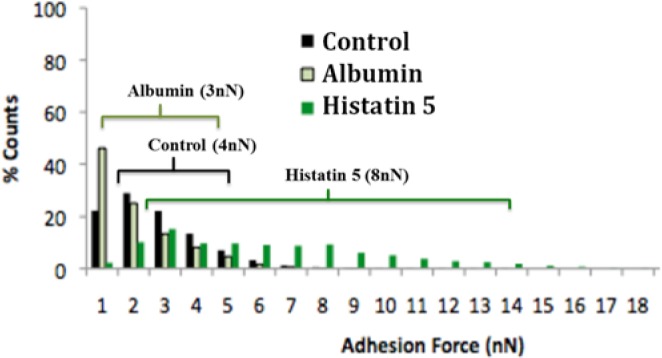
Distribution of adhesion forces between HA-albumin and HA-histatin 5 linked to the functionalized AFM tip. A control experiment with only streptavidin microsphere silica beads was also carried out. Each series incorporates ~9,000 force-distance curves acquired with 3 specimens. Horizontal lines demonstrate the range distribution of 90% for each condition. Median values are shown in parentheses.
Discussion
The composition, structure, and function of AEP represent a topic in oral biology that has gained significant interest in the past decade (Lendenmann et al., 2000; Hannig and Joiner, 2006; Garcia-Godoy and Hicks, 2008). Gaining an understanding of the adhesion forces of salivary proteins to enamel is key to the development of fundamental knowledge of the strength of these forces (Kambara and Norde, 1995). The experimental design of this study allowed us to gain insights with respect to the adhesion forces of H5 and albumin to HA. It can be anticipated that other pellicle proteins can affect the adhesion force of a particular protein because of the competition and synergism among all in vivo pellicle proteins during the dynamic event of pellicle formation. This is the first study to investigate adhesion forces between HA and a well-established in vivo pure pellicle protein. HA discs were used instead of enamel specimens, since HA discs provide a flat surface at the molecular level after being polished. The enamel surface, however, is not flat, and polishing could modify the surface energy and atomic composition. Analysis of our data demonstrated that the adhesion force between H5 and HA is stronger compared with that of the control (streptavidin-functionalized microspheres) and albumin. These results confirm that proteins with high affinity to HA, such as histatins, show strong molecular adhesive forces. Conversely, albumin is a salivary protein that does not exhibit high affinity for HA (Carlen et al., 1998), and thus does not demonstrate adhesion forces to HA as largely as those found for H5.
Recently, our group demonstrated that histatin 1 is able to maintain an intact structure after binding to HA, thus resisting proteolytic degradation within the oral cavity (McDonald et al., 2011). Since we found that a protein’s affinity to HA likely coincides with the strength of its molecular-scale adhesion properties, investigating how to modify or enhance the adhesive forces of salivary proteins (natural or synthetic) to inhibit their degradation in the oral cavity could potentially be used to develop novel therapeutic strategies that focus on the clinical application of salivary proteins and their derivatives.
There were 2 reasons to select H5 for this study. First, H5 is an established in vivo pellicle protein, and second, H5 is a small protein falling more into the size range of the peptides typically present in in vivo-formed AEP. The larger pellicle precursor proteins are mostly represented by fragments derived from their native structures (Siqueira and Oppenheim, 2009; Siqueira et al., 2010). Furthermore, many studies indicate that H5 exhibits potent antifungal activity against the pathogenic Candida albicans, which is the primary pathogen responsible for initiating oral candidiasis (Gyurko et al., 2000; Pusateri et al., 2009). Relevant to the adhesion of H5 is a recent discovery showing that this protein retains its antifungal effect against C. albicans after adsorption to HA (Vukosavljevic et al., 2012). Histatins and some other salivary proteins are well-known for their antibacterial functions. This raises the possibility for investigation of the benefits of augmentation of adhesive properties of salivary-protein-derived antimicrobial peptides against pathogenic oral micro-organisms. The development of a protein/peptide that exhibits antimicrobial properties and at the same time has superior adhesive properties to HA, could lead to the means of increasing therapeutic effects.
Adhesion forces between the protein of interest and a substrate can be used as an important quantitative measurement to evaluate the adsorption of proteins to a specific surface. It is important to develop an understanding of adhesion forces of physiologically important salivary proteins (i.e., H5, statherin, aPRP) to various oral surfaces. Once this knowledge is obtained, studies should be designed to understand factors that control the strength of these adhesive forces to surfaces within the oral cavity.
In conclusion, this study provided a measurement for the adhesion force between H5 and HA. This type of information can possibly be exploited to develop stable, protease-resistant, synthetic peptides for therapeutic applications against various oral diseases such as dental caries, periodontal disease, and fungal infections. In addition, this study demonstrates an experimental approach useful for the measurement of adhesion forces between a particular protein/peptide and, potentially, other oral surfaces, representing a methodology that can be applied to the understanding of the adsorption dynamics that is critical at the interphase between enamel and AEP.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
The authors acknowledge funding support from: the National Institutes of Health, National Institute of Dental and Craniofacial Research, and the National Institute of Allergy and Infectious Diseases (grants DE05672, DE07652, AI087803, and AI101067); the Natural Sciences and Engineering Research Council of Canada (NSERC grant #371813); and the Canadian Institutes of Health Research (CIHR grant #106657 and grant #97577). WLS is the recipient of a CIHR New Investigator Award (grant #113166).
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this manuscript.
References
- Agnihotri A, Siedlecki CA. (2004). Time-dependent conformational changes in fibrinogen measured by atomic force microscopy. Langmuir 20:8846-8852. [DOI] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. (1986). Atomic force microscope. Phys Rev Lett 56:930-933. [DOI] [PubMed] [Google Scholar]
- Botta AC, Duarte S, Jr, Paulin Filho PI, Gheno SM, Powers JM. (2009). Surface roughness of enamel and four resin composites. Am J Dent 22:252-254. [PubMed] [Google Scholar]
- Bowen WR, Hilal N, Lovitt RW, Wright CJ. (1998). Direct measurement of interactions between adsorbed protein layers using an atomic force microscope. J Colloid Interface Sci 197:348-352. [DOI] [PubMed] [Google Scholar]
- Carlen A, Bratt P, Stenudd C, Olsson J, Stromberg N. (1998). Agglutinin and acidic proline-rich protein receptor patterns May modulate bacterial adherence and colonization on tooth surfaces. J Dent Res 77:81-90. [DOI] [PubMed] [Google Scholar]
- Dufrêne YF. (2003). Recent progress in the application of atomic force microscopy imaging and force spectroscopy to microbiology. Curr Opin Microbiol 6:317-323. [DOI] [PubMed] [Google Scholar]
- Fang HH, Chan KY, Xu LC. (2000). Quantification of bacterial adhesion forces using atomic force microscopy (AFM). J Microbiol Methods 40:89-97. [DOI] [PubMed] [Google Scholar]
- Garcia-Godoy F, Hicks MJ. (2008). Maintaining the integrity of the enamel surface: the role of dental biofilm, saliva and preventive agents in enamel demineralization and remineralization. J Am Dent Assoc 139(Suppl):25S-34S. [DOI] [PubMed] [Google Scholar]
- Gyurko C, Lendenmann U, Troxler RF, Oppenheim FG. (2000). Candida albicans mutants deficient in respiration are resistant to the small cationic salivary antimicrobial peptide histatin 5. Antimicrob Agents Chemother 44:348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurko C, Lendenmann U, Helmerhorst EJ, Troxler RF, Oppenheim FG. (2001). Killing of Candida albicans by histatin 5: cellular uptake and energy requirement. Antonie van Leeuwenhoek 79:297-309. [DOI] [PubMed] [Google Scholar]
- Hannig M, Joiner A. (2006). The structure, function and properties of the acquired pellicle. Monogr Oral Sci 19:29-64. [DOI] [PubMed] [Google Scholar]
- Hay DI. (1975). Fractionation of human parotid salivary proteins and the isolation of an histidine-rich acidic peptide which shows high affinity for hydroxyapatite surfaces. Arch Oral Biol 20:553-558. [DOI] [PubMed] [Google Scholar]
- Hoh JH, Lal R, John SA, Revel JP, Arnsdorf MF. (1991). Atomic force microscopy and dissection of gap junctions. Science 253:1405-1408. [DOI] [PubMed] [Google Scholar]
- Holland NB, Marchant RE. (2000). Individual plasma proteins detected on rough biomaterials by phase imaging AFM. J Biomed Mater Res 51:307-315. [DOI] [PubMed] [Google Scholar]
- Jensen JL, Lamkin MS, Oppenheim FG. (1992). Adsorption of human salivary proteins to hydroxyapatite: a comparison between whole saliva and glandular salivary secretions. J Dent Res 71:1569-1576. [DOI] [PubMed] [Google Scholar]
- Kambara M, Norde W. (1995). Influence of fluoride applications on some physicochemical surface properties of synthetic hydroxyapatite and human dental enamel and its consequences for protein adsorption. Caries Res 29:210-217. [DOI] [PubMed] [Google Scholar]
- Karrasch S, Dolder M, Schabert F, Ramsden J, Engel A. (1993). Covalent binding of biological samples to solid supports for scanning probe microscopy in buffer solution. Biophys J 65:2437-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Nakaya K, Hayashi T, Hara M. (2009). Quantitative study of the gold-enhanced fluorescence of CdSe/ZnS nanocrystals as a function of distance using an AFM probe. Phys Chem Chem Phys 11:4403-4409. [DOI] [PubMed] [Google Scholar]
- Lendenmann U, Grogan J, Oppenheim FG. (2000). Saliva and dental pellicle—a review. Adv Dent Res 14:22-28. [DOI] [PubMed] [Google Scholar]
- McDonald EE, Goldberg HA, Tabbara N, Mendes FM, Siqueira WL. (2011). Histatin 1 resists proteolytic degradation when adsorbed to hydroxyapatite. J Dent Res 90:268-272. [DOI] [PubMed] [Google Scholar]
- Muller DJ, Schabert FA, Buldt G, Engel A. (1995). Imaging purple membranes in aqueous solutions at sub-nanometer resolution by atomic force microscopy. Biophys J 68:1681-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong QK, Sokolov I. (2007). Attachment of nanoparticles to the AFM tips for direct measurements of interaction between a single nanoparticle and surfaces. J Colloid Interface Sci 310:385-390. [DOI] [PubMed] [Google Scholar]
- Oppenheim FG, Yang YC, Diamond RD, Hyslop D, Offner GD, Troxler RF. (1986). The primary structure and functional characterization of the neutral histidine-rich polypeptide from human parotid secretion. J Biol Chem 261:1177-1182. [PubMed] [Google Scholar]
- Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, et al. (1988). Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem 263:7472-7477. [PubMed] [Google Scholar]
- Pelin IM, Piednoir A, Machon D, Farge P, Pirat C, Ramos SM. (2012). Adhesion forces between AFM tips and superficial dentin surfaces. J Colloid Interface Sci 376:262-268. [DOI] [PubMed] [Google Scholar]
- Pollock JJ, Denepitiya L, MacKay BJ, Iacono VJ. (1984). Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect Immun 44:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postollec F, Norde W, de Vries J, Busscher HJ, van der Mei HC. (2006). Interactive forces between co-aggregating and non-co-aggregating oral bacterial pairs. J Dent Res 85:231-234. [DOI] [PubMed] [Google Scholar]
- Pusateri CR, Monaco EA, Edgerton M. (2009). Sensitivity of Candida albicans biofilm cells grown on denture acrylic to antifungal proteins and chlorhexidine. Arch Oral Biol 54:588-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira WL, Salih E, Wan DL, Helmerhorst EJ, Oppenheim FG. (2008). Proteome of human minor salivary gland secretion. J Dent Res 87:445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira WL, Oppenheim FG. (2009). Small molecular weight proteins/peptides present in the in vivo formed human acquired enamel pellicle. Arch Oral Biol 54:437-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira WL, Margolis HC, Helmerhorst EJ, Mendes FM, Oppenheim FG. (2010). Evidence of intact histatins in the in vivo acquired enamel pellicle. J Dent Res 89:626-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siqueira WL, Custodio W, McDonald EE. (2012). New insights into the composition and functions of the acquired enamel pellicle. J Dent Res 91:1110-1118. [DOI] [PubMed] [Google Scholar]
- Toscano A, Santore MM. (2006). Fibrinogen adsorption on three silica-based surfaces: conformation and kinetics. Langmuir 22:2588-2597. [DOI] [PubMed] [Google Scholar]
- Toworfe GK, Composto RJ, Adams CS, Shapiro IM, Ducheyne P. (2004). Fibronectin adsorption on surface-activated poly(dimethylsiloxane) and its effect on cellular function. J Biomed Mater Res Part A 71:449-461. [DOI] [PubMed] [Google Scholar]
- Vadillo-Rodriguez V, Logan BE. (2006). Localized attraction correlates with bacterial adhesion to glass and metal oxide substrata. Environ Sci Technol 40:2983-2988. [DOI] [PubMed] [Google Scholar]
- Vadillo-Rodriguez V, Busscher HJ, Norde W, de Vries J, van der Mei HC. (2004). Atomic force microscopic corroboration of bond aging for adhesion of Streptococcus thermophilus to solid substrata. J Colloid Interface Sci 278:251-254. [DOI] [PubMed] [Google Scholar]
- van der Mei HC, Rustema-Abbing M, de Vries J, Busscher HJ. (2008). Bond strengthening in oral bacterial adhesion to salivary conditioning films. Appl Environ Microbiol 74:5511-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukosavljevic D, Custodio W, Del Bel Cury AA, Siqueira WL. (2012). The effect of histatin 5, adsorbed on PMMA and hydroxyapatite, on Candida albicans colonization. Yeast 29:459-466. [DOI] [PubMed] [Google Scholar]
- Wessel SW, Chen Y, Maitra A, van den Heuvel ER, Slomp AM, Busscher HJ, et al. (2014). Adhesion forces and composition of planktonic and adhering oral microbiomes. J Dent Res 93:84-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


